This randomized clinical trial examines the effects of a pharmacist-led medication management intervention with a social media component on improving medication adherence in adults with chronic heart failure in China.
Key Points
Question
Can a pharmacist-led management model improve medication adherence among patients with chronic heart failure (CHF) compared with a conventional management model?
Findings
In this randomized clinical trial involving 445 patients with CHF in China, patients who were assigned to a pharmacist-led management intervention showed modest improvement in medication adherence at 52 weeks compared with patients assigned to usual care.
Meaning
The findings indicate that a pharmacist-led management model is effective for improving medication adherence among patients with CHF.
Abstract
Importance
Poor medication adherence is associated with high morbidity and mortality among patients with chronic heart failure (CHF), which is particularly concerning in China.
Objective
To assess the effect of a pharmacist-led management model incorporating a social media platform vs usual care on medication adherence in patients with CHF.
Design, Setting, and Participants
This prospective, multicenter randomized clinical trial was conducted from March 2021 to May 2023, with a follow-up duration of 52 weeks. The trial was conducted in the cardiology wards of 5 hospitals in China. Participants were 18 years or older, had a CHF diagnosis, and were receiving stable medication. They were randomly assigned to either the intervention group (pharmacist-led management) or the control group (usual care) in a 1:1 ratio using a computer-generated random number table with concealed allocation via opaque envelopes. Intention-to-treat data analysis was performed from June 2023 to July 2024.
Intervention
The intervention group received a multimodal pharmaceutical intervention, including WeChat application–based communication and education, and a standardized follow-up visit from a pharmacist every month. The control group received the standardized follow-up visit from nurses every month.
Main Outcomes and Measures
The primary outcome was the proportion of days covered (PDC) by heart failure medication at 52 weeks.
Results
Among the 445 participants analyzed, 223 were assigned to the intervention group and 222 to the control group. These patients had a mean (SD) age of 63.2 (13.3) years and included 263 males (59.1%). A total of 333 patients (74.8%) had a New York Heart Association class III or IV heart failure, indicating severe limitations in physical activity. At 52 weeks, the intervention group had a significantly higher PDC for heart failure medication (8.1%; 95% CI, 5.5%-10.7%; P < .001) and a greater proportion of patients with PDC of 80% or greater (odds ratio, 0.34; 95% CI, 0.21-0.54; P < .001) compared with the control group.
Conclusions and Relevance
This randomized clinical trial found a modest improvement in medication adherence among patients with CHF who received the pharmacist-led management intervention vs usual care.
Trial Registration
Chinese Clinical Trial Registry Identifier: ChiCTR2000040232
Introduction
Chronic heart failure (CHF) is a complex clinical syndrome that affects approximately 37.7 million people and is a leading cause of morbidity and mortality worldwide.1,2,3 There are approximately 13.7 million adults with CHF in China.4 The standardized prevalence rates of heart failure are 0.57%, 3.86%, and 7.55% among individuals aged 25 to 64 years, 65 to 79 years, and 80 years or older, respectively.5 The case fatality rate of hospitalized patients with heart failure is approximately 4.1%.6 Thus, CHF places a heavy burden on individuals, families, and the health system.4,5,6,7 Guideline-directed medical therapy is the preferred and most effective way to reduce morbidity and mortality in patients with CHF. This treatment can improve the ejection fraction and quality of life.8
Poor medication adherence is an important factor in poor outcomes (eg, rehospitalization) in patients with CHF.9,10,11,12 In addition to needing guideline-recommended medications, patients with CHF often need medications for comorbidities and complications, increasing the risk of drug-related problems, such as drug interactions, adverse drug reactions, and poor compliance.13,14,15 A number of pharmacist-led or drug-based intervention studies on patients with CHF have shown that clinical pharmacists improve medication compliance, efficacy, and safety.16,17,18,19 To our knowledge, no studies have been reported on the effects of the pharmacist-led management model on medication adherence and efficacy among Chinese patients with CHF.
Instant messaging applications have gained popularity, with one such application in China reporting more than 1.2 billion monthly active users.20 This popular application is characterized by timeliness and convenience. It is simple to use and thus is not limited by age or cognitive behavior, making it suitable for health interventions in China.21,22,23,24 This messaging application has been used in studies on disease and health management in patients with chronic conditions, including AIDS and coronary heart disease, and has demonstrated good results. However, the its effectiveness in the management of patients with CHF needs to be verified.25,26,27 Therefore, we conducted a randomized clinical trial to assess the effect of a pharmacist-led management model incorporating a social media platform vs usual care on medication adherence in patients with CHF.
Methods
Design and Participants
This prospective, multicenter randomized clinical trial was conducted from March 2021 to May 2023 at the First Hospital of Hebei Medical University, the First Hospital of Handan, Cangzhou Central Hospital, the Fourth Hospital of Handan, and Hengshui People’s Hospital in China. The study was approved by the corresponding ethics committees at these hospitals. All participants provided written informed consent. We adhered to the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline. The trial protocol and statistical analysis plan are provided in Supplement 1.
We included patients with CHF who were admitted to one of the hospitals between March 2021 and April 2022. Inclusion criteria were (1) age 18 years or older; (2) a confirmed CHF diagnosis based on heart failure symptoms and signs, echocardiographic evidence of cardiac structural or functional abnormalities, and increased natriuretic peptide levels; (3) a stable heart failure medication regimen, indicating no acute heart failure exacerbation or medication adjustments in the week before study enrollment; and (4) the ability to understand and provide signed informed consent.
Procedures
After collecting baseline data, we categorized eligible patients with CHF into 3 groups based on left ventricular ejection fraction (LVEF): reduced LVEF (<40%), midrange LVEF (40%-49%), and preserved LVEF (≥50%). Following stratification, patients were randomly assigned to the intervention group (pharmacist-led management) or the control group (usual care) in a 1:1 ratio (Figure 1). The randomization process used a computer-generated random number table and was concealed using opaque envelopes. Although the study was open label for researchers and patients, assessors were blinded to the treatment assignments.
Figure 1. Flow Diagram of Study Participants.
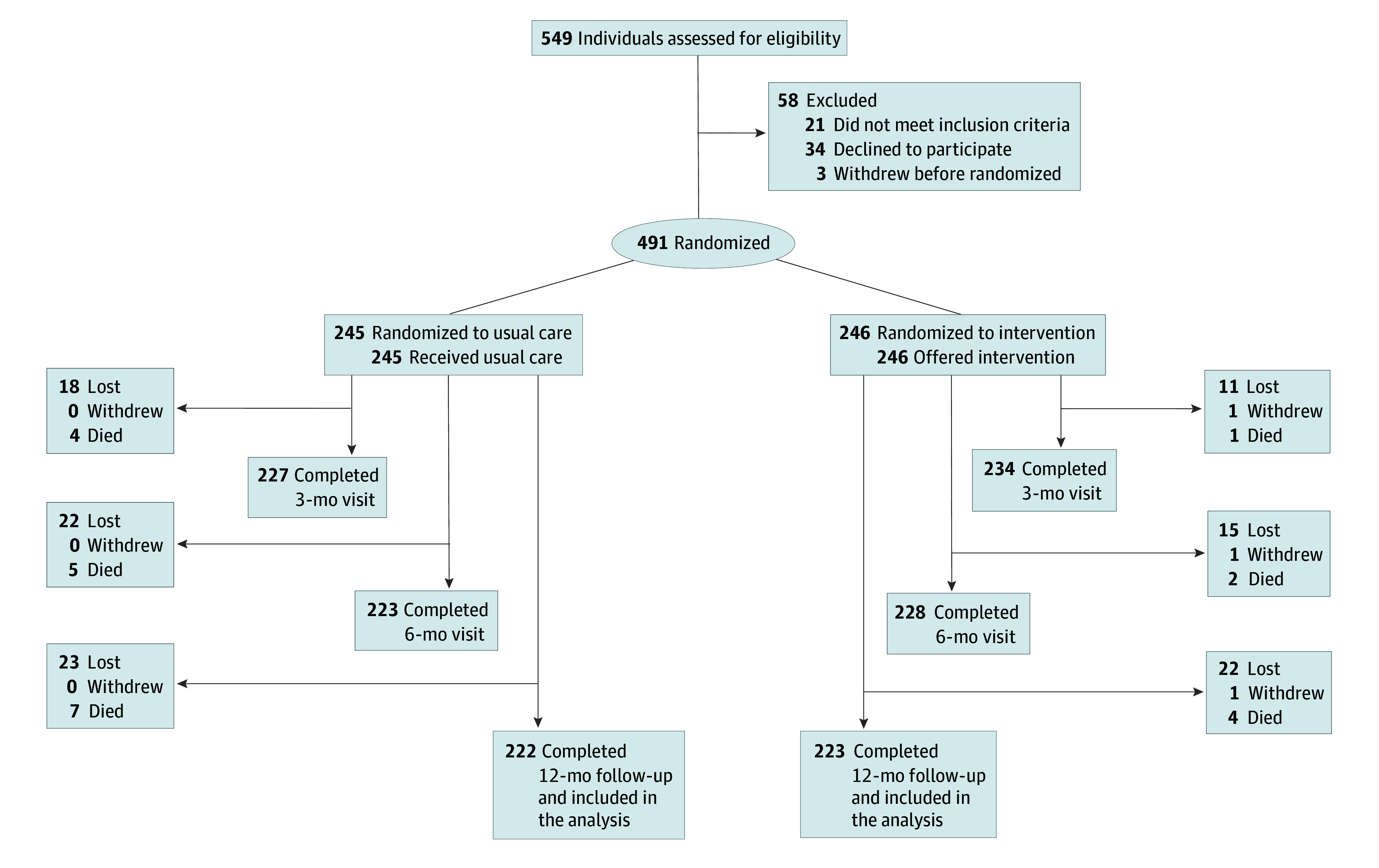
A total of 445 participants were allocated for analysis.
Usual Care and Intervention
Patients assigned to the control group underwent follow-up by nurses. The attending physicians prescribed relevant tests based on each patient’s clinical symptoms, adjusted the medication regimen, and issued a medication prescription for 4 weeks (plus or minus 7 days). Nurses reviewed the patients’ medication diaries, compared the diaries with the prescriptions, and inquired about any adverse events and new medications. The nurses instructed the patients to record each medication taken in their medication diary and scheduled the next follow-up visit.
For patients assigned to the intervention group, pharmacists conducted follow-up visits in accordance with the standardized procedures used by nurses for the control group along with the following additional components. Pharmacists (1) added the patient or their caregiver as a WeChat (Tencent Holdings Ltd) friend and created a group in the messaging application; (2) reminded patients to take their medication weekly via this application and responded to medication or disease-related inquiries; (3) provided patient education based on medication adherence, including introduction to misused, missed, newly added, or adjusted medications and information on the drug’s effects, necessity, dose and administration, possible adverse events, handling methods, and drug interactions; and (4) discussed any necessary adjustments to the medication regimen with the attending physician.
If a patient was hospitalized during the follow-up period, the management for both the intervention and control groups was the same. The attending physician determined whether the hospitalization was related to heart failure or other adverse events and continued with follow-up visits according to the standardized procedures.
Outcomes
Follow-up results were evaluated over a period of 52 weeks. The primary end point was the proportion of days covered (PDC) for heart failure medications at 52 weeks. The secondary end points were the proportion of patients with a PDC of 80% or greater at 52 weeks for heart failure medications and for 4 major drug classes: β-blockers, diuretics, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers (ACEIs or ARBs), and mineralocorticoid receptor antagonists (MRAs). We further analyzed the Morisky score (range: 0-8, with the highest score indicating the highest medication adherence), the Minnesota Living with Heart Failure Questionnaire (MLHFQ) score (range: 0-105, with the highest score indicating lowest quality of life), readmissions, composite end points of all-cause mortality, and days lost due to unplanned cardiovascular hospitalizations or death. The primary safety end point was the incidence of adverse drug events (ADEs).
Participants were thoroughly questioned about ADEs during each follow-up visit. Nurses and pharmacists collected information on ADEs from various sources, including verbal reports from patients or their families, log card records, medical records, and the national adverse drug reaction database, and then recorded this information in the data collection system for assessment by our research team. All recording and assessments of ADEs were conducted according to the standardized procedures for drug adverse reaction monitoring established by the State Administration for Market Regulation of China. Death data were obtained from hospital medical records, death certificates, household cancellation certificates, or cremation certificates provided by families as well as from the Chinese Centers for Disease Control and Prevention.
At the outset of the trial, we established a Data Management Committee composed of experts in the fields of pharmacy, clinical practice, and statistics. At the midpoint and conclusion of the trial, the Data Management Committee conducted a quality control audit of the data related to ADEs, mortality cases, and pharmacist interventions.
Sample Size Calculation
In a previous study,16 the overall SD of PDC for heart failure medications at 52 weeks was 14.9%. Assuming a mean (SD) difference of 4.0 (0.2) between the intervention and control groups and assuming α = .05 and β = 0.20, respectively, 218 cases were required per group. Assuming an estimated attrition rate of 10%, each group required 243 cases, for a total of 486 cases.
Statistical Analysis
To assess the balance between the control and intervention groups after randomization, we descriptively compared the baseline characteristics using 2-sample t tests, χ2 tests, Fisher exact tests, or Wilcoxon rank sum tests. Normally distributed continuous variables were described with the mean (SD), whereas nonnormally distributed variables were described using the median (IQR). Categorical variables were described using counts (percentages). To compare results between the intervention and control groups, independent-sample t tests or Wilcoxon rank sum tests were used for continuous variables. To control the type I error that may be introduced by multiple comparisons, we applied the Bonferroni correction method to all secondary end points. For categorical variables, χ2 tests or exact probability tests were used. Nelson-Aalen curves were plotted to assess the composite end point of heart failure hospitalization and mortality; differences between groups were compared using a log-rank test. P < .05 indicated statistical significance.
Subgroup analyses of the primary and secondary efficacy end points were performed to assess the consistency of intervention effects across the following subgroups: age (<75 vs ≥75 years), sex, heart rate at baseline (≤75 vs >75 bpm), New York Heart Association (NYHA) class (I or II [indicating mild limitations in physical activity] vs III or IV [indicating severe limitations in physical activity]), LVEF (<40% vs ≥40%), history of diabetes, level of disease burden (number of different medications at baseline), baseline adherence, and quality of life at baseline.
Intention-to-treat data analysis was performed from June 2023 to July 2024. All analyses used R, version 4.0.3 (R Project for Statistical Computing).
Results
Figure 1 depicts the flow of participants through the trial. A total of 549 patients were recruited, of whom 491 met the inclusion criteria and were randomized. After withdrawals, deaths, and loss to follow-up, 445 participants were analyzed, of whom 223 were assigned to the intervention group (received pharmacist-led management) and 222 to the control group (received usual care). All baseline characteristics were well balanced between the 2 groups (Table 1). Patients had a mean (SD) age of 63.2 (13.3) years and included 263 males (59.1%) and 182 females (40.9%). Among these patients, 333 (74.8%) had an NYHA class III or IV heart failure. Patients with heart failure often have multiple comorbidities. In this study, 246 patients (55.3%) had hypertension and 202 patients (45.4%) had coronary artery disease. For both groups, the median (IQR) Morisky score was 5.5 (4.0-7.8), and the median (IQR) MLHFQ score was 43.0 (36.0-54.8) in the entire sample.
Table 1. Baseline Characteristics of Participants.
| Characteristic | Patients, No. (%) | ||
|---|---|---|---|
| All (N = 445) | Intervention group (n = 223) | Control group (n = 222) | |
| Age, mean (SD), y | 63.2 (13.3) | 62.0 (13.30) | 64.3 (13.1) |
| Sex | |||
| Male | 263 (59.1) | 129 (57.8) | 134 (60.4) |
| Female | 182 (40.9) | 94 (42.2) | 88 (39.6) |
| BMI, mean (SD) | 23.5 (6.6) | 23.6 (6.9) | 23.4 (6.3) |
| SBP, mean (SD), mm Hg | 128.3 (23.9) | 126.8 (24.4) | 129.8 (23.3) |
| DBP, mean (SD), mm Hg | 81.5 (33.6) | 83.0 (44.9) | 80.0 (15.8) |
| Heart rate, mean (SD), bpm | 81.8 (19.8) | 82.3 (21.5) | 81.3 (18.0) |
| Smoking status | |||
| No smoking | 323 (72.6) | 159 (71.3) | 164 (73.9) |
| Current smoking | 63 (14.2) | 34 (15.2) | 29 (13.1) |
| Quit smoking | 57 (12.8) | 28 (12.6) | 29 (13.1) |
| Drinking status | |||
| No drinking | 349 (78.4) | 174 (78) | 175 (78.8) |
| Current drinking | 40 (9) | 21 (9.4) | 19 (8.6) |
| Quit drinking | 53 (11.9) | 26 (11.7) | 27 (12.2) |
| NYHA classa | |||
| I | 7 (1.6) | 4 (1.8) | 3 (1.4) |
| II | 32 (7.2) | 16 (7.2) | 16 (7.2) |
| III | 124 (27.9) | 67 (30.0) | 57 (25.7) |
| IV | 209 (47.0) | 99 (44.4) | 110 (49.5) |
| LVEF, % | |||
| <40 | 183 (41.1) | 91 (40.8) | 92 (41.4) |
| 40-49 | 114 (25.6) | 58 (26.0) | 56 (25.2) |
| ≥50 | 148 (33.3) | 74 (33.2) | 74 (33.3) |
| Medical history | |||
| Valvular disease | 42 (9.4) | 22 (9.9) | 20 (9.0) |
| Hypertension | 246 (55.3) | 124 (55.6) | 122 (55.0) |
| Hyperlipidemia | 22 (4.9) | 10 (4.5) | 12 (5.4) |
| CAD | 202 (45.4) | 97 (43.5) | 105 (47.3) |
| Diabetes | 97 (21.8) | 53 (23.8) | 44 (19.8) |
| Cardiomyopathy | 61 (13.7) | 32 (14.3) | 29 (13.1) |
| AMI | 17 (3.8) | 7 (3.1) | 10 (4.5) |
| Arrhythmia cordis | 97 (21.8) | 44 (19.7) | 53 (23.9) |
| TIA | 42 (9.4) | 20 (9.0) | 22 (9.9) |
| Medication | |||
| ACEI or ARB | 114 (25.6) | 62 (27.8) | 52 (23.4) |
| Diuretics | 367 (82.5) | 180 (80.7) | 187 (84.2) |
| β-blockers | 368 (82.7) | 184 (82.5) | 184 (82.9) |
| MRA | 374 (84.0) | 190 (85.2) | 184 (82.9) |
| ARNI | 245 (55.1) | 127 (57.0) | 118 (53.2) |
| SGLT-2i | 109 (24.5) | 53 (23.8) | 56 (25.2) |
| Lipid-modifying agent | 296 (66.5) | 150 (67.3) | 146 (65.8) |
| Cardiac glycoside | 175 (39.3) | 89 (39.9) | 86 (38.7) |
| Antidepressant | 66 (14.8) | 32 (14.3) | 34 (15.3) |
| Antithrombotic agent | 378 (84.9) | 190 (85.2) | 188 (84.7) |
| Oral antidiabetic or insulin | 167 (37.5) | 89 (39.9) | 78 (35.1) |
| Other medications | 394 (88.5) | 204 (91.5) | 190 (85.6) |
| Laboratory measurements | |||
| ALT, median (IQR), U/L | 22.0 (14.0-36.4) | 21.0 (13.9-40.8) | 22.0 (14.0-33.2) |
| AST, median (IQR), U/L | 24.0 (17.2-33.8) | 24.0 (17.0-34.0) | 24.0 (17.6-33.3) |
| Serum creatinine, median (IQR), mg/dL | 0.9 (0.7-1.1) | 0.9 (0.7-1.1) | 0.9 (0.8-1.1) |
| BUN, median (IQR), mg/dL | 18.2 (14.6-24.4) | 18.2 (14.3-23.0) | 18.2 (14.6-25.2) |
| LDL cholesterol, median (IQR), mg/dL | 96.5 (73.4-119.7) | 96.5 (77.2-119.7) | 92.7 (73.4-119.7) |
| LVEDD, median (IQR), mm | 54.0 (49.0-63.0) | 54.0 (49.0-63.0) | 54.0 (49.0-63.0) |
| LVESD, median (IQR), mm | 39.0 (32.0-53.0) | 40.0 (32.0-55.0) | 38.5 (32.0-51.0) |
| LVEF, median (IQR), % | 44.0 (33.3-54.8) | 44.0 (33.8-52.3) | 44.0 (33.0-55.0) |
| NT-proBNP, median (IQR), pg/mL | 2563.5 (954.8-6558.1) | 2516.5 (1068.2-6676.8) | 2668.2 (714.5-6141.0) |
| BNP, median (IQR), pg/mL | 379.7 (118.8-891.0) | 416.0 (124.0-951.6) | 374.4 (98.5-851.0) |
| Morisky scores, median (IQR) | 5.5 (4.0-7.8) | 5.5 (4.0-7.8) | 5.5 (4.0-8.0) |
| MLHFQ scores, median (IQR) | 43.0 (36.0-54.8) | 42.0 (36.0-53.0) | 44.0 (36.8-55.0) |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ALT, alanine aminotransferase; AMI, acute myocardial infarction; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibition; AST, aspartate aminotransferase; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BNP, B-type natriuretic peptide; bpm, beats per minute; BUN, serum urea nitrogen; CAD, coronary artery disease; DBP, diastolic blood pressure; LDL, low-density lipoprotein; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; LVEF, left ventricular ejection fraction; MLHFQ, Minnesota Living with Heart Failure Questionnaire (score range: 0-105, with the highest score indicating lowest quality of life); MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association (class I or II [indicating mild limitations in physical activity] vs III or IV [indicating severe limitations in physical activity]); NT-proBNP, N-terminal pro–B-type natriuretic peptide; SBP, systolic blood pressure; SGLT-2i, sodium-glucose cotransport protein 2 inhibitors; TIA, transient ischemic attack.
SI conversion factors: To convert ALT and AST to microkatal per liter, multiply by 0.0167; BNP to nanogram per liter, multiply by 1.0; BUN to millimoles per liter, multiply by 0.357; LDL cholesterol to millimoles per liter, multiply by 0.0259; serum creatinine to micromoles per liter, multiply by 88.4.
Values may not sum due to missing data. Baseline data were extracted from the medical records; however, this classification field is not required to be completed by physicians in China.
In this study, the calculation of PDC followed the steps outlined in previous research.16 Compared with the control group, the intervention group showed significant improvement in PDC for heart failure treatment at 52 weeks (8.1%; 95% CI, 5.5%-10.7%; P < .001) (Table 2). The PDC for diuretics was significantly higher in the intervention group vs the control group (11.6%; 95% CI, 7.1%-16.1%; P < .001). The differences in PDC for β-blockers (4.6%; 95% CI, 0.2%-9.0%; P = .04) and MRAs (12.8%; 95% CI, 8.5%-17.2%; P < .001) were also significant; in contrast, there was no significant difference in PDC for ACEIs or ARBs. The proportion of patients with a PDC of 80% or greater among patients with CHF was significant (odds ratio, 0.34; 95% CI, 0.21-0.54; P < .001). However, there were no statistically significant differences in the proportions of patients achieving adherence (PDC ≥80%) for ACEIs or ARBs and β-blockers. After application of the Bonferroni correction, although the calculated P values for diuretics and MRAs showed significant differences when compared with the corrected P value (.01), the conclusions for all secondary end points were consistent with the preliminary analysis results, indicating the robustness of the results.
Table 2. Primary and Secondary Outcomes.
| Outcome | Intervention group | Control group | Intervention effect (95% CI), % | OR (95% CI) | NNT | P value | ||
|---|---|---|---|---|---|---|---|---|
| No. (%) | Mean (SD), % | No. (%) | Mean (SD), % | |||||
| Primary | ||||||||
| Mean PDC | 223 (50.1) | 95.2 (17.9) | 222 (49.9) | 90.5 (24.5) | 8.1 (5.5 to 10.7) | NA | NA | <.001 |
| Secondary | ||||||||
| Mean PDC ≥80% | 190 (85.2) | NA | 147 (66.2) | NA | NA | 0.34 (0.21-0.54) | 5.27 | <.001 |
| ACEIs or ARBs | ||||||||
| PDC | 62 (54.4)a | 86.5 (28.8) | 52 (45.6)a | 88.6 (27.5) | −2.2 (−12.7 to 8.4) | NA | NA | .69 |
| PDC ≥80% | 49 (79.0)b | NA | 44 (84.6)b | NA | NA | 1.46 (0.55 to 3.85) | 17.9 | .44 |
| Diuretics | ||||||||
| PDC | 180 (49.0) | 94.6 (15.6) | 187 (51.0) | 83.0 (26.5) | 11.6 (7.1 to 16.1) | NA | NA | <.001 |
| PDC ≥80% | 161 (89.4) | NA | 126 (67.4) | NA | NA | 0.24 (0.14 to 0.43) | 4.5 | <.001 |
| β-blockers | ||||||||
| PDC | 184 (50.0) | 95.2 (17.9) | 184 (50.0) | 90.5 (24.5) | 4.63 (0.23 to 9.04) | NA | NA | .04 |
| PDC ≥80% | 170 (92.4) | NA | 159 (86.4) | NA | NA | 0.52 (0.26 to 1.04) | 16.7 | .06 |
| MRAs | ||||||||
| PDC | 190 (50.8) | 96.6 (12.1) | 184 (49.2) | 83.8 (27.9) | 12.8 (8.5 to 17.2) | NA | NA | <.001 |
| PDC ≥80% | 178 (93.7) | NA | 134 (72.8) | NA | NA | 0.18 (0.09 to 0.35) | 4.8 | <.001 |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; MRA, mineralocorticoid receptor antagonist; NA, not applicable; NNT, number needed to treat; OR, odds ratio; PDC, proportion of days covered.
The number of patients for each heart failure medication's PDC, and the proportion of patients in the control/intervention group relative to the total group for each heart failure medication.
The total number of patients receiving ACEIs or ARBs, and the proportion of patients who have PDC ≥ 80% relative to the total number of patients receiving ACEIs or ARBs.
Compared with the control group, the quality of life at 52 weeks was significantly improved in the intervention group, as reflected by a statistically meaningful reduction in median (IQR) MLHFQ scores (25.0 [12.0-40.0] vs 35.0 [20.0-44.0] points; difference, 10.0 points; P = .005) (eTable 1 in Supplement 2). We found a statistically significant difference in median (IQR) Morisky scores between the groups (7.8 [6.8-8.0] vs 7.3 [4.5-7.8] points; difference, 0.5 points; P < .001) (eTable 1 in Supplement 2), although no significant difference in safety outcomes was observed (eTable 2 in Supplement 2). Among the 445 participants, 165 (37.1%) experienced ADEs, of which 131 were judged as unrelated or unlikely to be related to the study. Among the 34 possibly or definitely related ADEs, 15 occurred in the control group (6 cases of gastrointestinal reactions, 4 male gynecomastia, 2 shortness of breath, 2 bleeding, and 1 liver injury).
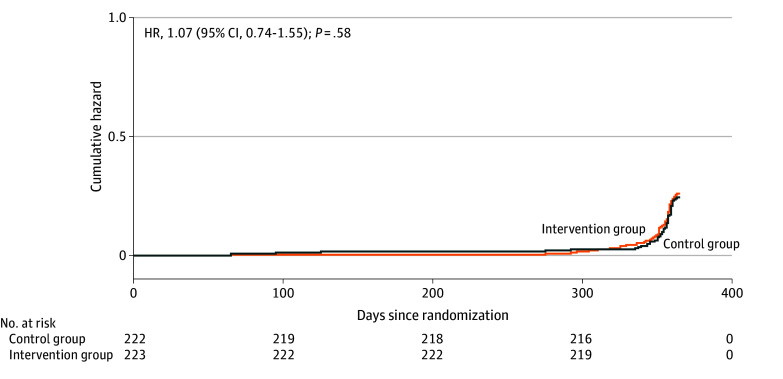
Within 52 weeks after randomization, 4 patients in the intervention group and 7 patients in the control group died. No significant differences between the 2 groups were observed in readmission rates, which were defined as unplanned cardiovascular hospitalizations, or all-cause deaths. However, there was no significant difference in the number of days lost due to unplanned cardiovascular hospitalizations or deaths between the 2 groups (eTable 3 in Supplement 2). The Nelson-Aalen plot for all-cause mortality or unplanned cardiovascular hospitalizations within 52 weeks after randomization is shown in Figure 2.
Figure 2. Nelson-Aalen Plot for Time to First Event During 52-Week Follow-Up.
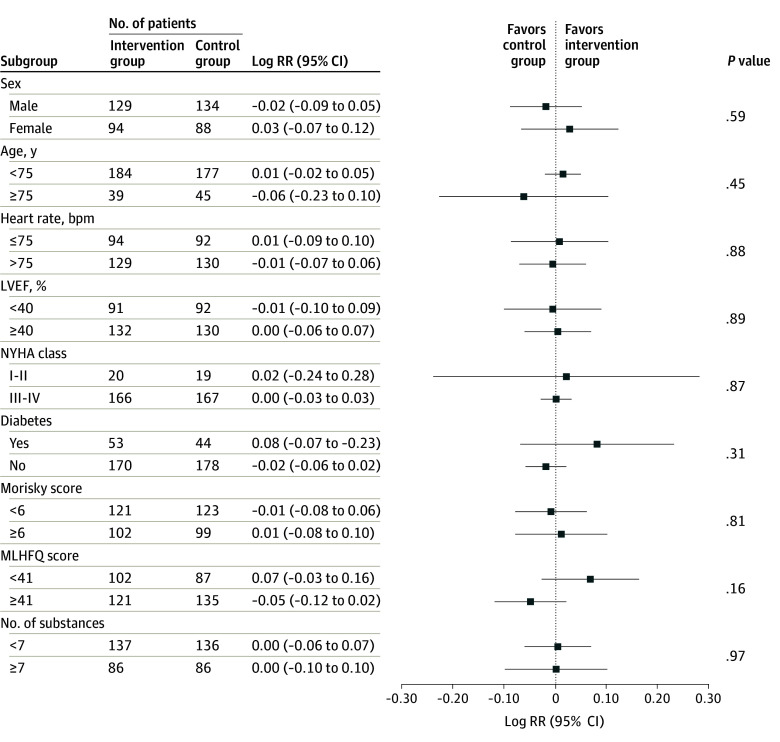
Among all subgroups examined, we identified 9 factors that had the potential to influence the primary efficacy end point. As previously mentioned, the intervention group compared with the control group showed improved medication adherence at 52 weeks. However, no significant interactions were observed across all subgroups (Figure 3).
Figure 3. Forest Plot of Sensitivity Analyses for the Primary Efficacy End Point.
bpm indicates beats per minute; LVEF, left ventricular ejection fraction; MLHFQ, Minnesota Living With Heart Failure Questionnaire; NYHA, New York Heart Association; and RR, relative risk.
Discussion
To our knowledge, this randomized clinical trial in the Chinese population was the first to investigate the effect of a multifaceted and interdisciplinary intervention led by clinical pharmacists on the management of patients with CHF. Although several trials have assessed the effectiveness of pharmacists in the treatment of patients with CHF, these trials have not been methodologically consistent.16,28,29,30,31,32 Their key conclusions suggest that the role of pharmacists in improving patient adherence and clinical outcomes is limited. Our findings are largely consistent with those of the past trials. While there was a statistically significant improvement in medication adherence among patients with CHF under the pharmacist-led management model, the improvement was modest. Moreover, no significant differences were observed in clinical outcomes such as readmission rates, all-cause deaths, and ADEs. We believe this finding may be due to the limited clinical effectiveness of the pharmacist intervention or the relatively short observation period, neither of which was sufficient to result in significantly different clinical outcomes. This finding suggests that more effective interventions need to be explored in future research to achieve substantial improvements in patient health outcomes. Considering that the trial’s recruitment period (March 2021 to April 2022) overlapped with the peak of the COVID-19 outbreak in Hebei Province by 2 months, we noticed a reduction in the number of new patients reported by the participating hospitals during that period. Additionally, the consultation time during follow-up visits was extended, and the frequency of medication changes increased. However, we observed that possibly due to the collective anxiety triggered by the COVID-19 pandemic, medication adherence in both groups of participants improved, a finding consistent with reports from health care workers in France and Switzerland.33,34 We speculate that this phenomenon of increased adherence under stress may be one of the factors in the high medication adherence observed in both the intervention and control groups in this trial.
Heart failure imposes considerable limitations on quality of life, and the success of heart failure treatment is closely related to improvements in quality of life.35 Poor quality of life among patients with heart failure is associated with an increased risk of all-cause mortality and a higher combined risk of heart failure–related death or hospitalization.36 Rasmussen et al37 found that patients with CHF who are in the terminal stage of various heart diseases often have comorbidities, and medication adherence is closely related to quality of life. This finding underscores the necessity of research such as the current trial. During the COVID-19 pandemic, the MLHFQ scores in the intervention group at 52 weeks were significantly reduced compared with baseline and significantly lower than MLHFQ scores in the control group. This result could potentially elucidate the benefit of messaging application–based follow-up frequency for the long-term outcomes of patients with CHF.
Patients with CHF are often older and have multiple comorbidities. Thus, they typically require the use of combined multiple medications, which increases the risk of drug-related adverse events.38,39 Based on their professional pharmaceutical knowledge, clinical pharmacists can provide answers to medication inquiries and offer guidance on dealing with adverse reactions, thereby reducing the occurrence of ADEs. In this study, there was no significant difference in the incidence of ADEs between the intervention and control groups. Similarly, Gurwitz et al40 found that clinical pharmacist-led interventions did not significantly reduce the incidence of drug-related adverse events or clinically significant medication errors. Other studies have indicated that pharmacist-led interventions may reduce the incidence of adverse reactions in chronic diseases.41,42,43 However, our findings underscore the necessity for larger sample sizes to confirm whether such interventions can decrease the prevalence of adverse reactions among patients with CHF.
The messaging application used in this trial is widely used in China, suggesting that the application may be useful for implementing mobile health interventions to improve medication adherence. Although some studies have been conducted in China using this application to improve self-management behaviors,22,24,44,45 none of them has specifically addressed medication adherence among patients with CHF. In this study, we leveraged the messaging application, a familiar platform for patients, to implement clinical pharmacist interventions in which pharmacists communicated with patients with CHF at any time. Educational materials on medication were regularly posted in the application’s chat groups, and weekly medication reminders prompted patients to take their medication. These measures were particularly important for patients who were confined to their homes during the COVID-19 pandemic. We speculate that the beneficial effects of the pharmacist-led management model may be related to the messaging application–based education. Previous studies have shown that the messaging application’s platform can provide individualized guidance, thereby enhancing patient adherence,26,46which is consistent with our findings. The scope of this trial can be extended to other terminal illnesses to alleviate the economic and lifestyle burdens of these serious diseases on patients and society. Furthermore, our experience with this pharmacist-led intervention has provided a standardized pathway for training grassroots pharmacists in rural areas of China, especially in the field of heart failure management, which helps to improve the quality of treatment and patient care levels in these regions.
Limitations
This study has several limitations. First, all participants were recruited from 5 hospitals in Hebei province. Although hospitals of different levels were included, the sample still may not be representative of the general population in China. Second, due to the uniqueness of the COVID-19 pandemic, some participants self-reported their medication use, which may have introduced variability in the results. Third, during data collection for the control group, some participants requested to communicate with nurses through the messaging application. However, we did not consider this effect in our analysis. Fourth, the absence of an attention control group may affect the accuracy of the assessment of the impact of the pharmacist-led management model on patients with heart failure.
Conclusions
In this randomized clinical trial, patients with CHF who received the pharmacist-led management intervention showed a significant improvement in medication adherence compared with patients who received usual care, but the improvement was modest. No significant differences were observed between these groups in terms of clinical outcomes, including readmission rates, all-cause mortality, and ADEs. Future research may need to examine more comprehensive intervention strategies to identify the impact of pharmacists on the incidence and mortality of CHF.
Trial Protocol and Statistical Analysis Plan
eTable 1. The Outcomes of Morisky Scores and MLHFQ Scores
eTable 2. Safety Outcomes
eTable 3. All-Cause Deaths and Unplanned Cardiovascular Hospitalizations During 52-Week Follow-Up
Data Sharing Statement
References
- 1.Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016;13(6):368-378. doi: 10.1038/nrcardio.2016.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163-2196. doi: 10.1016/S0140-6736(12)61729-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roth GA, Mensah GA, Johnson CO, et al. ; GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group . Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 Study. J Am Coll Cardiol. 2020;76(25):2982-3021. doi: 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hao G, Wang X, Chen Z, et al. ; China Hypertension Survey Investigators . Prevalence of heart failure and left ventricular dysfunction in China: the China Hypertension Survey, 2012-2015. Eur J Heart Fail. 2019;21(11):1329-1337. doi: 10.1002/ejhf.1629 [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Chai K, Du M, et al. Prevalence and incidence of heart failure among urban patients in China: a national population-based analysis. Circ Heart Fail. 2021;14(10):e008406. doi: 10.1161/CIRCHEARTFAILURE.121.008406 [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Zhang J, Butler J, et al. ; China-HF Investigators . Contemporary epidemiology, management, and outcomes of patients hospitalized for heart failure in China: results from the China Heart Failure (China-HF) registry. J Card Fail. 2017;23(12):868-875. doi: 10.1016/j.cardfail.2017.09.014 [DOI] [PubMed] [Google Scholar]
- 7.The Writing Committee of the Report on Cardiovascular Health and Diseases in China . Report on cardiovascular health and diseases in China 2022: an updated summary. Biomed Environ Sci. 2023;36(8):669-701. doi: 10.3967/bes2023.106 [DOI] [PubMed] [Google Scholar]
- 8.McDonagh TA, Metra M, Adamo M, et al. ; ESC Scientific Document Group . 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599-3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 9.Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119(23):3028-3035. doi: 10.1161/CIRCULATIONAHA.108.768986 [DOI] [PubMed] [Google Scholar]
- 10.Laufs U, Rettig-Ewen V, Böhm M. Strategies to improve drug adherence. Eur Heart J. 2011;32(3):264-268. doi: 10.1093/eurheartj/ehq297 [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald AA, Powers JD, Ho PM, et al. Impact of medication nonadherence on hospitalizations and mortality in heart failure. J Card Fail. 2011;17(8):664-669. doi: 10.1016/j.cardfail.2011.04.011 [DOI] [PubMed] [Google Scholar]
- 12.Schmidt S, Sheikzadeh S, Beil B, Patten M, Stettin J. Acceptance of telemonitoring to enhance medication compliance in patients with chronic heart failure. Telemed J E Health. 2008;14(5):426-433. doi: 10.1089/tmj.2007.0076 [DOI] [PubMed] [Google Scholar]
- 13.Roblek T, Deticek A, Leskovar B, et al. Clinical-pharmacist intervention reduces clinically relevant drug-drug interactions in patients with heart failure: a randomized, double-blind, controlled trial. Int J Cardiol. 2016;203:647-652. doi: 10.1016/j.ijcard.2015.10.206 [DOI] [PubMed] [Google Scholar]
- 14.Ewen S, Baumgarten T, Rettig-Ewen V, et al. Analyses of drugs stored at home by elderly patients with chronic heart failure. Clin Res Cardiol. 2015;104(4):320-327. doi: 10.1007/s00392-014-0783-2 [DOI] [PubMed] [Google Scholar]
- 15.Dempsey JT, Matta LS, Carter DM, et al. Assessment of drug therapy–related issues in an outpatient heart failure population and the potential impact of pharmacist-driven intervention. J Pharm Pract. 2017;30(3):318-323. doi: 10.1177/0897190016641491 [DOI] [PubMed] [Google Scholar]
- 16.Schulz M, Griese-Mammen N, Anker SD, et al. ; PHARM-CHF Investigators . Pharmacy-based interdisciplinary intervention for patients with chronic heart failure: results of the PHARM-CHF randomized controlled trial. Eur J Heart Fail. 2019;21(8):1012-1021. doi: 10.1002/ejhf.1503 [DOI] [PubMed] [Google Scholar]
- 17.Laufs U, Griese-Mammen N, Krueger K, et al. PHARMacy-based interdisciplinary program for patients with Chronic Heart Failure (PHARM-CHF): rationale and design of a randomized controlled trial, and results of the pilot study. Eur J Heart Fail. 2018;20(9):1350-1359. doi: 10.1002/ejhf.1213 [DOI] [PubMed] [Google Scholar]
- 18.Parajuli DR, Kourbelis C, Franzon J, et al. Effectiveness of the pharmacist-involved multidisciplinary management of heart failure to improve hospitalizations and mortality rates in 4630 patients: a systematic review and meta-analysis of randomized controlled trials. J Card Fail. 2019;25(9):744-756. doi: 10.1016/j.cardfail.2019.07.455 [DOI] [PubMed] [Google Scholar]
- 19.Tang AB, Brownell NK, Roberts JS, et al. Interventions for optimization of guideline-directed medical therapy: a systematic review. JAMA Cardiol. 2024;9(4):397-404. doi: 10.1001/jamacardio.2023.5627 [DOI] [PubMed] [Google Scholar]
- 20.Li X, Xu ZR, Tang N, et al. Effect of intervention using a messaging app on compliance and duration of treatment in orthodontic patients. Clin Oral Investig. 2016;20(8):1849-1859. doi: 10.1007/s00784-015-1662-6 [DOI] [PubMed] [Google Scholar]
- 21.Science on WeChat. Nat Methods. 2020;17(9):863. doi: 10.1038/s41592-020-0954-1 [DOI] [PubMed] [Google Scholar]
- 22.Zhou K, Wang W, Zhao W, et al. Benefits of a WeChat-based multimodal nursing program on early rehabilitation in postoperative women with breast cancer: a clinical randomized controlled trial. Int J Nurs Stud. 2020;106:103565. doi: 10.1016/j.ijnurstu.2020.103565 [DOI] [PubMed] [Google Scholar]
- 23.Tang J, Yang J, Liu Y, et al. Efficacy of WeChat-based online smoking cessation intervention (‘WeChat WeQuit’) in China: a randomised controlled trial. EClinicalMedicine. 2023;60:102009. doi: 10.1016/j.eclinm.2023.102009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X, Zhou X, Li H, Li J, Jiang H. The value of WeChat application in chronic diseases management in China. Comput Methods Programs Biomed. 2020;196:105710. doi: 10.1016/j.cmpb.2020.105710 [DOI] [PubMed] [Google Scholar]
- 25.Dong Y, Wang P, Dai Z, et al. Increased self-care activities and glycemic control rate in relation to health education via Wechat among diabetes patients: a randomized clinical trial. Medicine (Baltimore). 2018;97(50):e13632. doi: 10.1097/MD.0000000000013632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo Y, Xu Z, Qiao J, et al. Development and feasibility testing of an mHealth (text message and WeChat) intervention to improve the medication adherence and quality of life of people living with HIV in China: pilot randomized controlled trial. JMIR Mhealth Uhealth. 2018;6(9):e10274. doi: 10.2196/10274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ni Z, Wu B, Yang Q, Yan LL, Liu C, Shaw RJ. An mHealth intervention to improve medication adherence and health outcomes among patients with coronary heart disease: randomized controlled trial. J Med Internet Res. 2022;24(3):e27202. doi: 10.2196/27202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorsch MP, Farris KB, Rowell BE, Hummel SL, Koelling TM. The effects of the ManageHF4Life mobile app on patients with chronic heart failure: randomized controlled trial. JMIR Mhealth Uhealth. 2021;9(12):e26185. doi: 10.2196/26185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bell SP, Schnipper JL, Goggins K, et al. ; Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL-CVD) Study Group . Effect of pharmacist counseling intervention on health care utilization following hospital discharge: a randomized control trial. J Gen Intern Med. 2016;31(5):470-477. doi: 10.1007/s11606-016-3596-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kripalani S, Roumie CL, Dalal AK, et al. ; PILL-CVD (Pharmacist Intervention for Low Literacy in Cardiovascular Disease) Study Group . Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med. 2012;157(1):1-10. doi: 10.7326/0003-4819-157-1-201207030-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schumacher PM, Becker N, Tsuyuki RT, et al. The evidence for pharmacist care in outpatients with heart failure: a systematic review and meta-analysis. ESC Heart Fail. 2021;8(5):3566-3576. doi: 10.1002/ehf2.13508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas R, Huntley AL, Mann M, et al. Pharmacist-led interventions to reduce unplanned admissions for older people: a systematic review and meta-analysis of randomised controlled trials. Age Ageing. 2014;43(2):174-187. doi: 10.1093/ageing/aft169 [DOI] [PubMed] [Google Scholar]
- 33.Chagué F, Boulin M, Eicher JC, et al. Impact of lockdown on patients with congestive heart failure during the Coronavirus disease 2019 pandemic. ESC Heart Fail. 2020;7(6):4420-4423. doi: 10.1002/ehf2.13016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dietrich F, Polymeris AA, Verbeek M, et al. Impact of the COVID-19 lockdown on the adherence of stroke patients to direct oral anticoagulants: a secondary analysis from the MAAESTRO study. J Neurol. 2022;269(1):19-25. doi: 10.1007/s00415-021-10631-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allegrante JP, Wells MT, Peterson JC. Interventions to support behavioral self-management of chronic diseases. Annu Rev Public Health. 2019;40:127-146. doi: 10.1146/annurev-publhealth-040218-044008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kato N, Kinugawa K, Seki S, et al. Quality of life as an independent predictor for cardiac events and death in patients with heart failure. Circ J. 2011;75(7):1661-1669. doi: 10.1253/circj.CJ-10-1308 [DOI] [PubMed] [Google Scholar]
- 37.Rasmussen AA, Wiggers H, Jensen M, et al. Patient-reported outcomes and medication adherence in patients with heart failure. Eur Heart J Cardiovasc Pharmacother. 2021;7(4):287-295. doi: 10.1093/ehjcvp/pvaa097 [DOI] [PubMed] [Google Scholar]
- 38.Fleg JL, Aronow WS, Frishman WH. Cardiovascular drug therapy in the elderly: benefits and challenges. Nat Rev Cardiol. 2011;8(1):13-28. doi: 10.1038/nrcardio.2010.162 [DOI] [PubMed] [Google Scholar]
- 39.McAvay G, Allore HG, Cohen AB, Gnjidic D, Murphy TE, Tinetti ME. Guideline-recommended medications and physical function in older adults with multiple chronic conditions. J Am Geriatr Soc. 2017;65(12):2619-2626. doi: 10.1111/jgs.15065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gurwitz JH, Kapoor A, Garber L, et al. Effect of a multifaceted clinical pharmacist intervention on medication safety after hospitalization in persons prescribed high-risk medications: a randomized clinical trial. JAMA Intern Med. 2021;181(5):610-618. doi: 10.1001/jamainternmed.2020.9285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salgado TM, Moles R, Benrimoj SI, Fernandez-Llimos F. Pharmacists’ interventions in the management of patients with chronic kidney disease: a systematic review. Nephrol Dial Transplant. 2012;27(1):276-292. doi: 10.1093/ndt/gfr287 [DOI] [PubMed] [Google Scholar]
- 42.Gao L, Han Y, Jia Z, et al. Impact of continuous pharmaceutical care led by clinical pharmacists during transitions of care on medication adherence and clinical outcomes for patients with coronary heart disease: a prospective cohort study. Front Pharmacol. 2023;14:1249636. doi: 10.3389/fphar.2023.1249636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lapane KL, Hughes CM, Daiello LA, Cameron KA, Feinberg J. Effect of a pharmacist-led multicomponent intervention focusing on the medication monitoring phase to prevent potential adverse drug events in nursing homes. J Am Geriatr Soc. 2011;59(7):1238-1245. doi: 10.1111/j.1532-5415.2011.03418.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ye J, He L, Beestrum M. Implications for implementation and adoption of telehealth in developing countries: a systematic review of China’s practices and experiences. NPJ Digit Med. 2023;6(1):174. doi: 10.1038/s41746-023-00908-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu Y, Li Y, Li T, et al. New Path to Recovery and Well-Being: Cross-Sectional Study on WeChat Use and Endorsement of WeChat-Based mHealth Among People Living With Schizophrenia in China. J Med Internet Res. 2020;22(9):e18663. doi: 10.2196/18663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi B, Liu X, Dong Q, et al. The Effect of a WeChat-Based Tertiary A-Level Hospital Intervention on Medication Adherence and Risk Factor Control in Patients With Stable Coronary Artery Disease: Multicenter Prospective Study. JMIR Mhealth Uhealth. 2021;9(10):e32548. doi: 10.2196/32548 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eTable 1. The Outcomes of Morisky Scores and MLHFQ Scores
eTable 2. Safety Outcomes
eTable 3. All-Cause Deaths and Unplanned Cardiovascular Hospitalizations During 52-Week Follow-Up
Data Sharing Statement