Highlights
-
•
Methodology.
-
•
In a consecutive retrospective analysis of 190 patients treated with the Masquelet technique at the BG Klinikum Hamburg from January 2012 to January 2022, subgroup analysis for defect-specific features such as the extent and morphology of the defect were recorded, and their influence on the time to reach full weight-bearing of the affected limb was investigated.
-
•
Results and Conclusion:
-
•
A total of 217 defects were treated in 190 patients using the Masquelet technique. 70 % of all defects were in the tibia, followed by 22 % in the femur and only about 7 % in the upper extremity. The average length of all defects was 58 mm (+/- 31 mm), with the largest defect measuring 180 mm and the smallest measuring 20 mm. 89 % of the patients achieved full weight-bearing at the end of therapy. The average time from initiation of therapy to reaching safe full weight-bearing was 589 days. There was a significant correlation between defect length and time to reach full weight-bearing (p = 0.0134). These results could serve as a basis for creating a score for prognostics and evaluation of bone healing after treatment with the Masquelet technique. Additionally, the results could help guide indications for secondary stabilization using internal fixation.
Keywords: Osteomyelitis, Masquelet technique, Bone defect treatment, Infection
Abstract
Methodology
In a consecutive retrospective analysis of 190 patients treated with the Masquelet technique at the BG Klinikum Hamburg from January 2012 to January 2022, subgroup analysis for defect-specific features such as the extent and morphology of the defect were recorded, and their influence on the time to reach full weight-bearing of the affected limb was investigated.
Results and conclusion
A total of 217 defects were treated in 190 patients using the Masquelet technique. 70 % of all defects were in the tibia, followed by 22 % in the femur and only about 7 % in the upper extremity. The average length of all defects was 58 mm (+/- 31 mm), with the largest defect measuring 180 mm and the smallest measuring 20 mm. 89 % of the patients achieved full weight-bearing at the end of therapy. The average time from initiation of therapy to reaching safe full weight-bearing was 589 days. There was a significant correlation between defect length and time to reach full weight-bearing (p = 0.0134). These results could serve as a basis for creating a score for prognostics and evaluation of bone healing after treatment with the Masquelet technique. Additionally, the results could help guide indications for secondary stabilization using internal fixation.
Introduction
The restoration of bone defects after trauma or resection is often a complex and time-consuming process. Common techniques, such as segment transport, cannot be carried out in patients with traumatic brain injury and resulting noncompliance, after soft tissue flap transfer to the affected site or when no transferrable segment is available at the defect- site. Alternatively, fibula transfer is a single-stage alternative but is not recommended due to frequent infectious non-union and the need for microsurgical expertise [[1], [2], [3]]. As a result, the Masquelet technique, a two-stage procedure developed in the 1980s, offers a popular alternative. After debridement and stabilization, a cement spacer is placed at the defect site and induces a well vascularized membrane. After 4–6 weeks, the spacer is removed, and the defect is filled with an autologous bone graft [4,5]. But do size and configuration of the defect play a role? This study, conducted at BG Klinikum in Hamburg, Germany, retrospectively analyzed defects treated with the Masquelet technique conducting specific subgroup- analysis for defect size and configuration. As a functional outcome full weight and load bearing were chosen.
Materials and methods
Study design
The study employed a retrospective, consecutive series, single-arm cohort design, encompassing all patients treated with the Masquelet technique at BG Klinikum Hamburg (BGKH) from July 2011 to June 2021. Clinical and post-treatment electronic patient record data were meticulously collected and stored in a dedicated database, ensuring privacy by excluding personal identifiers. Ages were rounded, and no direct transfer of names, addresses, or birthdates occurred.
Patient inclusion criteria encompassed those aged 18 and above. The use of the Masquelet technique was indicated for defects longer than 3 cm and at least 2/3 of the circumference, failed segment transport, patient non-compliance or prior soft tissue flaps covering the defect. Ethical approval was obtained from the University of Lübeck EC on April 15th, 2021 (registration number 21–169). Subsequently, data extraction from the hospital information system followed in July 2021, and all data were pseudonymously recorded on an in-house spreadsheet.
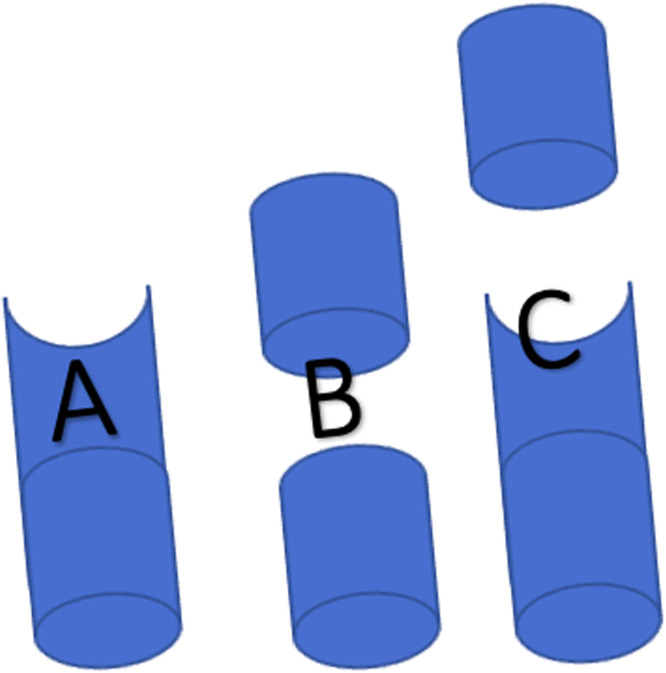
Besides demographic data, focus was placed on defect configuration with predefined subgroups (Fig. 1) and size, in relation to achievement of full weight and load bearing as well as the time until achievement.
Fig. 1.
Schematic of defect configurations as A semi- circular, B circular and C mixed.
Surgical technique
The surgical procedure, based on Masquelet et al.'s (2019) recommendations [4]. In the first step, potentially infected foreign material was removed, and an antibiotic-loaded Polymethylmethacrylate (PMMA) [4] spacer was implanted, securing bone length, axis, and rotation with external fixation; alternatively internal fixation was left in situ. Microbiological and histological samples were taken, and empirical antibiotic treatment initiated. Four to six weeks later, the PMMA spacer was removed, preserving the Masquelet membrane. The intramedullary canal was reconstructed, using gelatine sponge [6] and vancomycin-augmented allografts for larger defects. The grafting material, obtained from the pelvis or proximal tibia, was placed at the cortical defect site. Microbiological and histological regimens mirrored those in the first step.
Statistics
Statistical analysis was conducted using SAS statistical software (version 17) [7] and R statistics (version 3.5.1 and RStudio, version 1.1.456, both from R Consortium, Boston, MA, USA). Categorical data were presented as absolute values and percentages, while nominal data were expressed as means with corresponding percentages and standard deviations. Group differences for nominal data were assessed using Student's t-test, and chi-square test was employed for categorical parameters. The significance level (α) was set at 5 %, with p-values below 0.05 considered significant. The study adhered to the principles of the Declaration of Helsinki and achieved a power of 0.8.
Results
Patients
Demographic and patient data, categorized into two groups based on those who achieved full weight and load bearing and those who did not. Initially, 196 patients were treated with the Masquelet technique; however, 6 ongoing cases were excluded. Fourteen patients were lost to follow-up, and 5 patients passed away during treatment, resulting in a total of 171 patients. Among them, 113 (66 %) were male, and 58 (34 %) were female, with an age distribution of 52 +/−16 years (mean +/- SD), ranging from 18 to 83 years.
89 % of all patients reached full weight and load bearing.
Defect size and configuration
The mean length of all defects was 59 mm (+/−31 mm, min. 20 mm, max. 180 mm). Table 1 presents the distribution of defects, with 137 (70 %) located in the tibia, followed by 43 (22 %) in the femur, 10 (5 %) in the upper arm, 4 (2 %) in the forearm, and 1 (1 %) in the foot. Fig. 1 illustrates the configuration of defects, categorized into semi-circular (Fig. 1a), circular (Fig. 1b), and mixed defects (Fig. 1c). The prevalence of semi-circular defects was N = 73 (37 %), circular defects N = 87 (45 %), and mixed N = 35 (18 %).
Table 1.
Patients reaching full weight bearing versus not reaching full weight bearing regarding defect localization and size.
| No (N=18) | Yes (N=153) | Total (N=171) | |
|---|---|---|---|
| Localization of Defect | |||
| Femur | 2 (11 %) | 36 (24 %) | 38 (22 %) |
| Foot | 0 (0 %) | 1 (1 %) | 1 (1 %) |
| Forearm | 0 (0 %) | 3 (2 %) | 3 (2 %) |
| Humerus | 1 (6 %) | 8 (5 %) | 9 (5 %) |
| Tibia | 15 (83 %) | 105 (69 %) | 120 (70 %) |
| Size of Defect (mm) | |||
| Mean (SD) | 78 (29) | 57 (31) | 59 (31) |
| Range | 30 - 130 | 20 - 180 | 20 – 180 |
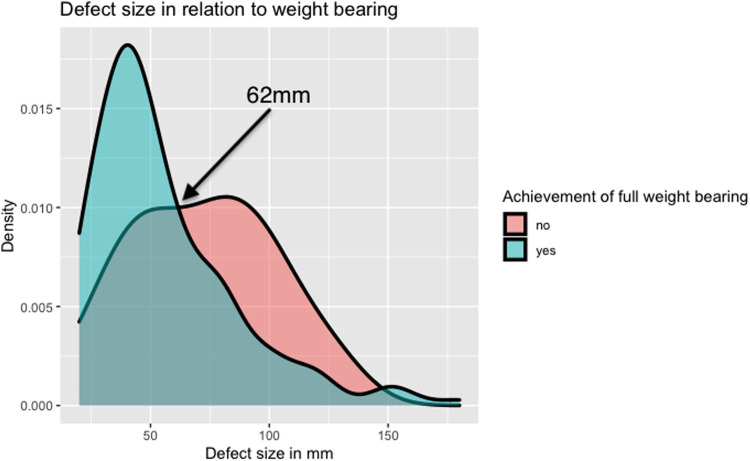
When defect size and the ability to achieve full weight bearing are plotted against each other, an intersection point can be seen at 62 mm (Fig. 2), defining the cut-off point for a negative treatment outcome and can hence be defied as the lower margin for a critical size defect.
Fig. 2.
Density plot of patients achieving full weight bearing versus not achieving full weight bearing in relation to defect size.
A non-parametric comparison of defect configurations using Bonferroni-adjusted p-values revealed no significant correlation between defect configurations and achieving full weight and load bearing, as outlined in Table 2.
Table 2.
Subgroup analysis on configuration of defects regarding significant differences for reaching full weight bearing.
| Defect configuration | Raw p-value | Bonferoni adjusted p-value |
|---|---|---|
| Semicircular vs. mixed | 0,0199 | 0,0597 |
| Semicircular vs. circular | 0,0962 | 0,2886 |
| Circular vs. mixed | 0,3323 | 0,9969 |
Discussion
Demographic data did such as age (mean 52years) and a predominantly male study population (66 %) did not differ from other cohort studies [8,9].
Achieving an 89 % rate of full weight and load bearing, our study demonstrated a higher rate of adequate union compared to the 80 % reported by Raven et al. It falls slightly below the union rates reported by Masquelet et al. (ranging from 79 % to 93 %) and Hsu et al. (90 %).
Within our study, there was a positive correlation between the overall defect size and the time required to achieve full weight and load bearing (p = 0.0134). Notably, a shift in the likelihood of a positive treatment outcome occurred at 62 mm, indicating a transition toward a negative outcome (Fig. 2). This finding, unique to our study, suggests that a defect size exceeding 62 mm can be deemed a critical threshold for patients undergoing bone reconstruction through the Masquelet technique.
This contrasts with Masquelet et al.'s observation that defect size exhibited independence from treatment outcomes, a claim not substantiated by statistical evidence [4]. A review by Nauth et al. [10] scrutinizing critical size bone defects in the literature yielded diverse results, ranging from 1 to 2 cm to a 50 % loss of bone circumference. Furthermore, Begue et al. [11] characterized defects surpassing a length of 5 cm as critical, positing that such cases are less amenable to conventional bone grafting and necessitate the application of the Masquelet technique.
Raven et al. reported a mean defect size of 44 mm, whereas the meta-analysis conducted by Hsu et al. documented an average of 55 mm, exhibiting comparable findings to the 59 mm reported in this study [8].
In our investigation, defect configurations were categorized as circular, semi-circular, and mixed (Fig. 1), with no significant correlation observed between these in a subgroup analysis configurations and the attainment of full weight and load bearing (Table 2). Stafford et al. adopted a similar approach, classifying defects into conical and cylindrical categories, emphasizing volumetric measurements [12]. Meanwhile, Karger et al. categorized defects as segmented (86 %) and beveled (14 %) [13], making direct comparisons challenging and underscoring the need for a universal nomenclature across studies.
The distribution of defects in our patient cohort is consistent with established clinical series such as the one by Masquelet et al. in their meta-analysis of clinical series involving more than 15 patients in 2019 [8]. Karger et al. [13] also reported a series of 84 posttraumatic long bone defects.
Conclusion
The Masquelet technique poses challenges in outcome assessment due to various influences and inherent difficulties in studying unmodifiable factors such as low patient compliance. While risk profiling through established non-union-scoring systems like NUSS Score [14] and Schmidmaier et al.'s [15] unvalidated risk profiling could enhance prospective studies, their integration into clinical practice remains limited.
The radiological healing process lacks objective criteria for guiding decisions on weight and load-bearing adjustments. Establishing a scoring system to complement expert opinions on radiologic bone healing would facilitate result comparisons across studies and treatment adaptation. Defining the treatment endpoint for a defect as full weight and load bearing, rather than solely osseous consolidation, is crucial.
Identifying defects larger than 62 mm as 'critical size defects' emphasizes the need for special attention. Potential adaptations of the Masquelet technique, such as exploring a three-step procedure involving initial defect reduction followed by two-step bone grafting with a secondary spacer, warrant further investigation.
CRediT authorship contribution statement
J. Frese: Writing – review & editing, Writing – original draft, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. AP Schulz: Supervision, Project administration, Data curation. B. Kowald: Data curation. U.J. Gerlach: Conceptualization. K.H. Frosch: Supervision, Methodology. R. Schoop: Validation, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
References
- 1.Arrington E.D., Smith W.J., Chambers H.G., Bucknell A.L., Davino N.A. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res. 1996;329:300–309. doi: 10.1097/00003086-199608000-00037. [DOI] [PubMed] [Google Scholar]
- 2.Iacobellis C., Berizzi A., Aldegheri R. Bone transport using the Ilizarov method: a review of complications in 100 consecutive cases. Strateg Trauma Limb Reconstr. 2010;5(1):17–22. doi: 10.1007/s11751-010-0085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herford A.S., Dean J.S. Complications in bone grafting. Oral Maxillofac Surg Clin N Am. 2011;23(3):433. doi: 10.1016/j.coms.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Masquelet A., Kanakaris N.K., Obert L., Stafford P., Giannoudis P.V. Bone repair using the masquelet technique. JBJS. 2019;101(11):1024–1036. doi: 10.2106/JBJS.18.00842. [DOI] [PubMed] [Google Scholar]
- 5.Careri S., Vitiello R., Oliva M., Ziranu A., Maccauro G., Perisano C. Masquelet technique and osteomyelitis: innovations and literature review. Eur Rev Med Pharmacol Sci. 2019;23(2):210–216. doi: 10.26355/eurrev_201904_17495. Suppl. [DOI] [PubMed] [Google Scholar]
- 6.Cho J.W., Kim J., Cho W.T., et al. Circumferential bone grafting around an absorbable gelatin sponge core reduced the amount of grafted bone in the induced membrane technique for critical-size defects of long bones. Injury. 2017;48(10):2292–2305. doi: 10.1016/j.injury.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Barr A. SAS statistical software. Published online August 2020.
- 8.Raven T., Moghaddam A., Ermisch C., et al. Use of Masquelet technique in treatment of septic and atrophic fracture nonunion. Injury. 2019;50:40–54. doi: 10.1016/j.injury.2019.06.018. [DOI] [PubMed] [Google Scholar]
- 9.Hsu C.A., Chen S.H., Chan S.Y., Yu Y.H. The induced membrane technique for the management of segmental tibial defect or nonunion: a systematic review and meta-analysis. BioMed Res Int. 2020;2020:1–9. doi: 10.1155/2020/5893642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nauth A., Schemitsch E., Norris B., Nollin Z., Watson J.T. Critical-size bone defects: is there a consensus for diagnosis and treatment? J Orthop Trauma. 2018;32:S7–S11. doi: 10.1097/BOT.0000000000001115. [DOI] [PubMed] [Google Scholar]
- 11.Begue T., Auregan J. European instructional lectures. Springer; 2014. Acute management of traumatic bone defects in the lower limb; pp. 71–83. [Google Scholar]
- 12.Stafford P.R., Norris B.L. Reamer-irrigator-aspirator bone graft and bi Masquelet technique for segmental bone defect nonunions: a review of 25 cases. Injury. 2010;41:S72–S77. doi: 10.1016/S0020-1383(10)70014-0. [DOI] [PubMed] [Google Scholar]
- 13.Karger C., Kishi T., Schneider L., Fitoussi F., Masquelet A.C. Treatment of posttraumatic bone defects by the induced membrane technique. Orthop Traumatol Surg Res. 2012;98(1):97–102. doi: 10.1016/j.otsr.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Calori G., Colombo M., Mazza E., et al. Validation of the non-union scoring system in 300 long bone non-unions. Injury. 2014;45:S93–S97. doi: 10.1016/j.injury.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 15.Schmidmaier G., Capanna R., Wildemann B., Beque T., Lowenberg D. Bone morphogenetic proteins in critical-size bone defects: what are the options? Injury. 2009;40:S39–S43. doi: 10.1016/S0020-1383(09)70010-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.