Abstract
Study Design
Observational comparative study.
Objective
To study the role of magnetic resonance spectroscopy (MRS) and T2 relaxometry (T2r) as imaging biomarkers for identifying early lumbar disc degeneration.
Methods
We evaluated 236 discs in normal volunteers and 215 discs in low back pain (LBP) patients by MRS and T2r to document the molecular spectra of various metabolites as well as disc hydration and collagen content, respectively. All volunteer discs were Pfirrmann grade 1 (PF1), whereas patients with LBP had PF 1 (n = 156) and PF 2 (n = 59). The study population was compared in three age groups: A (20-30 years), B (30-40 years), and C (40-50 years).
Results
T2r, an indicator of collagen and hydration, was higher in volunteers (121.8 ± 31.1), compared to PF 1 patients (110.68 ± 23.96) and PF 2 patients (90.15 ± 25.81) (P = 0.001). Proteoglycan assessed by MRS was more stable for volunteers (3.39 ± 1.69) and PF 1 patients (3.6 ± 1.69) but reduced in PF 2 patients (2.86 ± 1.47), showing that structural molecules did not alter within the PF 1. However, lactate and other metabolites showed a difference even within PF1 between volunteers and LBP patients. We were able to identify a unique subset of PF 1 that had a normal value of proteoglycan and T2r but altered metabolite distribution, which may represent early disc degeneration (DD).
Conclusion
MRS and T2r can be used as imaging biomarkers for early DD by identifying altered metabolic activity with an intact matrix.
Keywords: magnetic resonance spectroscopy, T2 relaxometry, imaging biomarkers, disc degeneration, lactate, Pfirrmann
Introduction
Low back pain imposes a major burden, whether in terms of societal expenses or personal suffering and has been identified as the leading cause of disability-related life years. 1 While 49% to 80% of the population experiences LBP at some point in their lives, the stated point prevalence estimates are closer to 35%. 2 The presence of disc degeneration 3 (DD), which is a natural component of ageing, has been linked to lower back pain (LBP) symptoms. According to Suthar et al., the leading cause of low back pain (LBP) is lumbar disc degeneration (LDD). 4 Furthermore, according to a meta-analysis by Brinjikji et al, lumbar degenerative changes detected on MRI are more common among adults 50 years of age or younger with back pain (54.8%–59.8%) than in asymptomatic individuals (31.5%–37.5%). 5
Detecting disc degeneration for the early diagnosis of patients with degenerative disc disease (DDD) is vital to facilitate optimal treatment, given that therapies are most efficacious in the early stages of the disease. Reversing disc degeneration has been approached from various angles—improving disc nutrition pharmacologically, infusion of stem cells, addition of growth factors, injection of plasma-rich proteins, etc.—but has not achieved sustained success to reach clinical implications.6-9 One of the main reasons may be the current difficulty in identifying early degeneration in vivo. The gold standard for identifying DDD is a morphological change observed by MRI imaging. Despite its high sensitivity, the identification of degeneration occurs once the process progresses, indicating that the DD has reached an irreversible stage. It then becomes important that the degeneration be identified at a very early stage, even before it is identifiable by routine MRI. 8
The integrity of any tissue depends on cellular activity and extra extracellular matrix. Alterations occur initially as changes in metabolism and energy cycles, and it becomes important to identify changes at the molecular level, which has been a challenge in in vivo situations.9,10 Newer MRI sequences like T2 relaxometry (T2r), T1rho, chemical exchange saturation transfer (CEST), diffusion, etc., now offer the possibility of identifying molecular changes in vivo.11-13 Researchers have found these sequences useful in documenting changes in collagen and proteoglycan during degeneration. MRS, on the other hand, is capable of detecting both structural and metabolic molecules. 14 In a group of volunteers and patients with low back pain, we used T2R and MRS to document structural molecules like collagen and proteoglycan, as well as metabolic molecules like lactate, alanine, propionic acid, and acetate. The study primarily aimed to investigate the effectiveness of MRS and T2r as imaging biomarkers in detecting degeneration at a stage of molecular-level changes prior to the progression of alterations in the matrix that routine MRI can diagnose.
Materials and Methods
Study Sample
This is an observational comparative study conducted from May 2021 to May 2022, following approval from the institutional review board (IRB No: 2020/09/11) and informed written consent from all participants. The study population included 57 normal volunteers and 75 patients with LBP. The patient population included participants with LBP with or without associated radiculopathy and neurological deficits. We excluded patients with a history of spinal trauma, tumours, infections, or previous spine surgery. Normal volunteers included patients with no previous history of low back pain or who had undergone treatment for LBP. The study subjects were classified into three groups based on their age: A (20-30 years), B (30-40 years), and C (40-50 years). All subjects had routine MRI, MRS, and T2r of the lumbar disc after standardization of the protocols.
MR Image Acquisition
Lumbar spine MRI was performed with a clinical 3-T scanner (Siemens Magneton, GMBH, Erlangen, Germany) using 16-channel phase array spine coils. Routine sequences were obtained by standard protocol.
T2 Relaxometry
For T2 mapping, a sagittal multi-echo spin echo sequence with a TR of 2000 ms, a TE of 13.80-110.40 ms (8 cycles), a matrix of 205 × 256, a 250 mm field of view, a 4 mm slice thickness, and a 0.8 mm intersection gap were used. The acquisition time was five minutes. The T2 map was generated by inline calculation software (MapIt; Siemens Medical Solutions, Erlangen, Germany).
Magnetic Resonance Spectroscopy
A single-voxel spectrum was obtained by a PRESS sequence with a three-pulse chemical shift selective (CHESS) saturation sequence for water suppression. TE/TR = 32 ms/1500 ms, number of data points = 1024, spectral width = 2000 Hz, suppressed echoes = 384. The total imaging time was 6-7 minutes per disc. A 5 × 18 × 16 mm3 voxel was placed at the centre of the intervertebral disc. Spectra were acquired with a full-width at half maximum (FWHM) < 15 Hz at 3T, corresponding to 0.12 ppm.
Post-Processing Protocol
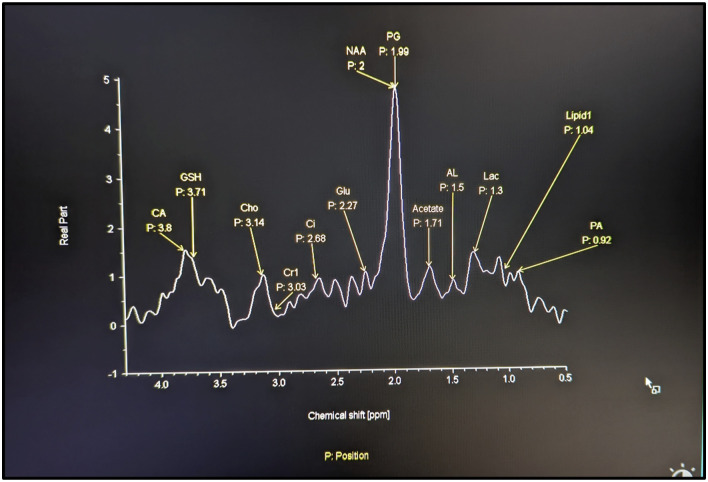
For MRS analysis, the peak value of the spectrum was considered for quantification. The molecules identified were proteoglycan, lactate, alanine, acetate, and propionic acid (Figure 1). We processed the spectral data offline using a Syngo workstation (Siemens Healthcare GMBH, Erlangen, Germany).
Figure 1.
Normal MRS spectrum of the lumbar IVD: The X-axis indicates the resonance frequency of the molecules indicated by chemical shift in parts by million (PPM) which is constant for each molecule. Y-axis gives the peak values of the molecules in area scanned and is the arbitrary estimate of concentration of the molecule. Resonance frequency of Proteoglycan is 2.02, lactate is 1.24, propionic acid is .92, alanine is 1.42, acetate is 1.69.
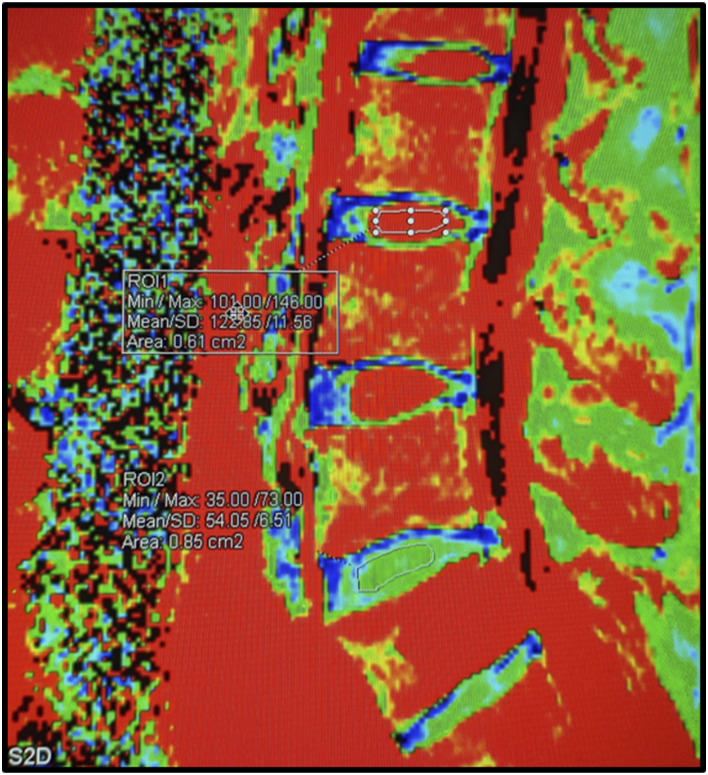
For T2 quantification, a region of interest of 1.3 mm2 was manually drawn on sagittal T2 R maps in the most central portion of the discs. We included only discs in control patients who had sufficient disc height for placing the ROI for assessing T2 Relaxometry. The average of three values from axial T2 was used for statistical analysis (Figure 2).
Figure 2.
T2 relaxometry of the disc: The post processed image obtained in the syngovia workstation. The ROI is drawn on the NP of each disc using the same area of circumference to maintain the uniformity. The L3/4 disc is normal with high water content in the NP (red in color) and T2r values of 122. Low water content noted in the AF (blue and green). The L5/S1 degenerated disc shows reduced water content in NP with T2R values of 54 (green). L3/4 and L5/S1 discs were Pfirrmann grade I and II respectively on T2 wt images.
Statistical Analysis
The significance between the groups was calculated by a one-way ANOVA with post-hoc Tukey using F statistics. The least significant difference (LSD) and honestly significant difference (HSD) were calculated grade-wise (k = 2; I, II) and age-wise (k = 3; 20-30, 30-40, 40-50 years).
Results
57 normal volunteers and 75 low back pain patients with ages ranging from 20 to 50 years were studied. A total of 451 discs (236 discs from volunteers) and 215 discs from LBP patients were studied. All discs in volunteers were PF 1, whereas in LBP patients, 156 discs were PF 1, and the remaining 59 discs were PF 2.
T2r, an indicator of collagen and hydration, showed a significantly higher value in volunteers (121.8 ± 31.1) when compared to LBP patients with PF1 (110.68 ± 23.96) and LBP patients with PF 2 (90.15 ± 25.81) (P = 0.001). Proteoglycan assessed by MRS was more stable in volunteers (3.39 ± 1.69) and LBP patients with PF1 (3.6 ± 1.69) but reduced in LBP patients with PF2 (2.86 ± 1.47) showing that structural molecules did not alter within the PF1 discs.
Amongst the metabolic molecules, the value of lactate in volunteers was 6.67 ± 2 compared with 7.86 ± 2.7 for LBP patients with PF1 (P = 0.01). Similarly, the other metabolites showed similar significant variation between volunteers and LBP patients with PF 1 (Table 1).
Table 1.
Demographic Variables, T2r, PG and Metabolites Across Three Study Populations.
| Variable | Volunteer (PF1) | LBP Patients (PF1) | LBP Patients (PF2) | P value |
|---|---|---|---|---|
| Age (years) | ||||
| 20-30 | 142 | 44 | 12 | 0.065 |
| 30-40 | 74 | 74 | 20 | |
| 40-50 | 20 | 38 | 27 | |
| Disc level | ||||
| L1-L2 | 56 | 41 | 11 | 0.143 |
| L2-L3 | 66 | 46 | 15 | |
| L3-L4 | 66 | 43 | 20 | |
| L4-L5 | 48 | 26 | 13 | |
| T2r | 121.83 ± 31.08 | 110.68 ± 23.96 | 90.15 ± 25.81 | 0.001 |
| PG | 3.39 ± 1.69 | 3.6 ± 1.69 | 2.86 ± 1.47 | 0.084 |
| Lactate | 6.67 ± 2.00 | 7.86 ± 2.70 | 4.4 ± 1.60 | 0.012 |
| PA | 4.96 ± 1.35 | 7.58 ±1.90 | 3.85 ± 1.10 | 0.001 |
| Alanine | 4.76 ± 1.50 | 4.88 ± 1.80 | 2.96 ± 1.40 | 0.023 |
| Acetate | 2.83 ± 1.00 | 2.59 ± 1.20 | 1.86 ± 1.00 | 0.034 |
PF- Pfirrmann, T2r- T2 relaxometry, LBP- Low back pain, PG- proteoglycan, PA- propionic acid. The p values highlighted in bold indicate that it is statistically significant (p-value: <0.05)
Subgroup Analysis Based on Age Groups
Subgroup analysis was performed in both volunteers and LBP patients based on different age groups: Group A (20-30 years), Group B (30-40 years), and Group C (40-50 years).
In Volunteers
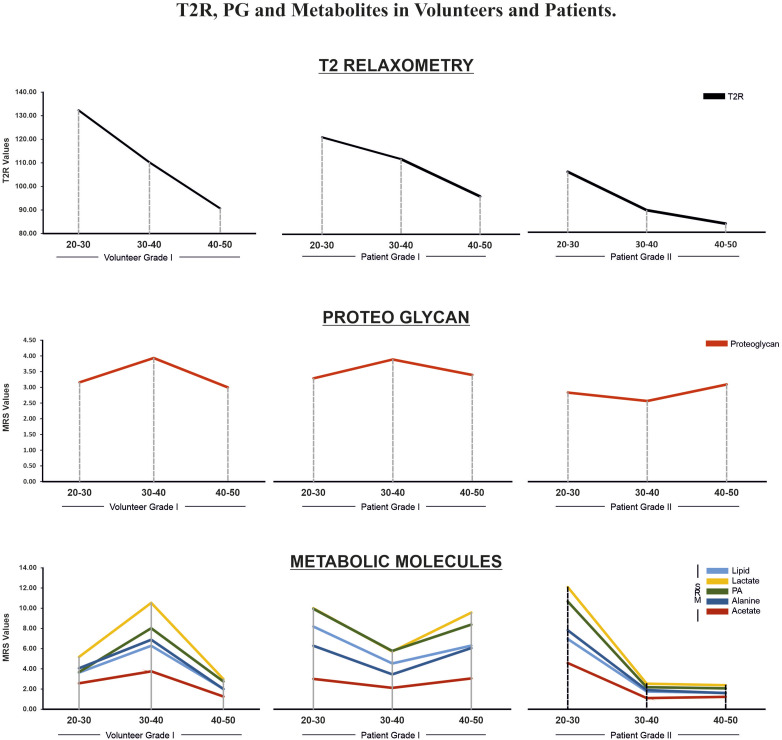
T2r showed the highest peak in group A (132.3 ± 33.7), followed by group B (110.1 ± 15.3), and group C (90.6 ± 14.4). The difference was significant across all age groups (P = 0.001). In contrast, proteoglycan values were 3.16 ± 1.6 in group A, followed by 3.93 ± 1.7 in group B, and 3.0 ± 1.5 in group C. The increase in group B was significant compared to other groups (P = 0.02) (Table 2 and Figure 3).
Table 2.
T2r, PG and Metabolites in Study Population Across Different Age Groups.
| Variables | Volunteer PF1 | LBP Patients (PF1) | LBP Patients (PF2) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Group A (20-30) | Group B (30-40) | Group C (40-50) | Group A (20-30) | Group B (30-40) | Group C (40-50) | Group A (20-30) | Group B (30-40) | Group C (40-50) | |
| T2r | 132.29 + 33.7 | 110 +15.6 | 90.61 + 14.4 | 121.19 + 27.4 | 111.91 + 21.2 | 96.09 + 17.1 | 105.86 + 35.0 | 89.46 + 23.9 | 83.67 + 19.8 |
| PG | 3.16 + 1.6 | 3.93 + 1.7 | 3.00 + 1.59 | 3.28 + 1.9 | 3.89 + 1.73 | 3.39 + 1.23 | 2.83 + 2.19 | 2.57 + 1.34 | 3.09 + 1.17 |
| Lactate | 5.17 + 1.9 | 10.53 + 2.5 | 3.00 + 1.7 | 9.99 + 5.2 | 5.73 + 2.2 | 9.57 + 2.3 | 12.08 + 6.2 | 2.53 + 1.25 | 2.38 + 1.3 |
| PA | 3.67 + 1.3 | 8.02 + 1.6 | 2.75 + 1.1 | 9.95 + 4.1 | 5.76 + 1.5 | 8.38 + 1.8 | 10.68 + 6.8 | 2.18 + 1.1 | 2.06 + 0.8 |
| Alanine | 4.05 + 1.4 | 6.88 + 1.7 | 2.00 + 1.3 | 6.28 + 4.1 | 3.45 + 1.6 | 6.06 + 1.4 | 7.82 ± 3.45 | 1.90 + 1.4 | 1.59 + 1 |
| Acetate | 2.57 + 1.0 | 3.74 + 1.2 | 1.25 + 1.0 | 3.00 + 1.8 | 2.11 + 1.2 | 3.05 + 1.0 | 4.58 + 2.4 | 1.10 + 1 | 1.22 + 1 |
Figure 3.
Comparison of T2r, PG and metabolites between PF1 of volunteers, and PF1 and PF2 of patients in each decade. T2r was significantly different across all age groups (P = 0.001). In contrast, proteoglycan values in 30-40 years was significantly increased compared to other decades (P = 0.02). Lactate showed a significant increase in 30-40 age group. Comparing PF1 of volunteers with patients there were no significant difference between the two in T2r and PG values across all age groups, however PF2 of patients had a lower T2r and PG value compared to the other two. In volunteers, lactate had a peak value at 30-40 year whereas in patients, the lactate and metabolites showed the highest values in 20-30 years after which it significantly decreased in 30-40-year group. Thereafter, there was a rise in lactate in 40-50 years group of PF1 of patients whereas there was continued decrease in PF2 group.
MRS clearly depicted the spectrum of the metabolic molecules lactate, alanine, acetate, and propionic acid. When analysed in volunteers across the age groups, lactate showed a significant increase in the 30-40 age group (10.53 ± 2.45) in comparison to the age group 20-30 (5.2 ± 1.95; P = 0.04). There was a decrease in lactate values in 40-50 years at 3 ± 1.7 (P = 0.02) (Table 2 and Figure 3).
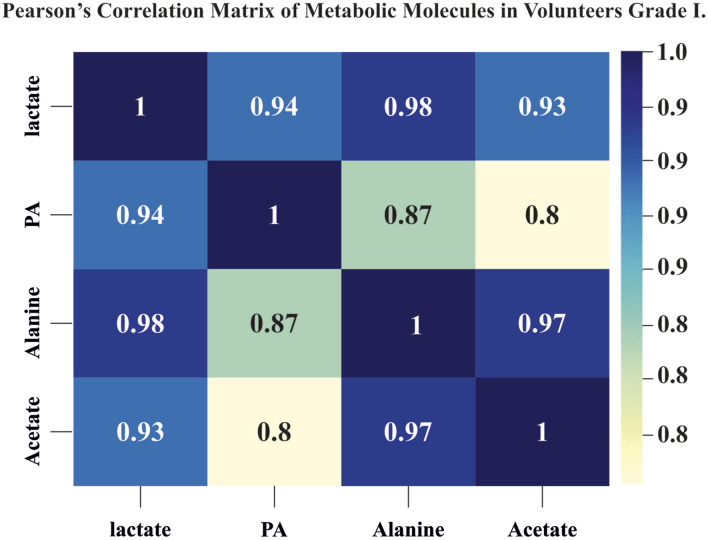
Alanine, acetate, and propionic acid showed a similar trend in their changes to lactate, with a Pearson’s correlation matrix ranging from 0.8 to 0.98, indicating a possible common metabolic pathway (Figure 4).
Figure 4.
Pearson’s correlation matrix of metabolic molecules in volunteer grade I: the lactate levels showed a significant positive correlation with values of alanine, acetate, and propionic acid showing the range of 0.8 to 0.97.
In LBP Patients
Comparing volunteers with LBP patients with PF1 showed no significant difference between the two with respect to T2r and PG values among all three groups. Whereas LBP patients with PF2 had a lower T2r and PG value compared to the other two (Table 2 and Figure 3).
Lactates and metabolites showed a different trend across the age groups in volunteers and LBP patients with PF2. In volunteers, lactate had a peak value at 30-40 years, resembling the anabolic peak of skeletal muscles. In patients, lactate and metabolites showed the highest values in group A, after which they showed a significant decrease in group B. Thereafter, there was a rise in lactate in group C, whereas there was a continued decrease in the PF2 group (Table 2 and Figure 3).
The study results highlight that among PF 1, there were two subsets, with one showing both normal structural and metabolic molecules (volunteers) and the other showing normal structural but altered metabolic molecules (LBP patients with PF 1). PF 2 discs, on the other hand, mostly had alterations in both structural and metabolic molecules.
Discussion
Magnetic resonance imaging (MRI) is the most widely used technique for assessing IVD status. It shows the hydration status and morphological characteristics of the disc based on proton density, water content, and biochemical composition. 15 The biochemical composition of the IVD at different phases of DDD has been studied in the recent decade using modern MRI techniques. These approaches investigate numerous characteristics, including relaxation durations, magnetization transfer (MT), spectroscopy, and the apparent diffusion coefficient (ADC). 16
T2r, T1rho, and CEST are useful to document collagen and proteoglycan content, as well as hydration, and MRS has been found to provide an accurate spectrum of both structural and metabolic molecules. 13 The ability of MRS to accurately define the absolute and relative molecular content has been well documented in the brain and prostrate but has not been extensively used in the spine.17-19 A recent study has successfully shown the ability of MRS to document the spectrum of the metabolites and the dynamic changes under different physiological conditions. 20 In a comparative study using MRS and provocative discography, Gornet et al found that MRS scores, using structural integrity markers (carbohydrate/collagen and PG) expected to decrease with disc degeneration and metabolites, namely alanine, lactate, and propionate, expected to increase with hypoxia and inflammation, could be obtained by quantifying spectral features from optimised clinical MRS data that identified painful discs in patients with chronic LBP with a sensitivity of 82% and a specificity of 88% when compared to provocative discography. 14 In our study, we deployed the sequences of T2r and MRS, as this combination would allow us to evaluate the entire milieu-interior by documenting the status of hydration, matrix proteins, and also the metabolites.
Pfirrmann Grade 1 has Metabolic Subsets
The most interesting observation of this study was that although PF1 was the same in both groups and across different age groups in routine MRI, there were considerable subsets when evaluated through T2r and MRS. In the volunteers, T2r was highest in the 20-30 age group, showing a gradual but not significant decrease in each decade, and proteoglycans showed no change in the three age groups. This clearly demonstrated that there was no significant alteration in the structural molecules and hydration among all PF1 discs in both LBP patients and volunteers, regardless of age. However, there were considerable differences in the metabolic molecules between both LBP patients and volunteers in the different age groups, showing that metabolic alterations precede changes in structural molecules. Metabolic changes alter the normal milieu, influencing both the energy cycle and protein formation, leading to degeneration.
Changes in Metabolites
Although lactate was the primadona of metabolic investigation, MRS was able to document other molecules such as alanine, acetate, and propionic acid. It was interesting to see that all four molecules have a similar trend of concentration, with Pearson’s correlation matrix coefficient ranging from 0.8 to 0.97. This indicates that all four molecules are involved in the same metabolic pathways, which is consistent with the newer concept of lactate and its actual role in disc and muscle metabolism. While the traditional concept was that lactate was a dead-end waste product, the accumulation of which led to a decreased pH and acidosis, 21 this can stop the production of proteoglycans, raise levels of inflammatory molecules like IL 1B and IL 6, and make the MMPS pathway work more, which can cause cells to age and matrix breakdown.10,22,23 However, there has been a complete shift in our understanding, and lactate is now documented to function as an energy molecule. 24 A recent study by Pushpa et al performed an MRS evaluation of lactate levels in discs in two diametrically opposite conditions of physiological stress. 20 Following intense, short-term, targeted low back exercises, they documented a significant decrease in the lactate spectrum in the disc, suggesting the use of lactate for fuel requirements.
In this study, we found alanine, acetate, and propionic acid to follow the changes in lactate in all study conditions. Wong et al have demonstrated that annulus fibrosis absorbs the lactate that NP produces through glycolysis, converts it to pyruvate, and then to acetyl CoA, which then enters the TCA cycle. 24 The pyruvate-acetyl CoA conversion produces alanine as one of its by-products. Other molecules, namely propionic acid and acetate, are also by-products of the pyruvate metabolism along with the TCA cycle. All of the above molecules changed consistently and linearly to lactate. This shows that they are all part of the TCA cycle in the energy metabolism of discs, not just involved in matrix production.
Lactate and other metabolic molecules showed considerable differences across age groups and between volunteers and patients. We observed that the lactate and metabolic increase in the 30-40 age group, indicating peak metabolic activity, is similar to the physiological peak observed in bone and muscle mass at this age. Subsequently, there is a decrease in metabolic activity in the 40-50 age group, corresponding to an overall decrease in anabolic activity observed elsewhere in the body. In PF Grade 1 patients, there was an alteration in that the peak lactate (9.9 ± 5.2) was in the 20-30 age group and then significantly reducing to 5.73 ± 2.15 and again raising high to 9.5 ± 2.25. According to Horner et al, 25 an increased lactate presence may also be explained by the accumulation that occurs with decreased cellular viability and activity. The increased lactate levels seen in LBP patients in the 20-30 age group probably indicate an early increase in pH with decreased cell viability. This reduces the metabolism in the 30-40 age group and then causes a progressive accumulation in the 40-50 age group.
Pfirrmann Grade 1 and Grade 2 Have Different Milieu-Interieur
The comparison of PF2’s T2r and MRS with those of PF1 revealed a clear distinction between the two groups. While the structural molecules, which are responsible for matrix integrity and disc hydration, were intact in PF 1, the matrix was significantly altered in PF 2. T2r showed no difference in PF 1 between volunteers and LBP patients, but a significant decrease in PF2.
However, even within PF1 discs, metabolic derangements and alterations were observed. The percentage of discs showing normal structural molecules but metabolic alterations was higher in volunteers compared to PF 1 of patients and PF 2 of patients. This clearly showed that MRS could be an effective radiological or imaging biomarker for identifying molecular changes before permanent matrix changes occurred.
We propose that the subset of Pfirrmann discs that showed altered metabolites but normal PG content and T2r may represent a unique imaging phenotype which are routinely missed on normal MRI sequences. This is important because it has been clearly shown that alterations in the mechanical pressure of the nucleus pulposus, which in turn is controlled by collagen and PG content, are detrimental to cellular viability and proliferation, leading to the failure of regenerative therapies. To date, the current practice has been to employ regenerative strategies only in degenerated discs identified by routine MRI when the opportunity has probably been lost. This situation was due to our inability to identify discs at the stage of molecular-level degeneration in vivo. Our study has shown that MRS and T2r can overcome this inability and shift the indication of regeneration therapies to the PF-1 disc, showing a molecular shift.
Limitation of the Study
The main limitation of the study is the limitation of performing the investigation in vivo on normal volunteers and patients, and hence the lack of a biochemical and pathological correlation. This is a one-point-time study, and it would have been interesting to see a follow-up of the discs showing metabolic alterations over a period of time, which will be one of the future aims of the study group.
Conclusion
Our study highlights that MRS and T2r are capable of identifying a subset within PF1 that exhibits metabolic alterations without structural changes. These discs may, in fact, be ideal for interventional therapies.
Acknowledgments
The authors thank Ms. Priya for her help in Data collection and maintenance.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Ganga Orthopedic Research and Education Foundation (GOREF). (GOREF-2019/11) (recognized by Department of Scientific and Industrial Research (DSIR) and cofunded by Department of Biotechnology (DBT), Govt of India, Grant reference no: BT/PR35631/MED/30/2186/2019.
Ethical Statement
Ethical Approval
Approved by the Institutional review board of Ganga Medical Center and Hospital, Coimbatore. (No: 2020/09/11).
ORCID iDs
Pushpa Bhari Thippeswamy https://orcid.org/0000-0001-8451-1729
Shanmuganathan Rajasekaran https://orcid.org/0000-0001-6043-006X
Karthik Ramachandran https://orcid.org/0000-0001-8194-4721
KS Sri Vijay Anand https://orcid.org/0000-0002-8885-5411
Ajoy Prasad Shetty https://orcid.org/0000-0001-5885-7152
Rishi Mugesh https://orcid.org/0000-0001-5817-4909
References
- 1.Murray CJ, Lopez AD. Measuring the global burden of disease. N Engl J Med. 2013;369(5):448-457. doi: 10.1056/NEJMra1201534 [DOI] [PubMed] [Google Scholar]
- 2.Maniadakis N, Gray A. The economic burden of back pain in the UK. Pain. 2000;84(1):95-103. doi: 10.1016/S0304-3959(99)00187-6 [DOI] [PubMed] [Google Scholar]
- 3.Meucci RD, Fassa AG, Faria NM. Prevalence of chronic low back pain: systematic review. Rev Saude Publica. 2015;49:1. doi: 10.1590/S0034-8910.2015049005874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suthar P, Patel R, Mehta C, Patel N. MRI evaluation of lumbar disc degenerative disease. J Clin Diagn Res. 2015;9(4):TC04-TC09. doi: 10.7860/JCDR/2015/11927.5761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brinjikji W, Diehn FE, Jarvik JG, et al. MRI findings of disc degeneration are more prevalent in adults with low back pain than in asymptomatic controls: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2015;36(12):2394-2399. doi: 10.3174/ajnr.A4498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckley CT, Hoyland JA, Fujii K, Pandit A, Iatridis JC, Grad S. Critical aspects and challenges for intervertebral disc repair and regeneration-harnessing advances in tissue engineering. JOR Spine. 2018;1(3):e1029. doi: 10.1002/jsp2.1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colombier P, Camus A, Lescaudron L, Clouet J, Guicheux J. Intervertebral disc regeneration: a great challenge for tissue engineers. Trends Biotechnol. 2014;32(9):433-435. doi: 10.1016/j.tibtech.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 8.Hegewald AA, Endres M, Abbushi A, et al. Adequacy of herniated disc tissue as a cell source for nucleus pulposus regeneration. J Neurosurg Spine. 2011;14(2):273-280. doi: 10.3171/2010.10.SPINE10223 [DOI] [PubMed] [Google Scholar]
- 9.Bibby SR, Urban JP. Effect of nutrient deprivation on the viability of intervertebral disc cells. Eur Spine J. 2004;13(8):695-701. doi: 10.1007/s00586-003-0616-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishihara H, Urban JP. Effects of low oxygen concentrations and metabolic inhibitors on proteoglycan and protein synthesis rates in the intervertebral disc. J Orthop Res. 1999;17(6):829-835. doi: 10.1002/jor.1100170607 [DOI] [PubMed] [Google Scholar]
- 11.Togao O, Hiwatashi A, Wada T, et al. A qualitative and quantitative correlation study of lumbar intervertebral disc degeneration using glycosaminoglycan chemical exchange saturation transfer, Pfirrmann grade, and T1-ρ. AJNR Am J Neuroradiol. 2018;39(7):1369-1375. doi: 10.3174/ajnr.A5657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishikawa T, Watanabe A, Kamoda H, et al. Evaluation of lumbar intervertebral disc degeneration using T1ρ and T2 magnetic resonance imaging in a rabbit disc injury model. Asian Spine J. 2018;12(2):317-324. doi: 10.4184/asj.2018.12.2.317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiong X, Zhou Z, Figini M, Shangguan J, Zhang Z, Chen W. Multi-parameter evaluation of lumbar intervertebral disc degeneration using quantitative magnetic resonance imaging techniques. Am J Transl Res. 2018;10(2):444-454. [PMC free article] [PubMed] [Google Scholar]
- 14.Gornet MG, Peacock J, Claude J, et al. Magnetic resonance spectroscopy (MRS) can identify painful lumbar discs and may facilitate improved clinical outcomes of lumbar surgeries for discogenic pain. Eur Spine J. 2019;28:674-687. doi: 10.1007/s00586-018-05873-3 [DOI] [PubMed] [Google Scholar]
- 15.Tertti M, Paajanen H, Laato M, Aho H, Komu M, Kormano M. Disc degeneration in magnetic resonance imaging. A comparative biochemical, histologic, and radiologic study in cadaver spines. Spine. 1991;16(6):629-634. doi: 10.1097/00007632-199106000-00006 [DOI] [PubMed] [Google Scholar]
- 16.Mallio CA, Vadalà G, Russo F, et al. Novel magnetic resonance imaging tools for the diagnosis of degenerative disc disease: a narrative review. Diagnostics. 2022;12(2):420. doi: 10.3390/diagnostics12020420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horská A, Barker PB. Imaging of brain tumors: MR spectroscopy and metabolic imaging. Neuroimaging Clin. 2010;20(3):293-310. doi: 10.1016/j.nic.2010.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinberg BD, Kuruva M, Shim H, Mullins ME. Clinical applications of magnetic resonance spectroscopy in brain tumors: from diagnosis to treatment. Radiol Clin. 2021;59(3):349-362. doi: 10.1016/j.rcl.2021.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JK, Kim DY, Lee YH, et al. In vivo differential diagnosis of prostate cancer and benign prostatic hyperplasia: localized proton magnetic resonance spectroscopy using external-body surface coil. Magn Reson Imaging. 1998;16(10):1281-1288. doi: 10.1016/s0730-725x(98)00110-6 [DOI] [PubMed] [Google Scholar]
- 20.Pushpa BT, Rajasekaran S, Easwaran M, et al. ISSLS PRIZE in basic science 2023: lactate in lumbar discs-metabolic waste or energy biofuel? Insights from in vivo MRS and T2r analysis following exercise and nimodipine in healthy volunteers. Eur Spine J. 2023;32(5):1491-1503. doi: 10.1007/s00586-023-07540-8 [DOI] [PubMed] [Google Scholar]
- 21.Ohshima H, Urban JP. The effect of lactate and pH on proteoglycan and protein synthesis rates in the intervertebral disc. Spine. 1992;17(9):1079-1082. doi: 10.1097/00007632-199209000-00012 [DOI] [PubMed] [Google Scholar]
- 22.Razaq S, Wilkins RJ, Urban JP. The effect of extracellular pH on matrix turnover by cells of the bovine nucleus pulposus. Eur Spine J. 2003;12(4):341-349. doi: 10.1007/s00586-003-0582-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bibby SR, Jones DA, Ripley RM, Urban JP. Metabolism of the intervertebral disc: effects of low levels of oxygen, glucose, and pH on rates of energy metabolism of bovine nucleus pulposus cells. Spine. 2005;30(5):487-496. doi: 10.1097/01.brs.0000154619.38122.47 [DOI] [PubMed] [Google Scholar]
- 24.Wang D, Hartman R, Han C, et al. Lactate oxidative phosphorylation by annulus fibrosus cells: evidence for lactate-dependent metabolic symbiosis in intervertebral discs. Arthritis Res Ther. 2021;23(1):145. doi: 10.1186/s13075-021-02501-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horner HA, Urban JP. 2001 volvo award winner in basic science studies: effect of nutrient supply on the viability of cells from the nucleus pulposus of the intervertebral disc. Spine (Phila Pa 1976). 2001;26(23):2543-2549. doi: 10.1097/00007632-200112010-00006 [DOI] [PubMed] [Google Scholar]