Abstract
Background:
Postoperative knee arthrofibrosis after arthroscopic ligament reconstruction is a serious complication. Among adolescents, risk factors for postoperative arthrofibrosis are not well characterized and the effectiveness of early manipulation under anesthesia (MUA) is not well established.
Purposes:
To identify risk factors for arthrofibrosis after arthroscopic knee ligament reconstruction in adolescent patients and to evaluate the safety and effectiveness of early MUA.
Study Design:
Case-control study; Level of evidence, 3.
Methods:
The charts of all adolescent patients (<19 years of age) who underwent early MUA (<3 months) for knee stiffness after anterior cruciate ligament (ACL) or medial patellofemoral ligament (MPFL) reconstructions between 2008 and 2021 were retrospectively reviewed. Patients were matched 2:1 with patients without MUA from the same study period. The primary outcome was the final range of motion (ROM) after MUA. Logistic regression analysis was performed to identify predictors of MUA.
Results:
A total of 25 patients (10 with ACL reconstruction and 15 with MPFL reconstruction) with a mean age of 14.8 ± 2.6 years were included for analysis. Overall, 44% were skeletally immature. Patients underwent MUA at a mean of 63.3 ± 19.5 days after the index surgery. The mean ROM improved significantly from 96.3°± 20.5° to 135°± 9.7° after MUA after a median follow-up of 8.1 months (interquartile range, 5.4-15.0 months). There were no complications associated with MUA, but 2 patients (8.0%) had MUA treatment failure. There were no differences in body mass index, type and frequency of associated procedures, or patellar height on lateral radiographs between the cohorts. The MUA cohort had statistically significant increased operative time, decreased preoperative motion, decreased ROM at 6 weeks postoperatively, and increased pain at 6 weeks postoperatively when compared with the non-MUA cohort. Regression analysis demonstrated that ROM at 6 weeks (OR: 0.83, 95% CI, 0.69-0.98, p = .034) was significantly associated with the need for MUA.
Conclusion:
The findings of this study suggest that early (<3 months) MUA is safe and effective in treating knee arthrofibrosis in adolescent patients. MUA is a treatment alternative for patients with restricted ROM at 6 weeks that may help them recover full ROM.
Keywords: knee ligaments, ACL; knee arthrofibrosis; manipulation under anesthesia; anterior cruciate ligament reconstruction; medial patellofemoral ligament reconstruction; pediatric sports medicine
Anterior cruciate ligament (ACL) reconstruction procedures have dramatically increased in the past few decades, with the largest increase among patients <15 years of age (924% between 1994 and 2006).2,9,29 Similarly, patellar injuries including patellofemoral instability account for approximately one-third (29.5%) of all knee injuries among high school athletes. 26 Not surprisingly, medial patellofemoral ligament (MPFL) reconstruction for patellar stabilization has steadily increased in the last decade. 27 With an increase in the incidence of ligament injuries and surgeries in adolescents, uncommon complications such as postoperative knee arthrofibrosis are increasingly being reported in these younger patients.19,20 Arthrofibrosis after ACL and MPFL reconstructions has been reported to occur in children at rates of 1.8% to 8.3%4,18,25 and 0.6% to 5.6%, 5 respectively.
While uncommon, knee arthrofibrosis is a serious complication after knee surgery. 18 In addition to pain and restriction in activities, it can lead to arthritis. 23 Histological studies have shown that organization and maturation of adhesive scar tissue progresses over 6 months, and early intervention in the initial months can break immature scars easily. 14 Thus, early intervention that begins with prompt diagnosis and an aggressive treatment approach is warranted to prevent established arthrofibrosis. 6 Current treatment strategies include manipulation under anesthesia (MUA) with or without lysis of adhesions (LOA). LOA can be challenging and may be associated with significant morbidity and compromise functional outcomes. 18 As such, strategies to treat arthrofibrosis without the need for LOA are preferred. However, there is a paucity in the literature on the safety and effectiveness of early MUA to treat knee arthrofibrosis and prevent the need for LOA, particularly in adolescent patients. Furthermore, identifying patients with postoperative stiffness who will ultimately develop arthrofibrosis can be challenging. Baghdadi et al 1 reported that patients who did not experience significant improvement in knee range of motion (ROM) in the first 5 to 8 weeks and had a total ROM deficit >50°, compared with the contralateral side, were associated with needing operative intervention for arthrofibrosis. Similarly, Ouweleen et al 19 reported a 14.7 increased odds of developing arthrofibrosis in patients who were unable to attain 90° of flexion 6 weeks after ACL reconstruction.
These findings underscore a critical time frame to intervene for patients at risk of developing arthrofibrosis. Previous reports on the effect of timing of intervention for arthrofibrosis have identified 3 months as a cutoff point; any intervention before 3 months to restore normal knee motion could be considered treatment for early arthrofibrosis, whereas efforts to regain motion after 3 months could be considered as treatment of established arthrofibrosis.7,10,11,16 The first aim of this study was to identify risk factors for arthrofibrosis after arthroscopic knee ligament reconstruction in adolescent patients. The second aim was to evaluate the effectiveness of early (<3 months) MUA to treat arthrofibrosis in these cases. We hypothesized that patients who underwent early MUA would regain normal ROM without complications or the need for LOA.
Methods
This study was approved by our institutional review board. A list of all ligamentous arthroscopic knee procedures between 2008 and 2021 was obtained to identify adolescent patients who had undergone ACL or MPFL reconstructions. From this cohort, patients who required MUA after the index procedure were identified. Patients >19 years of age, revision cases, those who underwent LOA without prior MUA, staged multiligament knee reconstruction, and charts with lack of proper documentation were excluded. Nineteen years was chosen based on the definition of “adolescent” by the World Health Organization. 24 Patients who met inclusion criteria were assessed for preoperative characteristics including age, body mass index (BMI), comorbidities, and associated injuries.
Skeletal maturity was evaluated on preoperative knee magnetic resonance imaging by assessing the distal femur and proximal tibia physes based on the study by the JUPITER (Justifying Patellar Instability Treatment by Results) study group. 8 In brief, if the low signal of the physis on an intermediate-weighted sequence could be visualized along the entire physis (without a central closure), then the physis was classified as open. Partially closed physes were classified as closing/closed. Patellar height (Caton-Deschamps index [CDI]) was assessed on preoperative and postoperative lateral knee radiographs. Intraoperative parameters including surgical technique, associated procedures, surgical time, and complications were recorded. A goniometer was used to assess preoperative and postoperative knee motion at each physical therapy visit. To identify risk factors for arthrofibrosis, patients’ age, sex, and procedure were matched in a 2:1 ratio to a control group of patients who regained full ROM after surgery without any intervention. The differences identified between cohorts, including age, race, length of surgery, and ROM before the index surgery and 6 weeks after the index surgery, were then selected as variables to include in the predictive model. For the second aim, the effectiveness of MUA was evaluated with the final ROM after MUA as the primary outcome. Secondary outcomes were complications of MUA, including failure of MUA, which was defined as the need for a second MUA or LOA. All surgeries and MUAs were performed by 1 of the 2 senior surgeons (E.J.W. and S.N.P.).
Surgical Technique
ACL reconstruction was performed with either hamstring (HS) tendon or quadriceps tendon autograft. All but 4 patients underwent a transphyseal or adult-type ACL reconstruction technique with suspensory fixation on the femoral side and interference screw fixation on the tibial side. The remaining 4 patients underwent all-epiphyseal ACL reconstruction with HS autograft utilizing the convergent tunnel technique. 13 Associated procedures included meniscal debridement/repair and/or chondroplasty/microfracture. All patients were enrolled in a routine postoperative ACL physical therapy protocol, starting in the first week after surgery.
MPFL reconstruction was performed with either HS tendon autograft or allograft. A single tendon graft was passed through a 3.5-mm bone tunnel that was made proximal to the equator of the patella. The femoral attachment point was identified on fluoroscopy per Schöttle point, and fixation was performed with an interference screw with the knee at >45° of flexion. Associated procedures included tibial tubercle osteotomy, chondral debridement/drilling, removal of loose body, or osteochondral fracture fixation. All patients were enrolled in a postoperative MPFL protocol with physical therapy starting within the first week of surgery.
There was no restriction in ROM after either ACL or MPFL reconstruction. If meniscal repair was performed, ROM was restricted from 0° to 90° for 6 weeks. In the study cohort, no patients underwent cartilage repair or regeneration. Associated microfracture and chondroplasty did not change the postoperative protocol.
MUA Indication and Technique
Patients who were believed to be at risk for needing early MUA were those who failed to meet knee motion goals of 90° of motion at 3 weeks and 120° of motion at 6 weeks. If knee ROM at 3 weeks was <90°, patients were prescribed nonsteroidal anti-inflammatory drugs, given a compression knee sleeve (if there was swelling), and counseled on the need for MUA if ROM did not improve. Home exercises and compliance with physical therapy were reinforced. If ROM was <120° by 6 weeks, an attempt was made to be more aggressive with education and physical therapy to try to regain flexion beyond 120°, and patients were given an additional 4 to 6 weeks to achieve it. MUA was indicated if patients were unable to achieve 120° of flexion at around 3 months. MUA was performed utilizing the technique described by Noyes et al. 17 In brief, under general anesthesia and muscle relaxation, patellar mobilization was first performed in all directions with the knee in extension. Then the hip was flexed to 90°, followed by slow and gradual knee flexion over 2 to 5 minutes, and hand over the proximal tibia. Separation of adhesions can be palpable and sometimes audible. The knee was then placed through slow, repeated cycles of flexion-extension with a gradual increase in knee flexion with each cycle. Once full or near-full knee flexion was obtained, a photograph was taken with the knee in full extension and flexion to share with the patient and family. If the patient had knee pain or swelling before MUA, then a mixture of 80 mg Depo-Medrol (methylprednisolone; Pfizer) and 3 mL 0.25% Marcaine (bupivacaine; Pfizer) was injected in the knee under sterile conditions. After MUA, arrangements were made for the patient to attend a physical therapy session on the day of the procedure, before being discharged home. After MUA, patients were encouraged to attend physical therapy sessions at least twice a week. Patients were seen back in the clinic at 2 to 3 weeks after MUA. Patients who experienced MUA failure, defined by having a final ROM <110° or requiring repeat MUA with or without LOA, were identified; 110° was chosen based on a functional study demonstrating a minimum of 110° ROM for normal everyday activity. 22
Statistical Analysis
Descriptive statistics are used to summarize the data, with means and standard deviations for normally distributed variables and medians with interquartile ranges (IQRs) for nonparametric data.
Specific analytical methods were applied to compare variables of interest within and between groups. Within the MUA cohort, changes in ROM and patellar height over time were evaluated using repeated-measures analysis of variance. To discern disparities between the MUA and non-MUA cohorts, either a paired t test or Wilcoxon signed-rank test (depending on data distribution) was conducted for continuous variables, and a chi-square test was performed for categorical variables.
Binary logistic regression was performed to assess predictors of MUA. The model was constructed as a fixed-effects model to allow for the estimation of the probability of experiencing MUA based on the values of the independent variables in the model. Factors identified as significant predictors in univariate analyses were further examined to assess their independent associations with MUA while adjusting for BMI, graft characteristics, time between injury and index surgery, patellar height, and associated procedures.
A significance threshold of P < .05 was utilized to denote statistical significance. Data was analyzed using IBM SPSS statistics software, version 28 ( IBM Corp.).
Results
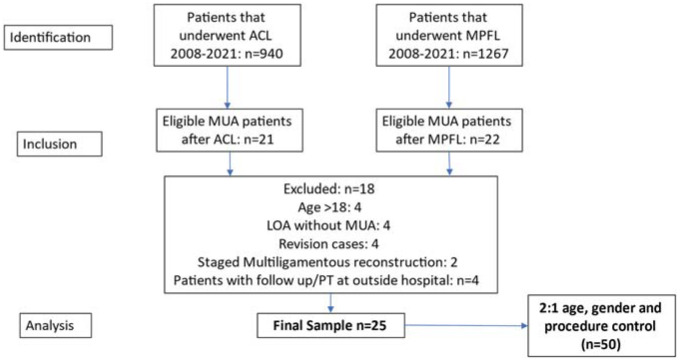
There were 940 ACL reconstructions and 1267 MPFL reconstructions during the study period. Of these, 21 patients (2.2%) with ACL reconstruction and 22 patients (1.7%) with MPFL reconstruction underwent MUA for limited ROM (Figure 1). After exclusion criteria were applied, a sample of 25 patients (10 with ACL reconstruction and 15 with MPFL reconstruction) was included for matched-pair analysis. The mean age was 14.8 ± 2.6 years, 52.0% were female, 44% had an open physis on magnetic resonance imaging, and the mean BMI was 24.3 ± 6.0 kg/m2 (Table 1). At the time of the index surgery, most patients (80.0%) required concomitant procedures including meniscal debridement/repair, chondroplasty/microfracture, or osteochondral fracture fixation. All patients followed standard postoperative protocols with early physical therapy and periodic clinical follow-up. Patients underwent MUA at a mean of 63.3 ± 19.5 days (range, 28-119 days) after the index surgery. The median follow-up was 8.1 months (IQR, 5.4-15.0 months).
Figure 1.
Flowchart of study population acquired from all anterior cruciate ligament (ACL) and medial patellofemoral ligament (MPFL) reconstructions between 2008 and 2021. LOA, lysis of adhesions; MUA, manipulation under anesthesia; PT, physical therapy.
Table 1.
Patient Characteristics a
| Value | |
|---|---|
| Total patients | 25 |
| Age, y | 14.8 ± 2.6 |
| Female sex | 52.0 |
| Race | |
| White | 40.0 |
| Black | 60.0 |
| BMI, kg/m2 | 24.3 ± 6.0 |
| Open physis on MRI | 44 |
| Time between index surgery and MUA, days | 63.3 ± 19.5 |
| Patellar index b | |
| Before index surgery | 1.16 ± 0.23 |
| Before MUA | 0.94 ± 0.17 |
| After MUA | 1.08 ± 0.20 |
| Type of injury | |
| ACL | 40.0 |
| MPFL | 60.0 |
| Laterality | |
| Right | 64.0 |
| Left | 36.0 |
| Steroid injection during MUA | 40.0 |
| Follow-up, mo, median (IQR) | 8.1 (5.4-15.0) |
| Failed MUA c | 8.0 |
Data are presented as mean ± SD or percentage unless otherwise indicated. ACL, anterior cruciate ligament; BMI, body mass index; IQR, interquartile range; MPFL, medial patellofemoral joint; MRI, magnetic resonance imaging; MUA, manipulation under anesthesia.
Normal Caton-Deschamps index: 0.6-1.2.
Second manipulation under anesthesia and/or requiring lysis of adhesions after manipulation under anesthesia.
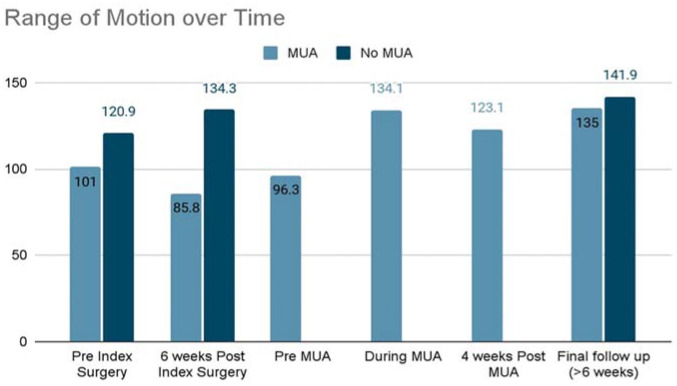
Analysis of ROM demonstrated significant differences in ROM over time (Figure 2). ROM achieved during MUA was significantly greater compared with before the index surgery (P < .001), 6 weeks after the index surgery (P < .001), and before MUA ROM (P < .001). ROM improvement slightly declined at 4 weeks after MUA, but subsequently increased significantly at >6 weeks after MUA (P = .002). Patellar height did not significantly change over time. Although CDI decreased between the index surgery and MUA, this did not reach statistical significance (1.16 ± 0.23 vs 0.94 ± 0.17; P = .304).
Figure 2.
Comparison of range of motion (degrees) over time between groups. MUA, manipulation under anesthesia.
Matched cohort analysis (Table 2) demonstrated a greater proportion of African American patients (60.0% vs 19.1%; P = .002) in the MUA cohort compared with the non-MUA cohort. There were no significant differences in BMI, the type and frequency of associated procedures, or radiographic patellar height between cohorts. However, the operative time of the index surgery was significantly longer in the MUA cohort compared with the non-MUA cohort (106.1 ± 20.3 vs 86.9 ± 27.2 minutes; P = .005). ROM was significantly less in the MUA cohort before the index surgery (101.0°± 52.6° vs 120.9°± 28.0°; P = .033) and 6 weeks after the index surgery (85.8°± 28.4° vs 134.3°± 12.3°; P < .001). However, ROM was similar between groups (135.0°± 10.4° vs 141.9°± 8.5°; P = .181) at the final follow-up. Overall, 28.6% of the MUA cohort reported pain 6 weeks after the index surgery compared with 12.8% of the non-MUA cohort (P = .114). Binary logistic regression analysis identified a significant association between ROM at 6 weeks after the index surgery (OR: 0.83, 95% CI, 0.69-0.98, p = .034) and the need for MUA to treat early arthrofibrosis (Table 3).
Table 2.
Matched Cohort Analysis a
| No MUA | MUA | P | |
|---|---|---|---|
| Total patients | 50 | 25 | |
| Age, y | 15.4 ± 1.9 | 14.8 ± 2.6 | .052 |
| Female sex | 56.0 | 52.0 | .510 |
| Race | |||
| White | 74.5 | 40.0 | .002 |
| Black | 19.1 | 60.0 | |
| Other | 6.4 | 0.0 | |
| BMI, kg/m2 | 24.1 ± 6.5 | 24.3 ± 6.0 | .230 |
| Associated procedures | |||
| None | 32.0 | 20.0 | .070 |
| Meniscal debridement | 2.0 | 20.0 | |
| Meniscal repair | 20.0 | 8.0 | |
| Chondroplasty/microfracture | 28.0 | 48.0 | |
| Loose body removal | 5.0 | 4.0 | |
| Hemiepiphysiodesis | 10.0 | 0.0 | |
| Osteotomy | 2.0 | 0.0 | |
| Graft type | |||
| Hamstring tendon | 88.0 | 92.0 | .657 |
| Quadriceps | 4.0 | 0.0 | |
| Bone patellar tendon | 2.0 | 0.0 | |
| Allograft b | 6.0 | 8.0 | |
| Graft size (ACL only), mm | 9.4 ± 1.1 | 9.3 ± 0.8 | .699 |
| Operative time, min | 86.9 ± 27.2 | 106.1 ± 20.3 | .005 |
| Time between injury and index surgery, days, median (IQR) | 46 (27.5-182.0) | 37.0 (23.8-75.0) | .145 |
| Patellar index c | |||
| Before index surgery | 1.13 ± 0.16 | 1.16 ± 0.23 | .462 |
| After index surgery | 1.08 ± 0.14 | 0.94 ± 0.17 d | .088 |
| Before MUA | — | 1.08 ± 0.13 | — |
| Pain 6 wk after index surgery | |||
| Mean reported VAS (0-10) | 0.26 | 1.43 | <.001 |
| Reporting pain | 12.8 | 28.6 | .114 |
| ROM, deg | |||
| Before index surgery | 120.9 ± 28.0 | 101.0 ± 52.6 | .033 |
| 7-10 days after index surgery | 72.5 ± 22.8 | 59.2 ± 29.4 | .059 |
| 6 wk after index surgery | 134.3 ± 12.3 | 85.8 ± 28.4 | <.001 |
Data are presented as mean ± SD or percentage unless otherwise indicated. Bold P values indicate statistical significance. ACL, anterior cruciate ligament; BMI, body mass index; IQR, interquartile range; MUA, manipulation under anesthesia; ROM, range of motion; VAS, visual analog scale. Dashes indicate no data available. Blank cell indicates no p value for sample size between cohorts as this was a 2:1 match study.
Allograft only used for medial patellofemoral ligament reconstruction.
Normal Caton-Deschamps index: 0.6-1.2.
Before manipulation under anesthesia.
Table 3.
Factors Associated With Manipulation Under Anesthesia a
| OR | 95% CI | P | |
|---|---|---|---|
| Age, y | 0.825 | 0.34-1.9 | .664 |
| Race (reference: African American) | 2.72 | 0.07-112.61 | .377 |
| Operative time | 1.01 | 0.91-1.12 | .229 |
| Range of motion 6 wk after index surgery | 0.83 | 0.69-0.98 | .034 |
Bold P value indicates statistical significance. OR, odds ratio.
MUA treatment failed in 2 patients (8.0%). One patient who underwent MPFL reconstruction required LOA 5 months after MUA for ROM of 10° to 100°. This patient had a grade 2 chondral injury in the medial patellar facet and underwent debridement at the time of the index surgery. The initial MUA was performed 90 days after the index surgery. The ROM after LOA was 0° to 120°. This patient continued to experience tightness in early flexion due to anterior placement of the femoral tunnel, and MPFL was subsequently revised 2 years after the index surgery. Another patient who experienced MUA failure had undergone an all-epiphyseal ACL reconstruction and lateral meniscus debridement. First, MUA was performed 76 days after the index procedure. After the first MUA, this patient had an ROM of 40° to 100° and underwent a repeat MUA with subsequent extension casting. The final ROM at 16 months was 2° to 140°.
Discussion
The rates of postoperative knee fibrosis after ACL (2.2%) and MPFL (1.7%) reconstruction in the present study are consistent with those in other reports in the literature, and fortunately this is not a common event. Arthrofibrosis after ACL reconstruction has been reported to occur in children at a rate of 1.8% to 8.3%.4,18,25 Limited ROM after MPFL reconstruction can occur because of graft malposition, but true arthrofibrosis has been reported to occur in 0.6% to 5.6% of cases in the literature. 5
Several recent studies have reported on the risk factors associated with arthrofibrosis after ACL reconstruction in adolescent patients. In 1 study, the use of patellar tendon autograft, decreased ROM at 6 weeks, and female sex were predictive of arthrofibrosis after ACL reconstruction in children. 19 While we did not find differences in concomitant procedures, sex, graft type, or age between groups, the MUA cohort was associated with a greater proportion of African American patients, longer operative time, worse pain after surgery, and greater restriction in ROM before the index surgery and at 6 weeks after the index surgery. However, regression analysis identified ROM at 6 weeks as predictive of the need for MUA to treat early arthrofibrosis. Graft size has also been found to contribute to arthrofibrosis after ACL reconstruction. Su et al 25 reported that a 10-mm graft diameter was independently associated with a 3.2 times increased odds of arthrofibrosis after ACL reconstruction. In the present study, graft size did not appear to be associated with the need for MUA. The mean ACL graft diameter did not differ between groups (no MUA: 9.4 ± 1.1 mm vs MUA: 9.3 ± 0.8 mm; P = .699), and all grafts ranged from 8 mm to 10.5 mm. While graft size is an important consideration for ACL reconstruction in children and adolescent patients, a size threshold has not been established, and other factors are also likely to contribute to the development of knee arthrofibrosis.
Uncontrolled pain may play a role in the limitation of movement, 12 and there is some evidence that successful postoperative pain control can reduce the incidence of postsurgical fibrosis of the knee. 3 Pain at 6 weeks was more prevalent in the MUA cohort among patients with ACL and MPFL reconstructions. The senior author (S.N.P.) routinely recommends a compression sleeve if swelling is present and anti-inflammatory medication in patients with an ROM <90° at their first follow-up visit (3-4 weeks). However, if ROM does not improve by 6 weeks after the index surgery, patients may be at risk for needing early MUA to prevent arthrofibrosis becoming established with dense scar tissue and the need for more invasive procedures, like LOA. This approach is supported by other studies in the literature that have reported improved ROM and prevention of arthrofibrosis when MUA is performed within 3 months of the index surgery.10,11,17 In addition to LOA being an invasive procedure to regain ROM, the outcomes of LOA are inferior when compared with MUA. LOA is associated with more morbidity, more complications, and less improvement in ROM when compared with MUA. 7
Identifying patients at risk of developing knee arthrofibrosis can be challenging, as many patients complain of knee pain or stiffness early in the postoperative period. In a recent report, Baghdadi et al 1 demonstrated that an ROM deficit of >50° compared with the contralateral side 5 to 8 weeks after ACL reconstruction has high specificity (93%) and sensitivity (89%) for needing surgical intervention to improve ROM. Despite early recognition, MUA with or without LOA was performed at a mean of 3.2 months (range, 2.1-8 months) after the index surgery. Furthermore, of the 18 patients who required intervention, 6 (33%) underwent MUA with LOA. The present study adds to this body of knowledge, demonstrating that early MUA (6-11 weeks) can treat early arthrofibrosis without the need of LOA with a >90% success rate. The initial MUA treatment was considered to have failed for 2 patients (8.0%). One patient underwent an all-epiphyseal ACL reconstruction and required a repeat MUA and subsequent extension casting for an ROM of 40° to 100°. While this patient had both flexion and extension limitations, MUA is more appropriate for flexion-type deficits, and extension deficits may be better treated with other treatments, like extension casting. 20 Meanwhile, the other patient underwent MPFL reconstruction with HS tendon autograft. MUA was performed for an ROM deficit of 10° to 100°. This patient had a grade 2 chondral injury in the medial patellar facet and underwent debridement at the time of the index surgery. The ROM after LOA was 0° to 120°. This patient continued to experience tightness in early flexion due to anterior placement of the femoral tunnel, and the MPFL was subsequently revised 2 years after the index surgery. It is important to recognize tunnel malposition as a possible cause of postoperative knee ROM deficit, as MUA may not be effective in the setting of graft malposition.
There were no complications associated with MUA. It is presumed that early MUA can safely break up initial scar tissue before thick fibrous bands are formed and before maturation of scar tissue. 14 Timing of MUA in the present study is perhaps the biggest difference compared with reports in which iatrogenic physeal injury was observed during MUA at 18 to 24 weeks. 28 Haller et al 10 recommended early (<3 months) MUA as patients who underwent MUA at 2.9 months regained normal motion compared with patients who underwent MUA at 4.9 months. Similarly, Evans et al 7 reported successful results when MUA was performed at 2.6 months.
Patella infera has previously been identified as a radiographic finding associated with knee arthrofibrosis.12,21 In the present study, patellar height, measured by CDI, was not significantly different between matched groups. While CDI slightly decreased between the index surgery and MUA, this did not reach statistical significance. Furthermore, patellar height did not significantly differ after MUA. Patella infera may be a useful diagnostic tool for established arthrofibrosis when there is patellar tendon scarring; however, if arthrofibrosis is located in other areas of the knee, it may not be apparent. Furthermore, the patients who underwent MUA likely did not have established arthrofibrosis and therefore patellar height may not be helpful as an indicator of early knee arthrofibrosis.
Limitations
There are several limitations inherent to the retrospective nature of this study. There was no standardized technique for ACL and MPFL reconstruction, and concomitant procedures were done on a case-by-case basis based on the surgeons’ expertise. However, the postoperative rehabilitation protocol was standardized for all patients. In addition, we recognize that certain concomitant procedures such as osteotomies and meniscal repairs may be associated with a prolonged (approximately 6 weeks) limitation in ROM to protect the repair. In this scenario, the timing of ROM goals may have to be modified before recommending MUA. In general, if the patient is making progress in ROM, MUA may not have to be performed. However, in cases in which there is no improvement in ROM, MUA can be performed and is likely more effective if performed within 3 months of the index surgery. 15 Tunnel malposition can be a cause of limited knee ROM after MPFL and ACL reconstruction. Tunnel position was not assessed or evaluated, and it is possible that the limited ROM in some patients was due to tunnel malposition. However, in our experience, MUA is not effective at treating ROM deficits in patients with tunnel malposition. Knee ROM measurements were obtained from the physical therapy encounters to have consistency in measuring technique as goniometers are not routinely used in the clinic by all providers. Patient-reported outcomes were not collected, and it is possible that even after restoration of motion, they may have suboptimal outcomes. There is no definition of arthrofibrosis based on time, 12 and a 3-month threshold was chosen as an arbitrary time point to define arthrofibrosis. However, there is a difference between early and established arthrofibrosis from a pathophysiological standpoint, and the findings of this study propose that MUA is effective in early arthrofibrosis. Follow-up was also limited to a median of 8.1 months. While ROM is unlikely to decrease after 6 months, it is possible that MUA can stretch the graft, which can lead to greater failure rates at 2 years. Postoperative knee fibrosis is a rare occurrence, and the number of patients with MUA after single-ligament reconstruction surgery is low. This limits the ability to study this type of complication to retrospective studies with inherent limitations. Regardless, increasing awareness and improvement in management of this condition will help better characterize risk factors to prevent postoperative knee fibrosis.
Conclusion
The findings of this study demonstrate that early knee arthrofibrosis after knee arthroscopy can be safely and successfully treated with MUA in adolescent patients. The mean time to MUA after the index surgery was approximately 2 months, well below the recommended 3-month threshold for MUA reported in the literature. There were no complications associated with MUA. Concomitant procedures such as meniscal debridement/repair and cartilage preservation techniques were common in the MUA population; however, the type and rate of these procedures were similar in the non-MUA cohort. Matched cohort analysis also highlighted significant differences in presurgery and early postoperative ROM that may help surgeons predict the need for early MUA for patients undergoing knee ligament reconstruction procedures. ROM achieved during MUA is likely the maximum ROM that patients will achieve; however, it may take >6 weeks after MUA to determine ultimate improvement in ROM. Although further research is warranted to better characterize risk factors for knee arthrofibrosis in adolescent patients, early recognition and management with early MUA is a safe and effective strategy to help adolescent patients regain full ROM without invasive LOA.
Footnotes
Final revision submitted April 19, 2024; accepted May 14, 2024.
One or more of the authors has declared the following potential conflict of interest or source of funding: A.M.L. has received education payments from Piedmont Plus Innovation and hospitality payments from NuVasive. W.P. has received education payments from Pinnacle and hospitality payments from Stryker. E.J.W. has received education payments from Legacy Ortho. S.N.P. has received consulting fees from Pfizer. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from Cincinnati Children's Hospital (2022-0180).
References
- 1. Baghdadi S, Ganley TJ, Wells L, Lawrence JTR. Early identification of arthrofibrosis in adolescents following anterior cruciate ligament reconstruction is associated with the need for subsequent surgery: a matched case-control study. Arthroscopy. 2022;38(7):2278-2286. [DOI] [PubMed] [Google Scholar]
- 2. Buller LT, Best MJ, Baraga MG, Kaplan LD. Trends in anterior cruciate ligament reconstruction in the United States. Orthop J Sports Med. 2015;3(1):2325967114563664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cosgarea AJ, Sebastianelli WJ, DeHaven KE. Prevention of arthrofibrosis after anterior cruciate ligament reconstruction using the central third patellar tendon autograft. Am J Sports Med. 1995;23(1):87-92. [DOI] [PubMed] [Google Scholar]
- 4. Cruz AI Jr, Fabricant PD, McGraw M, Rozell JC, Ganley TJ, Wells L. All-epiphyseal ACL reconstruction in children: review of safety and early complications. J Pediatr Orthop. 2017;37(3):204-209. [DOI] [PubMed] [Google Scholar]
- 5. Drez D Jr, Edwards TB, Williams CS. Results of medial patellofemoral ligament reconstruction in the treatment of patellar dislocation. Arthroscopy. 2001;17(3):298-306. [DOI] [PubMed] [Google Scholar]
- 6. Ekhtiari S, Horner NS, de Sa D, et al. Arthrofibrosis after ACL reconstruction is best treated in a step-wise approach with early recognition and intervention: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2017;25(12):3929-3937. [DOI] [PubMed] [Google Scholar]
- 7. Evans KN, Lewandowski L, Pickett A, Strauss JE, Gordon WT. Outcomes of manipulation under anesthesia versus surgical management of combat-related arthrofibrosis of the knee. J Surg Orthop Adv. 2013;22(1):36-41. [DOI] [PubMed] [Google Scholar]
- 8. Fabricant PD, Heath MR, Veerkamp M, et al. Reliability of radiologic assessments of clinically relevant growth remaining in knee MRI of children and adolescents with patellofemoral instability: data from the JUPITER cohort. Orthop J Sports Med. 2021;9(4):2325967121991110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fabricant PD, Kocher MS. Management of ACL injuries in children and adolescents. J Bone Joint Surg Am. 2017;99(7):600-612. [DOI] [PubMed] [Google Scholar]
- 10. Haller JM, Holt DC, McFadden ML, Higgins TF, Kubiak EN. Arthrofibrosis of the knee following a fracture of the tibial plateau. Bone Joint J. 2015;97-B(1):109-114. [DOI] [PubMed] [Google Scholar]
- 11. Issa K, Banerjee S, Kester MA, Khanuja HS, Delanois RE, Mont MA. The effect of timing of manipulation under anesthesia to improve range of motion and functional outcomes following total knee arthroplasty. J Bone Joint Surg Am. 2014;96(16):1349-1357. [DOI] [PubMed] [Google Scholar]
- 12. Kalson NS, Borthwick LA, Mann DA, et al. International consensus on the definition and classification of fibrosis of the knee joint. Bone Joint J. 2016;98-B(11):1479-1488. [DOI] [PubMed] [Google Scholar]
- 13. Lykissas MG, Nathan ST, Wall EJ. All-epiphyseal anterior cruciate ligament reconstruction in skeletally immature patients: a surgical technique using a split tibial tunnel. Arthrosc Tech. 2012;1(1):e133-e139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mariani PP, Santori N, Rovere P, Della Rocca C, Adriani E. Histological and structural study of the adhesive tissue in knee fibroarthrosis: a clinical-pathological correlation. Arthroscopy. 1997;13(3):313-318. [DOI] [PubMed] [Google Scholar]
- 15. Marquez-Lara A, Padget W, Wall EJ, Parikh SN. Manipulation under anesthesia is safe and effective for management of early postoperative knee arthrofibrosis in adolescent patients. J Pediatr Orthop. 2024;44(1):e84-e90. [DOI] [PubMed] [Google Scholar]
- 16. Namba RS, Inacio M. Early and late manipulation improve flexion after total knee arthroplasty. J Arthroplasty. 2007;22(6 Suppl 2):58-61. [DOI] [PubMed] [Google Scholar]
- 17. Noyes FR, Berrios-Torres S, Barber-Westin SD, Heckmann TP. Prevention of permanent arthrofibrosis after anterior cruciate ligament reconstruction alone or combined with associated procedures: a prospective study in 443 knees. Knee Surg Sports Traumatol Arthrosc. 2000;8(4):196-206. [DOI] [PubMed] [Google Scholar]
- 18. Nwachukwu BU, McFeely ED, Nasreddine A, et al. Arthrofibrosis after anterior cruciate ligament reconstruction in children and adolescents. J Pediatr Orthop. 2011;31(8):811-817. [DOI] [PubMed] [Google Scholar]
- 19. Ouweleen AJ, Hall TB, Finlayson CJ, Patel NM. Predictors of arthrofibrosis after pediatric anterior cruciate ligament reconstruction: what is the impact of quadriceps autograft? J Pediatr Orthop. 2021;41(7):395-399. [DOI] [PubMed] [Google Scholar]
- 20. Pace JL, Nasreddine AY, Simoni M, Zurakowski D, Kocher MS. Dynamic splinting in children and adolescents with stiffness after knee surgery. J Pediatr Orthop. 2018;38(1):38-43. [DOI] [PubMed] [Google Scholar]
- 21. Paulos LE, Rosenberg TD, Drawbert J, Manning J, Abbott P. Infrapatellar contracture syndrome. An unrecognized cause of knee stiffness with patella entrapment and patella infera. Am J Sports Med. 1987;15(4):331-341. [DOI] [PubMed] [Google Scholar]
- 22. Rowe PJ, Myles CM, Walker C, Nutton R. Knee joint kinematics in gait and other functional activities measured using flexible electrogoniometry: how much knee motion is sufficient for normal daily life? Gait Posture. 2000;12(2):143-155. [DOI] [PubMed] [Google Scholar]
- 23. Shelbourne KD, Urch SE, Gray T, Freeman H. Loss of normal knee motion after anterior cruciate ligament reconstruction is associated with radiographic arthritic changes after surgery. Am J Sports Med. 2012;40(1):108-113. [DOI] [PubMed] [Google Scholar]
- 24. Singh JA, Siddiqi M, Parameshwar P, Chandra-Mouli V. World Health Organization guidance on ethical considerations in planning and reviewing research studies on sexual and reproductive health in adolescents. J Adolesc Health. 2019;64(4):427-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Su AW, Storey EP, Lin SC, et al. Association of the graft size and arthrofibrosis in young patients after primary anterior cruciate ligament reconstruction. J Am Acad Orthop Surg. 2018;26(23):e483-e489. [DOI] [PubMed] [Google Scholar]
- 26. Swenson DM, Collins CL, Best TM, Flanigan DC, Fields SK, Comstock RD. Epidemiology of knee injuries among U.S. high school athletes, 2005/2006-2010/2011. Med Sci Sports Exerc. 2013;45(3):462-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Uimonen MM, Repo JP, Huttunen TT, Nurmi H, Mattila VM, Paloneva J. Surgery for patellar dislocation has evolved towards anatomical reconstructions with assessment and treatment of anatomical risk factors. Knee Surg Sports Traumatol Arthrosc. 2021;29(6):1944-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vander Have KL, Ganley TJ, Kocher MS, Price CT, Herrera-Soto JA. Arthrofibrosis after surgical fixation of tibial eminence fractures in children and adolescents. Am J Sports Med. 2010;38(2):298-301. [DOI] [PubMed] [Google Scholar]
- 29. Werner BC, Yang S, Looney AM, Gwathmey FW., Jr. Trends in pediatric and adolescent anterior cruciate ligament injury and reconstruction. J Pediatr Orthop. 2016;36(5):447-452. [DOI] [PubMed] [Google Scholar]