Abstract
Leiomyomas are uncommon tumors of the gastrointestinal system, representing around 0.03% to 0.05% of all rectal tumors. They tend to have a benign biological behavior and are mostly asymptomatic. When leiomyomas are large in size, they can cause symptoms and complications, including abdominal pain, perforation, intestinal obstruction, and bleeding. We herein present a case of a 57-year-old male patient presenting for colonoscopic evaluation following a positive screening with a fecal occult blood test. The patient was found to have a 10 mm pedunculated polyp at the level of the recto-sigmoid junction, which was removed by hot snare polypectomy and was found to have spindle cells that were positive for desmin, consistent with the rare diagnosis of recto-sigmoid leiomyoma. That being said, this case evokes a rare entity that endoscopists should keep in mind when approaching a recto-colonic polyp.
Keywords: Recto-colonic leiomyoma, spindle cell tumors, gastrointestinal stromal tumor (GIST), leiomyosarcoma, case report
Learning Objectives
Include leiomyoma in the differential diagnosis when a polyp is encountered during colonoscopic evaluation.
Differentiate between leiomyoma and GIST and its malignant counterpart, leiomyosarcoma.
Get acquainted with current treatment modalities and follow-up protocols tackled in medical literature.
Introduction
Recto-colonic leiomyomas are benign tumors that constitute around 3% of gastrointestinal smooth muscle tumors. 1 Most leiomyomas originate from the muscularis mucosa, while some of them originate from the muscularis propria. Gastrointestinal (GI) leiomyomas are commonly encountered incidentally in the esophagus and stomach during upper endoscopy but rarely during colonoscopic evaluation.1,2 Recto-colonic leiomyomas have a preponderance to males over the age of 50. Notably, leiomyomas are discovered incidentally and are asymptomatic in most cases. 3 Abdominal pain and hemorrhage are symptoms that can rarely be triggered by recto-colonic leiomyomas. Leiomyomas predominantly originate from the muscularis mucosa, which poses a challenge for complete endoscopic removal. Therefore, surgical excision remains the cornerstone for the removal of leiomyomas. 4 They are benign with an excellent prognosis because they do not recur once removed. 5 We present a case of a 59-year-old male patient presenting for colonoscopic evaluation due to a positive fecal occult blood test (FOBT) who was found to have a 10 mm pedunculated polyp at the level of recto-sigmoid junction, corroborating a diagnosis of colo-rectal leiomyoma on immunohistochemical staining.
The uniqueness of this case is brought about by a colo-rectal leiomyoma masquerading as a “traditional” pedunculated polyp. This article is valuable as it highlights the importance of endoscopic resection in completely removing leiomyomas. In other words, endoscopic modality is deemed as sufficient and efficacious as its surgical counterpart in resecting leiomyomas.
Case Presentation
A 59-year-old male patient presented for colonoscopy following a positive FOBT. The patient reported chronic constipation, for which he takes laxatives and denies abdominal pain, hematemesis, hematochezia or melena. He did not undergo a previous colonoscopic screening for colon cancer. He does not have any past surgical history, nor does he have a family history of colon cancer. Physical exam was unrevealing and blood tests were normal. In addition, Computed Tomography (CT) scan of the abdomen and pelvis was unremarkable. Colon cancer screening with FOBT was positive. A colonoscopy was performed, whereby findings included a 10 mm short-pedicle polyp at the level of the recto-sigmoid junction (Figure 1A). Methylene blue, a biologically inert blue color staining dye, was used as a lifting agent and the polyp was subsequently removed by hot snare polypectomy (Figure 1B), followed by three hemoclips insertion. The polyp was retrieved and sent to histopathologic analysis for further characterization. Hemostasis was attained and no rapid post-polypectomy bleeding occurred. Immunohistochemical staining revealed spindle cells with eosinophilic cytoplasm that stained positively for smooth muscle actin and desmin and negatively for CD117 (Figure 2A and B), corroborating a diagnosis of leiomyoma.
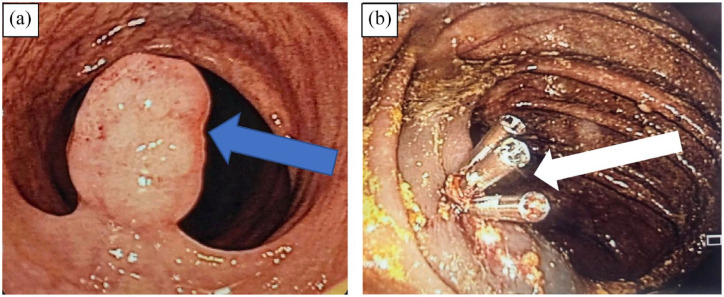
Figure 1.
(A) Recto-sigmoid junction: a 10 mm non-bleeding pedunculated polyp with smooth contour was visualized (blue arrow) and (B) polyp was removed via hot snare polypectomy and three hemoclips were subsequently applied to decrease the risk of post-polypectomy bleeding (white arrow).
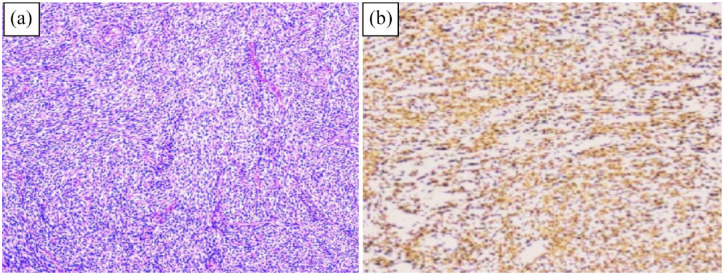
Figure 2.
(A) H&E stain revealing smooth muscle tumor exhibiting no evidence of atypia or increased mitotic activity with an eosinophilic cytoplasm and (B) immunohistochemical staining revealing expression of desmin in the recto-sigmoid leimyoma.
Discussion
Recto-colonic leiomyomas are also termed recto-colonic smooth muscle tumors. They are rare GI tumors and predominantly found in the transverse and sigmoid colon, respectively.1,2 Leiomyomas have a predilection for males above the age of 60 and they tend to have a benign behavior in most cases. However, less commonly, some leiomyomas are biologically aggressive and have malignant potential. 3 Histological appearance, mitotic count, site and size dictate the malignant potential of recto-colonic leiomyomas.3,5 When leiomyomas exhibit high cellular atypia, they are termed “symplastic leiomyomas.”
Upon histochemical evaluation, leiomyomas stain positively for smooth muscle actin and desmin and negatively for CD34, CD117, and S100 protein. It is pivotal to differentiate between leiomyoma and GIST because they resemble each other under light microscopy. 6 GISTs exhibit a myriad of biological behaviors, with all forms harboring the possibility of metastatic potential. 7 Thus, it is essential to discriminate between leiomyoma and GIST. From the same token, it is paramount to distinguish leiomyoma from its malignant counterpart, leiomyosarcoma. The latter has the following features: larger size, more aggressive behavior and a more pronounced malignant potential. Multiple components come into play when differentiating leiomyoma from leiomyosarcoma: cellularity, nuclear pleomorphism, necrosis, tumor size, and the number of mitotic figures.4,7 Table 1 summarizes the immunohistochemical schema for the differentiation of spindle cell tumors of the GI tract.
Table 1.
Distinction of spindle cell tumors of the GI tract through immunohistochemical schema.
| Immunohistochemical marker | Leiomyoma | Leiomyosarcoma | GIST | Schwannoma |
|---|---|---|---|---|
| Desmin | + | + | − | − |
| CD117 | − | − | + | − |
| DOG-1 | − | − | + | − |
| CD34 | − | − | + | − |
| S100 protein | − | − | − | + |
| SMA* | + | + | − | − |
Abbreviations: DOG-1, discovered on GIST-1; GIST, gastrointestinal stromal tumor.
Alpha smooth muscle actin.
Leiomyomas are predominantly asymptomatic and discovered incidentally during colonoscopic evaluation. However, they can cause symptoms such as abdominal pain and occult bleeding, as was exemplified in our reported case.
To date, no consensus has been reached regarding a standardized treatment for colo-rectal leiomyomas. Treatment of leiomyomas can be either surgical or endoscopic and should ensure treatment-free margins.3,4 Treatment modalities for colo-rectal leiomyomas include: transanal excision, endoscopic resection, lower anterior resection and abdomino-perineal amputation.2,3 Surgical excision is favored when large pedunculated polyps and sessile leiomyomas are encountered during colonoscopy.5,7 Wedge colonic resection is reserved for lesions greater than 5 cm in size. 7 Pedunculated polyps less than 2 cm are amenable to endoscopic resection. Cold forceps biopsy, endoscopic mucosal resection (EMR) and snare polypectomies are frequently used endoscopic modalities when removing recto-colonic leiomyomas.4,6 They usually exhibit a higher safety profile because they have lower rates of post-polypectomy bleeding and perforation. 1 Leiomyomas are submucosal lesions; therefore, they should be lifted during resection using submucosal injection techniques. A “positive lift sign” indicates a superficial lesion and fosters a complete endoscopic resection. On the other hand, a “negative lift sign” represents a deeper lesion and circumvents the use of endoscopic modalities. It is worth noting that EMR techniques can ensure the removal of the tumor in its entirety.1,5 In our case, rectal leiomyoma was removed via hot snare polypectomy. Histological findings corroborated a diagnosis of leiomyoma originating from the muscularis mucosa with benign features and no leiomyoma tissue remaining at the resection margin. That being said, extended follow-up is warranted to confirm a disease-free status. Endoscopic ultrasound (EUS), tomography and flexible digestive endoscopy are gold-standard modalities employed when an extended follow-up for an excised leiomyoma is needed.1,7
Leiomyoma of the rectosigmoid junction was reported by Husain et al 7 who described in 2004 a case of a polyp encountered at the level of the recto-sigmoid junction, which was found to be a leiomyoma upon histopathologic analysis. The polyp was removed by conventional snare polypectomy in the absence of complications. Furthermore, leiomyomas have a preponderance to either the sigmoid or the descending colon. Therefore, our patient evokes a rare case of leiomyoma lodging at the level of the recto-sigmoid junction which was successfully removed via conventional snare polypectomy.
On one hand, this case highlights endoscopic resection as a valid alternative to invasive surgery. Endoscopic resection is more cost-effective and associated with a faster recovery time than its surgical counterpart. On the other hand, EUS was not employed to further delineate the depth of invasion of the polyp. For instance, EUS plays a key role in the diagnosis of leiomyomas by providing echographic features that define the lesion extent. It is pivotal to determine the depth of invasion of recto-colonic leiomyomas because depth impacts management. 5
This case poses a challenge to differentiate between “traditional” polyps and leiomyomas endoscopically. Thus, further studies are warranted to allow for a better detection of leiomyomas endoscopically and to tailor adequate resection techniques that ensure complete removal of colo-rectal leiomyomas.
Conclusion
In conclusion, leiomyomas are rare recto-colonic entities discovered incidentally during colonoscopic evaluation. Recto-sigmoid leiomyomas are exceedingly rare. They predominantly behave in a benign fashion and are asymptomatic in most cases. Leiomyomas have an excellent prognosis and generally do not recur, provided that no residual leiomyomatous tissue is left following removal. Gastroenterologists should be vigilant enough to consider leiomyomas in their differential diagnosis when approaching a polyp during routine colonoscopy. Diagnosing leiomyomas endoscopically remains a daunting challenge, as they are often misdiagnosed as “adenomatous polyps.” This article ought to trigger further studies to elucidate how to approach leiomyomas during endoscopy and to develop standardized treatment protocols that optimize therapeutic outcomes.
Footnotes
Author Contributions: Karam Karam, Houssein Chebbo, Sarah Saleh, Sarah Jalloul, Johny Salem, Karim Al Halabi: Data curation, investigation and writing original draft. Elias Fiani: Data curation, investigation, supervising and editing original draft and he is the manuscript guarantor.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Informed Consent: A signed written informed consent was obtained from the patient prior to writing the manuscript attesting permission to publish the clinical history.
ORCID iD: Elias Fiani  https://orcid.org/0000-0003-3204-9098
https://orcid.org/0000-0003-3204-9098
References
- 1. Miettinen M, Sarlomo-Rikala M, Sobin LH. Mesenchymal tumors of muscularis mucosae of colon and rectum are benign leiomyomas that should be separated from gastrointestinal stromal tumors—a clinicopathologic and immunohistochemical study of eighty-eight cases. Mod Pathol. 2001;14:950–956. [DOI] [PubMed] [Google Scholar]
- 2. Lee SH, Huh GY, Cheong YS. A case of endoscopic resection of a colonic semipedunculated leiomyoma. J Korean Soc Coloproctol. 2011;27:215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sagnotta A, Sparagna A, Uccini S, Mercantini P. Giant extraluminal leiomyoma of the colon: rare cause of symptomatic pelvic mass. Int Surg. 2015;100:805–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heidet L, Boye E, Cai Y, et al. Somatic deletion of the 5′ ends of both the COL4A5 and COL4A6 genes in a sporadic leiomyoma of the esophagus. Am J Pathol. 1998;152:673–678. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1858389/ [PMC free article] [PubMed] [Google Scholar]
- 5. Bramis GD, Bramis J, Golematis B. Leiomyoma of the sigmoid colon. Intern Surg. 1974;59:184–185. [PubMed] [Google Scholar]
- 6. Badipatla KR, Kamireddy C, Niazi M, Nayudu SK. Cecal leiomyoma: can we attempt endoscopic resection? Gastroenterol Res. 2016;9:105–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Husain N, Botchu R, Shahabdeen MM, Schofield J, South LM. Leiomyoma of the rectosigmoid junction in an adult. Intern J Surg. 2004;7. [Google Scholar]