Abstract
Background
This study aimed to (1) summarise research on the impact of peer support interventions aimed at improving psychosocial functioning among cancer survivors, and (2) identify key components for developing a support intervention for patients with a rare cancer living in rural, regional or remote areas.
Methods
A comprehensive search of EMBASE, MEDLINE, PsycINFO, CINAHL, and the Cochrane Library identified papers that examined peer support interventions: (i) for rare cancer patients, or (ii) for those living in rural, regional or remote locations, or (iii) that provided support online or via telehealth. After screening, data on study characteristics, intervention components and impact on psychosocial functioning were extracted. Quality assessment was conducted, and findings were synthesised narratively.
Results
A total of 23 unique studies were included, primarily exploring peer support for middle-aged females with a breast cancer diagnosis. Interventions were online or telephone-based, targeting a range of psychosocial outcomes with significant improvements found for coping abilities and loneliness. The most impactful interventions involved online, group formats facilitated by healthcare professionals. There were limited data on rare cancers and rural populations.
Conclusions
Few studies have explored peer support interventions for those diagnosed with a rare cancer living in rural, regional or remote areas. Evidence shows mixed impact on psychosocial functioning for cancer survivors, yet promising elements of peer support that can be translated to rare cancer patients living in rural, regional or remote areas.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13023-024-03477-3.
Keywords: Cancer, Rare cancer, Psychosocial functioning, Peer support, Digital health, Rural, Regional and remote health
Background
In 2020, there was an estimated 18.1 million cancer cases around the world [1], accounting for one in six deaths globally [2]. People diagnosed with cancer often experience adverse psychosocial outcomes [3]. For example, up to a third of cancer patients in acute care hospitals were found to have a diagnosed mental health disorder requiring treatment [4]. Cancer patients also report significant physical, emotional and social challenges which affect overall wellbeing and quality of life [5].
There is a growing appreciation of the need for community based, peer-to-peer support, rooted in patient activation and patient empowerment principles [6]. Peer support is defined as ‘a system of giving and receiving help founded on key principles of respect, shared responsibility, and an agreement of what is helpful’ [7, page 6]. Peer support can be provided one-on-one or through groups [6, 8]. Groups can be led by either trained professionals or by cancer patients, the latter with or without formal supervision and training in cancer support or group facilitation [6, 8]. Increasingly, owing in part to the covid-19 pandemic, peer support is delivered remotely, either via telephone or using online forums or videoconferencing software [9]. Peer support is considered particularly effective when programs are developed in collaboration with individuals who have lived experience, including cancer survivors and carers [10].
There is growing recognition of the importance of peer support [11]. Indeed, in several reviews the impact of peer support for cancer patients on a range of psychological outcomes has been demonstrated [6, 8, 11, 12, 51]. Findings have shown peer support to decrease anxiety, depression, social isolation and stigma, and increase understanding of cancer-related information, hope and optimism, psychological empowerment, stress management skills and quality of life [12]. Additionally, high workloads for healthcare professionals, as well as a recent shift towards patient empowerment, has led to an increased interest in supporting self-management of psychosocial outcomes by cancer patients [6].
Despite a large volume of research exploring the benefits of peer support for cancer patients in general, there is little research that focuses specifically on the benefits of peer support for those diagnosed with a rare form of cancer. Rare cancers are defined as those with an incidence rate of less than 6 cases in 100,000 people per annum [13]. Despite the label ‘rare’, one in five cancer patients is diagnosed with a rare tumour type, and more than 85% of all identified tumour types can be considered rare [14]. Patients diagnosed with a rare cancer face a more challenging illness trajectory than those with a common cancer, including delays in diagnosis, receiving incorrect treatment, and having limited access to clinical trials [15]. Moreover, the 5-year survival rate for individuals with a rare cancer is notably lower at 52% compared to 69% for those with a common cancer [16]. Recent evidence suggests that rare cancer patients also experience worse psychosocial outcomes than those with a common cancer, including higher prevalence of suicide and PTSD [17].
In addition to the significant challenges rare cancer patients deal with following their diagnosis, those living in regions far from the main population centres are challenged in accessing clinical, psychological, and informational support. There is little research exploring the impact of peer support on psychosocial functioning for cancer patients living in rural, regional or remote areas. Recent data has indicated that cancer patients living further away from metropolitan centres are at a higher risk of dying within five years of diagnosis [18]. Like rare cancer patients, rural cancer patients encounter numerous challenges in accessing care such as; limited availability of treatments and support providers, transportation barriers, financial challenges, and restricted access to clinical trials [19].
Those diagnosed with a rare cancer and who live in a rural, regional or remote area are therefore doubly challenged, yet there is a lack of evidence regarding how support can best be tailored to meet their needs. Therefore, the aims of the present study were to: (1) summarise research on the impact of peer support interventions aimed at improving psychosocial functioning among cancer survivors, and (2) identify key components for inclusion in a support intervention for patients with a rare cancer living in rural, regional or remote areas. By addressing these gaps, healthcare providers and policymakers can improve the quality of life and overall outcomes for patients with a rare cancer living in rural, regional, or remote areas.
Methods
Search strategy
This review adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [20]. Eligible studies were identified through comprehensive searches conducted in the following databases: EMBASE, MEDLINE, PsycINFO, CINAHL, and the Cochrane Library. The searches were carried out in September 2023 and updated in February 2024. There were no restrictions placed on publication date.
The search strategy was structured following the PICO format (Population/Intervention/Comparison/Outcome). The population of interest encompassed individuals of all age groups diagnosed with cancer. The primary intervention of interest was peer support, and the key outcomes were assorted measures of quality of life and psychosocial functioning. The search strategy was collaboratively developed in conjunction with a librarian consultant and incorporated terms related to the following key concepts: cancer AND peer support AND psychosocial outcomes AND (rare OR rural OR online). For a detailed overview of the complete search strategy, please refer to Supplement 1.
Eligibility criteria
Studies were eligible for inclusion if: (1) they were published in peer-reviewed journals, encompassing any study design, and written in English; (2) the full text was accessible; (3) they included human participants of any age diagnosed with any type of cancer; (4) they incorporated an intervention utilising some form of peer support for cancer patients, with at least one of the following three characteristics: patients were diagnosed with a rare cancer, patients lived in rural, regional or remote areas, or the intervention was conducted remotely, and (5) the study reported at least one psychosocial outcome (e.g., quality of life, well-being, anxiety, depression, loneliness, isolation, or other mental health issue).
Studies were excluded from the review if: (1) it was not possible to segregate data related to cancer patients from data involving other participants, or where less than 30% of participants had a cancer diagnosis (a figure deemed appropriate by the research team to allow sufficient focus on cancer), (2) it did not focus on rural, regional, or remote patients—the intervention was not conducted remotely, and it was not possible to segregate data specific to rare cancer patients from other cancer patients, with at least 30% of participants having a rare cancer diagnosis (as above, a figure deemed by the research team to indicate sufficient focus on rare cancers) (3) the peer support program comprised a physical intervention (even where psychosocial outcomes were reported) (4) no original findings were reported (e.g., reviews, book chapters, clinical guidelines, letters, study protocols, editorials, erratum/corrections, obituaries), and/or (5) no peer review was performed (e.g., conference/meeting abstracts, dissertations and theses).
The definition of peer support was broad, encompassing diverse types and formats, including both synchronous and asynchronous support, peer education programs, and support groups facilitated by professionals with the aim of promoting peer support among cancer patients. Remote peer support interventions included online meetings, telehealth, mobile health (mHealth), mobile phone applications, and online discussion forums. The classification of rare cancers adhered to the RARECARE definition [13], defining them as having an incidence rate of fewer than 6 cases per 100,000 persons per year.
Study selection and risk of bias
The systematic review process incorporated use of ASReview, a machine learning tool designed for systematic reviews [21]. This tool requires users to label studies as relevant or irrelevant regarding a specific research question. These data train the tool to identify relevant papers. The initial screening paper phase involved the first author (LH) assessing 1,132 articles in ASReview. Building on a previous study on the efficacy of semi-automated screening tools, including ASReview [22], and guided by researchers’ input, a decision was made to exclude articles deemed irrelevant after 100 consecutive instances of non-eligibility. This process resulted in a total of 802 records deemed relevant. These were imported into the systematic review software Covidence, where all title and abstracts were screened by two authors (involving LH, NZ & SD). The full texts of potentially eligible articles were obtained and screened independently by two authors (involving LH, SD, ES, CW, TF & EY). Any disparities that arose between the authors at any stage of the selection process were resolved through discussion.
Due to the range of included study types in this review, a number of quality assessment tools were used; the Cochrane Risk of Bias tool [23], the Quality Assessment Tool for Before-After (Pre-Post) Studies With No Control Group [24], the CASP Qualitative Studies Checklist [25], and the JBI Critical Appraisal Checklist for Analytical Cross Sectional Studies [26]. Risk of bias assessment was conducted concomitantly with data extraction and was performed in duplicate by LH, RH, JVV & TF.
Data extraction and synthesis
A data extraction template was created using Covidence and was piloted by LH. Data were extracted by LH, RH, JVV & TF, with all studies being checked by a second author. Any conflicts were resolved through discussion. Data extraction involved recording information pertaining to study details (e.g., authors, year, country), methods (e.g., study design, outcome measures), participants (e.g., number of participants, age, gender, education level, working status, ethnicity, relationship status), disease characteristics (e.g., diagnosis), intervention characteristics (e.g., mode, format, communication type, frequency, length, facilitator, patient or carer involvement in creation) and outcomes (e.g., quality of life, well-being, anxiety, depression, loneliness, isolation, or other mental health issues). A narrative synthesis was applied to included studies.
Results
Study characteristics and risk of bias
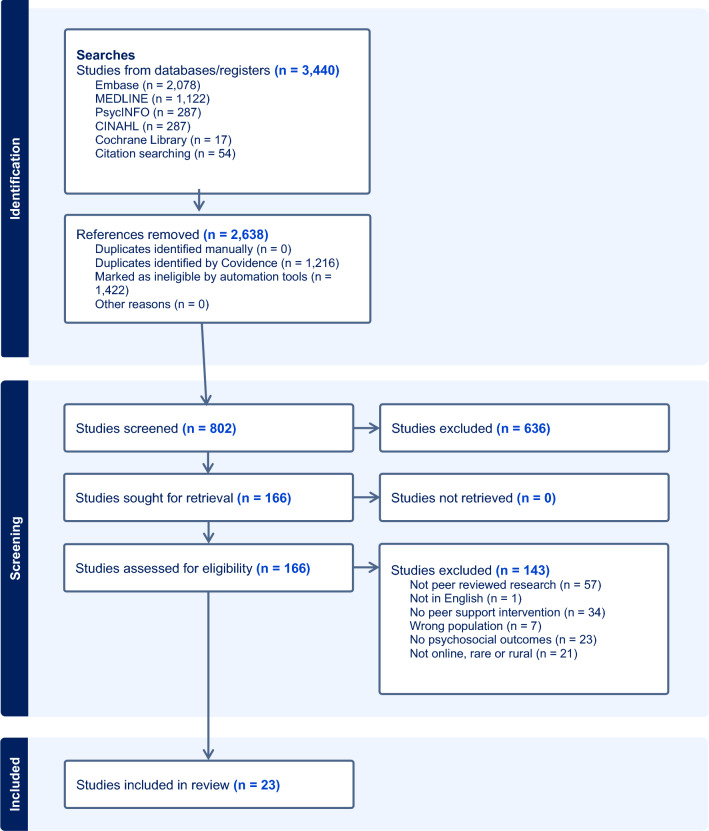
Of the 802 identified records, 23 unique studies were included in the review based on full text screening. Data from two studies were merged together because they included the same sample, and therefore there are two references for one study included in the review [40, 41]. See Fig. 1 for the PRISMA flow chart of included studies and Table 1 for details of the studies included.
Fig. 1.
PRISMA flow diagram
Table 1.
Characteristics of included studies
| References | Year | Country | Study design | N | Gender | Mean age | Diagnosis | Online, rare or rural? | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Akers et al. [27] | 2021 | UK | Qualitative | 11 | 100% male | 67 | Penile cancer | Rare cancers | Loneliness |
| Bender et al. [28] | 2022 | Canada | Quasi-experimental | 34 | Not reported | Not reported | Prostate cancer | Online or telephone | Anxiety, depression, quality of life, perceived social support |
| Canella et al. [29] | 2023 | Switzerland | Mixed methods | 165 | 24% male | 44.8 | Mixed | Online or telephone. Included rare cancer patients | Coping |
| Changrani et al. [30] | 2008 | United States | Randomised controlled trial | 68 | 0% male | 46.8 | Breast cancer | Online or telephone | Depression, quality of life, personal growth |
| Crane-Okada et al. [31] | 2012 | United States | Randomised controlled trial | 142 | 0% male | 61.8 | Breast cancer | Online or telephone | Anxiety, coping, perceived social support |
| Gotay et al. [32] | 2007 | United States | Randomised controlled trial | 305 | 0% male | Not reported | Breast cancer | Online or telephone | Depression, distress |
| Harmon et al. [33] | 2021 | United States | Cross-sectional observational | 102 | 0% male | 59.2 | Breast cancer | Online or telephone | Anxiety, coping |
| Hirayama et al. [34] | 2022 | Japan | Cross-sectional observational | 47 | 57% male | Not reported | Mixed | Online or telephone. Included rare cancer patients | Coping |
| Houts et al. [35] | 1986 | United States | Randomised controlled trial | 32 | 0% male | 49.8 | Gynaecologic | Online or telephone. Included rare cancer patients | Depression, distress |
| Hoybye et al. [36] | 2010 | Denmark | Randomised controlled trial | 794 | 11% male | 54.1 | Mixed | Online or telephone. Included rare cancer patients | Depression, distress |
| Kaal et al. [37] | 2018 | Netherlands | Cross-sectional observational | 66 | 62% male | 29.8 | Mixed | Online or telephone. Included rare cancer patients | Loneliness |
| Klemm [38] | 2012 | United States | Longitudinal | 50 | 0% male | 52.2 | Breast cancer | Online or telephone | Depression |
| Kosugi et al. [39] | 2021 | Japan | Cross-sectional observational | 334 | 20% male | 43.1 | Mixed | Online or telephone. Included rare cancer patients | Loneliness |
| Lepore et al. [40, 41]1 | 2019 | United States | Randomised controlled trial | 183 | 0% male | 52.3 | Breast cancer | Online or telephone | Anxiety, depression, distress |
| Lieberman and Lepore [42] | 2017 | United States | Quasi-experimental | 370 | 0% male | 46.2 | Breast cancer | Online or telephone | Depression |
| Osei et al. [43] | 2013 | United States | Randomised controlled trial | 40 | 100% male | 67.2 | Prostate cancer | Online or telephone | Quality of life |
| Salzer et al. [44] | 2010 | United States | Randomised controlled trial | 78 | 0% male | Not reported | Breast cancer | Online or telephone | Distress, quality of life |
| Sansom-Daly et al. [45] | 2021 | Australia | Randomised controlled trial | 40 | 48% male | 20.6 | Mixed |
Online or telephone Included 30% living in regional locations. Included rare cancer patients |
Anxiety, coping, depression |
| Setoyama, Yamazaki and Namayama [46] | 2011 | Japan | Cross-sectional observational | 220 | 0% male | Not reported | Breast cancer | Online or telephone | Anxiety, depression |
| Toija et al. [47] | 2019 | Finland | Randomised controlled trial | 260 | 0% male | 60 | Breast cancer | Online or telephone | Quality of life |
| van Erp et al. [48] | 2023 | Netherlands | Quasi-experimental | 10 | 40% male | 25.1 | Mixed | Online or telephone. Included rare cancer patients | Distress, quality of life |
| Vilhauer, McClintock and Matthews [49] | 2010 | United States | Randomised controlled trial | 30 | 0% male | 52.7 | Breast cancer | Online or telephone | Depression, wellbeing |
| Winzelberg et al. [50] | 2003 | United States | Randomised controlled trial | 72 | 0% male | 49.5 | Breast cancer | Online or telephone | Anxiety, depression, PTSD, stress |
1Data merged from two separate papers with the same sample
Of the twenty-three studies included in this review, twelve were conducted in the United States [30–33, 35, 38, 40, 42–44, 49, 50], three in Japan [34, 39, 46], two in The Netherlands [37, 48] and one each in Australia [45], Canada [28], Denmark [36], Finland [47], Switzerland [29] and the UK [27]. Most (83%) were conducted from 2010, with 35% of studies conducted from 2020 indicating a rapid increase in scholarly attention to this topic.
The included studies comprised data from a total of 3,453 participants. Of the studies that reported gender (k = 22) and age (k = 18), overall, 89% of participants were women and the average age of participants was 40.8 years (SD = 12.7). The majority of the sample were married or partnered (58.7%, k = 13), participants were well educated, with 64.1% of the sample having completed some form of study beyond high school (k = 7), and around half of the sample (51%, k = 10) were employed. Of the seven studies which included details on participant ethnicity, an average of 91% of participants were Caucasian.
Nearly half of included studies (k = 12) focussed on women with breast cancer [30–33, 38, 40–42, 44, 46, 47, 49, 50]. Seven studies included participants with a range of cancer diagnoses [29, 34, 36, 37, 39, 45, 48]. Two studies focused on men with prostate cancer [28, 43]. One study focussed on gynaecologic cancers [35], and one on penile cancer [27]. The latter was the only paper that focused specifically on a rare cancer population, meeting the inclusion criteria for rare cancer [27]. Eight additional papers, which met eligibility criteria for online interventions, included data from rare cancer patients, but it was not possible to separate this data from that of other cancer patients [29, 34–37, 39, 45, 48].
No papers specifically focused on a rural population. Although no studies formally met the inclusion criteria for including rural, regional or remote participants, one study [45] reported that 30% of participants lived in inner or outer regional areas and another [49] reported that 16% of participants lived in a rural location. Nearly all studies (k = 22) focussed on online or telephone interventions [28–50].
Twelve studies utilised a randomised controlled trial design [30–32, 35, 36, 40, 41, 43–45, 47, 49, 50], and of these, two were assessed as low risk of bias, four as unsure and six as high risk of bias. Five studies used a cross-sectional observational design [33, 34, 37, 39, 46]. Risk of bias was considered high across these studies in areas such as sample details, objective measurements and a lack of consideration for confounding factors. Three studies used a quasi-experimental design (pre-post) [28, 42, 48] and were all assessed as ‘fair’ risk of bias. One study used a qualitative design [27] and was appraised as low risk of bias. One study used a mixed methods design and showed a high risk of bias in its quantitative components but low risk of bias in its qualitative components [29]. Finally, one study utilised a longitudinal design (38), which was appraised as high risk of bias.
Studies explored the impact of peer support interventions on a variety of psychosocial outcomes. The most common outcomes included depression (k = 12) [28, 30, 32, 35, 36, 38, 40–42, 45, 46, 49, 50], anxiety (k = 7) [28, 31, 33, 40, 41, 45, 46, 50], distress (k = 6) [32, 35, 36, 40, 41, 44, 48], quality of life (k = 6) [28, 30, 43, 44, 47, 48] and coping (k = 5) [29, 31, 33, 34, 45]. Fewer studies explored loneliness (k = 3) [27, 37, 39], perceived social support (k = 2) [28, 31], wellbeing (k = 1) [49], personal growth (k = 1) [30], PTSD (k = 1) [50] and stress (k = 1) [50].
Intervention characteristics
The most common form of peer support (k = 11) was online forums [33, 36–39, 42–44, 46, 49, 50]. Online and/or in-person group meetings were used in six studies [27, 30, 34, 45, 48]. One study used a combination of online meetings and an online forum [40, 41]. Online and/or telephone one-to-one support were used in five studies [28, 31, 32, 35, 47]. One study explored online cancer survival stories [29] (See Table 2).
Table 2.
Characteristics of interventions
| References | Type of peer support | Mode of delivery | Format | Communication type | Frequency | Length of intervention | Facilitator | Lived experience involvement |
|---|---|---|---|---|---|---|---|---|
| Akers et al. [27] | In-person group meetings | In-person | Group | Synchronous | Monthly | 1–2 years | Health professional (Nurse) | None |
| Bender et al. [28] | Online/telephone one-to-one support | In-person; Online; Telephone | One-to-one | Synchronous | Variable | 1–3 months | Cancer survivor | Some |
| Canella et al. [29] | Online cancer survival stories | Online | One-to-one | Asynchronous | 24-h availability | Not reported | No facilitator | Some |
| Changrani et al. [30] | Online group meetings | Online | Group | Synchronous | Weekly | 30 weeks | Trained non-healthcare professional | Some |
| Crane-Okada et al. [31] | Telephone one-to-one support | Telephone | One-to-one | Synchronous | Weekly | 7–12 months | Trained non-healthcare professional | None |
| Gotay et al. [32] | Telephone one-to-one support | Telephone | One-to-one | Synchronous | Weekly | 4–8 sessions | Cancer survivor | None |
| Harmon et al. [33] | Online forum | Online | Group | Asynchronous | 24-h availability | 7–12 months | Trained non-healthcare professional | None |
| Hirayama et al. [34] | Online group meetings | Online | Group | Synchronous | Monthly | Not reported | Trained non-healthcare professional | None |
| Houts et al. [35] | Telephone one-to-one support | Telephone | One-to-one | Synchronous | Variable | 3 calls | Cancer survivor | Some |
| Hoybye et al. [36] | Online forum | Online | Group | Synchronous | 24-h availability | 13 months | No facilitator | None |
| Kaal et al. [37] | Online forum | Online | Group | Asynchronous | 24-h availability | Not reported | Trained non-healthcare professional | Some |
| Klemm [38] | Online forum | Online | Group | Asynchronous | 24-h availability | 12 weeks | Health professional (Social worker) | None |
| Kosugi et al. [39] | Online forum | Online | Group | Asynchronous | 24-h availability | Not reported | Trained non-healthcare professional | None |
| Lepore et al. [40, 41]1 | Online group meetings; online forum | Online | Group | Synchronous | Weekly | 6 weeks | Health professional (Not specified) | None |
| Lieberman and Lepore [42] | Online forum | Online | Group | Asynchronous | Variable | 6 months | Health professional (Not specified) | None |
| Osei et al. [43] | Online forum | Online | Group | Asynchronous | 3 times a week | 1–3 months | Not reported | None |
| Salzer et al. [44] | Online forum | Online | Group | Asynchronous | Variable | 7–12 months | No facilitator | None |
| Sansom-Daly et al. [45] | Online group meetings | Online | Group | Asynchronous | Weekly | 1–3 months | Health professional (Psychologist) | None |
| Setoyama, Yamazaki and Namayama [46] | Online forum | Online | Group | Asynchronous | Variable | Not reported | Not reported | None |
| Toija et al. [47] | Telephone one-to-one support | Telephone | One-to-one | Synchronous | Variable | Not reported | Cancer survivor | None |
| van Erp et al. [48] | Online group meetings | Online | Group | Synchronous | Weekly | 6 weeks | Health professional (Psychologist) | Some |
| Vilhauer, McClintock and Matthews [49] | Online forum | Online | Group | Asynchronous | 24-h availability | 2 + years | No facilitator | None |
| Winzelberg et al. [50] | Online forum | Online | Group | Asynchronous | 24-h availability | 12 weeks | Health professional (Mental health practitioner) | None |
1Data merged from two separate papers with the same sample
Seventeen of the interventions were delivered online [29, 30, 33, 34, 36–46, 48–50], four over the telephone [31, 32, 35, 47], one in person [27], and one had a blended format, with online, over the telephone and in-person delivery [28]. Seventeen interventions used a group format [27, 30, 33, 34, 36–46, 48–50] and six used a one-to-one format [28, 29, 31, 32, 35, 47]. Twelve interventions were asynchronous [29, 33, 37–39, 42–46, 49, 50] and eleven were synchronous [27, 28, 30–32, 34–36, 40, 41, 47, 48]. Of the synchronous interventions, five occurred weekly [30–32, 40, 41, 48], three occurred with a variable frequency [28, 35, 47] and two occurred monthly [27, 34]. Duration of interventions were: 1–3 months (k = 7) [28, 38, 40, 41, 43, 45, 48, 50], 4–6 months (k = 1) [42], 7–12 months (k = 4) [30, 31, 33, 44], 1–2 years (k = 2) [27, 36], 2 + years (k = 1) [49], or of a variable length (k = 3) [29, 32, 35]. The remaining five studies did not report the duration of the intervention [34, 37, 39, 46, 47]. Of the 21 studies where the facilitator was reported, 33% of interventions were facilitated by health professionals (e.g. nurse, social worker, psychologist) [27, 38, 40–42, 45, 48, 50], 29% were facilitated by trained non-professionals [30, 31, 33, 34, 37, 39], 19% were facilitated by cancer survivors [28, 32, 35, 47] and 19% had no facilitator [29, 36, 44, 49]. Only six studies (26%) reported involving individuals with lived experience in the design and development of the intervention [28–30, 35, 37, 48].
Interventions offered topics for discussion as well as a more open agenda to be led by patients. Combining data across both, the most commonly discussed topics included relationships with others (k = 8), physical symptoms and side effects (k = 7), emotions (k = 4), information needs, sexuality, spirituality, the future, day to day life and existentialism (k = 3) and practical matters, medical team and self-esteem (k = 2).
The impact of peer support interventions on psychosocial outcomes
This section reports on aggregated results across all research designs (randomised controlled trials, quasi-experimental designs, cross-sectional observational and qualitative studies).
Depression
Of the twelve studies that examined the impact of peer support on depression, mixed findings were reported. Six studies (50%) revealed no significant difference in depression symptoms following intervention [28, 30, 32, 35, 38, 49]. One study [36] found less improvement in depression in the intervention group than the control group 6 months post-intervention. Three studies reported a significant reduction in depression following the intervention [40–42, 50], while an additional study showed an improvement in depression symptoms, although significance was not reported [45]. Finally, one study [46] found that conflict in online forums was associated with an increase in depression scores, whilst emotional expression was associated with a decrease.
Anxiety
Seven studies explored the impact of peer support on anxiety. The majority of studies (k = 4) found that peer support had no significant impact on anxiety [28, 31, 45, 50], with one exception finding a significant decrease in anxiety post-intervention [40, 41]. One study found that 10% of peer support participants reported an increase in fear and anxiety [33]. In another study [46] using peer support forums for emotional support, advice and insight was associated with a reduction in anxiety.
Distress
In the six studies that examined the impact of peer support on distress, mixed findings were reported. Three studies (50%) showed no changes in distress [32, 35, 36]. Two studies [40, 41, 48] showed a significant improvement in distress, although one of these was measured immediately post-chat [40, 41]. Another study showed an improvement in distress, however the sample size was too small to conclude whether this was significant [44].
Quality of life
Six studies reported on the impact of peer support on quality of life. The majority of studies (k = 5) found no statistically significant impact [28, 30, 43, 47, 48]. One study [44] found a decreased quality of life in the intervention compared to the control group, though this did not reach statistical significance.
Coping
Six studies explored the impact of peer support on coping with cancer, suggesting a positive impact. Three studies found that participants agreed that the peer support intervention had improved their ability to cope with cancer [29, 33, 34]. Two studies found that those who had received an intervention were more likely to use positive coping strategies following the intervention than those who had received care as usual [31, 45]. Finally, in one study [49], those in the control group showed a decline in ability to cope with breast cancer, which led to a significant difference in coping between intervention and control group at follow-up.
Loneliness
There were largely positive findings for the impact of peer support on loneliness; one study [37] found that around half of participants in an online forum agreed ‘I do not feel lonely anymore’, ‘I have good contact with peers’ and ‘I make new friends’. Another reported that men who attended peer support group meetings spoke about enjoying the opportunity to make friends and share cancer-related experiences [27]. Conversely, one study found that weekly active users of an online peer support group were equally as likely to be classified as belonging to a ‘high loneliness group’ and a ‘low loneliness group’ [39].
Perceived social support
Two studies [28, 31] explored the impact of peer support on social support. Neither found that peer support had a significant impact on perceived social support.
Wellbeing, personal growth, PTSD and stress
Three studies examined the impact of peer support interventions on wellbeing, personal growth, or PTSD and stress. One study [49] found no differences in wellbeing scores between intervention and control group participants at a one month and two month follow-up. In another controlled study [30], non-significant findings were also reported on the impact of peer support on personal growth. However, in this study, those who had received the intervention showed improvements in seeing new possibilities and increased feelings of strength. In a study which examined the impact of peer support on PTSD and stress [50], those who used online forums showed a significant improvement in PTSD symptoms and a reduction in stress.
The most impactful components of peer support
Of the included interventions, thirteen resulted in improvements in at least one outcome [27, 29, 31, 33, 34, 37, 40–42, 44, 45, 48–50]. Narrative synthesis identified common features of the most successful interventions, which were defined as those that either showed improvements in multiple outcomes or had only significant improvements without any null findings. This resulted in eight interventions [27, 29, 34, 37, 40–42, 45, 50]. The majority (k = 7) of these interventions were delivered online [29, 34, 37, 40–42, 45, 50] in a group format (k = 7) [27, 34, 37, 40–42, 45, 50] and were facilitated by a healthcare professional (k = 5) [27, 40–42, 45, 50]. The interventions were varied in terms of their frequency, communication type and length of intervention.
Nine studies did not find peer support to have a significant impact on any psychosocial outcomes [28, 30, 32, 35, 36, 38, 39, 43, 47]. Although these interventions were varied in their characteristics, some common features emerged. Nearly half (k = 4) were delivered via telephone in a one-to-one format and were facilitated by individuals with lived experience [28, 32, 35, 47].
Peer support for rare cancer patients
In the only study focussed exclusively on rare cancer patients [27], the impact of an in-person monthly peer support group meeting was evaluated qualitatively. Six themes were identified: developing friendships, peer support, sharing experiences, support from the clinical team, receiving information and raising awareness. Overall, participants reported that peer support was helpful in decreasing loneliness in men in this penile cancer patient’s cohort.
In eight studies [29, 34–37, 39, 45, 48], patients with a rare cancer were included in the sample, although it was not possible to separate their results from those of common cancer patients. Across the full sample, peer support was found to have a positive impact on depression [45], distress [48], coping [29, 34, 45] and loneliness [37].
Peer support for patients living in rural, regional or remote areas
Two studies reported the rurality of their participants. In these studies, only 30% [45] and 16% [49] of participants lived in rural, regional or remote areas. Both studies evaluated the impact of online peer support groups, though one focused on breast cancer patients [49] and the other focussed on young adult cancer survivors with various diagnoses [45]. The breast cancer peer support program used asynchronous online forums [49], while the program for young adult cancer survivors involved six weekly synchronous videoconference group sessions [45].. The study on young adult cancer survivors did not report any location-specific findings. However, qualitative findings from the breast cancer study indicated that online provision of the peer support intervention was helpful because it made groups easily accessible for those who lived in rural areas [49].
Additionally, one study, which did not report participants rurality, included a qualitative finding relevant to participant location. In contrast to the breast cancer findings, the study on men diagnosed with rare penile cancer revealed that participants were willing to travel up to four hours to attend in-person support groups.
Discussion
Main findings
In this review, peer support interventions aimed at improving psychosocial functioning among cancer survivors were explored to identify key components for inclusion in a peer support intervention suitable for patients with a rare cancer living in rural, regional or remote areas. A total of 23 unique studies were included in the review. Included interventions comprised of online forums, group meetings and one-to-one support. Of the included interventions, thirteen resulted in improvements in at least one outcome, with the most significant outcomes being coping and loneliness. The majority of these interventions were delivered online, in a group format, and were facilitated by a healthcare professional. Finally, there is not enough data available to report conclusively on the evidence for peer support for rare cancer survivors, living in rural, regional or remote areas.
Interpretation of findings
In this review, peer support services fell into one of three categories: online forums, group meetings, and one-to-one support. Facilitators included health professionals, trained non-professionals, and cancer survivors, aligning with existing typologies of peer support based on delivery mode and facilitator type [8, 51]. Few interventions involved individuals with lived experience in their design, despite its importance for ensuring relevancy, acceptability and effectiveness [10]. This contradicts complex intervention development frameworks that emphasise involving individuals with lived experience [52].
The impact of peer support services on psychosocial outcomes was mixed, with improvements mainly identified in coping and loneliness. Previous reviews also found mixed results for the impact of peer support programs on psychosocial outcomes for cancer patients [51]. These mixed findings may be due to the heterogeneity of peer support interventions and the varied outcomes tested in the included studies, which make it difficult to compare results [53].
Online support groups facilitated by a healthcare professional led to the most improved psychosocial outcomes, while one-to-one telephone support with a cancer survivor had the least favourable impact. These findings partially support previous studies that found one-to-one face-to-face and group internet peer support groups to be most effective [51] but contradict others suggesting one-to-one face-to-face interventions are beneficial [12]. In one study, more than half of prostate cancer patients preferred face-to-face peer connections over internet-based ones [54]. Still, other studies suggest no differences in outcomes between face-to-face and online peer support groups, though user profiles may differ [55].
Research on peer support for rare cancer patients is limited. This review found that peer support was typically accessed by well-educated, middle-aged white females diagnosed with breast cancer. This supports previous research which has found this group benefits most frequently from peer support [51]. Indeed, economic analysis indicates more funding is invested into research and treatment of breast cancer than other cancer types [56]. Notably, the one study which examined the impact of a peer support program for people with rare cancer found several qualitative improvements for men with penile cancer [27]. Together, the findings suggest the need for further research and resources invested into examining the utility of peer support for broader groups of people diagnosed with cancer, including men, and those with rare cancer diagnoses.
This review highlights the lack of research involving cancer survivors living in rural, regional, and remote areas. Only two of the included studies reported on the rurality of participants, and none specifically met the inclusion criteria for targeting rural, regional, and remote populations. The findings suggested that online peer support can improve accessibility for those living remotely, though qualitative evidence indicated that participants are still willing to travel for in-person sessions. Despite limited research on psychosocial interventions for non-metropolitan areas, studies not specifically addressing geographic impact are still valuable. Research shows rural and urban cancer patients have similar psychosocial needs [57], suggesting these findings may generalise to this group. Additionally, studies on peer support for other rare diseases, focussing on sharing clinical information and personal experiences, have shown positive outcomes [58], suggesting peer support may also have an impact for rare cancer patients. This review includes peer support programs primarily delivered online or via telehealth, formats beneficial for patients with less common cancers [59], and those in geographically distant locations [60].
Strengths and limitations
This study adds to our understanding of the impact of peer support on cancer patients. Whilst previous reviews have explored this topic, the current review focusses only on peer support groups that were either a) delivered online b) targeted rare cancer patients or c) targeted rural, regional and remote cancer patients. The search strategy and study selection process had several strengths. Firstly, the review adhered to the PRISMA guidelines, ensuring transparency and methodological rigour. The use of the ASReview tool for initial screening helped streamline the study selection process. Duplicate screening of titles and abstracts, as well as resolving conflicts through discussion, ensured the reliability of study selection.
However, there were also limitations. Restricting the search to studies written in English may have introduced language bias. Additionally, the review did not include grey literature, which could have provided valuable insights. Further, the findings of this review may be constrained due to methodological flaws of the included studies; less than half of the included studies utilised a randomised controlled trial design, and many included studies were found to be at high risk of bias. Finally, the majority of included studies focussed on peer support provided to women with breast cancer. It has been suggested that peer support may have fewer profound impacts on this population due to the abundance of support that is already available for breast cancer patients, and the findings may therefore not extend to patients with other cancers, particularly less common or rare cancers [51].
Implications for research and practice
This review found that peer support programs typically targeted well-educated, middle-aged white females with breast cancer. Future peer support programs should aim to reach a broader range of demographics including those diagnosed with a rare cancer, and those living in rural, regional and remote areas.. Additionally, there was a notable lack of involvement of people with lived experience (i.e., patients and their carers) in the design of these interventions. Future efforts should focus on co-designing peer support programs, ensuring that those with lived experience are involved to enhance the relevance and acceptability of these programs for the target users.
The findings suggest that online group programs facilitated by healthcare professionals may produce the best outcomes, although results were inconclusive. More research is needed to determine which type of peer support is most effective. Well-designed, rigorous trials with sufficient power to detect meaningful differences in important psychosocial outcomes are also required, ensuring that adverse events are examined. Finally, interventions tailored specifically for rare cancer patients living in rural, regional, and remote areas are needed to address the current lack of research and investment in this population..
Conclusion
Peer support is primarily delivered online in a group setting to patients with breast cancer. Although evidence of the impact of peer support services on various psychosocial outcomes is mixed, coping abilities and loneliness appeared to improve with peer support. Peer support that is delivered by a healthcare professional in an online group setting may lead to more positive outcomes than one-to-one telephone support provided by individuals with lived experience. However, there is a need for more research to determine which type of peer support yields the best results; the question remains of what works, for whom and why. Importantly, the findings highlight the lack of research and investment in peer support for those diagnosed with rare cancers living in rural, regional, or remote locations. Given the significant challenges that this unique, yet large, population faces, it is crucial to develop an intervention that serves the needs of this group.
Supplementary Information
Acknowledgements
The authors wish to thank Angela Johns-Hayden (AJH) for her input to the search strategy.
Author contributions
LH, SFAD, NZ, CC, EY, CW and ES contributed to the study conception and design. LH, SFAD, NZ, CC, EY, RH, JVV, TF, CW and ES contributed to data collection and analysis. The first draft of the manuscript was written by LH and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Grants-in-Aid Scheme administered by Cancer Council Victoria.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Cancer Research Fund International. Worldwide cancer data [Available from: https://www.wcrf.org/cancer-trends/worldwide-cancer-data/#:~:text=Find%20information%20about%20world%20cancer,and%208.8%20million%20in%20women.
- 2.World Health Organisation. Cancer 2022 [updated 3rd February, 2022. Available from: https://www.who.int/news-room/fact-sheets/detail/cancer.
- 3.Mitchell AJ, Chan M, Bhatti H, Halton M, Grassi L, Johansen C, et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol. 2011;12(2):160–74. [DOI] [PubMed] [Google Scholar]
- 4.Singer S, Das-Munshi J, Brähler E. Prevalence of mental health conditions in cancer patients in acute care—a meta-analysis. Ann Oncol. 2010;21(5):925–30. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Ou L, Hollis SJ. A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Serv Res. 2013;13(1):1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dennis C-L. Peer support within a health care context: a concept analysis. Int J Nurs Stud. 2003;40(3):321–32. [DOI] [PubMed] [Google Scholar]
- 7.Mead S, Hilton D, Curtis L. Peer support: a theoretical perspective. Psychiatr Rehabil J. 2001;25(2):134–41. [DOI] [PubMed] [Google Scholar]
- 8.Dunn J, Steginga SK, Rosoman N, Millichap D. A review of peer support in the context of cancer. J Psychosoc Oncol. 2003;21(2):55–67. [Google Scholar]
- 9.Harkin LJ, Beaver K, Dey P, Choong KA. Secret groups and open forums: Defining online support communities from the perspective of people affected by cancer. Digital Health. 2020. 10.1177/2055207619898993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grynne A, Browall M, Fristedt S, Ahlberg K, Smith F. Integrating perspectives of patients, healthcare professionals, system developers and academics in the co-design of a digital information tool. PLoS ONE. 2021;16(7): e0253448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajkhowa A, Williams A, O’Connor K, Taylor K. Cancer peer support groups in australia: a review of consumer resources. J Consumer Health Internet. 2023;27(4):443–67. [Google Scholar]
- 12.Hu J, Wang X, Guo S, Chen F, Wu Y-Y, Ji F-J, et al. Peer support interventions for breast cancer patients: a systematic review. Breast Cancer Res Treatment. 2019;174:325–41. [DOI] [PubMed] [Google Scholar]
- 13.Gatta G, Van Der Zwan JM, Casali PG, Siesling S, Dei Tos AP, Kunkler I, et al. Rare cancers are not so rare: the rare cancer burden in Europe. Eur J Cancer. 2011;47(17):2493–511. [DOI] [PubMed] [Google Scholar]
- 14.de Heus E, Duijts SFA, van der Zwan JM, Kapiteijn E, van Dijkum EJMN, van Herpen CML, et al. The gap between rare and common cancers still exists: results from a population-based study in the Netherlands. Eur J Cancer. 2022;167:103–11. [DOI] [PubMed] [Google Scholar]
- 15.de Heus E, van der Zwan JM, Husson O, Frissen AR, van Herpen CML, Merkx MAW, et al. Unmet supportive care needs of patients with rare cancer: a systematic review. Eur J Cancer Care. 2021;30(6): e13502. [DOI] [PubMed] [Google Scholar]
- 16.DeSantis CE, Kramer JL, Jemal A. The burden of rare cancers in the United States. CA A Cancer J Clin. 2017;67(4):261–72. [DOI] [PubMed] [Google Scholar]
- 17.Low CE, Loke S, Pang GE, Sim B, Yang VS. Psychological outcomes in patients with rare cancers: a systematic review and meta-analysis. Eclinicalmedicine. 2024;72:102631. 10.1016/j.eclinm.2024.102631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Australian Institute of Health and Welfare. Cancer in Australia 2019. Canberra: AIHW; 2019.
- 19.Charlton M, Schlichting J, Chioreso C, Ward M, Vikas P. Challenges of rural cancer care in the United States. Oncology (Williston Park). 2015;29(9):633–40. [PubMed] [Google Scholar]
- 20.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, The PRISMA, et al. statement: an updated guideline for reporting systematic reviews. Int J Surgery. 2020. 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van De Schoot R, De Bruin J, Schram R, Zahedi P, De Boer J, Weijdema F, et al. An open source machine learning framework for efficient and transparent systematic reviews. Nat Machine Intell. 2021;3(2):125–33. [Google Scholar]
- 22.Ros R, Bjarnason E and Runeson P. A Machine Learning Approach for Semi-Automated Search and Selection in Literature Studies. In: Proceedings of EASE’17, Karlskrona, Sweden, June 15–16, 2017. 10.1145/3084226.3084243
- 23.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Heart, Lung and Blood Institute. Study Quality Assessment Tools [Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- 25.Critical Appraisal Skills Programme. CASP Qualitative Studies Checklist 2024 [Available from: https://casp-uk.net/checklists/casp-qualitative-studies-checklist-fillable.pdf.
- 26.Moola SZ, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. Systematic reviews of etiology and risk. InJoanna Briggs Institute reviewer’s manual. Adelaide, Australia: The Joanna Briggs Institute. 2017 (Vol. 5, pp. 217-69)
- 27.Akers C, Plant H, Riley V, Alnajjar HM, Muneer A. Exploring penile cancer survivors’ motivations and experiences of attending a support group: eUROGEN study. Int J Urol Nurs. 2021;15(1):20–6. [Google Scholar]
- 28.Bender JL, Flora PK, Soheilipour S, Dirlea M, Maharaj N, Parvin L, et al. Web-based peer navigation for men with prostate cancer and their family caregivers: a pilot feasibility study. Curr Oncol. 2022;29(6):4285–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canella C, Inderbitzin M, Oehler M, Witt CM, Barth J. Cancer survival stories: Perception, creation, and potential use case. Health Exp Int J Public Particip Health Care Health Policy. 2023;26(4):1551–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Changrani J, Lieberman M, Golant M, Rios P, Damman J, Gany F. Online cancer support groups: experiences with underserved immigrant Latinas. Prim Psychiatry. 2008;15(10):55–62. [Google Scholar]
- 31.Crane-Okada R, Freeman E, Kiger H, Ross M, Elashoff D, Deacon L, et al. Senior peer counseling by telephone for psychosocial support after breast cancer surgery: effects at six months. Oncol Nursing Forum. 2012;39(1):78–89. [DOI] [PubMed] [Google Scholar]
- 32.Gotay CC, Moinpour CM, Unger JM, Jiang CS, Coleman D, Martino S, et al. Impact of a peer-delivered telephone intervention for women experiencing a breast cancer recurrence. J Clin Oncol. 2007;25(15):2093–9. [DOI] [PubMed] [Google Scholar]
- 33.Harmon DM, Young CD, Bear MA, Aase LA, Pruthi S. Integrating online community support into outpatient breast cancer care: Mayo Clinic Connect online platform. Digital Health. 2021. 10.1177/20552076211048979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirayama T, Kojima R, Udagawa R, Yanai Y, Ogawa Y, Tanaka M, et al. A hospital-based online patients support program, online adolescent and young adult hiroba, for adolescent and young adult cancer patients at a designated cancer center in Japan. J Adolesc Young Adult Oncol. 2022;11(6):588–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Houts PS, Whitney CW, Mortel R, Bartholomew MJ. Former cancer patients as counselors of newly diagnosed cancer patients. J Natl Cancer Inst. 1986;76(5):793–6. [PubMed] [Google Scholar]
- 36.Hoybye MT, Dalton SO, Deltour I, Bidstrup PE, Frederiksen K, Johansen C. Effect of Internet peer-support groups on psychosocial adjustment to cancer: a randomised study. Br J Cancer. 2010;102(9):1348–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaal SEJ, Husson O, van Dartel F, Hermans K, Jansen R, Manten-Horst E, et al. Online support community for adolescents and young adults (AYAs) with cancer: user statistics, evaluation, and content analysis. Patient Prefer Adherence. 2018;12:2615–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klemm P. Effects of online support group format (Moderated vs Peer-Led) on depressive symptoms and extent of participation in women with breast cancer. CIN - Comput Informatics Nursing. 2012;30(1):918. [DOI] [PubMed] [Google Scholar]
- 39.Kosugi K, Nishiguchi Y, Miura T, Fujisawa D, Kawaguchi T, Izumi K, et al. Association between loneliness and the frequency of using online peer support groups among cancer patients with minor children: a cross-sectional web-based study. J Pain Symptom Manage. 2021;61(5):955–62. [DOI] [PubMed] [Google Scholar]
- 40.Lepore SJ, Rincon MA, Buzaglo JS, Golant M, Lieberman MA, Bauerle Bass S, et al. Digital literacy linked to engagement and psychological benefits among breast cancer survivors in Internet-based peer support groups. Eur J Cancer Care. 2019;28(4): e13134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lepore SJ, Buzaglo JS, Lieberman MA, Golant M, Greener JR, Davey A. Comparing standard versus prosocial internet support groups for patients with breast cancer: a randomized controlled trial of the helper therapy principle. J Clin Oncol. 2014;32(36):4081–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lieberman MA, Lepore S. Fighting spirit expressed by women with breast cancer in professional-and peer-led online support groups: effects on outcomes. Madridge J Cancer Study Res. 2017;1(1):08–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osei DK, Lee JW, Modest NN, Pothier PKT. Effects of an online support group for prostate cancer survivors: a randomized trial. Urol Nurs. 2013;33(3):123–33. [PubMed] [Google Scholar]
- 44.Salzer MS, Palmer SC, Kaplan K, Brusilovskiy E, Have TT, Hampshire M, et al. A randomized, controlled study of Internet peer-to-peer interactions among women newly diagnosed with breast cancer. Psychooncology. 2010;19(4):441–6. [DOI] [PubMed] [Google Scholar]
- 45.Sansom-Daly UM, Wakefield CE, Ellis SJ, McGill BC, Donoghoe MW, Butow P, et al. Online, group-based psychological support for adolescent and young adult cancer survivors: results from the recapture life randomized trial. Cancers. 2021;13(10):2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Setoyama Y, Yamazaki Y, Namayama K. Benefits of peer support in online Japanese breast cancer communities: differences between lurkers and posters. J Med Internet Res. 2011;13(4): e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toija AS, Kettunen TH, Leidenius MHK, Vainiola THK, Roine RPA. Effectiveness of peer support on health-related quality of life in recently diagnosed breast cancer patients: a randomized controlled trial. Supportive Care Cancer. 2019;27(1):123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Erp LME, Maurice-Stam H, Beek LR, Kremer LCM, den Hartogh JG, van Gorp M, et al. Online cognitive-behavioral group intervention for young adult survivors of childhood cancer: a pilot study. J Psychosoc Oncol. 2023;41(5):518–38. [DOI] [PubMed] [Google Scholar]
- 49.Vilhauer RP, McClintock MK, Matthews AK. Online support groups for women with metastatic breast cancer: a feasibility pilot study. J Psychosoc Oncol. 2010;28(5):560–86. [DOI] [PubMed] [Google Scholar]
- 50.Winzelberg AJ, Classen C, Alpers GW, Roberts H, Koopman C, Adams RE, et al. Evaluation of an internet support group for women with primary breast cancer. Cancer Interdiscip Int J Am Cancer Soc. 2003;97(5):1164–73. [DOI] [PubMed] [Google Scholar]
- 51.Hoey LM, Ieropoli SC, White VM, Jefford M. Systematic review of peer-support programs for people with cancer. Patient Educ Couns. 2008;70(3):315–37. [DOI] [PubMed] [Google Scholar]
- 52.Chammas A, Quaresma M, Mont’Alvão C. A closer look on the user centred design. Procedia Manufact. 2015;3:5397–404. [Google Scholar]
- 53.Bellamy C, Schmutte T, Davidson L. An update on the growing evidence base for peer support. Ment Health Soc Incl. 2017;21(3):161–7. [Google Scholar]
- 54.Boyes A, Turon H, Hall A, Watson R, Proietto A, Sanson-Fisher R. Preferences for models of peer support in the digital era: a cross-sectional survey of people with cancer. Psychooncology. 2018;27(9):2148–54. [DOI] [PubMed] [Google Scholar]
- 55.Huber J, Muck T, Maatz P, Keck B, Enders P, Maatouk I, et al. Face-to-face vs. online peer support groups for prostate cancer: a cross-sectional comparison study. J Cancer Survivorship Res Practice. 2018;12(1):1–9. [DOI] [PubMed] [Google Scholar]
- 56.Kamath SD, Kircher SM, Benson AB. Comparison of cancer burden and nonprofit organization funding reveals disparities in funding across cancer types. J Natl Compr Canc Netw. 2019;17(7):849–54. [DOI] [PubMed] [Google Scholar]
- 57.Van Der Kruk SR, Butow P, Mesters I, Boyle T, Olver I, White K, et al. Psychosocial well-being and supportive care needs of cancer patients and survivors living in rural or regional areas: a systematic review from 2010 to 2021. Support Care Cancer. 2022;30:1021–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Depping MK, Uhlenbusch N, Härter M, Schramm C, Löwe B. Efficacy of a brief, peer-delivered self-management intervention for patients with rare chronic diseases: a randomized clinical trial. JAMA Psychiat. 2021;78(6):607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reid Rudy R, Rosenfeld LB, Galassi JP, Parker J, Schanberg R. Participants’ perceptions of a peer-helper, telephone-based social support intervention for melanoma patients. Health Commun. 2001;13(3):285–305. [DOI] [PubMed] [Google Scholar]
- 60.Curran VR, Church JG. A study of rural women’s satisfaction with a breast cancer self-help network. J Telemed Telecare. 1999;5(1):47–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.