Abstract
Background
Urine biomarkers are crucial for monitoring patient responses in treating urological pathologies, including non-muscle invasive bladder cancer (NMIBC). Yet, analysing urine biomarkers poses several challenges, including ensuring specimen stability during transportation and analytical processing. This prospective feasibility study aimed to investigate how urinary leukocytes and proteins are impacted by storing and refrigerating urine.
Methods
Stability of leukocytes from four healthy donors (HD) spiked into urine supernatants was analyzed for up to 72 h at 4°C. Urine samples from five NMIBC patients undergoing BCG treatment were divided into two portions, followed by either immediate processing or overnight refrigeration. Urinary cell content and soluble factors were analyzed by multiparameter flow cytometry and Luminex®, respectively.
Results
We confirmed the stability of healthy donor peripheral blood leukocytes spiked into cell-free urine supernatants from healthy donors or untreated bladder cancer patients for up to 72 h under refrigeration at + 5℃. Additionally, we conducted immune cell and proteomic analysis from urine samples obtained from five NMIBC patients receiving Bacillus Calmette-Guérin (BCG) therapy either processed immediately or after overnight refrigeration. We successfully demonstrated that leukocyte and protein composition remain stable in refrigerated urine from BCG-treated NMIBC for 24 h. This included granulocytes, monocytes, and T cells, as well as total protein, creatinine and 46 additional individual immune-related mediators.
Conclusions
This work demonstrates the compatibility of refrigerated urine shipment from the collection sites to analytical laboratories with both immunophenotyping and proteomic analysis and establishes clear logistical benefits for numerous clinical settings focused on monitoring patient immune responses in the urine matrix.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12894-024-01673-8.
Keywords: Biomarkers, Bladder cancer, Immunophenotyping, Non-muscle invasive bladder cancer, Proteomics, Stability, Urinary leukocytes, Urine
Background
Immunotherapy has revolutionised the treatment of cancer [1], and bacterial immunotherapy became a golden standard for a non-muscle invasive bladder cancer (NMIBC) treatment for decades [2]. Yet, most patients do not benefit from these treatments.
Indeed, while BCG therapy is one of the most successful immunotherapies, approximatively 50% of patients do not respond to the treatment [3], partly due to immune regulatory mechanism [4]. Tracking cancer patient responses to treatment traditionally relies on measuring biomarkers from either tumour tissue or peripheral blood, both of which can be complex and invasive to sample over time. Understanding the dynamic changes in the immune compartment, especially at the local level, is crucial to uncovering the mechanisms of response or non-responsiveness, thereby unlocking the full potential of immunotherapy [1, 5, 6].
While systemic immunomonitoring for response to cancer immunotherapy is well-explored [7, 8], the immunomonitoring of local responses remains underexplored [5, 9, 10]. Using urine as a non-invasive biomarker matrix is an effective alternative that has the potential to substantially improve the patient experience. Urine protein profiling for detection of NMIBC by multiplex technologies is standardised [11, 12], and monitoring of immune responses to BCG therapy in NMIBC is well-documented [10, 13], but sample processing and stability questions persist. And despite limited research, studies on immunophenotyping urinary leukocytes in bladder cancer immunotherapy have shown its potential in predicting treatment response and understanding the therapy’s mechanism of action [14–17].
Thus, understanding urine sample stability for local immune response monitoring in NMIBC immunotherapy is critical. There is a perception that urine sample processing must occur at the collection site, which is often impractical in multicentre clinical trials where sample processing typically happens at a central lab, necessitating a shipping period of at least 24 h [18, 19].
Therefore, this study aims to assess the optimal timeframe for urine sample stability during transit from a clinical site to an analytical laboratory. The goal is to determine the sample quality window for effective processing and analysis of cellular and protein urine composition and to offer practical insights for streamlined logistics in clinical trials for NMIBC and other urological conditions.
Methods
Study design and participants
Leukocytes were obtained from the buffy coat blood product material from healthy donors collected by NHS Blood and Transplantation (NHSBT; n = 4, London, UK). Cell-free urine supernatants were obtained from healthy donor (HD; n = 2) or untreated bladder cancer patients (n = 2) (Fidelis Research, Sophia, Bulgaria).
Non muscle invasive bladder cancer (NMIBC) patients (n = 5) receiving Bacillus Calmette-Guérin (BCG) therapy at Lausanne University Hospital (CHUV), Switzerland, were prospectively recruited for this study (Table S1). Due to the exploratory nature of the study, no formal hypothesis on the sample size was made. At the time of inclusion, NMIBC patients were starting standard BCG therapy involving 6-weekly intravesical instillations of OncoTICE® BCG (2 to 8 × 108 colony forming units; Merck Sharp & Dohme AG) in line with European Association of Urology (EAU) guidelines and at the shared decision of both the physicians and patients. Urine samples were self-collected at the Lausanne University Hospital 2–3 h following BCG instillation (second urination) (Table S1). All patients provided written informed consent before taking part.
The study was approved by the Health Research Authority, London—Stanmore Research Ethics Committee (19/LO/0179; 257743) and the Ethics Committee of the Canton de Vaud in Switzerland (#2019–00564).
Isolation of leukocytes from healthy donors and spiking into cell-free urine supernatant
Buffy coat was recovered from the NHSBT bag into a 50 mL tube and centrifuged (800xg, 10 min, room temperature (RT)). The leukocyte layer and plasma were collected and washed with Hanks’ Balanced Salt Solution (HBSS; no Ca2+ , no Mg2 +) by centrifugation (300xg, 10 min, RT). Red blood cells (RBC) were removed by lysis with RBC lysis buffer (Biolegend®). Leukocytes were washed again and re-suspended in Dulbecco’s phosphate buffered saline (D-PBS, Gibco) with 0.5% bovine serum albumin (BSA; Sigma-Aldrich) for cell counting.
Cell-free urine supernatant was thawed at 4°C. Before adding leukocytes, cell-free urine was centrifuged (300xg, 10 min, 4°C), then filtered with a 0.2 μm syringe filter to remove any protein aggregates or cell debris. The leukocytes were spiked into urine cell-free supernatants at three different concentrations (103, 104 or 105 cells/mL of urine supernatant) and analysed by flow cytometry either immediately or after 24, 48 or 72 h at 4℃ (Fig. 1a).
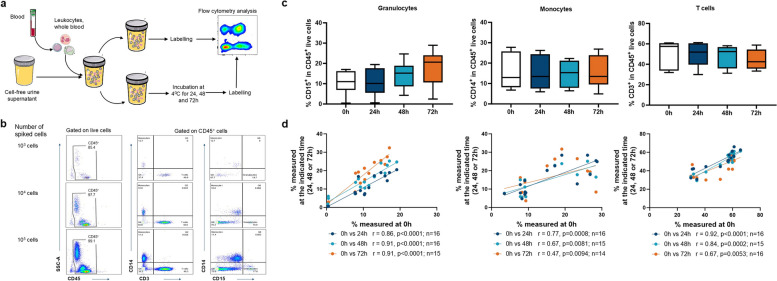
Fig. 1.
Extended leukocyte sample quality window defined for up to 72h in refrigerated urine matrix. a Experimental protocol of the healthy donor leukocytes spiking experiments. Blood leukocytes from four healthy donors were spiked at three concentrations (103, 104 or 105 cells/mL) into cell-free urine supernatants obtained from either healthy donors (n = 2) or untreated NMIBC patients (n = 2) and analysed by flow cytometry either fresh or after incubation for 24h, 48h or 72h at 4°C. This figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license. b Gating strategy from urine spiked with different numbers of leukocytes (103, 104 or 105 cells): after debris, doublets and dead cells exclusion, frequencies of monocytes (CD45+CD14+CD3−), T cells (CD45+CD3+CD14−) and granulocytes (CD45+CD15+CD14−) were determined. Since no difference in stability was observed between different concentrations of spiked leukocytes, these were therefore treated as technical replicates. c Frequency of granulocytes (CD15+), monocytes (CD14+) and T cells (CD3+) from spiked leukocytes after 0h, 24h, 48h or 72h at 4℃. Comparisons were made using an ordinary one-way ANOVA with Dunnett’s multiple comparisons test (with no statistically significant differences). Graphs include n = 6 independent leukocyte/urine matrix conditions per timepoint. d Correlations between the frequencies of immune cell subsets determined in fresh samples (0h) and samples refrigerated for 24h, 48h or 72h. A Spearman r test with two-tailed P-value was performed
Processing of urine samples from BCG-treated NMIBC patients
Upon collection, patient urine samples were split into two tubes followed by either immediate processing or overnight refrigeration for 24h at 4℃ (Fig. 2a). Both tubes were centrifuged (1500 revolutions per minute (RPM), 5 min) and the resulting cell pellet was counted, stained (as per below) and fixed before flow cytometry acquisition. The urine cell-free supernatant was frozen at -80℃ for batch proteomic analysis (Fig. 2a).
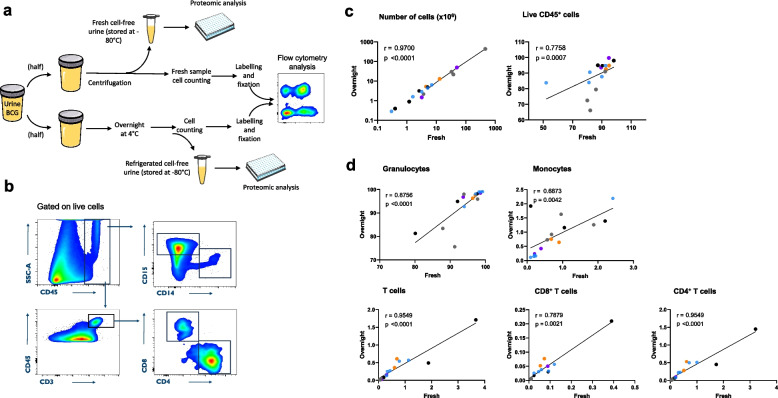
Fig. 2.
Urinary leukocyte stability in overnight refrigerated BCG-treated patients’ samples. a Experimental protocol for processing urine from BCG-treated non-muscle invasive bladder cancer patients. Urine samples were split in two tubes followed by either immediate analysis or overnight refrigeration at 4°C. Frequencies of urinary immune cell subsets were then assessed by flow cytometry. This figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license. b Representative gating strategy to identify urinary CD8+ and CD4+ T cells (CD45+CD3+CD8+ or CD4+, respectively), granulocytes (CD45+CD15+CD14−) and monocytes (CD45+CD15−CD14+), after debris, doublets and dead cells exclusion. c Correlation of total absolute cell number, live leukocyte frequency (CD45+ cells) and d immune cell subset frequencies measured in fresh versus overnight incubated samples (n = 16) from BCG-treated non muscle invasive bladder cancer patients. The Spearman r test with two-tailed P test was performed. Each colour represents an individual patient
Flow cytometry analysis
Cell-surface antigens were stained for 20 min at 4°C in the staining buffer (PBS with 0.2% BSA and 2 mM ethylenediaminetetraacetic acid [EDTA]) and an amine reactive dye (aqua live/dead stain kit, Life Technologies, Carlsbad, CA, USA) or propidium iodide (PI) (Invitrogen™) was used for dead cell exclusion. Fc-Receptor Blocking Reagent (Miltenyi Biotec) was used to increase staining specificity by blocking non-specific antibody binding.
Spiked leukocytes were stained with anti-human CD45-BV605™ (BioLegend®, mouse IgG1, clone HI30), anti-human CD3-PE (BioLegend®, mouse IgG1, clone UCHT1), anti-human CD14-BV421™ (BioLegend®, mouse IgG1, clone HCD14) and anti-human CD15-AF700 (BioLegend®, mouse IgM, clone HI98).
The following monoclonal antibodies were applied at pre-determined optimal concentrations to stain leukocytes in urine samples from BCG-treated patients: anti-human CD45-PE (eBioscience™, mouse IgG1, clone 2D1), anti-human CD15-PerCP/Cy5.5 (BioLegend®, mouse IgG1, clone W6D3), anti-human CD14-PE-Cy7 (eBioscience™, mouse IgG1, clone 61D3), anti-human CD3-AF700 (BioLegend®, mouse IgG2a, clone HIT3a), anti-human CD4-APC (BioLegend®, mouse IgG2b, clone OKT4) and anti-human CD8-AF488 (BioLegend®, mouse IgG1, clone SK1).
Stained samples were acquired on a Gallios (Beckman Coulter™) or Attune (Thermo Fisher Scientific) flow cytometer followed by analysis on FlowJo™ version 10.8.1 software (FlowJo LLC, Ashland, OR, USA).
Protein quantification
The Quick Start™ Bradford Protein Assay Kit 2 (Bio-Rad) was used to measure total protein in urine according to manufacturer’s instructions. Briefly, urine was diluted 1:10 and 1:50 with sterile D-PBS (Gibco). A standard curve was built using the albumin standard. The 1 × dye reagent provided in the kit was added to each diluted sample and reference standard in a microplate format, incubated at room temperature for not less than 10 min, and absorbances were measured at 595nm with a Spark microplate reader (Tecan).
Creatinine quantification
A creatinine assay kit was used to measure creatinine in urine according to manufacturer’s instructions (Oxford Biomedical Research®). Briefly, urine was diluted 1:10 with sterile distilled water and a standard curve was prepared in a microplate using creatinine. Two sequential absorbance readings were performed at 490 nm using a Spark microplate reader (Tecan) to determine the creatinine concentration (in mg/dL) in the samples. The first reading was performed after adding alkaline picrate solution (initial reading) and a second reading was performed after addition of acid reagent (final reading). The difference in absorbance upon subtracting the final reading from the initial reading for each standard and sample was directly proportional to the creatinine concentration.
Multiplex assay
Multiplex protein immunoassays were conducted using both the 48-plex (Merck Millipore) and 3-plex TGF-beta (Merck Millipore) assay kits according to manufacturer’s instructions. For the 48-plex assay, urine was diluted 1:3 with assay diluent. For the 3-plex TGF-beta assay, urine was acidified with 1N HCl for 10 min and the reaction was stopped by the addition of 1N NaOH. Acidified urine was diluted 1:3 with assay diluent. Samples were read on the Luminex™ xMAP™ INTELLIFLEX system (Merck, Germany). Acquired multiplex data were initially analysed using Belysa® software (Millipore Merck, version 1.0.19). After excluding any values below the limit of detection from analysis and accepting paired samples with a minimum of seven samples, the following analytes were excluded from the analysis: IL-1β, IL-7, IL-17E/IL-25, IL-22 and PDGF-AB/BB. To facilitate comparison between analytes, concentrations were normalised in the range of 0 to 1 using the Python numpy package (Supplementary online content).
Statistical analysis
GraphPad Prism version 10.0.3 (275) for Windows (GraphPad Software, USA) was used for statistical analysis of flow cytometry, total protein and urine creatinine data, as well as for plotting of raw bead array data.
Comparisons between groups for immune cell analysis to distinguish the change in cell frequencies with refrigeration time were conducted using a one-way ANOVA mixed-effects model with Dunnett’s multiple comparison test.
Correlation analysis was performed using the Spearman non-parametric r test with a two-tailed P value. The normality assumption for correlation analysis was assessed via the Shapiro–Wilk normality test.
Group analysis for multiplex assays was conducted using a mixed-effect model (REML) with Sidak multiple comparisons test. For proteomic correlation analysis, the Spearman rho test with two-tailed P test was calculated using the Python stats module of the SciPy library (1.11.1). Correlation for each analyte was done using Jupyter Notebooks (version 6.5.4 executed on Python 3.10.12) with the support of the packages: pandas (2.0.3), scipy (1.11.1), matplotlib (3.7.2) and numpy (1.25.1). The full python code is provided in Supplementary online content.
Results
Extended leukocyte sample quality window defined for up to 72h in refrigerated urine matrix
An initial controlled assessment of the urine sample quality window was conducted by spiking healthy donor or untreated NMIBC patient urine cell-free supernatants with healthy donor leukocytes (at 3 different concentrations: 103, 104 and 105 cells/mL) obtained from buffy coat products. Spiked samples were processed immediately or refrigerated at 4°C for 24, 48 or 72 h and the frequency of granulocytes (CD15+), monocytes (CD14+) and T cells (CD3+) was determined by flow cytometry (Fig. 1a, b, and Methods), as these immune cell subsets are the most frequent in urine from BCG-treated patients [17]. Interestingly, granulocyte, monocyte and T cell frequencies were stable up to 72h post-refrigeration compared to fresh (0h) sample (Fig. 1c). Notably, since no difference in stability was observed between different quantities of spiked blood leukocytes, these were treated as technical replicates. In addition, the frequency of each immune cell subset analysed prior to refrigeration significantly correlated with the frequency measured at each timepoint after refrigeration (Fig. 1d). Taken together, these findings indicated that storing urine samples at 4°C for up to 72 h did not significantly alter their immune cell content.
Urinary leukocytes are stable in overnight refrigerated BCG treated NMIBC patient urine samples
To confirm our observations in the clinical setting, urine samples were prospectively collected from five BCG-treated NMIBC patients following intravesical instillations (2–3 h after BCG instillation) throughout the induction treatment regimen (Table S1). Collected patient urine specimens were split into two samples: one was analysed by flow cytometry immediately after collection (fresh), while the other half was analysed following overnight (24h) refrigeration (Fig. 2a and Methods). As shown in Fig. 2C, total urine cell numbers as well as live CD45+ leukocytes showed high recovery rates with a strong positive correlation between fresh versus overnight refrigerated samples (n = 16). These results appear to be consistent across patients, despite the small size of the cohort. Similarly, all studied urinary immune cell subset frequencies, including granulocytes, monocytes, total T cells and CD8+ and CD4+ T cells, demonstrated remarkable stability upon refrigeration at 4°C compared to the paired sample freshly analysed (Fig. 2d).
Total protein and creatinine remain stable following overnight refrigeration of urine
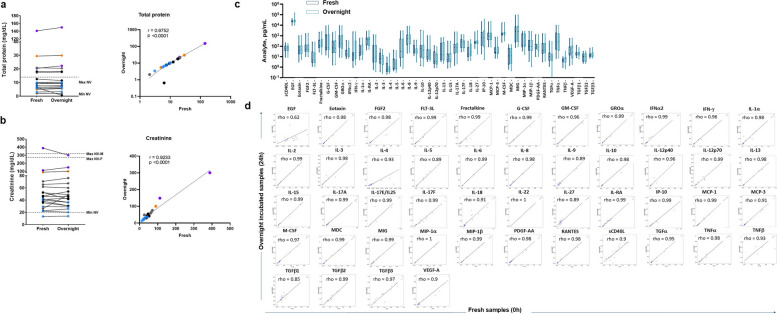
In parallel, proteomic analysis of 51 soluble factors was performed in BCG-treated NMIBC patient urine supernatants (n = 20), frozen immediately after collection or frozen after overnight refrigeration (Fig. 2a and Methods). As illustrated in Fig. 3a and b, comparable levels of total protein and creatinine were measured for samples frozen either immediately or following overnight refrigeration. Further, the cytokine, chemokine, and growth factor profiles in urine supernatants from BCG-treated NMIBC patients remained comparable between fresh and overnight refrigerated samples (Fig. 3c), which was confirmed by correlation analysis for all detected inflammatory mediators (Fig. 3d).
Fig. 3.
Proteomic profiling in BCG-treated NMIBC patient urine samples upon refrigeration. Comparison of total protein (a) and creatinine (b) concentrations between urine supernatant samples (n = 20) from BCG-treated NMIBC patients freshly frozen versus frozen after overnight refrigeration. A non-parametric paired t test (left panel, with no statistically significant differences) and a Spearman r test with two-tailed P-value (right panel) were performed. Each colour represents an individual patient. The dotted lines represent the minimum (Min NV) and maximum (Max NV) normal values in single void urine samples, serving as a reference control for our results. c, d Comparison of the urinary concentration of each detected soluble factor from BCG-treated NMIBC patients between freshly frozen versus frozen after overnight refrigeration. Analytes, namely IL-1b, IL-7, IL-17E/IL-25, IL-22, and PDGF-AB/BB, that were below the limit of detection or had paired samples from fewer than seven samples were not plotted. No significant differences were observed between measurements for each analyte in the group analysis using a mixed-effect model (REML) with a Sidak multiple comparisons test (c). Correlations between fresh and overnight refrigerated at 4℃ cell-free urine supernatants from BCG-treated non-muscle invasive bladder cancer patients for each measured analyte, and the Spearman rho test with two-tailed P test was calculated using the Python stats module of the SciPy library (d)
Discussion
Urine contains multiple components that can be used as effective biomarkers and can potentially reflect dynamic changes in the urological tumour microenvironment, including immune cells and proteins [20, 21]. Voided urine sampling is thus a simple, non-invasive procedure that may generate a highly informative source of biomarkers allowing for regular monitoring of treatment and disease, especially bladder cancer [11, 22, 23], and can potentially increase patient enrolment and retention in clinical trials. However, the limited knowledge about the stability of urine samples during storage and transit poses a challenge for their use in clinical trials, where samples must be analysed by a central laboratory.
Tumour tissue and peripheral blood are traditionally considered key samples for tracking biomarkers in oncology patients. Yet urine samples represent a non-invasive and readily available patient output that can provide similarly valuable clinical insights, especially in the urology setting [14–17, 24]. Extensive research has investigated stability and storage considerations for assays involving tumour tissue and peripheral blood [7, 8, 18], while established standards for voided urine samples remain limited. Existing studies mainly focus on urinary metabolites/electrolytes, with limited analysis of cytokines and no exploration of the urinary cell compartment.
We examined the stability of leukocytes from healthy donors spiked into either healthy donor or NMBIC patient urine supernatants, as well as the stability of immune cell and protein content in prospectively collected urine samples from NMIBC patients undergoing BCG treatment. We successfully show for the first time that storing urine at + 2 to + 8°C, without preservatives, for up to at least 24 h does not substantially affect the viability or recovery of total urine immune cells, and we were able to extend this stability window up to 72 h based on the result of spiking urine with whole blood leukocytes. Moreover, frequencies of monocytes, granulocytes and T cells remain unaffected in refrigerated urine. This discovery presents promising new logistical avenues for clinical trials or ongoing treatments involving the immunophenotyping of urinary leukocytes for biomarker research.
We also demonstrate that total protein and creatinine levels, together with cytokine, chemokine and growth factor profiles, remain stable in the overnight refrigerated urine matrix. Recently, Chang et al. explored the stability of urinary proteins in a 12-marker panel under different temperature regimens for 48h [19]. This study highlighted that the refrigerated storage of urine did not lead to loss of any of the twelve investigated proteins. Our findings are in line with this research, demonstrating robust protein recovery in overnight refrigerated specimens in a large-scale proteomic analysis.
Overall, our study confirms the feasibility of the immunophenotyping and proteomic analysis of refrigerated urine samples within a 24h sample quality window, that might be extended up to 72h. However, further long-term stability studies testing different refrigeration conditions are warranted.
This study has two main limitations. Although we analysed a substantial number of samples (16 for cellular and 20 for proteomic analyses), they were collected only from 5 BCG-treated patients. Besides, this small cohort may not faithfully represent the NMIBC population well, since 4 patients only had a carcinoma in situ (CIS). Future studies investigating larger cohorts will be needed to confirm our results.
Conclusions
By showing that whole urine samples can be overnight transported at 4℃ without undergoing any substantial decay in their quality, our results demonstrated that urine cellular and soluble factor biomarker analysis can be reliably implemented in decentralised or multicentred clinical trials. Consequently, this may potentially enhance patient enrolment and retention in clinical trials.
Supplementary Information
Acknowledgements
We would like to thank Stephanie Swift of Swift Science Writing (York, UK) for medical writing and editorial support associated with manuscript development. We are obliged to all patients for their dedicated collaboration and to healthy blood donors. Authors acknowledge Prof. Beat Roth and Dr. Ilaria Lucca (Department of Urology, University hospital of Lausanne, Switzerland) for the collection of urine samples. We also thank the Flow Cytometry Facility at University of Lausanne for its contribution in data acquisition.
Abbreviations
- BCG
Bacillus Calmette-Guérin
- NMIBC
Non-Muscle Invasive Bladder Cancer
- ON
Overnight
- RT
Room temperature
Authors’ contributions
Author contributions # Laurent Derré (LDC – Laurent Derré, CHUV) and Livija Deban (LDP – Livija Deban, Prokarium) contributed equally to this work and share last authorship. AS, LDC, LDP developed the study concept, designed and supervised the research; CP, SR, SDP, VC acquired the data; AS, CP, MBC, LDC performed data analysis; AS, LDC, LDP interpreted data and wrote the manuscript. All authors contributed to the study and agreed to its publication. AS had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This work was supported by Prokarium Ltd.
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Ethics approval and consent to participate
The study was approved by the Health Research Authority, London—Stanmore Research Ethics Committee (19/LO/0179; 257743) and the Ethics Committee of the Canton de Vaud in Switzerland (#2019–00564). All patients provided written informed consent before taking part.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Laurent Derré and Livija Deban contributed equally to this work and share last authorship.
References
- 1.Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20(11):651–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Unsworth-White SR, Kitchen MO, Bryan RT. Immunotherapy for non-muscle-invasive bladder cancer: from the origins of BCG to novel therapies. Future Oncol. 2022;18(1):105–15. [DOI] [PubMed] [Google Scholar]
- 3.Babjuk M, Burger M, Capoun O, Cohen D, Comperat EM, Dominguez Escrig JL, Gontero P, Liedberg F, Masson-Lecomte A, Mostafid AH, et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur Urol. 2022;81(1):75–94. [DOI] [PubMed] [Google Scholar]
- 4.Schneider AK, Chevalier MF, Derre L. The multifaceted immune regulation of bladder cancer. Nat Rev Urol. 2019;16(10):613–30. [DOI] [PubMed] [Google Scholar]
- 5.Mushtaq MU, Papadas A, Pagenkopf A, Flietner E, Morrow Z, Chaudhary SG, Asimakopoulos F. Tumor matrix remodeling and novel immunotherapies: the promise of matrix-derived immune biomarkers. J Immunother Cancer. 2018;6(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rui R, Zhou L, He S. Cancer immunotherapies: advances and bottlenecks. Front Immunol. 2023;14:1212476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Britten CM, Janetzki S, van der Burg SH, Gouttefangeas C, Hoos A. Toward the harmonization of immune monitoring in clinical trials: quo vadis? Cancer Immunol Immunother. 2008;57(3):285–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartmann FJ, Babdor J, Gherardini PF, Amir ED, Jones K, Sahaf B, Marquez DM, Krutzik P, O’Donnell E, Sigal N, et al. Comprehensive immune monitoring of clinical trials to advance human immunotherapy. Cell Rep. 2019;28(3):819-831 e814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joseph M, Enting D. Immune responses in bladder cancer-role of immune cell populations, prognostic factors and therapeutic implications. Front Oncol. 2019;9:1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamat AM, Li R, O’Donnell MA, Black PC, Roupret M, Catto JW, Comperat E, Ingersoll MA, Witjes WP, McConkey DJ, et al. Predicting response to intravesical bacillus calmette-guerin immunotherapy: are we there yet? A systematic review. Eur Urol. 2018;73(5):738–48. [DOI] [PubMed] [Google Scholar]
- 11.Laukhtina E, Shim SR, Mori K, D’Andrea D, Soria F, Rajwa P, Mostafaei H, Comperat E, Cimadamore A, Moschini M, et al. Diagnostic accuracy of novel urinary biomarker tests in non-muscle-invasive bladder cancer: a systematic review and network meta-analysis. Eur Urol Oncol. 2021;4(6):927–42. [DOI] [PubMed] [Google Scholar]
- 12.Murakami K, Pagano I, Furuya H, Daskivich T, Mori D, Rosser CJ. Clinical utility of oncuria, a multiplexed liquid biopsy for the non-invasive detection of bladder cancer-a pilot study. Diagnostics (Basel). 2022;12(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamat AM, Briggman J, Urbauer DL, Svatek R, Nogueras Gonzalez GM, Anderson R, Grossman HB, Prat F, Dinney CP. Cytokine Panel for Response to Intravesical Therapy (CyPRIT): nomogram of changes in urinary cytokine levels predicts patient response to bacillus calmette-guerin. Eur Urol. 2016;69(2):197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertolo M, Baumgart S, Durek P, Peddinghaus A, Mei H, Rose T, Enghard P, Grutzkau A. Deep phenotyping of urinary leukocytes by mass cytometry reveals a leukocyte signature for early and non-invasive prediction of response to treatment in active lupus nephritis. Front Immunol. 2020;11:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chevalier MF, Schneider AK, Cesson V, Dartiguenave F, Lucca I, Jichlinski P, Nardelli-Haefliger D, Derre L. Conventional and PD-L1-expressing regulatory t cells are enriched during BCG therapy and may limit its efficacy. Eur Urol. 2018;74(5):540–4. [DOI] [PubMed] [Google Scholar]
- 16.Wong YNS, Joshi K, Khetrapal P, Ismail M, Reading JL, Sunderland MW, Georgiou A, Furness AJS, Ben Aissa A, Ghorani E, et al. Urine-derived lymphocytes as a non-invasive measure of the bladder tumor immune microenvironment. J Exp Med. 2018;215(11):2748–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chevalier MF, Trabanelli S, Racle J, Salome B, Cesson V, Gharbi D, Bohner P, Domingos-Pereira S, Dartiguenave F, Fritschi AS, et al. ILC2-modulated T cell-to-MDSC balance is associated with bladder cancer recurrence. J Clin Invest. 2017;127(8):2916–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elliott P, Peakman TC, Biobank UK. The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int J Epidemiol. 2008;37(2):234–44. [DOI] [PubMed] [Google Scholar]
- 19.Chang C, Obeid W, Thiessen-Philbrook H, Parikh CR. Sample processing and stability for urine biomarker studies. J Appl Lab Med. 2021;6(6):1628–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chakraborty A, Dasari S, Long W, Mohan C. Urine protein biomarkers for the detection, surveillance, and treatment response prediction of bladder cancer. Am J Cancer Res. 2019;9(6):1104–17. [PMC free article] [PubMed] [Google Scholar]
- 21.Armenta-Castro A, Nunez-Soto MT, Rodriguez-Aguillon KO, Aguayo-Acosta A, Oyervides-Munoz MA, Snyder SA, Barcelo D, Saththasivam J, Lawler J, Sosa-Hernandez JE, et al. Urine biomarkers for Alzheimer’s disease: a new opportunity for wastewater-based epidemiology? Environ Int. 2024;184:108462. [DOI] [PubMed] [Google Scholar]
- 22.Soria F, Droller MJ, Lotan Y, Gontero P, D’Andrea D, Gust KM, Roupret M, Babjuk M, Palou J, Shariat SF. An up-to-date catalog of available urinary biomarkers for the surveillance of non-muscle invasive bladder cancer. World J Urol. 2018;36(12):1981–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuiverloon TC, Nieuweboer AJ, Vekony H, Kirkels WJ, Bangma CH, Zwarthoff EC. Markers predicting response to bacillus Calmette-Guerin immunotherapy in high-risk bladder cancer patients: a systematic review. Eur Urol. 2012;61(1):128–45. [DOI] [PubMed] [Google Scholar]
- 24.Suh J, Yuk HD, Jeong CW, Kwak C, Kim HH, Ku JH. Pyuria as a predictive marker of bacillus Calmette-Guerin unresponsiveness in non-muscle invasive bladder cancer. J Clin Med. 2021;10(17):3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.