Abstract
Purpose
Immunotherapy is a promising treatment for cancers but should be optimized for malignant gliomas. Because of immune privilege feature of the brain, local administration of immunotherapy may be a promising strategy for malignant glioma treatment. Identification of patients who may benefit from local immunotherapy is essential.
Methods
We retrospectively reviewed the clinicopathological characteristics and outcomes of six malignant glioma patients who received local administration of autologous cytokine-induced killer (CIK) cells through Ommaya reservoirs implanted into the tumor resection cavity. Profiles of tumor genome, transcriptome and immune microenvironment were also investigated by genomic target sequencing, RNA sequencing, electrochemiluminescence assay and immunohistochemistry (IHC) staining.
Results
Four patients died from tumor progression and the overall survival ranged from 10.0 to 33.9 months. Remarkably, two patients, including one diagnosed as diffuse hemispheric glioma H3 G34-mutant (G34-DHG, WHO grade 4) and the other diagnosed as astrocytoma (IDH1 mutation, WHO grade 3) survived more than 20 years without evidence of recurrence. The distinctive clinical feature of the two long-term survivors was tumor gross total resection (GTR) before CIK therapy. NTRK1 mutation was uniquely present and 353 genes were differentially expressed in the long-term survivors compared with the short-term survivors. These differential expression genes were highly associated with immune function. Electrochemiluminescence assay and IHC staining revealed higher expressions of cytokines and lower infiltrations of tumor-associated macrophages in the tumors of the long-term survivors.
Conclusion
These findings suggest that certain patients diagnosed as malignant gliomas, including G34-DHG (WHO grade 4), can acquire long-term survival after local immunotherapy. Tumor GTR before local immunotherapy and relatively weaker immunosuppressive tumor microenvironment are the favorable factors for long-term survival. Larger, controlled studies with standardized treatment protocols, including consistent use of GTR, are warranted to further evaluate the potential benefits of locally delivered immunotherapy.
Keywords: Glioma, Immunotherapy, Long-term survival
Key points
1. Two out of six malignant glioma patients, including one patient diagnosed with G34-DHG (WHO grade 4), receiving local immunotherapy with CIK cells survived more than 20 years.
2. Tumor GTR before local immunotherapy and relatively weaker immunosuppressive tumor microenvironment are the favorable factors for long-term survival.
Importance of the study
Immunotherapy has not yet achieved satisfactory results in malignant gliomas. One of the major obstacles is the immune privilege feature of the brain. Local administration of immunotherapy may be a promising strategy for malignant glioma treatment. Here, we report a cohort of six malignant glioma patients receiving immunotherapy with local administration of autologous CIK cells. Two patients, including one diagnosed as G34-DHG (WHO grade 4) and the other diagnosed as astrocytoma (IDH1 mutation, WHO grade 3) following the 2021 WHO Classification of Tumors of the Central Nervous System, survived more than 20 years without recurrence. To our knowledge, this is the longest survival that was reported among patients with G34-DHG (WHO grade 4), which indicated that G34-DHG (WHO grade 4) patients may benefit from local immunotherapy. In further analyses, we found that the two long-term survivors received tumor GTR before CIK therapy and possessed higher expressions of cytokines and lower infiltrations of tumor-associated macrophages in the tumors. These findings shed light on the selection of eligible malignant glioma patients for local immunotherapy.
Introduction
Malignant gliomas are the most prevalent malignant primary brain tumors [1]. The standard treatment currently involves surgical tumor reduction followed by simultaneous chemoradiation and additional chemotherapy [2]. Despite these efforts, the prognosis remains poor, with 5-year survival rates below 10% for glioblastoma (GBM) and under 25% for anaplastic astrocytoma [1, 3]. Therefore, developing new treatment strategies for malignant gliomas is of critical importance.
Immunotherapy, which induces anti-tumor effect either through activating the host’s immune response or transferring immune effector cells into patients, has emerged as a promising treatment for cancer patients [4]. Cytokine-induced killer (CIK) cells are a heterogeneous cell population comprising predominantly CD3+CD56+ T cells, with smaller fractions of CD3+CD56– T cells and CD3–CD56+ NK cells [5]. These cells can be generated in vitro from peripheral blood mononuclear cells (PBMCs), bone marrow or cord blood in the presence of defined cytokines [6]. The unique phenotype endows CIK cells with both the antigen-specific cytotoxicity characteristic of T cells and the non-MHC-restricted cytotoxicity typical of NK cells. Therefore, CIK cells can target tumor cells more effectively than standard CD8+ T cells [7, 8].
The immune privilege feature of the brain is one of the major obstacles impeding immunotherapy for malignant gliomas. The direct introduction of immune cells into the tumor cavity after tumor resection bypasses the blood-brain barrier (BBB) and therefore achieves a much higher local concentration of effector cells compared with intravenous infusion. We previously demonstrated the cytotoxic activity of CIK cells against glioma cells in vitro and reported the therapeutic effect of local administration of autologous CIK cells through Ommaya reservoirs implanted into the tumor cavity following tumor resection in a cohort of six patients with malignant gliomas [9].
In the present study, we retrospectively reviewed the clinicopathological characteristics and outcomes of these six malignant glioma patients. Remarkably, two patients have survived more than 20 years without evidence of recurrence. The favorable factors for long-term survival were further analyzed by comparing the clinicopathological characteristics and profiles of the tumor genome, transcriptome and immune microenvironment between the long-term and short-term survivors.
Materials and methods
Clinical data collection and follow-up
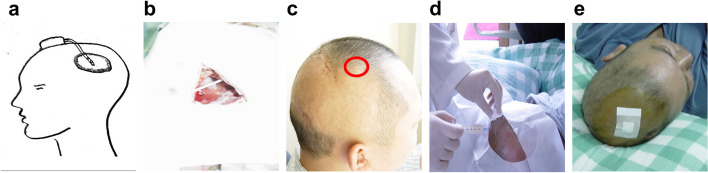
Between November 2002 and March 2004, six malignant glioma patients underwent tumor resection and a subcutaneous Ommaya reservoir was implanted with its catheter into the resection cavity during surgery at Sun Yat-sen University Cancer Center (Fig. 1a–b). Clinical data, including sex, age at enrollment, history of diagnosis, history of treatment, location of lesions, operation date, extent of resection (EOR), postoperative diagnoses, postoperative treatments and side effect of CIK therapy, were retrospectively reviewed. The EOR was qualitatively determined by the residual enhancing tumor in T1-enhanced scans of postoperative magnetic resonance imaging (MRI). Gross total resection (GTR) was defined as the absence of residual enhancing tumor, and subtotal resection (STR) was defined as an enhancing tumor residue that constituted less than 50%.
Fig. 1.
Injection of autologous CIK cells into the tumor cavity via the Ommaya reservoir. a The schematic of placement of Ommaya reservoir. b The catheter of the Ommaya reservoir was implanted into the resection cavity during surgery. c The red circle indicated the reservoir implanted under the scalp. d Autologous CIK cells were injected into the tumor cavity via the Ommaya reservoir under sterile conditions. e The puncture was covered with a dressing patch after injection of autologous CIK cells
Regular MRI scans were performed every 3 months in the first 2 years after the CIK therapy, semi-annually between the third and fifth years, and annually thereafter. The last date of follow-up was December 31, 2023. Survival status of all patients was determined from clinical attendance records or direct telecommunication with the patients or their families. Overall survival (OS) was calculated from the operation date before CIK therapy to the time of patient death or the follow-up deadline.
Protocol of CIK Therapy
Human CIK cells were prepared as described in previous studies [6]. Each patient's PBMCs were used to generate autologous CIK cells. There was no cross-patient use of PBMCs. Peripheral blood was collected from patients at least 1 month postoperatively or 2 months after adjuvant chemotherapy or radiotherapy. PBMCs were isolated and primed in RPMI-1640 culture medium supplemented with recombinant human IFN-γ (1000 IU/mL), IL-2 (300 IU/mL), IL-1 (100 IU/mL) and CD3 monoclonal antibody (50 ng/mL). Fresh culture medium supplemented with IL-2 (300 IU/mL) was replaced every 3 days for approximately 2 weeks. CIK cells reach a maximum total cell number and cytotoxic capacity between 14 and 21 days of culture [10]. The phenotype of CIK cells was analyzed by flow cytometry [9]. CIK cells were confirmed to be free of bacteria and fungus contamination before transfusion.
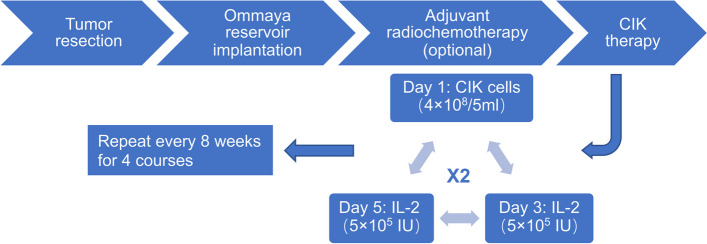
After preparation, autologous CIK cells were injected into the tumor cavity via the Ommaya reservoir under sterile conditions (Fig. 1c–e). A 2-week treatment schedule for a course of CIK therapy consisted of twice weekly infusions of 4 × 108 CIK cells followed by infusions of recombinant human IL-2 (5 × 105 IU) every other day. CIK therapy was repeated every 8 weeks for 4 courses unless tumor recurrence or intolerable toxicity (Fig. 2).
Fig. 2.
Schema of treatment. After tumor resection, a subcutaneous Ommaya reservoir was implanted with its catheter into the resection cavity. Adjuvant radiochemotherapy was optional based on the patient's condition. A 2-week treatment schedule was administered for a course of CIK therapy consisting of twice weekly infusions of 4 × 108 CIK cells followed by infusions of recombinant human IL-2 (5 × 105 IU) every other day. The CIK therapy was repeated every 8 weeks for 4 courses
Pathological diagnosis
The pathological diagnoses of all patients were updated using the 2021 WHO Classification of Tumors of the Central Nervous System [11]. Patients’ pathological sections were reviewed by an experienced pathologist. Molecular features of the tumors were analyzed by isolating genomic DNA from formalin-fixed and paraffin-embedded (FFPE) tumor tissues and analyzing the samples by next generation sequencing (NGS) targeting 131 glioma-related genes and chromosomal variation. The O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation status was examined using a methylation-specific polymerase chain reaction as previously described [12].
DNA extraction and library preparation
Genomic DNA (gDNA) was extracted from FFPE tissues using the Genomic DNA Tissue Extraction Kit (Concert®). NGS tests targeting 131 glioma-related genes were performed at Simcere Diagnostics CO. (Nanjing, China) following the manufacturer’s instructions. In brief, 200 ng gDNA was sheared into 200–300 bp fragments with combined bisulfite restriction assay. Indexed paired-end adaptors for Illumina platform were synthesized by Integrated DNA Technologies (IDT). End repair, A-tailing and adaptor ligation of sheared DNA were performed with reagents from the KAPA Hyper DNA Library Prep kit (Roche Diagnostics). Unligated adaptors were removed by the size selection function of Agencourt AMPure XP beads (Beckman Coulter) and the ligation products were PCR amplified to form a prelibrary for hybridization.
Library sequencing and bioinformatics analysis
Prepared DNA libraries were sequenced on the Illumina NovaSeq6000 platform (Illumina, San Diego, CA, USA) and generated 150 bp paired end reads. The principle of sequencing is sequencing by synthesis. Raw sequencing reads were assessed with FastQC for quality metrics. Adapters and low-quality bases were trimmed using Trimmomatic. Cleaned reads were aligned to the human reference genome (hg38) using BWA-MEM with default settings. Aligned reads were sorted and duplicates were marked using SAMtools and Picard to reduce PCR artifacts. Base quality score recalibration was performed using GATK with known variant sites to correct systematic sequencing errors. Somatic variants were identified using GATK Mutect2 in tumor-only mode, incorporating a panel of normals and germline resource to minimize false positives. Variants were filtered using GATK FilterMutectCalls to obtain high-confidence calls. Filtered variants were annotated with Ensembl's Variant Effect Predictor (VEP) to predict functional impacts and gene associations. Analysis focused on a custom panel of 131 mutations in genes commonly implicated in GBM.
RNA Sequencing (RNAseq)
Freshly frozen tumor tissues preserved in liquid nitrogen were used for RNAseq. Total RNA was extracted using the Trizol reagent kit (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol. RNA quality was assessed on an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA) and checked using RNase-free agarose gel electrophoresis. After total RNA was extracted, eukaryotic mRNA was enriched by Oligo (dT) beads, while prokaryotic mRNA was enriched by removing rRNA with the Ribo-Zero™ Magnetic Kit (Epicentre, Madison, WI, USA). The enriched mRNA was fragmented into short fragments using fragmentation buffer and reverse transcribed into cDNA with random primers. Second-strand cDNA was synthesized by DNA polymerase I, RNase H, dNTP and buffer. cDNA fragments were purified with the QiaQuick PCR extraction kit (Qiagen, Venlo, the Netherlands), end repaired, poly(A) added and ligated to Illumina sequencing adapters. The ligation products were size selected by agarose gel electrophoresis, PCR amplified and sequenced (150 PE, approximately 60 megabyte reads for each sample) using the Illumina HiSeq2500 by Gene Denovo Biotechnology Co. (Guangzhou, China). The sequences were aligned to the UCSC hg38 reference genome using RNA-Star (v2.4.2a). Gene expression abundance was calculated and normalized by RSEM (v1.3.3). Differential expression analysis was performed by DESeq2 (v1.44.0).
Determination of cytokine concentration in tumors
Freshly frozen tumor tissues preserved in liquid nitrogen were subjected to an electrochemiluminescence assay for 10 cytokines (K15049D-1, MSD) following the manufacturer’s recommendations.
Immunohistochemistry (IHC) Staining
Archived FFPE blocks used in pathological diagnosis were de-paraffinized, rehydrated and treating with 3% H2O2 at 37 °C for 10 min. The sections were subjected to antigen retrieval in antigen retrieval buffer (citrate buffer pH 6.0 or EDTA buffer pH 8.0 at 100 °C for 2 min in a pressure cooker). After blocking non-specific antigens with 5% goat serum for 1 h at room temperature, the sections were incubated with antibodies against CD3 (ZA-0503, ZSGB-Bio), CD68 (ZM-0060, ZSGB-Bio), CD86 (91,882, CST), or CD163 (ZM-0428, ZSGB-Bio) overnight at 4 °C, followed by incubation with secondary antibody at room temperature for 30 min. The slides were washed in PBS and stained with 3,3-diaminobenzidine (DAB). Finally, the sections were counterstained using Mayer’s hematoxylin, dehydrated and mounted. The average percentage of positive cells under three high power fields was determined as the final expression score of each section.
Statistical analysis
Student’s t test (unpaired, two-tailed) was performed using Prism software for statistical analysis between groups. p < 0.05 indicated statistical significance.
Results
Clinicopathological characteristics of patients
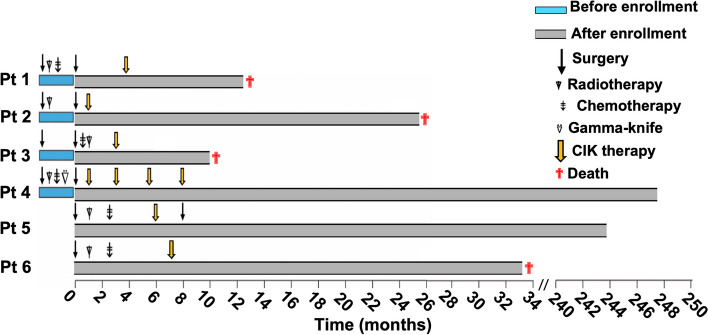
The clinicopathological characteristics of the six patients are summarized in Table 1. The patients’ age ranged from 25 to 58 years and the median age was 38 years. At enrollment, four patients had histories of malignant gliomas treated by GTR with or without subsequent adjuvant radiotherapy or chemotherapy and developed new single lesions in the brain. The other two patients were newly diagnosed with single brain lesions and did not have histories of anti-tumor therapy. The clinical event timeline is shown in Fig. 3.
Table 1.
Clinicopathological characteristics of patients
| Characteristics | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 |
|---|---|---|---|---|---|---|
| History of diagnosis | A (IDH1 mutation, WHO grade 4) | A (IDH1 mutation, WHO grade 4) | A (IDH1 mutation, WHO grade 3) | A (IDH1 mutation, WHO grade 3) | NA | NA |
| History of treatment | GTR + RT + (VP-16 + Me-CCNU)#8 | GTR + RT | GTR | GTR + RT + (VM-26 + Me-CCNU)#1 + (VM-26 + DDP)#3 + Gamma-knife#1 | None | None |
| Location of lesions | R-Frontal-parietal lobe | L-Temporal lobe | R-Temporal lobe | L-Frontal-parietal lobe | L-Temporal-parietal lobe | R-Parietal-occipital lobe |
| Operation date | 2002/11/21 | 2003/3/28 | 2003/7/2 | 2003/9/3 | 2003/12/12 | 2004/3/16 |
| EOR | STR | STR | STR | GTR | GTR | STR |
| Postoperative diagnosis | Recurrent A (IDH1 mutation, WHO grade 4) | Recurrent A (IDH1 mutation, WHO grade 4) | Recurrent A (IDH1 mutation, WHO grade 3) | Radionecrosis | G34-DHG (WHO grade 4) | A (IDH1 mutation, WHO grade 4) |
| Postoperative treatment | CIK#1 | CIK#1 | (VM-26 + BCNU)#1 + RT + CIK#1 | CIK#4 | RT + (VM-26 + DDP)#2 + CIK#1 + 2nd OP | RT + (VM-26 + DDP)#2 + CIK#1 |
| Side effect of CIK therapy | High fever | High fever, encephaledema | Low fever, encephaledema | None | Encephaledema | High fever, encephaledema |
| CTCAE v5.0 Grade | 2 | 3 | 3 | None | 3 | 3 |
| Death date | 2003/11/30 | 2005/4/30 | 2004/4/27 | Alive | Alive | 2006/12/27 |
| OS (months) | 12.5 | 25.5 | 10.0 | 247.5 | 244.1 | 33.9 |
A Astrocytoma, EOR Extent of resection, G34-DHG Diffuse hemispheric glioma H3 G34-mutant, GTR Gross total resection, L Left, NA Not applicable, OP Operation, R, right, RT Radiotherapy, STR Subtotal resection
Fig. 3.
Clinical event timeline. Four patients died from progressive disease during follow-up, and two patients survived more than 20 years without evidence of recurrence. Pt, patient; blue bars, unquantified representation of period before enrollment into this study; gray bars, quantified representation of period after enrollment into this study
The two cases of newly-diagnosed malignant gliomas were pathologically confirmed as diffuse hemispheric glioma H3 G34-mutant (G34-DHG, WHO grade 4) and astrocytoma (IDH1 mutation, WHO grade 3). The patients postoperatively received adjuvant radiotherapy followed by two courses of chemotherapy (VM-26 + DDP) and then a course of CIK therapy. Of the two patients, Patient 5 received a GTR for G34-DHG (WHO grade 4) before CIK therapy and a second resection for radiographic pseudoprogression after CIK therapy. He survived more than 244 months without evidence of recurrence at the end of follow-up. In contrast, Patient 6 who received a STR for astrocytoma (IDH1 mutation, WHO grade 4) before CIK therapy died from tumor progression at 33.9 months postoperatively.
For the four patients with histories of malignant gliomas, three patients underwent STR for their newly developed lesions, which were pathologically confirmed as recurrent astrocytoma (IDH1 mutation, WHO grade 4) or astrocytoma (IDH1 mutation, WHO grade 3). Among the three patients postoperatively diagnosed with recurrent diseases, one received a course of adjuvant chemotherapy (VM-26 + BCNU) followed by radiotherapy and a course of CIK therapy, and two only received a course of CIK therapy without other adjuvant therapies. Patient 4 who had a history of astrocytoma (IDH1 mutation, WHO grade 3) received a GTR for newly-developed lesion in the left frontal-parietal lobe, and this lesion was pathologically diagnosed as radionecrosis. Postoperatively, the patient received four courses of CIK therapy without other adjuvant therapies. Similar to Patient 5, Patient 4 who was also void of residual enhancing tumor in T1-enhanced scans of MRI before CIK therapy survived more than 247 months without evidence of recurrence at the end of follow-up. The other three patients who received STR for their recurrent diseases died from tumor progression during follow-up; the OS of these patients ranged from 10.0 to 25.5 months.
During the CIK therapy, all patients except Patient 4 experienced fever or symptoms of encephaledema, including headache, vomiting or neurological dysfunction. The severity of the side effects was evaluated by Common Terminology Criteria for Adverse Events (CTCAE) v5.0. The side effects of Patient 4 and Patient 1 were scored as Grade 1 and 2, and those of other patients were scored as Grade 3. While mannitol and dexamethasone efficiently relieved these side effects, only Patient 4 finished four courses of CIK therapy as planned. All other patients were unable to manage the side effects and received only one course of the CIK therapy. Other toxicities, including leukopenia, rash, diarrhea and anorexia, were not observed.
CIK cell viability in the resection cavity
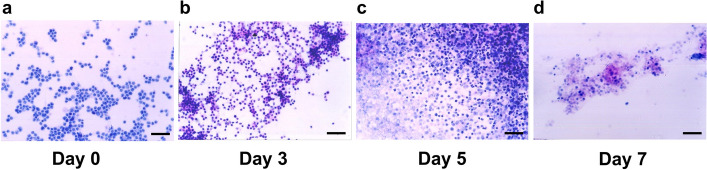
To trace the viability of CIK cells in the resection cavity, we extracted 1 ml of liquid from the resection cavity via the Ommaya reservoir on Day 3, Day 5 and Day 7 after injection of CIK cells. The liquid was subject to cytology smears. The morphology of these cells were assessed by two experienced pathologists and compared with that of the Day 0 samples representing freshly generated CIK cells prior to injection. On Day 0, cells exhibit small, round nuclei with scant cytoplasm, consistent with lymphocytes or NK cells, which are not typically present in brain tissue. On Day 3, almost all CIK cells maintained intact morphology with preserved nuclear membranes and no signs of apoptosis or necrosis, indicating the viability of CIK cells was similar with that before injection (Day 0) (Fig. 4a–b). On Day 5, cellular disintegration was observed in a proportion of CIK cells (Fig. 4c). On Day 7, the cellular structures of most CIK cells were disintegrated (Fig. 4d). These results indicated that CIK cells mostly survived in the resection cavity less than 1 week.
Fig. 4.
Cytology smears of CIK cells. a CIK cells before injection into the resection cavity. b–d CIK cells extracted from the resection cavity via the Ommaya reservoir on Day 3, Day 5 and Day 7 after injection. Bar represents 20 µm
MRI after Local Administration of CIK Cells
The dynamic features of MRI after injecting CIK cells into the resection cavity were investigated for the two long-term survivors and one of the short-term survivors.
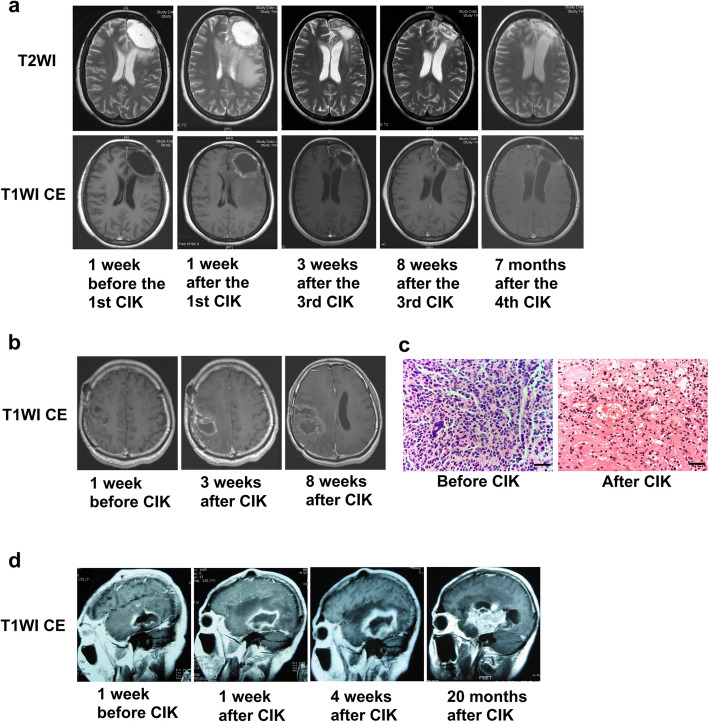
For Patient 4 who completed four courses of CIK therapy and achieved a long-term survival without evidence of recurrence, ring enhancement around the resection cavity and edema of the surrounding brain tissue were observed on MRI at 1 week after the first course of CIK therapy (Fig. 5a). The ring enhancement and edema resolved in the following several months, and the resection cavity also gradually shrank.
Fig. 5.
MRI after Local Administration of CIK Cells. a Pseudoprogression featured as ring enhancement of the resection cavity and edema of surrounding brain tissue was observed in a long-term survivor (Patient 4) at 1 week after local administration of CIK cells and gradually resolved during the following several months. b Pseudoprogression resulted in mild mass effect in another long-term survivor (Patient 5) after local administration of CIK cells. c For Patient 5, two operations were performed to resect the tumor before the CIK therapy and the pseudoprogression after the CIK therapy. HE staining showed that the tissue samples obtained before and after the CIK therapy were tumor and inflammatory necrosis with lymphocytes infiltration, respectively. Bar represents 10 µm. d True tumor progression occurred in a short-term survivor (Patient 2) after local administration of CIK cells
For Patient 5, who was the other long-term survivor, ring enhancement around the resection cavity also occurred after the CIK therapy and increased gradually. At 8 weeks after CIK therapy, the ring enhancement resulted in mild mass effect (Fig. 5b). Therefore, a second operation was performed to remove the ring enhancement. Unlike the tumor tissue resected before the CIK therapy, the ring enhancement formed after the CIK therapy was pathologically confirmed as inflammatory necrosis with lymphocyte infiltration (Fig. 5c).
For Patient 2 who died of tumor progression at 25.5 months postoperatively, the ring enhancement around resection cavity had already existed before the CIK therapy because of the STR for the tumor. After the CIK therapy, the ring enhancement was more obvious than those observed in Patient 4 and Patient 5. Moreover, the ring enhancement enlarged continuously and finally formed a blocky enhancement, indicating tumor recurrence (Fig. 5d).
Molecular features of tumors
As shown in Table 2, the most common genomic alterations were IDH1 mutation and TP53 mutation. IDH1 R132H point mutation occurred in five of the six patients, while Patient 5 harbored H3F3A G34R mutation and was diagnosed with G34-DHG (WHO grade 4). TP53 mutation also occurred in five patients; various mutations were detected. ATRX mutation occurred in four patients. Notably, NTRK1 mutation was uniquely present in the two long-term survivors. Additionally, Patient 6 harbored a number of chromosomal variations, which were rarely detected in other patients. MGMT promoter methylation and unmethylation were evenly distributed in the long-term and short-term survivors. All patients showed microsatellite stability.
Table 2.
Molecular characteristics of patients
| Characteristics | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 |
|---|---|---|---|---|---|---|
| SNV and INDEL | ARID2 p.A810P | ATM p.H1568D/M900V | CIC p.S721A | ATRX p.R1739* | ATRX p.F2113Sfs*9 | BRCA2 p.A2786T |
| ATRX p.N1283Ifs*63 | ATRX p.P842Qfs*27 | DNMT3A p.G532S | CHEK2 p.H371Y | FLT4 p.E1336K | ERBB3 p.R488Q | |
| BRCA1 p.G1350S | ERBB2 p.R985H | EPCAM p.Q204H | FGFR4 p.P136L | H3F3A p.G34R | HMCN1 p.I5618T | |
| FAT1 p.Y2690C/S2310C | IDH1 p.R132H | FUBP1 p.Y58Wfs*18 | HMCN1 p.R4397Q | KIT p.I744T | IDH1 p.R132H | |
| IDH1 p.R132H | SMARCA4 p.A130T | IDH1 p.R132H | IDH1 p.R132H | MLH1 p.R217C | MYCN p.P44L | |
| IRS2 p.P614L | TP53 p.R273H | NOTCH1 p.F357del | NTRK1 p.D79G | NTRK1 p.H268Q | PDGFRB p.T88I | |
| JAK2 p.E665D | PIKCR1 p.K448del | PTCH1 p.T416S | PDGFRA p.S377R | VEGFB p.R77C | ||
| KLF4 p.A188E | PTCH1 p.E165del | TP53 p.Y234H | PDGFRB p.Q443R | YAP1 p.P335L | ||
| NOTCH1 p.D1815G | TERT promoter C228T | TP53 p.R342* | ||||
| PLCG1 p.E1115K | TP53 p.N131del | |||||
| TP53 p.H214R/R175H | ||||||
| CNV | CDK6 amp | CCND2 amp | CCND2 amp | CDK4 amp | ||
| CDKN2A del | CDK4 amp | CDK6 amp | PTEN del | |||
| CDKN2B del | CDKN2B del | |||||
| MYC amp | MYC amp | |||||
| NAB2 amp | ||||||
| Chromosomal variation | 19q Chr del | 9p24.3-p21.2 del | 7p-q Chr amp | 10q11.21-q25.3 del | ||
| 10q26.12-q26.3 del | ||||||
| 10q26.3 amp | ||||||
| 11p15.5-p11.12 del | ||||||
| 12q12-q13.3 del | ||||||
| 12q14.1-q24.33 del | ||||||
| 19q Chr del | ||||||
| 19q11-q12 del | ||||||
| 19q13.2-q13.43 del | ||||||
| 21q11.2-q22.3 amp | ||||||
| 22q11.1-q13.33 del | ||||||
| 3p26.3-p11.1 del | ||||||
| 3q11.1-q29 del | ||||||
| 5p15.33-p12 del | ||||||
| 5q11.1-q35.3 del | ||||||
| MGMT promoter | Unmethylation | Methylation | Methylation | Unmethylation | Methylation | Unmethylation |
| MSI | MSS | MSS | MSS | MSS | MSS | MSS |
INDEL Small insertion and deletion, MSI Microsatellite instability, MSS Microsatellite stable, SNV Single nucleotide variant
Tumor transcriptome profiles
To investigate the tumor transcriptome profiles of the long-term and short-term survivors, freshly frozen tumor tissues of Patient 2, Patient 3, Patient 5 and Patient 6 were acquired and subjected to RNAseq. Because freshly frozen tumor tissue of Patient 4 was unavailable, only Patient 5 represented the long-term survival group. Patient 2, Patient 3 and Patient 6 were assigned to the short-term survival group.
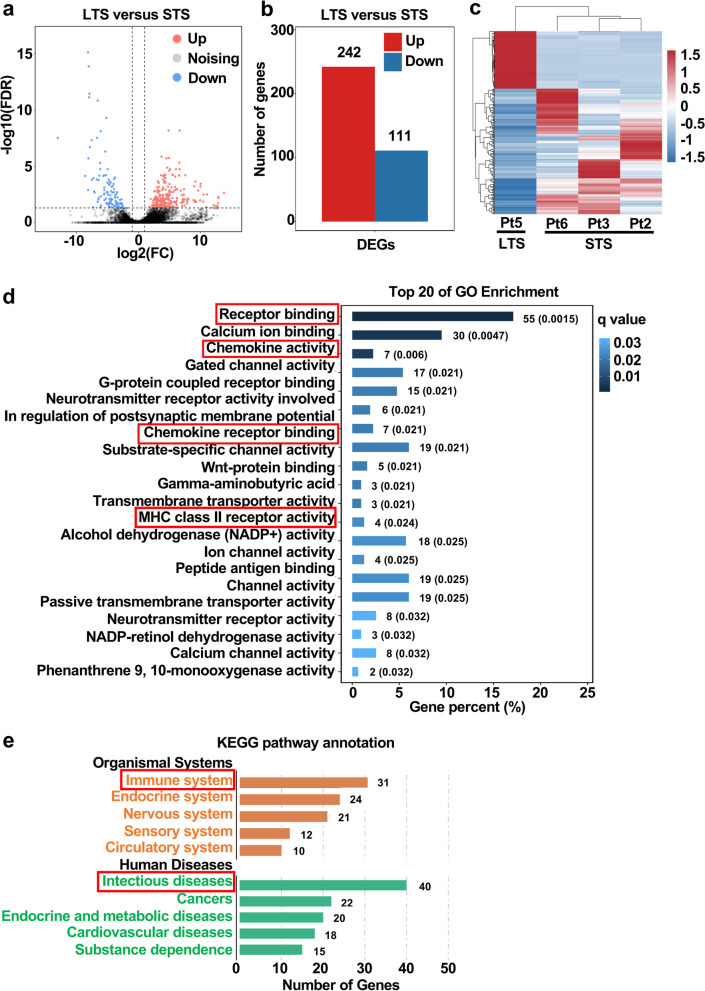
A total of 353 genes were differentially expressed (false discovery rate (FDR) < 0.05 and log2 fold change (FC) ≥ 1) in the tumor of the long-term survivor compared with tumors of the short-term survivors, including 242 markedly up-regulated genes and 111 down-regulated gene (Fig. 6a–b). Hierarchical clustering clearly showed the difference of the transcriptome profiles between long-term and short-term survivors (Fig. 6c). Functional annotations of GO enrichment indicated the differentially expressed genes (DEGs) were highly associated with immune function, such as receptor binding, chemokine activity, chemokine receptor binding and MHC class II receptor activity (Fig. 6d). KEGG pathway demonstrated that these DEGs were also corelated with immune function; the results showed that 31 and 40 DEGs were enriched in immune system and infectious diseases, respectively (Fig. 6e).
Fig. 6.
Tumor transcriptome profiles of the long-term and short-term survivors (total n = 1 long-term survivor and 3 short-term survivors). a–b A total of 353 genes were differentially expressed (false discovery rate (FDR) < 0.05 and log2 fold change (FC) ≥ 1) in the tumor of the long-term survivor compared with the tumors of the short-term survivors. Red, up-regulated; blue, down-regulated. c Hierarchical clustering of differentially expressed genes in tumors of the long-term and short-term survivors. Red, up-regulated; blue, down-regulated. Each column represents a patient; each row represents a differentially expressed gene. d GO functional enrichment analyses for differentially expressed genes in the tumor of the long-term survivor. e KEGG pathway enrichment analyses for differentially expressed genes in the tumor of the long-term survivor. LTS, long-term survivor; STS, short-term survivor; DEG, differential expression gene; Pt, patient
Expressions of cytokines in tumors
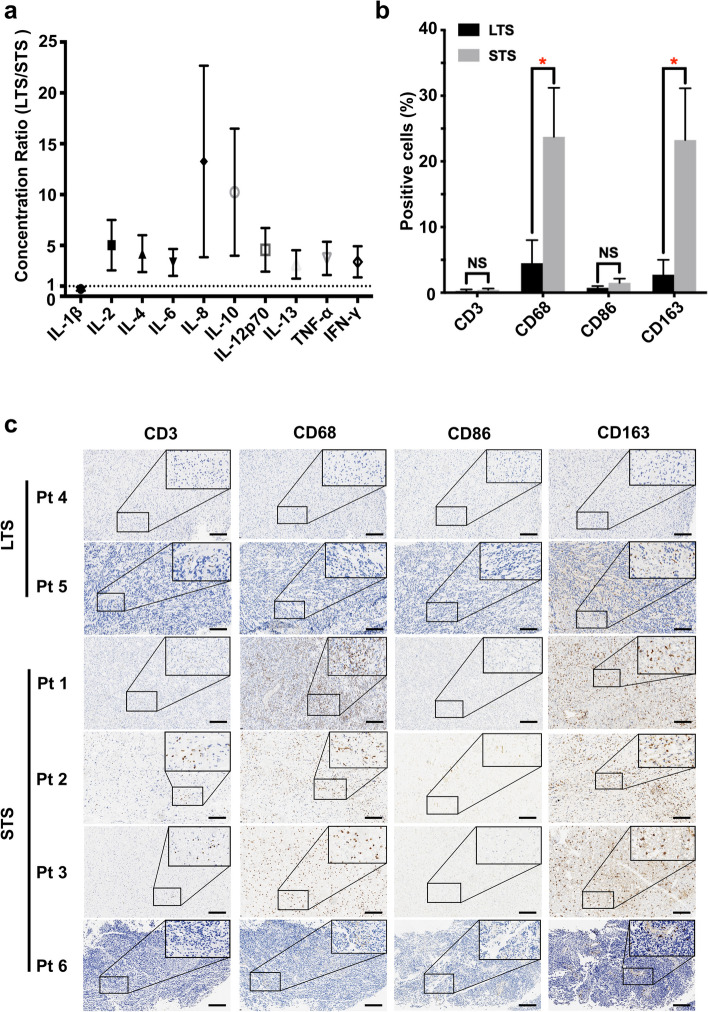
RNAseq showed that the DEGs of tumors of the long-term and short-term survivors were mainly enriched in immune function. We thus next tested the expressions of 10 cytokines in tumors of all patients except Patient 4 whose freshly frozen tumor tissue was unavailable. The expressions of all cytokines except IL-1B were higher in the tumor of Patient 5 who was a long-term survivor comparing with those of the four short-term survivors (Fig. 7a). Among the 10 cytokines, the expressions of IL-8 and IL-10 were the most discriminative and were 10 times higher in the tumor of the long-term survivor (Fig. 7a).
Fig. 7.
Expressions of cytokines and infiltrations of immune cells in tumors of the long-term and short-term survivors. a Ratio of the cytokine concentrations in the tumor of the long-term survivor to that of the short-term survivors (total n = 1 long-term survivor and 4 short-term survivors, mean ± SEM). b–c Tumor sections were stained with anti-CD3, CD68, CD86 and CD163 antibodies (total n = 2 long-term survivor and 4 short-term survivors). b Average percentages of positive cells under five high power fields are shown (mean ± SEM). *, P < 0.05; P values were determined by Student’s t test. c Representative images of (b) are shown. LTS, long-term survivor; STS, short-term survivor; Pt, patient; Bar represents 100 µm
T Cells and macrophage infiltration in tumors
The classification of “hot” (inflamed) and “cold” (non-inflamed) tumors was initially developed for colorectal cancer [13, 14], and the quantity of tumor-infiltrating lymphocytes (TILs) is meaningful for predicting the response to immunotherapy. Tumor-associated macrophages (TAMs), which are a major population of the non-neoplastic cells in glioma, play a pivotal role in tumor progression, immunosuppression and therapy resistance [15–19]. Therefore, we performed IHC to analyze T cells and macrophage infiltration in tumors of all patients.
As shown in Fig. 7b–c, the infiltrations of CD3+ T cells were low and comparable in the tumors of the long-term and short-term survivors, while CD68+ macrophages were significantly more abundant in tumors of the short-term survivors (P < 0.05). To determine the subtype of these infiltrating macrophages, we further analyzed the expressions of CD86 and CD163 (Fig. 7b–c). The expression of CD86, which is a hallmark of anti-tumor macrophages, was low and comparable in tumors of the long-term and short-term survivors. However, the expression of CD163, which is a hall mark of TAMs, was higher in tumors of the short-term survivors (P < 0.05).
Discussion
CIK therapy is a cancer immunotherapy with promising antitumoral activity and moderate toxicity. In the past decade, 106 clinical trials, which included 10,225 patients and more than 30 kinds of cancers, investigated the antitumor effects of CIK cells [20]. Overall, 27 trials showed significantly improved progression-free survival (PFS) and OS, and 9 trials reported a significantly increased 5-year survival rate in lung cancer, hepatocellular carcinoma, pancreatic cancer, colorectal cancer, gastric cancer, nasopharyngeal carcinoma, melanoma, renal cell carcinoma, ovarian cancer and breast cancer [20]. While CIK cell immunotherapy has been widely studied and has proven to be effective for extracranial tumors, very few clinical studies on malignant gliomas have been undertaken to date. In 2017, a multicenter, randomized, open-label, phase III study for newly-diagnosed GBM in Korea reported the addition of CIK therapy to standard chemoradiotherapy with temozolomide (TMZ) improved PFS, but not OS [21]. In the present study, two of six malignant glioma patients receiving local immunotherapy with CIK cells survived more than 20 years without recurrence. While the significance was limited by the small sample size and non-controlled design, the achievement of long-term survival in two of six malignant glioma patients is promising.
One of the two long-term survivors was diagnosed as G34-DHG (WHO grade 4), a new subtype of WHO grade 4 gliomas following the 2021 WHO Classification of Tumors of the Central Nervous System [22]. The median age at diagnosis and median OS of G34-DHG (WHO grade 4) patients were 15.8 years and 17.3 months, respectively [23]. In this study, the G34-DHG (WHO grade 4) patient was in his 30 s at diagnosis and survived more than 20 years after receiving local CIK therapy. To our best knowledge, this is the longest survival reported regarding G34-DHG (WHO grade 4) patients. Both the extent of tumor resection and whether the tumor is primary or recurrent are significant factors influencing OS. The G34-DHG (WHO grade 4) patient in our study had a primary tumor and GTR. However, even with GTR and a primary tumor, the patient survived significantly longer than the median OS for patients with G34-DHG (WHO grade 4), suggesting that factors beyond GTR and tumor status may have contributed to the extended survival. We hypothesize that the combination of GTR, primary tumor status, and local CIK therapy may have synergistically contributed to the prolonged survival. Previously, we reported a G34-DHG (WHO grade 4) patient with a tumor that harbored MUC16 mutation and significant immune infiltration and who survived more than 75 months [24]. Notably, the patient diagnosed as G34-DHG (WHO grade 4) also has a hot tumor immunomicroenvironment. Since the targeted sequencing panel used in this study did not cover MUC16, the MUC16 mutation was undetermined in this G34-DHG (WHO grade 4) patient. These finding indicated the G34-DHG (WHO grade 4) with a hot tumor immunomicroenvironment would be a good candidate for immunotherapy.
The distinctive clinical feature of the two long-term survivors in this study was the absence of residual enhancing tumor in T1-enhanced scans of MRI before the CIK therapy. Further analyses showed that higher expressions of cytokines and lower infiltrations of TAMs were present in the tumors of the long-term survivors. These results indicated that malignant glioma patients with a relatively weaker immunosuppressive tumor microenvironment (TME) could benefit more from local immunotherapy with CIK cells after tumor GTR.
Invasive growth is the main biological feature of malignant gliomas. The infiltration of tumor cells into the surrounding brain tissue makes malignant gliomas hard to be removed thoroughly by resection. However, GTR can reduce the tumor burden to the greatest extent, which creates an optimal condition for adjuvant therapy. While the enhancing tumor in T1-enhanced scans of MRI of the two long-term survivors in the present study was removed before the CIK therapy, there were still scattered tumor cells at tumor borders theoretically. Therefore, we speculated that CIK therapy suppressed tumor recurrence by further wiping out the scattered tumor cells and converting the immunosuppressive TME by which the tumor cells live. In contrast, all short-term survivors underwent STR before CIK therapy. The residual tumor mass after STR might be a formidable task for adjuvant therapies, including CIK therapy.
The dose of CIK cells and mode of administration varied between different studies because of the distinctly therapeutic regimes and patient health conditions. Most studies intravenously infused autologous CIK cells with a cell number ranging from 8 × 108 to 8 × 1010 per infusion [20]. Considering the local recurrence of malignant gliomas and immune privilege of brain, we directly injected CIK cells with a concentration of 4 × 108 per 5 ml into the resection cavity via Ommaya reservoirs. Hayes et al. reported that the local administration of lymphokine-activated killer (LAK) cells combined with recombinant human IL-2 into the resection cavity via Ommaya reservoirs provided malignant glioma patients with a promising prognosis [25]. However, the significance of this study was also limited by the small sample size and non-controlled design [25]. Additionally, intraventricular delivery of drugs for leptomeningeal metastasis by Ommaya reservoirs was reported to be associated with an increased risk of neurotoxicity [24]. Therefore, patients whose ventricles are opened during tumor resection are though to be ineligible for local immunotherapy postoperatively.
CIK therapy was generally reported to be tolerable with minor side effects. The side effects were mainly CTCAE Grade I–II toxicities such as fever, chills, fatigue, headache and skin rash [20]. However, the toxicity of CIK therapy was relatively obvious in the present study. Four of six patients experienced symptoms of encephaledema and thereby reported CTCAE Grade III toxicity. Severe side effects can result from the administration of IL-2 [26, 27]. The most prominent side effect of high-dose IL-2 therapy is vascular leak syndrome (VLS), characterized by increased endothelial cell permeability and the movement of fluid into the extravascular spaces [28]. Therefore, the encephaledema of our patients may result from VLS induced by IL-2 in brain parenchyma. While we tried to increase the viability of CIK cells in the resection cavity by using IL-2, CIK cells still mostly survived less than 1 week. Therefore, the necessity of administration of IL-2 should be reevaluated carefully in future studies.
The inflammatory response in brain parenchyma could mimic radiological features of tumor progression with increased enhancement and edema. Pseudoprogression featured as ring enhancement, edema or even mild mass effect would transiently occur after local immunotherapy with CIK cells and gradually be resolved during the following several months. Histopathology also confirmed the infiltration of lymphocytes but not mitotically active tumor cells in the pseudoprogression. In contrast, the ring enhancement of true tumor progression would increase continuously and finally form an obvious tumor mass. These findings were consistent with those reported in a previous study in which local immunotherapy with TILs and IL-2 was used in six patients with recurrent malignant gliomas [29]. The pseudoprogression following immunotherapy for a brain tumor was also noticed by iRANO (immunotherapy response assessment in neuro-oncology) committee. Currently, iRANO criteria suggest that in patients with early findings suggesting progression within the first 6 months of immunotherapy regimen but without substantial neurological decline, therapy should be continued and a confirmation of radiographic progression by follow-up imaging should be sought 3 months after the initial radiographic evidence of progressive disease [30].
A major challenge in using immunotherapy for malignant gliomas is an immunosuppressive TME [31]. In the present study, the tumors of the long-term survivors contained higher expressions of cytokines and lower infiltrations of TAMs, indicating a relatively weaker immunosuppressive TME that would be favorable for immunotherapy. TAMs represent up to 50% of the cells in the TME of brain tumor [32–34]. Their predominantly immunosuppressive roles in the TME of malignant gliomas facilitate the progression of malignant gliomas [35, 36]. These results supported that our long-term survivors may benefit from their low infiltration of TAMs.
Among the 10 cytokines that we tested, the expressions of IL-8 and IL-10 were elevated to the greatest extent in the tumor of the long-term survivor. Unlike TAMs, the role of IL-8 in predicting the response to immunotherapy for cancer patients was controversial. IL-8 was originally described as a chemokine whose main function is the attraction of a polymorphonuclear inflammatory leukocyte infiltrate [37]. IL-8 expression is frequently elevated in tumor cell lines and tissues, as well as in peripheral blood obtained from cancer patients [38–40]. Thus, IL-8 exerts various functions, including angiogenesis, survival signaling for cancer stem cells and attraction of myeloid cells [38]. Some studies reported that an increased level of IL-8 in peripheral blood was correlated with prolonged OS in cancer patients treated with immune checkpoint inhibitor, while others reported opposite results [41].
IL-10 plays a major role in modulating the activity of infiltrating immune cells in malignant gliomas, predominantly conferring an immunosuppressive action. However, several studies reported that IL-10 could have an immunostimulatory effect [42–48], particularly in regulating the behavior of CD8+ cytotoxic T cells. IL-10 stimulates the proliferation of CD8+ T cells in vitro and is necessary for inducing T cell cytotoxicity against tumor cells and the release of molecules necessary for antigen presentation [45, 46]. In addition to CD8+ T cells, Th1 CD4+ T cells require IL-10 to adopt anti-tumor functions in vitro [47]. Notably, the stimulatory role of IL-10 could depend on the presence of other pro-inflammatory cytokines in the TME. This was demonstrated in an in vivo study where enhanced T cell infiltration into the tumor accompanied with a more robust anti-tumor effect was observed in mice transplanted with glioma cells expressing IL-10 and IL-2 compared with those transplanted with tumor cells producing only IL-10 or IL-2 [48]. These findings suggested a synergy of the up-regulated cytokines in the tumor of the long-term survivor, including IL-10 and IL-2, facilitating the anti-tumor effect of CIK therapy in the present study.
This study had several limitations. First of all, the significance of this study was mainly limited by the small sample size and the non-controlled design. The side effects of local administration of CIK cells followed by recombinant human IL-2 were intolerable for most patients. Local administration of immune cells without additional cytokines should be considered in future studies. Additionally, the favorable factors for long-term survival were investigated preliminarily. Despite these limitations, the achievement of long-term survival in two of six malignant glioma patients was still attractive.
In conclusion, certain patients diagnosed as malignant gliomas, including G34-DHG (WHO grade 4), can acquire long-term survival after local immunotherapy. Tumor GTR before local immunotherapy and a relatively weaker immunosuppressive TME may be the favorable factors for long-term survival. Since our results are hypothesis-generating, larger, controlled studies with standardized treatment protocols, including consistent use of GTR, are warranted to further evaluate the potential benefits of locally delivered immunotherapy.
Acknowledgements
We thank Simcere Diagnostics Co. (Nanjing, China) for their assistance with the analyses of the molecular features of tumors.
Abbreviations
- BBB
Blood–brain barrier
- CIK
Cytokine-induced killer cells
- CTCAE
Common Terminology Criteria for Adverse Events
- DEGs
Differentially expressed genes
- EOR
Extent of resection
- FDR
False discovery rate
- FFPE
Formalin-fixed and paraffin-embedded
- FPKM
Fragments per kilobase million
- GBM
Glioblastoma
- gDNA
Genomic DNA
- GTR
Gross total resection
- IHC
Immunohistochemistry
- iRANO
Immunotherapy response assessment in neuro-oncology
- MGMT
O6-methylguanine-DNA methyltransferase
- MRI
Magnetic resonance imaging
- NGS
Next generation sequencing
- OS
Overall survival
- PBMC
Peripheral blood mononuclear cells
- STR
Subtotal resection
- TAMs
Tumor-associated macrophages
- TILs
Tumor-infiltrating lymphocytes
- TME
Tumor microenvironment
- TMZ
Temozolomide
- VLS
Vascular leak syndrome
Authors’ contributions
All authors made substantial contributions to the conception or design of the study; the acquisition analysis, or interpretation of data; or drafting or revising the manuscript. All authors approved the manuscript. YM, HD, ZH, ZC and YC designed the study and analyses. YM, WH, KS, XZ and ZC provided clinicopathologic data and tumor specimens. Experiments were performed t by YM, HD, YC, JX, YL, and RL. Data analysis was performed by YM, HD, ZH, ZC, YC and CZ. The manuscript and figures were prepared by HD, ZH, ZC, YC and CZ with input from all authors. The study was supervised by YM.
Funding
This work was supported by the National Natural Science Foundation of China (82303805, 82373386, 82203129), and the GuangDong Basic and Applied Basic Research Foundation (2020A1515110069, 2020A1515110203).
Data availability
The datasets generated during the current study are available in the Genome Sequence Archive (GSA) at https://ngdc.cncb.ac.cn/gsa-human/, accessible with Accession Number HRA009437 (https://bigd.big.ac.cn/gsa-human/browse/HRA009437). Additionally, the data and materials from this research are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Medical Ethics Committees of SYSUCC (Reference No. B2023-198–01). Informed consent to participate was obtained from all participants in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hao Duan, Zhenqiang He, Zhenghe Chen and Yukun Chen contributed equally to this work.
References
- 1.Ostrom QT, Price M, Neff C, Cioffi G, Waite KA, Kruchko C, et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015–2019. Neuro Oncol. 2022;24(Suppl 5):v1–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96. [DOI] [PubMed] [Google Scholar]
- 3.Smoll NR, Hamilton B. Incidence and relative survival of anaplastic astrocytomas. Neuro Oncol. 2014;16(10):1400–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim M, Xia Y, Bettegowda C, Weller M. Current state of immunotherapy for glioblastoma. Nat Rev Clin Oncol. 2018;15(7):422–42. [DOI] [PubMed] [Google Scholar]
- 5.Pievani A, Borleri G, Pende D, Moretta L, Rambaldi A, Golay J, et al. Dual-functional capability of CD3+CD56+ CIK cells, a T-cell subset that acquires NK function and retains TCR-mediated specific cytotoxicity. Blood. 2011;118(12):3301–10. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt-Wolf GD, Negrin RS, Schmidt-Wolf IG. Activated T cells and cytokine-induced CD3+CD56+ killer cells. Ann Hematol. 1997;74(2):51–6. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt-Wolf IG, Lefterova P, Mehta BA, Fernandez LP, Huhn D, Blume KG, et al. Phenotypic characterization and identification of effector cells involved in tumor cell recognition of cytokine-induced killer cells. Exp Hematol. 1993;21(13):1673–9. [PubMed] [Google Scholar]
- 8.Introna M, Correnti F. Innovative Clinical Perspectives for CIK Cells in Cancer Patients. Int J Mol Sci. 2018;19(2). [DOI] [PMC free article] [PubMed]
- 9.Lin L, Mu Y, Chen Z. Preliminary study of local immunotherapy with autologous cytokine-induced killer cells for glioma patients %J Chinese Journal of Clinical Oncology. 2008;5(4).
- 10.Jiang J, Xu N, Wu C, Deng H, Lu M, Li M, et al. Treatment of advanced gastric cancer by chemotherapy combined with autologous cytokine-induced killer cells. Anticancer Res. 2006;26(3B):2237–42. [PubMed] [Google Scholar]
- 11.Wendel P, Reindl LM, Bexte T, Kunnemeyer L, Sarchen V, Albinger N, et al. Arming Immune Cells for Battle: A Brief Journey through the Advancements of T and NK Cell Immunotherapy. Cancers (Basel). 2021;13(6). [DOI] [PMC free article] [PubMed]
- 12.Esteller M, Hamilton SR, Burger PC, Baylin SB, Herman JG. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res. 1999;59(4):793–7. [PubMed] [Google Scholar]
- 13.Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18(3):197–218. [DOI] [PubMed] [Google Scholar]
- 14.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–4. [DOI] [PubMed] [Google Scholar]
- 15.Morantz RA, Wood GW, Foster M, Clark M, Gollahon K. Macrophages in experimental and human brain tumors. Part 1: Studies of the macrophage content of experimental rat brain tumors of varying immunogenicity. J Neurosurg. 1979;50(3):298–304. [DOI] [PubMed]
- 16.Hambardzumyan D, Gutmann DH, Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci. 2016;19(1):20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4(1):71–8. [DOI] [PubMed] [Google Scholar]
- 18.Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27(4):462–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ostuni R, Kratochvill F, Murray PJ, Natoli G. Macrophages and cancer: from mechanisms to therapeutic implications. Trends Immunol. 2015;36(4):229–39. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Schmidt-Wolf IGH. Ten-year update of the international registry on cytokine-induced killer cells in cancer immunotherapy. J Cell Physiol. 2020;235(12):9291–303. [DOI] [PubMed] [Google Scholar]
- 21.Kong DS, Nam DH, Kang SH, Lee JW, Chang JH, Kim JH, et al. Phase III randomized trial of autologous cytokine-induced killer cell immunotherapy for newly diagnosed glioblastoma in Korea. Oncotarget. 2017;8(4):7003–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021;23(8):1231–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crowell C, Mata-Mbemba D, Bennett J, Matheson K, Mackley M, Perreault S, et al. Systematic review of diffuse hemispheric glioma, H3 G34-mutant: Outcomes and associated clinical factors. Neurooncol Adv. 2022;4(1):vdac133. [DOI] [PMC free article] [PubMed]
- 24.Hu W, Duan H, Zhong S, Zeng J, Mou Y. High frequency of PDGFRA and MUC family gene mutations in diffuse hemispheric glioma, H3 G34-mutant: a glimmer of hope? J Transl Med. 2022;20(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayes RL. The cellular immunotherapy of primary brain tumors. Rev Neurol (Paris). 1992;148(6–7):454–66. [PubMed] [Google Scholar]
- 26.Pachella LA, Madsen LT, Dains JE. The Toxicity and Benefit of Various Dosing Strategies for Interleukin-2 in Metastatic Melanoma and Renal Cell Carcinoma. J Adv Pract Oncol. 2015;6(3):212–21. [PMC free article] [PubMed] [Google Scholar]
- 27.Acquavella N, Kluger H, Rhee J, Farber L, Tara H, Ariyan S, et al. Toxicity and activity of a twice daily high-dose bolus interleukin 2 regimen in patients with metastatic melanoma and metastatic renal cell cancer. J Immunother. 2008;31(6):569–76. [DOI] [PubMed] [Google Scholar]
- 28.MacDonald A, Wu TC, Hung CF. Interleukin 2-Based Fusion Proteins for the Treatment of Cancer. J Immunol Res. 2021;2021:7855808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith MM, Thompson JE, Castillo M, Cush S, Mukherji SK, Miller CH, et al. MR of recurrent high-grade astrocytomas after intralesional immunotherapy. AJNR Am J Neuroradiol. 1996;17(6):1065–71. [PMC free article] [PubMed] [Google Scholar]
- 30.Okada H, Weller M, Huang R, Finocchiaro G, Gilbert MR, Wick W, et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol. 2015;16(15):e534–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang M, Oh IY, Mahanty A, Jin WL, Yoo JS. Immunotherapy for Glioblastoma: Current State, Challenges, and Future Perspectives. Cancers (Basel). 2020;12(9). [DOI] [PMC free article] [PubMed]
- 32.Klemm F, Maas RR, Bowman RL, Kornete M, Soukup K, Nassiri S, et al. Interrogation of the Microenvironmental Landscape in Brain Tumors Reveals Disease-Specific Alterations of Immune Cells. Cell. 2020;181(7):1643–60 e17. [DOI] [PMC free article] [PubMed]
- 33.Venteicher AS, Tirosh I, Hebert C, Yizhak K, Neftel C, Filbin MG, et al. Decoupling genetics, lineages, and microenvironment in IDH-mutant gliomas by single-cell RNA-seq. Science. 2017;355(6332). [DOI] [PMC free article] [PubMed]
- 34.Andersen BM, Faust Akl C, Wheeler MA, Chiocca EA, Reardon DA, Quintana FJ. Glial and myeloid heterogeneity in the brain tumour microenvironment. Nat Rev Cancer. 2021;21(12):786–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xuan W, Lesniak MS, James CD, Heimberger AB, Chen P. Context-Dependent Glioblastoma-Macrophage/Microglia Symbiosis and Associated Mechanisms. Trends Immunol. 2021;42(4):280–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei J, Chen P, Gupta P, Ott M, Zamler D, Kassab C, et al. Immune biology of glioma-associated macrophages and microglia: functional and therapeutic implications. Neuro Oncol. 2020;22(2):180–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol. 1994;56(5):559–64. [PubMed] [Google Scholar]
- 38.Alfaro C, Sanmamed MF, Rodriguez-Ruiz ME, Teijeira A, Onate C, Gonzalez A, et al. Interleukin-8 in cancer pathogenesis, treatment and follow-up. Cancer Treat Rev. 2017;60:24–31. [DOI] [PubMed] [Google Scholar]
- 39.Kim JH. Interleukin-8 in the Tumor Immune Niche: Lessons from Comparative Oncology. Adv Exp Med Biol. 2020;1240:25–33. [DOI] [PubMed] [Google Scholar]
- 40.Kosmopoulos M, Christofides A, Drekolias D, Zavras PD, Gargalionis AN, Piperi C. Critical Role of IL-8 Targeting in Gliomas. Curr Med Chem. 2018;25(17):1954–67. [DOI] [PubMed] [Google Scholar]
- 41.Wang M, Zhai X, Li J, Guan J, Xu S, Li Y, et al. The Role of Cytokines in Predicting the Response and Adverse Events Related to Immune Checkpoint Inhibitors. Front Immunol. 2021;12:670391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Emmerich J, Mumm JB, Chan IH, LaFace D, Truong H, McClanahan T, et al. IL-10 directly activates and expands tumor-resident CD8(+) T cells without de novo infiltration from secondary lymphoid organs. Cancer Res. 2012;72(14):3570–81. [DOI] [PubMed] [Google Scholar]
- 43.Xi J, Xu M, Song Z, Li H, Xu S, Wang C, et al. Stimulatory role of interleukin 10 in CD8(+) T cells through STATs in gastric cancer. Tumour Biol. 2017;39(5):1010428317706209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dennis KL, Saadalla A, Blatner NR, Wang S, Venkateswaran V, Gounari F, et al. T-cell Expression of IL10 Is Essential for Tumor Immune Surveillance in the Small Intestine. Cancer Immunol Res. 2015;3(7):806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mumm JB, Emmerich J, Zhang X, Chan I, Wu L, Mauze S, et al. IL-10 elicits IFNgamma-dependent tumor immune surveillance. Cancer Cell. 2011;20(6):781–96. [DOI] [PubMed] [Google Scholar]
- 46.Groux H, Bigler M, de Vries JE, Roncarolo MG. Inhibitory and stimulatory effects of IL-10 on human CD8+ T cells. J Immunol. 1998;160(7):3188–93. [PubMed] [Google Scholar]
- 47.De Vleeschouwer S, Spencer Lopes I, Ceuppens JL, Van Gool SW. Persistent IL-10 production is required for glioma growth suppressive activity by Th1-directed effector cells after stimulation with tumor lysate-loaded dendritic cells. J Neurooncol. 2007;84(2):131–40. [DOI] [PubMed] [Google Scholar]
- 48.Book AA, Fielding KE, Kundu N, Wilson MA, Fulton AM, Laterra J. IL-10 gene transfer to intracranial 9L glioma: tumor inhibition and cooperation with IL-2. J Neuroimmunol. 1998;92(1–2):50–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during the current study are available in the Genome Sequence Archive (GSA) at https://ngdc.cncb.ac.cn/gsa-human/, accessible with Accession Number HRA009437 (https://bigd.big.ac.cn/gsa-human/browse/HRA009437). Additionally, the data and materials from this research are available from the corresponding author upon reasonable request.