Abstract
Background
This study aimed to investigate the therapeutic effects of vibration therapy for improving upper extremity motor impairment, function, and disability recovery in people with stroke.
Design
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines. PubMed, EMBASE, the Cochrane Library Database, Physiotherapy Evidence Database (PEDro), China Knowledge Resource Integrated Database, and Google Scholar were searched from inception to May 31, 2024. Randomized controlled trials (RCTs) that evaluated the effects of vibration therapy on upper extremity motor impairment, function, and disability recovery post-stroke were analyzed.
Setting and participants
Participants with a diagnosis of stroke with hemiplegia (or hemiparesis) were recruited.
Methods
Methodological quality assessment was performed using the PEDro quality score. Upper extremity motor impairment, function, and disability were the primary outcomes. Upper extremity motor impairment was measured using the Fugl-Meyer Assessment scale and other methods. Upper extremity functions were evaluated using the Wolf Motor Function test or other tools assessing manipulative activities. Disability was assessed using the Functional Independence Measure, Barthel index, and other methods.
Results
Overall, 30 RCTs including 1621 people with stroke were selected. Compared with the control, vibration therapy exerted significant effects on upper extremity motor impairment [standardized mean difference (SMD) = 1.19; p < 0.00001)], function (SMD = 0.62; p < 0.00001), and disability recovery (SMD = 1.01; p < 0.00001). The subgroup analysis revealed that focal vibration therapy (SMD = 2.14) had favorable effects on disability recovery compared with whole-body vibration therapy (SMD = 2.0). Interventions lasting 4–8 weeks showed significant improvements in motor impairment (SMD = 1.19), motor function (SMD = 0.57), and disability (SMD = 0.84); additionally, the effects of vibration therapy combined with conventional rehabilitation (SMD = 1.03) were superior to those of vibration therapy alone (SMD = 0.21).
Conclusions
Vibration therapy may be a reliable rehabilitation program to improve upper extremity motor functions and disabilities. Furthermore, vibration therapy should be performed at the earliest possibility after stroke for at least 4–8 weeks.
Trial registration The protocol of this study was registered with PROSPERO (Registration number: CRD42022301119).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12984-024-01515-6.
Keywords: Stroke, Upper extremity, Quality of life, Vibration therapy, Motor function, Disability
Background
Brief Summary Vibration therapy (VT) significantly reduces upper extremity (UE) motor impairment, enhances UE motor function, and improves disability outcomes in people with stroke. Combining VT with standard rehabilitation is recommended, starting as early as possible after a stroke and continuing for at least 8 weeks. Both low and high vibration frequencies are effective, but focal muscle vibration is particularly beneficial for disability recovery.
Feeding, dressing, and writing are the most common activities of daily living that require motor function and participation of the upper limbs [1, 2]. These activities are essential for maintaining independence and quality of life. Neurologic disorders such as stroke, which often results in hemiparesis, where one side of the body becomes weak or paralyzed, may lead to poor motor function, muscle weakness, spasticity in paretic limbs, and disability [1, 2].
Full recovery of motor function and disabilities of the upper limbs occurs in less than 20% of people with stroke undergoing rehabilitation programs [1, 3]. Hence, many of these people with stroke have poor motor function and disability in the upper limbs, affecting their quality of life substantially [4, 5]. This impairment often necessitates long-term rehabilitation and support to manage daily activities [2, 3]. Consequently, independence and social participation can be significantly compromised for these individuals.
Compared with previous rehabilitation programs, vibration therapy (VT) stimulates muscle activity through the excitation of the tonic vibration reflex which activates efferent Ia, resulting in α-motor neuron excitation to generate muscle fiber strength and induce motor function performance [6, 7]. This implies that weak muscle activity and motor function in paretic limbs could be improved using VT. Recent studies investigated the effects of the whole-body vibration (WBV) on improving disabilities in the upper limbs for people with stroke by asking them to sit on a chair and place their hands on the WBV platform [8, 9]. After 4 weeks of the intervention, the people with stroke in the experimental group treated with WBV showed better motor function improvements in the upper limbs than those in the control group undergoing a traditional rehabilitation program [8, 9]. Furthermore, several studies have developed focal muscle vibration (FMV) to improve motor function and disabilities in people with stroke [10–12].
However, vibration force transmission from WBV or FMV to the targeted upper limbs is different being a complex process that could be influenced by various biomechanical mechanisms and result in different outcomes according to the type of vibration application [13, 14]. Previous studies often used vibrations < 20 Hz for muscle relaxation and reduction of spasticity [15, 16]. Research also shows that vibrations in the range of 20–30 Hz can improve gait balance [17]. Since the resonance frequencies of some important human organs are between 5 and 20 Hz, previous studies have considered 20 Hz as a safety threshold for vibration frequency [18].
Hence, optimal vibration protocols to improve motor function and disabilities should be established with strong evidence before applying them to improve motor and functional recovery in individuals with stroke in the clinical setting [7, 19, 20]. Therefore, it is crucial to develop an optimal evidence-based VT protocol for improving motor function and disability recovery to help clinical therapists enhance upper limb recovery in people with stroke.
Despite the potential of VT, its use in the upper extremities (UEs) and benefits on function and disability recovery have rarely been discussed; furthermore, evidence-based treatment effects are not well-established. Therefore, this meta-analysis aimed to investigate the effects of VT protocols on UE motor impairment, function, and disability recovery in people with stroke.
Methods
Design
The present study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines [21]. The protocol of this study was registered with PROSPERO (Registration number: CRD42022301119) and the need for written informed consent was waived because of its retrospective design.
Articles were screened and obtained through a comprehensive electronic search of online databases, including PubMed, EMBASE, the Cochrane Library, the Physiotherapy Evidence Database (PEDro), the China Knowledge Resource Integrated Database, and Google Scholar, from their inception to May 31, 2024. Secondary sources included papers in related systemic reviews. No restriction regarding language or time of publication was applied. Two authors (HJC and CDL) independently searched for relevant articles, screened them, and extracted data. Any disagreement between the authors was resolved through discussion. If a consensus was not reached, another team member (CHY), who served as an arbitrator, was consulted.
Search strategy
Our key search terms were “stroke” OR “cerebral vascular accident” OR “hemiplegia” OR “hemiparesis” AND “vibration” AND “upper extremity.” The search formulas for each database are detailed in Supplementary Table 1.
Selection criteria of the studies
Studies were eligible if they met the following criteria: randomized controlled trials (RCTs) that explored the effectiveness of VT for UEs; had adult participants with a diagnosis of ischemic or hemorrhagic stroke; the experimental group underwent VT with or without post-stroke standard rehabilitation (SR) (SR referred to conventional physiotherapy or occupational therapy, which increased UE strength, improved joint flexibility, and enhanced function); the control group received placebo vibratory stimuli, SR, or a combination of both; and those that performed a validated measurement of changes in UE motor impairment, function, or disability before and after the interventions. The definitions of these outcomes are provided in the g subsections below.
The exclusion criteria were studies that used an animal model, case reports or case series, and studies that were prospectively designed trials without a comparison group.
Outcome measures
UE motor impairment, function, and disability were the primary outcomes in the present study. UE motor impairment was measured using the Fugl-Meyer Assessment scale [22, 23] or other tools assessing UE motor impairment. UE functions were evaluated using the Wolf Motor Function test [24] or other tools assessing manipulative activities. Disability was assessed using the Functional Independence Measure [25], Barthel index [26], and other tools.
Data extraction
The following data were extracted from each included trial (Table 1): characteristics of the study sample and design, including the group design, the age, sex, diagnosis, stroke type, and disease onset duration in individuals with stroke; characteristics of the interventions (i.e., type of VT, treatment duration, and number of sessions); measurement time points; and main outcome measures. The follow-up duration was categorized into four types: immediate (< 1 month), short-term (≥ 1 month, < 3 months), medium-term (≥ 3 months, < 6 months), and long-term (≥ 6 months) [27]. When multiple time points were reported within the same timeframe, the longest period was selected for analysis. One author (HJC) extracted the relevant data from the included trials, and another author (CDL) reviewed the extracted data. Any disagreement between the two authors was resolved through discussion and a third author (CHY) was consulted if a consensus could not be reached. If a trial had more than one therapeutic or control intervention, each comparison was considered to be independent in the meta-analyses [28].
Table 1.
Summary of the characteristics of the included studies
| Study, first author date | Country (area) | Groups | Age (y)* | Sex (F/M) | N | Type of stroke | Dx duration (month) | Brunnstrom stage | Exercise intervention | Measured time point (week) | Primary outcome results | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Frequency × duration | |||||||||||
| Ahn | Korea | EG: VT | 58.7 ± 7.1 | 18/12 | 30 | IS, HS | 3.4 ± 1.1 | 2.53 ± 0.52 | WBV | 5 d/wk × 4 wk | Baseline | MFT |
| 2019 | (Asia) | CG: SR | 60.7 ± 5.9 | 17/16 | 30 | 2.8 ± 1.1 | 2.40 ± 0.51 | (20 sessions) | Posttest: 4 | |||
| Alp | Turkey | EG: VT + SR | 61.2 ± 11.0 | 0/10 | 10 | IS, HS | 18 (12–48) | ≥ 3 | WBV | 3 d/wk × 4 wk | Baseline | FIM |
| 2018 | (Asia) | CG: PLA + SR | 62.9 ± 8.2 | 2/9 | 11 | 24 (6–60) | (12 sessions) | Mid-test: 1 | ||||
| Posttest: 4, 12, 24 | ||||||||||||
| Annino | Italy | EG: VT + SR | 67.8 ± 8.3 | 5/14 | 19 | IS, HS | 33.6 ± 3.7 | NR | FMV | 3 d/wk × 8 wk | Baseline | BI, MT |
| 2019 | (Europe) | CG: SR | 69.4 ± 10.4 | 3/15 | 18 | 33.5 ± 3.7 | (24 sessions) | Posttest: 8 | ||||
| Calabrò | America | EG: VT + SR | 66.0 ± 5.0 | 5/5 | 10 | IS | 5 ± 2 | NR | FMV | 5 d/wk × 8 wk | Baseline | FMA |
| 2017 | CG: PLA + SR | 67.0 ± 4.0 | 6/4 | 10 | 6 ± 2 | (40 sessions) | Posttest: 8 | FIM | ||||
| Follow-up: 12 | ||||||||||||
| Caliandro | Italy | EG: VT + SR | 57.4 ± 12.8 | 8/20 | 28 | IS, HS | 100.7 ± 82.8 | NR | FMV | 3 d/wk × 1 wk | Baseline | WFM |
| 2012 | (Europe) | CG: PLA + SR | 61.9 ± 15.7 | 7/14 | 21 | 96.4 ± 66.8 | (3 sessions) | Posttest: 1 | ||||
| Follow-up: 5 | ||||||||||||
| Casale | Italy | EG: VT + SR | 64.7 ± 5.4 | 6/9 | 15 | IS, HS | Chronic | NR | FMV | 5 d/wk × 2 wk | Baseline | MFT |
| 2014 | (Europe) | CG: PLA + SR | 65.1 ± 5.8 | 6/9 | 15 | (≥ 6) | (10 sessions) | Posttest: 2 | ||||
| Follow-up: 4 | ||||||||||||
| Celletti | Italy | EG 1: VT + SMS | 43 (31–68) | 2/4 | 6 | IS, HS | 72 (24–396) | NR | FMV | 3 d/wk × 1 wk | Baseline | WFM |
| 2017 | (Europe) | EG 2: VT + SR | 43 (30–57) | 2/4 | 6 | 30 (30–48) | (3 sessions) | Posttest: 1 | MFT | |||
| CG: SR | 62.5 (46–69) | 2/4 | 6 | 66 (46–84) | ||||||||
| Cordo | USA | EG: VT + SMS | 56.3 ± 12.7 | 15/29 | 44 | IS, HS | 11.8 ± 5.7 | NR | FMV | 2–3 d/wk × 6–10 wk | Baseline | FMA |
| 2022 | (America) | CG: PLA + SMS | 57.7 ± 12.9 | 16/23 | 39 | 11.3 ± 5.0 | (18 sessions) | Posttest: 10 | MFT | |||
| Da-Silva | UK | EG: VT | 73 (65–80) | 8/6 | 14 | IS | 0.9 (0.4–1.6) | NR | FMV | 7 d/wk × 4 wk | Baseline | MFT, BI |
| 2019 | (Europe) | CG: PLA | 69 (61–80) | 12/7 | 19 | 0.9 (0.6–1.1) | (28 sessions) | Posttest: 4 | ||||
| Follow-up: 8 | ||||||||||||
| Feng | China | EG: VT + VR | 60.2 ± 2.9 | 20/31 | 51 | IS, HS | Acute | NR | FMV | 5 d/wk × 4 wk | Baseline | FMA, BI |
| 2019 | (Asia) | CG: VR | 59.8 ± 4.3 | 19/32 | 51 | (20 sessions) | Posttest: 4 | |||||
| Hsu | Taiwan | EG: VT + SMS | 53.6 ± 12.4 | NR | 10 | IS, HS | 28.9 ± 23.5 | NR | FMV | 2 d/wk × 6 wk | Baseline | FMA |
| 2021 | (Asia) | CG: SMS | 61.7 ± 8.4 | 9 | 24.9 ± 15.3 | (12 sessions) | Posttest: 6 | MFT | ||||
| Follow up: 18 | ||||||||||||
| Study, first author date | Country (area) | Groups | Age (y)* | Sex (F/M) | N | Type of stroke | Dx duration (month) | Brunnstrom stage | Exercise intervention | Measured time point (week) | Primary outcome results | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Frequency × duration | |||||||||||
| Lee | Korea | EG 1: VT + SR | 58.5 ± 11.8 | 5/10 | 15 | IS, HS | 8.1 ± 4.9 | NR | WBV | 3 d/wk × 4 wk | Baseline | FMA, WFM |
| 2016 | (Asia) | EG 2: VT | 59.2 ± 7.7 | 10/5 | 15 | 7.9 ± 4.2 | (12 sessions) | Posttest: 4 | ||||
| CG: SR | 60.2 ± 6.7 | 6/9 | 15 | 6.7 ± 3.9 | ||||||||
| Li | China | EG: VT + SR | 55.0 ± 10.9 | 2/12 | 14 | IS, HS | 1.5 ± 0.3 | 2-4 | WBV | 5 d/wk × 3 wk | Baseline | FMA |
| 2020 | (Asia) | CG: SR | 56.9 ± 9.9 | 4/10 | 14 | 1.5 ± 0.2 | (15 sessions) | Posttest: 3 | ||||
| Liu | China | EG: VT + SR | 57.8 ± 5.3 | 9/21 | 30 | IS, HS | 2.8 ± 0.3 | 2.86 ± 0.59 | FMV | 5 d/wk × 4 wk | Baseline | FMA, BI |
| 2022 | (Asia) | CG: PLA + SR | 58.5 ± 4.2 | 10/20 | 30 | 2.7 ± 0.2 | 2.94 ± 0.45 | (20 sessions) | Posttest: 4 | |||
| Lu | China | EG: VT + SR | 55.3 ± 6.6 | 12/18 | 30 | IS, HS | 3.4 ± 1.2 | 2-3 | WBV | 5 d/wk × 8 wk | Baseline | FMA |
| 2017 | (Asia) | CG: SR | 55.1 ± 6.3 | 13/17 | 30 | 3.3 ± 1.3 | (40 sessions) | Posttest: 8 | BI | |||
| Lu | China | EG: VT + SR | 54.7 ± 4.3 | 16/23 | 39 | IS, HS | 0.5 ± 0.1 | 3-5 | FMV | 5 d/wk × 4 wk | Baseline | FMA, BI |
| 2021 | (Asia) | CG: SR | 54.5 ± 4.3 | 15/24 | 39 | 0.5 ± 0.1 | (20 sessions) | Posttest: 4 | FIM | |||
| Meng | China | EG: VT + SR | 54.7 ± 4.3 | 35/25 | 60 | IS, HS | 1.2 ± 0.2 | NR | FMV | 3 d/wk × 4 wk | Baseline | FMA, BI |
| 2020 | (Asia) | CG: SR | 54.5 ± 4.3 | 29/31 | 60 | 1.2 ± 0.3 | (12 sessions) | Posttest: 4 | ||||
| Oliveira | Brazil | EG 1: VT | 60.1 (55–65) | 13/8 | 7 | NR | ≥ 12 | ≥ 4 | FMV | 3 d/wk × 4 wk | Baseline | WFM, MFT, |
| 2018 | (Europe) | CG 1: MrT | 7 | (12 sessions) | Posttest: 4 | Mobility Index | ||||||
| CG 2: SR | 7 | |||||||||||
| Seo | American | EG: VT + SR | 61.0 ± 10.0 | 1/5 | 6 | IS | 84 ± 84 | NR | FMV | 3 d/wk × 2 wk | Baseline | WFM |
| 2019 | CG: PLA + SR | 64.0 ± 8.0 | 4/2 | 6 | 36 ± 24 | (6 sessions) | Posttest: 2 | MFT | ||||
| Follow up: 6 | ||||||||||||
| Song | China | EG: VT + SR | 77.0 ± 10.5 | 16/24 | 40 | IS, HS | Subacute | 1-3 | FMV | 5 d/wk × 3 wk | Baseline | FMA, BI |
| 2018 | (Asia) | CG: SR | 75.0 ± 9.8 | 13/27 | 40 | (15 sessions) | Posttest: 3 | |||||
| Tavernese | Italy | EG: VT + SR | 58.9 ± 14.7 | 3/21 | 24 | IS, HS | Chronic | 4.5 (3.0–5.3) | FMV | 5 d/wk × 2 wk | Baseline | MFT |
| 2013 | (Europe) | CG: SR | 58.3 ± 12.4 | 2/18 | 20 | (≥ 6) | 4.3 (3.0–5.0) | (10 sessions) | Posttest: 2 | |||
| Toscano | Italy | EG: VT + SR | 64.7 ± 17.2 | 2/10 | 10 | IS, HS | Acute | NR | FMV | 1 session/d × 3 d | Baseline | FMA, MFT |
| 2019 | (Europe) | CG: PLA + SR | 69.5 ± 7.3 | 6/6 | 12 | (≤3 days) | (3 sessions) | Posttest: 1 | ||||
| Wang | China | EG: VT + SR | 48.4 ± 6.9 | 3/14 | 17 | IS, HS | 1.6 ± 0.3 | 1.6 ± 0.3 | WBV | 6 d/wk × 4 wk | Baseline | FMA, BI |
| 2018 | (Asia) | CG: SR | 49.2 ± 7.3 | 3/13 | 16 | 1.6 ± 0.3 | 1.7 ± 0.3 | (24 sessions) | Mid-test: 2 | |||
| Study, first author year | Country (area) | Groups | Age (y)* | Sex (F/M) | N | Type of stroke | Dx duration (month) | Brunnstrom stage | Exercise intervention | Measured time point (week) | Primary outcome results | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Frequency × duration | |||||||||||
| Wang | China | EG: VT + EMGB | 61.3 ± 8.0 | 21/39 | 60 | IS, HS | Subacute | NR | WBV | 5 d/wk × 4 wk | Baseline | FMA, BI |
| 2021 | (Asia) | CG: EMGB | 58.7 ± 9.3 | 15/45 | 60 | (20 sessions) | Posttest: 4 | |||||
| Wei | China | EG 1: VT + SR | 59.2 ± 11.3 | 7/25 | 32 | IS, HS | Subacute | 1.6 ± 0.7 | FMV | 7 d/wk × 4 wk | Baseline | FMA |
| 2019 | (Asia) | CG 1: PLA + SR | 60.4 ± 10.4 | 5/20 | 25 | 2.0 ± 1.4 | (28 sessions) | Posttest: 4 | FIM | |||
| CG 2: SR | 63.1 ± 10.3 | 4/23 | 27 | 1.8 ± 1.4 | Follow up: 8, 12 | |||||||
| Wu | China | EG: VT + SR | 58.1 ± 6.8 | 21/22 | 43 | IS, HS | 1.4 ± 0.1 | 2-4 | FMV | 5 d/wk × 8 wk | Baseline | FMA, BI |
| 2016 | (Asia) | CG: SR | 58.4 ± 6.3 | 20/23 | 43 | 1.4 ± 0.1 | (40 sessions) | Posttest: 8 | ||||
| Wu | China | EG: VT + SR | 59.3 ± 5.7 | 16/14 | 30 | IS, HS | 1.6 ± 0.2 | NR | WBV | 5 d/wk × 6 wk | Baseline | FMA, BI |
| 2022 | (Asia) | CG: SR | 58.7 ± 6.4 | 12/18 | 30 | 1.5 ± 0.2 | (30 sessions) | Posttest: 6 | ||||
| Yang | China | EG 1: VT + MIT | 63.2 ± 3.0 | 16/14 | 30 | IS, HS | 1.2 ± 0.1 | NR | WBV | 6 d/wk × 8 wk | Baseline | FMA, BI |
| 2022 | (Asia) | EG 2: VT | 62.4 ± 3.9 | 12/18 | 30 | 1.2 ± 0.1 | (48 sessions) | Posttest: 8 | ||||
| CG: MIT | 63.8 ± 2.7 | 16/14 | 30 | 1.2 ± 0.2 | ||||||||
| Yuan | China | EG: VT + SR | 58.7 ± 5.4 | 6/17 | 23 | IS, HS | 1.6 ± 0.3 | 1-2 | FMV | 5 d/wk × 4 wk | Baseline | FMA, MFT |
| 2018 | (Asia) | CG: PLA + SR | 59.2 ± 4.7 | 8/15 | 23 | 1.6 ± 0.3 | (20 sessions) | Posttest: 4 | ||||
| Zhu | China | EG 1: VT + SR | 65.0 ± 5.7 | 8/12 | 20 | IS, HS | 3.7 ± 1.4 | 2-3 | FMV | 7 d/wk × 3 wk | Baseline | FMA |
| 2017 | (Asia) | EG 2: VT | 62.0 ± 6.5 | 10/10 | 20 | 3.5 ± 1.5 | (21 sessions) | Posttest: 3 | ||||
| CG: SR | 67.0 ± 7.9 | 13/7 | 20 | 4.1 ± 2.3 | ||||||||
Values are presented as means ± standard deviations or medians (ranges or interquartile ranges)
Data present the sample mean value
F, female; M, male; Dx, disease; EG, experimental group; CG, control group; VT, vibration therapy; SR, standard rehabilitation; PLA, placebo; SMS, sensorimotor stimulation; MrT, mirror therapy; NR, not reported; IS, ischemic stroke; HS, hemorrhagic stroke; WBV, whole-body vibration; FMV, focal muscle vibration; MFT, motor function test; WFM, Wolf Motor Function test; FIM, functional independence measure; BI, Barthel index; VR, virtual reality
Assessment of bias risks and methodological quality of the included studies
Quality assessment was performed using the PEDro quality score to assess the risk of bias. Methodological quality (MQ) of all the included studies was independently assessed by two researchers in accordance with the PEDro classification scale, which is a valid measure of the MQ of clinical trials [29]. The PEDro scale scores 10 items including: random allocation (selection bias), concealed allocation (selection bias), similarity at baseline, subject blinding (performance bias), therapist blinding (performance bias), assessor blinding (detection bias), more than 85% follow-up for at least one key outcome (attrition bias), intention-to-treat analysis (attrition bias), inter-group statistical comparison for at least one key outcome, and point and variability measures for at least one key outcome. Each item is scored as either 1 for present or 0 for absent, and a total sum score ranging 0–10 is obtained by summation of all 10 items. Based on the PEDro score, the MQ of the included RCTs was rated as high (≥ 7/10), medium (4–6/10), or low (≤ 3/10) [30].
Data synthesis and analysis
We computed effect sizes for each study separately for the primary outcome measures (UE motor impairment, function, and disability). Primary outcome measures were defined as a pooled estimate of the mean difference in change between the mean of the treatment and the control groups. Analyses based on change scores (i.e., change from baseline) were performed to partially correct for inter-participant variability [31]. If the included studies did not present data in the manner calculated in this study, the corresponding author of the respective publication was contacted to request the research data. If no response was received, the calculations were performed using the following method: change scores were extracted whenever the mean and standard deviation (SD) of the changes were available. If the exact variance of the paired difference was not derivable, a conservative estimation was performed by assuming an intra-participant correlation coefficient of 0.7 between the baseline and post-test measured data [32]. If the SD was not reported, it was estimated by p-values or 95% confidence intervals (CIs). If the data was presented as medians (full ranges or interquartile ranges), they were re-calculated algebraically from the trial data to estimate the sample mean and the SD [31, 33, 34]. Owing to the diversity of measurement tools among studies for UE outcomes, all the extracted outcome data were calculated as standard mean differences (SMDs) with 95% CIs for sufficient comparability of effect sizes. We categorized the magnitude of SMDs in accordance with the following version of Cohen’s criteria [35]: trivial (d < 0.10), small (0.10 £ d < 0.25), medium (0.25 £ d < 0.40), and large (d ≥ 0.40).
Subgroup analyses were performed to identify potential factors that may affect treatment effects. These factors included the MQ level, disease stage, intervention design (i.e., monotherapy or adjunct therapy), intervention parameters, and treatment duration. The disease stage based on the time period since the onset of stroke was classified as acute (< 3 months), subacute (≥ 3 months, < 6 months), and chronic (≥ 6 months) [27]. All subgroup differences were tested for significance, and an I2 statistic was computed to estimate the degree of subgroup variability. Potential publication bias was assessed using Egger’s regression asymmetry test [36]. This meta-analysis was performed using Review Manager Software 5.4 (The Nordic Cochrane Centre, Copenhagen, Denmark).
Results
Trial flow
Figure 1 presents a flowchart of the selection process. Through the electronic and manual literature search, we identified a total of 438 articles. After removing duplicates, we reviewed the titles and abstracts of 172 studies to assess their eligibility; subsequently, 40 were considered to be relevant for full-text assessment. The final sample comprised 30 RCTs [8–12, 37–61], which were published between 2012 and 2024.
Fig. 1.
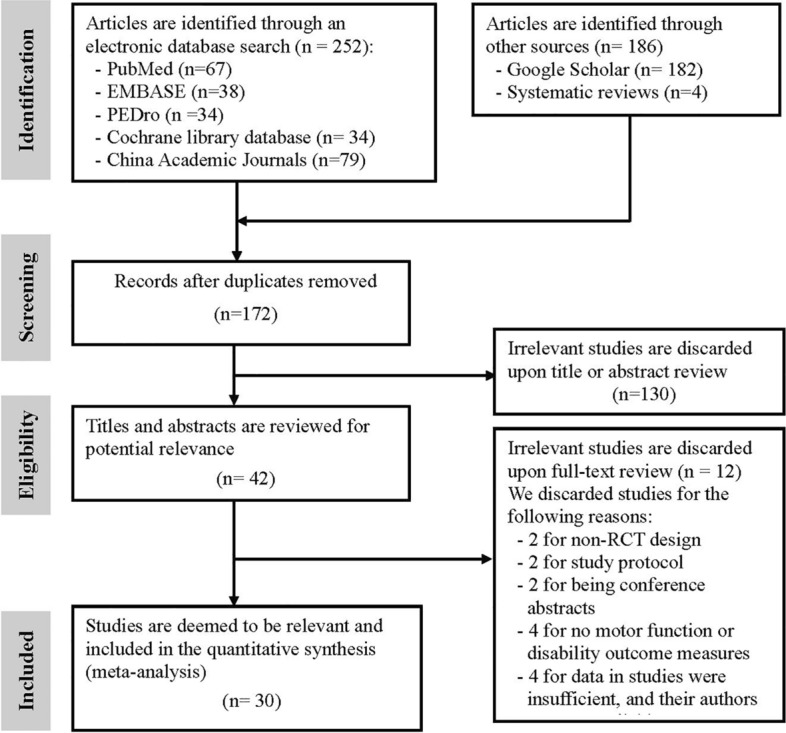
Flowchart of study selection. PEDro Physiotherapy Evidence Database
Study characteristics
Table 1 summarizes the study characteristics and demographic data of the participants with stoke in the included RCTs. In total, 1,621 participants who had a diagnosis of stroke with hemiplegia (or hemiparesis) were recruited, with a mean age of 60.4 years (range: 43.0–77.0 years). Twelve RCTs [42–45, 47, 48, 53, 54, 57–60] enrolled people with stroke who had acute onset stroke from a few days to 3 months, whereas the other 6 RCTs [9, 46, 51, 55, 56, 61] and 12 RCTs [8, 10–12, 30, 37–41, 50, 52] enrolled people with stroke in the subacute (disease duration: < 6 months) and chronic stage (mean disease duration: 6–100 months), respectively.
Most of the included RCTs used VT as an adjunct therapy with SR [8, 10, 11, 37–40, 42–48, 50–54, 56–58, 60, 61] or other active treatments [12, 40, 41, 55, 59], whereas 6 RCTs [8, 9, 30, 42, 59, 61] used VT as monotherapy. Most of the included RCTs conducted an immediate or short-term follow-up within 3 months, four RCTs had a medium-term follow-up duration of 12–18 weeks [10, 12, 37, 56], and only one RCT had a long-term follow-up period of 6 months [37].
Protocol of VT
A summary of the protocols for VT is presented in Table 1, and details of the protocol for VT are presented in Supplementary Table 2. Regarding the vibration mode, most of the included RCTs used FMV [10–12, 30, 38–43, 45, 47, 48, 50–53, 56, 57, 60, 61], whereas nine used WBV [8, 9, 37, 44, 46, 54, 55, 58, 59]. The most frequently used brand for FMV research was Panasonic (n = 2) [43, 57], with the model being EV2610 (n = 2) [43, 57]. The most frequently used brand for WBV research was Novotec Medical GmbH (n = 4) [8, 9, 44, 58], with the model being Galileo Med M Plus (n = 2) [44, 58].
The frequency of the vibratory wave applied ranged from 3 to 500 Hz among the included trials. Generally, seven RCTs [8, 9, 44, 45, 54, 55, 58] used low-frequency VT (≤ 20 Hz), and the other 23 RCTs [10–12, 30, 37–43, 46, 47, 50–53, 55–57, 59–61] employed a high-frequency VT (> 20 Hz). The duration of each vibration application ranged from 5 min to 3 h per training day. VT was mostly performed immediately following SR [8, 12, 37–39, 42, 43, 45–47, 51, 52, 54, 57–59], although three RCTs [10, 50, 56] used VT simultaneously with SR and two RCTs prescribed VT prior to each SR session [9, 11]. All people with stroke received VT in the seated [8–10, 12, 30, 41, 44, 45, 48, 50, 51, 54, 59, 61], supine [11, 40, 43, 47, 51–53, 57, 61], or standing position [37, 46, 55, 58, 59]. In addition, nine RCTs [11, 39, 40, 44, 50–53, 61] had a treatment duration of less than 4 weeks (3–21 sessions), whereas 13 RCTs [8, 9, 12, 30, 37, 42, 43, 45, 47, 48, 54–56, 60] and seven RCTs [10, 38, 41, 46, 57–59] employed 4-week (12–28 sessions) and 8-week (18–48 sessions) interventions of VT, respectively. Furthermore, in most of the included RCTs, the people with stroke in the control group received no treatment related to VT, whereas 11 RCTs [10, 11, 37, 39, 41, 42, 45, 50, 53, 56, 60] conducted placebo VT for the people with stroke in the control group.
Risk of bias in the included studies
Individual PEDro scores are displayed in Supplementary Table 3. Overall, the MQ assessment revealed that 14 (46.7%) and 16 (54.3%) of the 30 included RCTs were classified as high [8, 10–12, 37, 39–41, 45, 50, 52, 53, 56, 60] and medium [9, 30, 38, 42–44, 46–48, 51, 54, 55, 57–59, 61], respectively, with a median PEDro score of 6/10 (range: 5/10–9/10). The inter-rater reliability of the cumulative PEDro scores was acceptable with an intraclass correlation coefficient of 0.88 (95% CI 0.74–0.95). All of the 30 included RCTs used random allocation, similarity at the baseline, and point estimates and variability; 6 of the 14 high-quality RCTs [11, 12, 40, 52, 56, 61] performed allocation concealment, whereas no medium-quality RCT did. Overall, the people with stroke and assessors were blinded to the study group allocations in 11 RCTs [10, 11, 37, 39, 41, 42, 45, 50, 53, 56, 60] and 13 RCTs [8, 10–12, 37–42, 50, 52, 53, 56], respectively; in addition, therapist blinding was used in 2 high-quality RCTs [50, 53], indicating potential performance bias.
Effectiveness on UE motor impairment
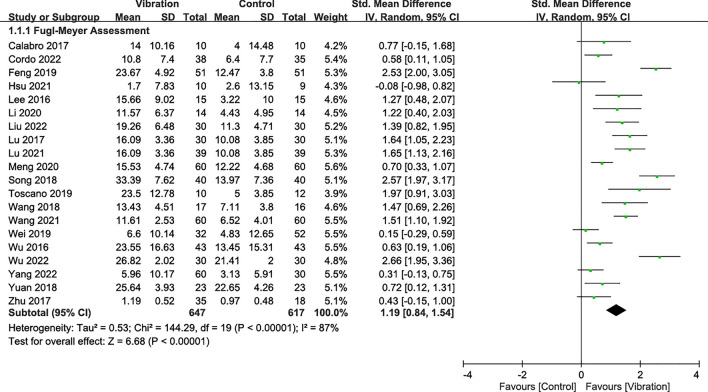
Upper-limb motor impairment was assessed using the Fugl-Meyer Assessment scale (in 20 RCTs [8, 10, 12, 41, 43–48, 51, 53–61]). The combined analysis showed that VT exerted significant effects on decreases in UE motor impairment with a pooled SMD of 1.19 (95% CI 0.84–1.54; p < 0.00001; I2 = 87%) during the overall follow-up period regardless of the type of vibration, intervention mode, and disease stage (Fig. 2).
Fig. 2.
Forest plot showing the effects of vibration therapy on upper-extremity motor impairment
Results of subgroup analyses for the MQ level, disease stage, and intervention factors (i.e., treatment design, vibration type, vibration frequency, treatment duration, and follow-up time) are presented in Supplementary Table 4. Significant differences in the effect of VT on UE motor impairment were observed between the subgroups based on the follow-up duration (I2: 90%; p < 0.00001) and treatment design (I2: 91%; p = 0.0009). Vibration was effective across all disease stages, including acute (SMD = 1.35), subacute (SMD = 1.25), and chronic (SMD = 0.64), with no significant differences between them. Both FMV (SMD = 1.07) and WBV (SMD = 1.39) showed significant effects, with no differences between them. Vibration frequencies ≤ 20 Hz (SMD = 1.46) and > 20 Hz (SMD = 1.05) were both effective, with no significant differences between them. However, the benefit of adjunct therapy was significant, with an SMD of 1.18. Significant effects in favor of VT were observed in the immediate (SMD = 1.51) and short-term follow-up periods (SMD = 1.12), but not in the medium-term follow-up period. Intervention durations of less than 4 weeks (SMD = 1.51), 4–8 weeks (SMD = 1.19), and 8 weeks or more (SMD = 0.89) showed significant improvements in motor impairment. Additionally, people with stroke had better outcomes (SMD = 1.32) in response to VT combined with SR than those who received VT alone (SMD = 0.39).
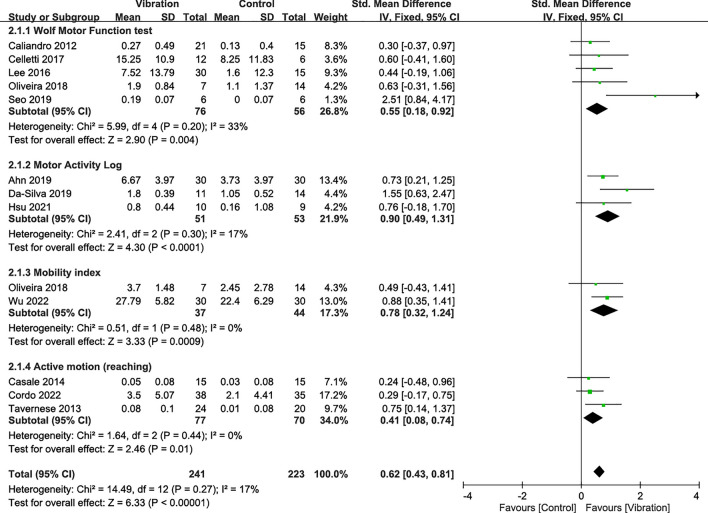
Effectiveness on UE motor function
UE motor function was assessed using the Wolf Motor Function test (5 RCTs [8, 11, 30, 40, 50]), Motor Activity Log (3 RCTs [9, 12, 42]), mobility index (2 RCTs [49, 58]), and active arm motion tests (3 RCTs [39, 41, 52]). Meta-analysis results showed that VT obtained favorable effects on score changes in the Wolf Motor Function test (SMD = 0.55; p = 0.004), Motor Activity Log test (SMD = 0.90; p < 0.0001), mobility index (SMD = 0.78; p = 0.0009), and active arm motion tasks (SMD = 0.41; p = 0.01) compared with the control groups (Fig. 3). The combined analysis showed that VT exerted significant effects on increases in UE motor function with a pooled SMD of 0.62 (95% CI 0.43–0.81; p < 0.00001) during the overall follow-up period regardless of the type of vibration, intervention mode, and disease stage (Fig. 3). Subgroup analysis results showed no significant factor affecting treatment effects on UE motor function (Supplementary Table 4). However, only the 4–8 weeks duration (SMD = 0.57) demonstrated slightly superior outcomes in motor function (Supplementary Table 4).
Fig. 3.
Forest plot showing the effects of vibration therapy on upper-extremity motor function
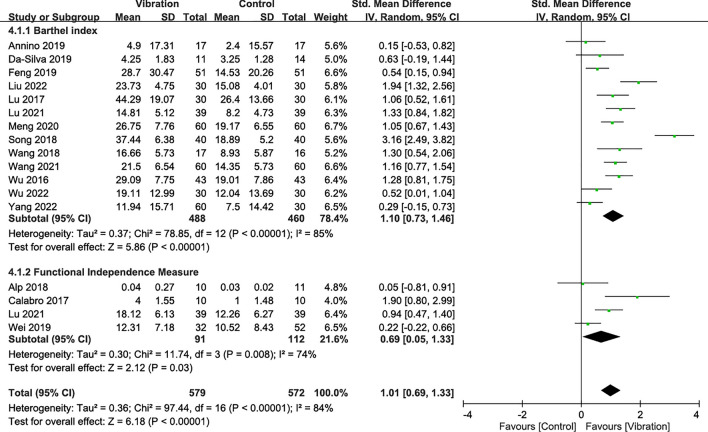
Effectiveness on disability
A total of 16 RCTs reported disability outcomes that were assessed by the Functional Independence Measure (4 RCTs [10, 37, 47, 56]) and Barthel index (13 RCTs [38, 42, 43, 45–48, 51, 54, 55, 57–59]). Meta-analysis results showed that VT had favorable effects on score changes regarding the Barthel index (SMD = 1.10; p < 0.00001) and the Functional Independence Measure (SMD = 0.69; p = 0.03) compared with the control groups (Fig. 4). The combined results showed that VT achieved significantly greater changes in disability indices with an SMD of 1.01 (95% CI 0.69–1.33; p < 0.00001; I2: 84%) than the controls, regardless of the follow-up duration, intervention design, and type of vibration (Fig. 4).
Fig. 4.
Forest plot showing the effects of vibration therapy on disability
Subgroup analysis results indicated significant differences between the treatment designs (I2: 82.6%; p = 0.02) and vibration types (I2: 84.7%; p = 0.01; Supplementary Table 4). People with stroke appeared to obtain favorable effects (SMD = 1.03) in response to VT combined with SR compared with those who received VT alone (SMD = 0.21). In addition, FMV (SMD = 2.14) appeared to achieve better treatment effects than WBV (SMD = 0.75). For disability, interventions lasting 4–8 weeks (SMD = 0.84) and 8 weeks or more (SMD = 0.87) yielded better results (Supplementary Table 4).
Side effects and compliance
No clinically relevant adverse events, side effects (e.g., pain), or serious complications were reported after VT interventions in the included RCTs. Furthermore, good tolerance and compliance for FMV therapy was reported by three RCTs [42, 50, 52].
Publication bias
The funnel plots of the effect sizes of each primary outcome measure are presented in Supplementary Fig. 1. No substantial asymmetry was identified by visual inspection of the funnel plots for UE motor impairment, UE motor function, and disability. The Egger’s linear regression test for each main outcome did not indicate any evidence of obvious reporting bias among the comparisons (All p > 0.05).
Discussion
Regardless of whether people with stroke are in the acute, subacute, or chronic stage, FMV and WBV are effective for improving upper extremity motor impairment and enhancing motor function. Local vibration acts as a source of proprioceptive stimuli, inducing somatosensory and sensorimotor improvements through cortical reorganization, although disability benefits are only seen in the acute and subacute stages. VT can effectively improve upper limb motor impairments and function in people with chronic stroke. However, to enhance their independence in daily activities, a more comprehensive approach may be needed, potentially including improvements in environmental adaptation and cognitive skills and addressing psychosocial factors. Neuroplasticity significantly declines in the chronic stage after stroke, which necessitates the use of multiple therapies to promote overall recovery. In this regard, adjunct therapy combined with VT is more effective than monotherapy, particularly in improving functional impairments in the chronic stage, and could help enhance recovery outcomes in poeple with stroke.
Disease stage, treatment design, and vibration type
In the disease stage, whether the person is in the acute, subacute, or chronic stage, using FMV or WBV is effective for improving motor impairment and enhancing motor function, with no difference between the two treatments. VT could be regarded as proprioceptive training that can induce meaningful somatosensory and sensorimotor functional improvements by means of cortical reorganization [62]. However, in terms of disability, benefits are only noticeable in the acute and subacute stages.
The tools that are used to assess disability status (such as the Functional Independence Measure or Barthel Index) focus on overall functionality [25, 26], rather than on a single motor function. Previous studies have suggested that specific training can significantly improve motor function in people with stroke but these improvements do not necessarily translate directly into independence in activities of daily living [63, 64]. In recent years, some studies have started to focus on reablement, a rehabilitation approach conducted in the individuals' living environment to help them relearn and regain essential life skills [65, 66]. VT may effectively improve motor impairments and enhance motor function in people with chronic stroke, but improving their independence in daily activities may require a more comprehensive approach. In addition to rehabilitation, enhancing environmental adaptation and cognitive abilities and addressing psychosocial factors are crucial considerations.
The treatment design of VT shows that adjunct therapy benefits are superior to those of monotherapy. Previous systematic reviews have also suggested that adjunct therapy is more effective than monotherapy in people with stroke. Neuroplasticity is higher in the early rehabilitation stage after a stroke, but in the chronic stage, the adaptability and self-repair capacity of the nervous system decrease significantly [67, 68]. Different therapies may have synergistic effects, enhancing overall recovery by addressing different mechanisms of stroke recovery [69]. Particularly for improving disability in the chronic stage, vibration therapy should be considered as part of adjunct therapy.
Vibration frequency, intervention duration, and follow-up duration
Although both low (≤ 20 Hz) and high (> 20 Hz) frequencies can improve motor impairment and enhance motor function, as well as disability, with no significant difference between the two strategies, it appears that low frequency offers better benefits. Previous studies have indicated that VT can stimulate the excitation of the neuromuscular system and muscle strength [8]. Vibration can enhance the function of the spinal cord and cerebral cortex by stimulating Ia afferent signals from muscle spindles [10, 40]. Previous studies have also suggested that low-frequency vibration interventions can trigger somatosensory feedback to improve limb function and alleviate spasticity symptoms in people with stroke [16, 70].
Intervention times of 4–8 weeks and longer than 8 weeks are both effective for motor impairment, enhancing motor function, and disability, but intervention times of less than 4 weeks are only helpful for improving motor impairment. Previous meta-analyses on vibration also suggest that short-term vibration interventions are not helpful for reducing spasticity in people with stroke, with the effect size increasing gradually with the duration of vibration [18]. The effectiveness of training is closely related to the length of the training time; sufficient training time is necessary to achieve significant therapeutic effects [71]. Short VT intervention times may not provide adequate stimulation, thus limiting the effectiveness of the treatment.
Vibration intervention has significant effects in the short term (< 1 month and 1–3 months), but no sustained benefits were observed during the follow-up period of ≥ 3 months. Previous studies have indicated that insufficient training makes limb paralysis one of the major challenges faced by people with stroke after discharge [56]. People with stroke who frequently use the affected arm after discharge tend to experience better recovery in arm function [56]. Previous meta-analyses have indicated that high-intensity training post-stroke is crucial, and the more intense the training, the better the effect. An important principle in motor learning is repeated practice; when neurons are activated for a long duration simultaneously, the connections among them become stronger [72]. This suggests that in people with stroke, maintaining therapeutic effects in the long term may require continuous training interventions.
Side effects and compliance
In this meta-analysis, no clinically relevant adverse effects were reported in the collected studies and the training protocols were well tolerated in two studies [50, 53]. The impact induced by high dosage vibration was reported to increase the risk of deteriorating osteoporosis, lower back pain, and complementary disability [73]; thus, the target location and direction of therapeutic equipment are crucial to prevent damage. Moreover, dizziness or muscle soreness were noted in 2.4–3.6% of people with stroke undergoing VT with stroke and cerebral palsy, respectively [74, 75]. WBV was reported to induce warm feet, dizziness, severe hip discomfort, and jaw or neck pain due to vibration [6]. A frequency higher than 20 Hz accompanied with a greater G value caused resonance, trauma, and dizziness, whereas that higher than 30 Hz led to discomfort and damage of fragile bones [74, 76].
Limitations
Our study had several limitations. First, function and disability are interrelated, and while clinical assessment tools have primary measurement goals, they cannot solely assess motor function or disability elements alone. The mixed type of outcome results may have been a confounding factor. Second, more than two motor function outcome measures were used in some studies, which may have affected the results of the analysis. To maintain low heterogeneity, the Fugl-Meyer Assessment scale results were considered important and selected for the analysis.
Conclusions
Our meta-analysis indicates that VT for improving upper limb function and disability recovery, especially when combined with SR, is both a reliable and safe therapeutic method. Initiating VT as early as possible post-stroke and a minimum of 4–8 weeks of VT is necessary to achieve improvements in upper extremity motor function after stroke. Both low and high vibration frequencies are effective, with FMV showing superior results compared with WBV, particularly for disability recovery. The effectiveness of VT is also influenced by factors such as the type of vibration, intervention mode, and follow-up duration. Maintaining the therapeutic effects of vibration therapy in the long term may require continuous interventions for people with stroke.
Supplementary Information
Acknowledgements
This study was supported by the National Health Research Institutes, Ministry of Science and Technology and National Science and Technology Council of Taiwan. The findings and conclusions in this paper are those of the authors, who are responsible for its contents. We also thank Editage who provided English editing assistance.
Abbreviations
- CI
Confidence interval
- CIs
Confidence intervals
- FMV
Focal muscle vibration
- MQ
Methodological quality
- PEDro
Physiotherapy evidence database
- RCTs
Randomized controlled trials
- SD
Standard deviation
- SMD
Standard mean difference
- SMDs
Standard mean differences
- SR
Standard rehabilitation
- UE
Upper extremity
- UEs
Upper extremities
- VT
Vibration therapy
- WBV
Whole-body vibration
Author contributions
Study concept and design: YHL, HJC, CDL, PJC, XMW, CHY, PYC, CHL; Acquisition of data: YHL, HJC, CDL, CHY, PYC; Analysis and interpretation of data: CDL, CHY; Drafting of the manuscript: YHL, HJC, CDL, PJC, XMW, PYC, CHL; Critical revision of the manuscript for important intellectual content: YHL, HJC, CDL, CHL.
Funding
This work was supported by the National Health Research Institutes, Taiwan (13A1-CGGP21-051, 13A1-PHGP17CG-055). This study was also supported by the Ministry of Science and Technology (MOST: 110-2221-E-038-016; MOST 111-2221-E-038-015) and National Science and Technology Council (NSTC 112-2622-E-038-005) of Taiwan. The funders played no role in the design, data collection, analysis, or interpretation of the data, or in writing the manuscript.
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The protocol of this study was registered with PROSPERO (Registration number: CRD42022301119) and the need for written informed consent was waived owing to its retrospective design.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yueh-Hsun Lu, Hung-Ju Chen and Chun-De Liao contributed equally to this study.
References
- 1.Lai CH, Sung WH, Chiang SL, Lu LH, Lin CH, Tung YC, et al. Bimanual coordination deficits in hands following stroke and their relationship with motor and functional performance. J Neuroeng Rehabil. 2019;16:101. 10.1186/s12984-019-0570-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu WJ, Lin LF, Chiang SL, Lu LH, Chen CY, Lin CH. Impacts of stroke on muscle perceptions and relationships with the motor and functional performance of the lower extremities. Sensors (Basel). 2021. 10.3390/s21144740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hendricks HT, van Limbeek J, Geurts AC, Zwarts MJ. Motor recovery after stroke: a systematic review of the literature. Arch Phys Med Rehabil. 2002;83:1629–37. 10.1053/apmr.2002.35473. [DOI] [PubMed] [Google Scholar]
- 4.Giroud M, Jacquin A, Béjot Y. The worldwide landscape of stroke in the 21st century. Lancet. 2014;383:195–7. 10.1016/s0140-6736(13)62077-2. [DOI] [PubMed] [Google Scholar]
- 5.De Wit L, Theuns P, Dejaeger E, Devos S, Gantenbein AR, Kerckhofs E, et al. Long-term impact of stroke on patients’ health-related quality of life. Disabil Rehabil. 2017;39:1435–40. 10.1080/09638288.2016.1200676. [DOI] [PubMed] [Google Scholar]
- 6.Cochrane DJ. Vibration exercise: the potential benefits. Int J Sports Med. 2011;32:75–99. 10.1055/s-0030-1268010. [DOI] [PubMed] [Google Scholar]
- 7.Alam MM, Khan AA, Farooq M. Effect of whole-body vibration on neuromuscular performance: a literature review. Work. 2018;59:571–83. 10.3233/WOR-182699. [DOI] [PubMed] [Google Scholar]
- 8.Lee JS, Kim CY, Kim HD. Short-term effects of whole-body vibration combined with task-related training on upper extremity function, spasticity, and grip strength in subjects with poststroke hemiplegia: a pilot randomized controlled trial. Am J Phys Med Rehabil. 2016;95:608–17. 10.1097/PHM.0000000000000454. [DOI] [PubMed] [Google Scholar]
- 9.Ahn JY, Kim H, Park CB. Effects of whole-body vibration on upper extremity function and grip strength in patients with subacute stroke: a randomised single-blind controlled trial. Occup Ther Int. 2019;2019:5820952. 10.1155/2019/5820952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calabrò RS, Naro A, Russo M, Milardi D, Leo A, Filoni S, et al. Is two better than one? Muscle vibration plus robotic rehabilitation to improve upper limb spasticity and function: a pilot randomized controlled trial. PLoS ONE. 2017;12: e0185936. 10.1371/journal.pone.0185936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caliandro P, Celletti C, Padua L, Minciotti I, Russo G, Granata G, et al. Focal muscle vibration in the treatment of upper limb spasticity: a pilot randomized controlled trial in patients with chronic stroke. Arch Phys Med Rehabil. 2012;93:1656–61. 10.1016/j.apmr.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Hsu HY, Kuan TS, Tsai CL, Wu PT, Kuo YL, Su FC, et al. Effect of a novel perturbation-based pinch task training on sensorimotor performance of upper extremity for patients with chronic stroke: a pilot randomized controlled trial. Arch Phys Med Rehabil. 2021;102:811–8. 10.1016/j.apmr.2020.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Santos LMM, Oliveira ACC, Fonseca SF, Silva AF, Santos JNV, Souza ALC, et al. Whole-body vibration exercise in different postures on handgrip strength in healthy women: a cross-over study. Front Physiol. 2020;11: 469499. 10.3389/fphys.2020.469499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiiski J, Heinonen A, Järvinen TL, Kannus P, Sievänen H. Transmission of vertical whole body vibration to the human body. J Bone Miner Res. 2008;23:1318–25. 10.1359/jbmr.080315. [DOI] [PubMed] [Google Scholar]
- 15.Choi E-T, Kim Y-N, Cho W-S, Lee D-K. The effects of visual control whole body vibration exercise on balance and gait function of stroke patients. J Phys Ther Sci. 2016;28:3149–52. 10.1589/jpts.28.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Q, Zheng S, Li S, Zeng Y, Chen L, Li G, et al. Efficacy and safety of whole-body vibration therapy for post-stroke spasticity: a systematic review and meta-analysis. Front Neurol. 2023;14:1074922. 10.3389/fneur.2023.1074922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin Y, Wang J, Yu Z, Zhou L, Liu X, Cai H, et al. Does whole-body vibration training have a positive effect on balance and walking function in patients with stroke? A meta-analysis. Front Hum Neurosci. 2022;16:1076665. 10.3389/fnhum.2022.1076665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng D, Lei W, Kong Y, Ma F, Zhao K, Ye X, et al. Effects of vibration therapy for post-stroke spasticity: a systematic review and meta-analysis of randomized controlled trials. Biomed Eng OnLine. 2023;22:121. 10.1186/s12938-023-01176-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang M, Pang MYC. Muscle activity and vibration transmissibility during whole-body vibration in chronic stroke. Scand J Med Sci Sports. 2019;29:816–25. 10.1111/sms.13408. [DOI] [PubMed] [Google Scholar]
- 20.Sañudo B, Taiar R, Furness T, Bernardo-Filho M. Clinical approaches of whole-body vibration exercises in individuals with stroke: a narrative revision. Rehabil Res Pract. 2018;2018:8180901. 10.1155/2018/8180901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015: Elaboration and explanation. BMJ. 2015;350: g7647. 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 22.Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. 10.2340/1650197771331. [PubMed] [Google Scholar]
- 23.Sullivan KJ, Tilson JK, Cen SY, Rose DK, Hershberg J, Correa A, et al. Fugl-Meyer assessment of sensorimotor function after stroke: standardized training procedure for clinical practice and clinical trials. Stroke. 2011;42:427–32. 10.1161/STROKEAHA.110.592766. [DOI] [PubMed] [Google Scholar]
- 24.Wolf SL, Catlin PA, Ellis M, Archer AL, Morgan B, Piacentino A. Assessing wolf motor function test as outcome measure for research in patients after stroke. Stroke. 2001;32:1635–9. 10.1161/01.str.32.7.1635. [DOI] [PubMed] [Google Scholar]
- 25.Keith RA, Granger CV, Hamilton BB, Sherwin FS. The functional independence measure: a new tool for rehabilitation. Adv Clin Rehabil. 1987;1:6–18. [PubMed] [Google Scholar]
- 26.Collin C, Wade DT, Davies S, Horne V. The barthel adl index: a reliability study. Int Disabil Stud. 1988;10:61–3. 10.3109/09638288809164103. [DOI] [PubMed] [Google Scholar]
- 27.Overman MJ, Binns E, Milosevich ET, Demeyere N. Recovery of visuospatial neglect with standard treatment: a systematic review and meta-analysis. Stroke. 2024;55:2325–39. 10.1161/strokeaha.124.046760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins J. Cochrane handbook for systematic reviews of interventions. Version 5.1. 0. The cochrane collaboration.www.cochrane-handbook.org. Accessed Mar 2011.
- 29.Wu CH, Chen KT, Hou MT, Chang YF, Chang CS, Liu PY, et al. Prevalence and associated factors of sarcopenia and severe sarcopenia in older Taiwanese living in rural community: the tianliao old people study 04. Geriatr Gerontol Int. 2014;14(Suppl 1):69–75. 10.1111/ggi.12233. [DOI] [PubMed] [Google Scholar]
- 30.Briani RV, Ferreira AS, Pazzinatto MF, Pappas E, De Oliveira SD, Azevedo FM. What interventions can improve quality of life or psychosocial factors of individuals with knee osteoarthritis? A systematic review with meta-analysis of primary outcomes from randomised controlled trials. Br J Sports Med. 2018;52:1031–8. 10.1136/bjsports-2017-098099. [DOI] [PubMed] [Google Scholar]
- 31.Higgins JPT, Li T, Deeks JJ. 2021. Choosing effect measures and computing estimates of effect. In: Higgins JPT (Eds). Cochrane handbook for systematic reviews of interventions version 6.2. London, United Kingdom: Cochrane.
- 32.Rosenthal R. Meta-analytic procedures for social research. Newbury Park: Sage Publications; 1993. [Google Scholar]
- 33.Higgins JPT, Deeks JJ, Altman DG. Chapter 16. Special topics in statistics. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0. London: The Cochrane Collaboration; 2011. [Google Scholar]
- 34.Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27:1785–805. 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 35.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 36.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alp A, Efe B, Adalı M, Bilgiç A, Demir Türe S, Coşkun Ş, et al. The impact of whole body vibration therapy on spasticity and disability of the patients with poststroke hemiplegia. Rehabil Res Pract. 2018;2018:8637573. 10.1155/2018/8637573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Annino G, Alashram AR, Alghwiri AA, Romagnoli C, Messina G, Tancredi V, et al. Effect of segmental muscle vibration on upper extremity functional ability poststroke: a randomized controlled trial. Medicine (Baltim). 2019;98: e14444. 10.1097/MD.0000000000014444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casale R, Damiani C, Maestri R, Fundarò C, Chimento P, Foti C. Localized 100 Hz vibration improves function and reduces upper limb spasticity: a double-blind controlled study. Eur J Phys Rehabil Med. 2014;50:495–504. [PubMed] [Google Scholar]
- 40.Celletti C, Sinibaldi E, Pierelli F, Monari G, Camerota F. Focal muscle vibration and progressive modular rebalancing with neurokinetic facilitations in post- stroke recovery of upper limb. Clin Ter. 2017;168:e33–6. 10.7417/CT.2017.1979. [DOI] [PubMed] [Google Scholar]
- 41.Cordo P, Wolf S, Rymer WZ, Byl N, Stanek K, Hayes JR. Assisted movement with proprioceptive stimulation augments recovery from moderate-to-severe upper limb impairment during subacute stroke period: a randomized clinical trial. Neurorehabil Neural Repair. 2022;36:239–50. 10.1177/15459683211063159. [DOI] [PubMed] [Google Scholar]
- 42.Da-Silva RH, Moore SA, Rodgers H, Shaw L, Sutcliffe L, van Wijck F, et al. Wristband accelerometers to motivate arm exercises after stroke (waves): a pilot randomized controlled trial. Clin Rehabil. 2019;33:1391–403. 10.1177/0269215519834720. [DOI] [PubMed] [Google Scholar]
- 43.Feng CM, Wei R, Xu F, Zhang WN. Analysis of the effects of repetitive local muscle vibration therapy combined with virtual reality rehabilitation training on hand function, upper limb function, clinical effect in stroke patients with hemiplegia. Chin J Front Med Sci. 2019;11:50–3. 10.12037/YXQY.2019.10-10. [Google Scholar]
- 44.Li HB, Zhou MW. Effects of whole body vibration training on upper limb motor function in hemiplegic patients with subacute stroke. Chin J Rehabil Med. 2020;35:1055–60. 10.3969/j.issn.1001-1242.2020.09.006. [Google Scholar]
- 45.Liu BX, Hu C, Wang X. Effects of upper extremity compression vibration combined with sling exercise therapy on glenohumeral subluxation and function in stroke patients. Chin J Rehabil. 2022;37:200–3. 10.3870/zgkf.2022.04.002. [Google Scholar]
- 46.Lu SY. Influence of vertical vibration training on muscular tension and motor function of upper limbs in stroke hemiplegia. Chin J Convalescent Med. 2017;26:35–8. 10.13517/j.cnki.ccm.2017.01.013. [Google Scholar]
- 47.Lu Y, Li HJ. Effects of repetitive local muscle vibration combined with mirror visual feedback training on upper limb function and finger mobility in stroke hemiplegic patients. Health Med Res Pract. 2021;18:90–4. 10.11986/j.issn.1673-873X.2021.03.018. [Google Scholar]
- 48.Meng HC, Wang ZT, Wang T, Chen YX, Wang XN, Chang YX, et al. Ultrasound-guided shock wave and vibration training for the treatment of muscle spasm after brain stroke. Stroke Nerv Dis. 2020;27:767–71. 10.3969/j.issn.1007-0478.2020.06.014. [Google Scholar]
- 49.Oliveira MDCB, Silva DRC, Cortez BV, Coêlho CKDS, Silva FMSE, de Oliveira GBVP, et al. Mirror and vibration therapies effects on the upper limbs of hemiparetic patients after stroke: a pilot study. Rehabil Res Pract. 2018;2018:6183654. 10.1155/2018/6183654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seo NJ, Woodbury ML, Bonilha L, Ramakrishnan V, Kautz SA, Downey RJ, et al. Therabracelet stimulation during task-practice therapy to improve upper extremity function after stroke: a pilot randomized controlled study. Phys Ther. 2019;99:319–28. 10.1093/ptj/pzy143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song ZM. Influence of muscle vibration combined with acupuncture and conventional rehabilitation on rehabilitation effect of post-stroke shoulder-hand syndrome. Chin J Ration Drug Use. 2018;15:64–6. 10.3969/j.issn.2096-3327.2018.07.020. [Google Scholar]
- 52.Tavernese E, Paoloni M, Mangone M, Mandic V, Sale P, Franceschini M, et al. Segmental muscle vibration improves reaching movement in patients with chronic stroke. A randomized controlled trial. NeuroRehabilitation. 2013;32:591–9. 10.3233/NRE-130881. [DOI] [PubMed] [Google Scholar]
- 53.Toscano M, Celletti C, Viganò A, Altarocca A, Giuliani G, Jannini TB, et al. Short-term effects of focal muscle vibration on motor recovery after acute stroke: a pilot randomized sham-controlled study. Front Neurol. 2019;10:115. 10.3389/fneur.2019.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang GS, Guo GH, Li Z, Liang YZ. The influence of the whole body vibration therapy based on Bobath concept on upper limb spasticity in stroke patients with hemiplegia. Clin Med Eng. 2018;25:733–5. 10.3969/j.issn.1674-4659.2018.06.0733. [Google Scholar]
- 55.Wang SF, Wang SL, Li CJ, Ran X. Effect of whole body vibration training combined with electromyographic biofeedback therapy on rehabilitation for stroke patients with hemiplegia. J Nurs Sci. 2021;36:16–8. 10.3870/j.issn.1001-4152.2021.09.016. [Google Scholar]
- 56.Wei WXJ, Fong KNK, Chung RCK, Cheung HKY, Chow ESL. ‘Remind-to-move’ for promoting upper extremity recovery using wearable devices in subacute stroke: a multi-center randomized controlled study. IEEE Trans Neural Syst Rehabil Eng. 2019;27:51–9. 10.1109/TNSRE.2018.2882235. [DOI] [PubMed] [Google Scholar]
- 57.Wu C. Efficacy of local vibration training in rehabilitating the upper limb spasticity and function of patients with cerebral infarction hemiplegia. J Health Res. 2016;36:539–41. 10.3969/j.issn.1674-6449.2016.05.019. [Google Scholar]
- 58.Wu Y, Fu DQ, Yan XL. Effect of whole body vibration training on function of upper limb in patients with stroke recovery. Chongqing Med. 2022;51:1886–9. 10.3969/j.issn.1671-8348.2022.11.017. [Google Scholar]
- 59.Yang KQ, Zhang L, Zhang CY, Chen CC, Qi H, Zhao B. Effect of mirror image therapy combined with whole body vibration training on unilateral spatial neglect after stroke. RM J Fujian Univ Tradit Chin Med. 2022;32:56–61. 10.3724/SP.J.1329.2022.01009. [Google Scholar]
- 60.Yuan XM, Qu HY. Effect of repetitive focal muscle stimulation on upper limb function recovery after stroke. Chin J Rehabil Theor Pract. 2018;24:938–41. 10.3969/j.issn.1006-9771.2018.08.013. [Google Scholar]
- 61.Zhu CE, Yu B, Zhang W, Chen WH, Qi Q. Observation on clinical efficacy of muscle vibration therapy combined with conventional rehabilitation treating shoulder-hand syndrome after stroke. Chin J Rehabil Med. 2017;32:902–6. 10.3969/j.issn.1001-1242.2017.08.009. [Google Scholar]
- 62.Aman JE, Elangovan N, Yeh IL, Konczak J. The effectiveness of proprioceptive training for improving motor function: a systematic review. Front Hum Neurosci. 2014;8:1075. 10.3389/fnhum.2014.01075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet. 2011;377:1693–702. 10.1016/S0140-6736(11)60325-5. [DOI] [PubMed] [Google Scholar]
- 64.Choi JU, Kang SH. The effects of patient-centered task-oriented training on balance activities of daily living and self-efficacy following stroke. J Phys Ther Sci. 2015;27:2985–8. 10.1589/jpts.27.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chiu EC, Chi FC, Chen PT. Investigation of the home-reablement program on rehabilitation outcomes for people with stroke: a pilot study. Medicine (Baltimore). 2021;100: e26515. 10.1097/md.0000000000026515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen PT, Chiu EC. Reablement of instrumental activities of daily living for patients with stroke: a randomized crossover trial. Am J Occup Ther. 2024;78:7802180160. 10.5014/ajot.2024.050288. [DOI] [PubMed] [Google Scholar]
- 67.Nudo RJ. Recovery after brain injury: mechanisms and principles. Front Hum Neurosci. 2013;7:887. 10.3389/fnhum.2013.00887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krakauer JW. Motor learning: its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol. 2006;19:84–90. 10.1097/01.wco.0000200544.29915.cc. [DOI] [PubMed] [Google Scholar]
- 69.Pollock A, Baer G, Campbell P, Choo PL, Forster A, Morris J, et al. Physical rehabilitation approaches for the recovery of function and mobility following stroke. Cochrane Database Syst Rev. 2014;2014:CD001920. 10.1002/14651858.CD001920.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pollock RD, Woledge RC, Mills KR, Martin FC, Newham DJ. Muscle activity and acceleration during whole body vibration: effect of frequency and amplitude. Clin Biomech (Bristol, Avon). 2010;25:840–6. 10.1016/j.clinbiomech.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 71.Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol. 2009;8:741–54. 10.1016/S1474-4422(09)70150-4. [DOI] [PubMed] [Google Scholar]
- 72.Veerbeek JM, van Wegen E, van Peppen R, van der Wees PJ, Hendriks E, Rietberg M, et al. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS ONE. 2014;9: e87987. 10.1371/journal.pone.0087987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bovenzi M. Metrics of whole-body vibration and exposure-response relationship for low back pain in professional drivers: a prospective cohort study. Int Arch Occup Environ Health. 2009;82:893–917. 10.1007/s00420-008-0376-3. [DOI] [PubMed] [Google Scholar]
- 74.Pang MY, Lau RW, Yip SP. The effects of whole-body vibration therapy on bone turnover, muscle strength, motor function, and spasticity in chronic stroke: a randomized controlled trial. Eur J Phys Rehabil Med. 2013;49:439–50. [PubMed] [Google Scholar]
- 75.Cheng HYK, Ju YY, Chen CL, Chuang LL, Cheng CH. Effects of whole body vibration on spasticity and lower extremity function in children with cerebral palsy. Hum Mov Sci. 2015;39:65–72. 10.1016/j.humov.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 76.Lau RWK, Yip SP, Pang MYC. Whole-body vibration has no effect on neuromotor function and falls in chronic stroke. Med Sci Sports Exerc. 2012;44:1409–18. 10.1249/MSS.0b013e31824e4f8c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.