Abstract
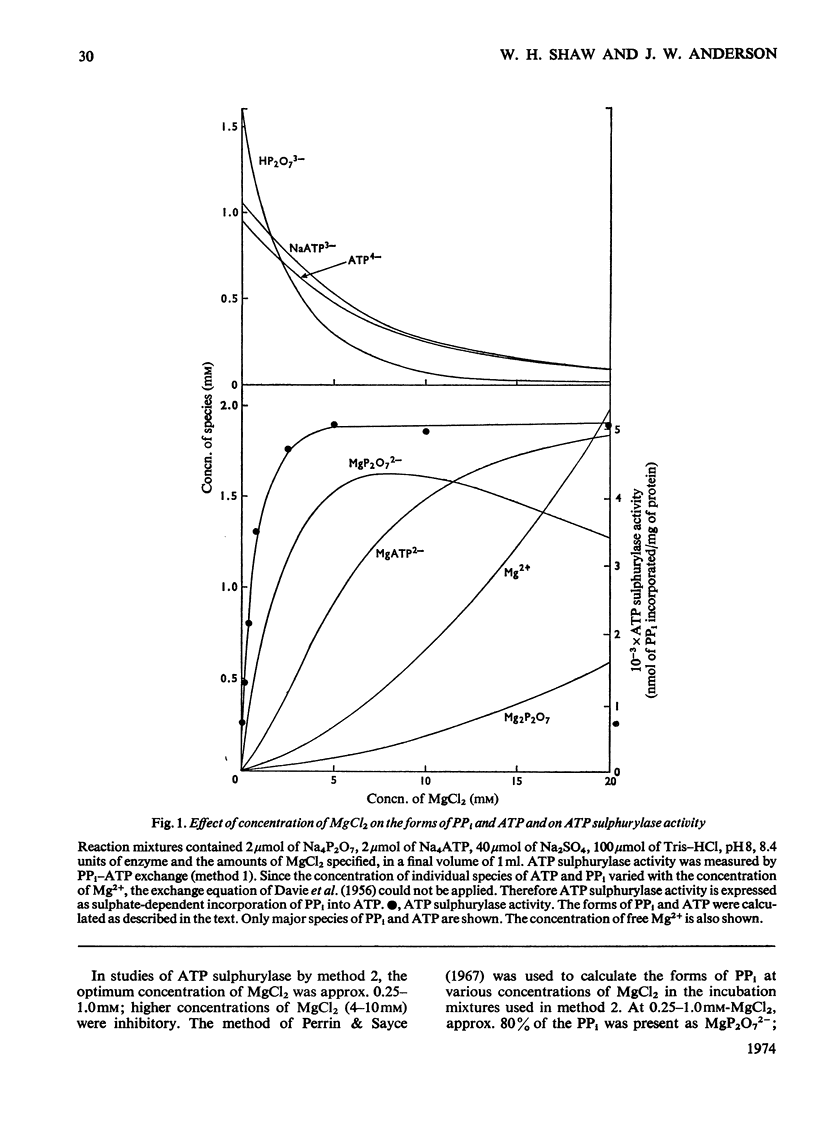
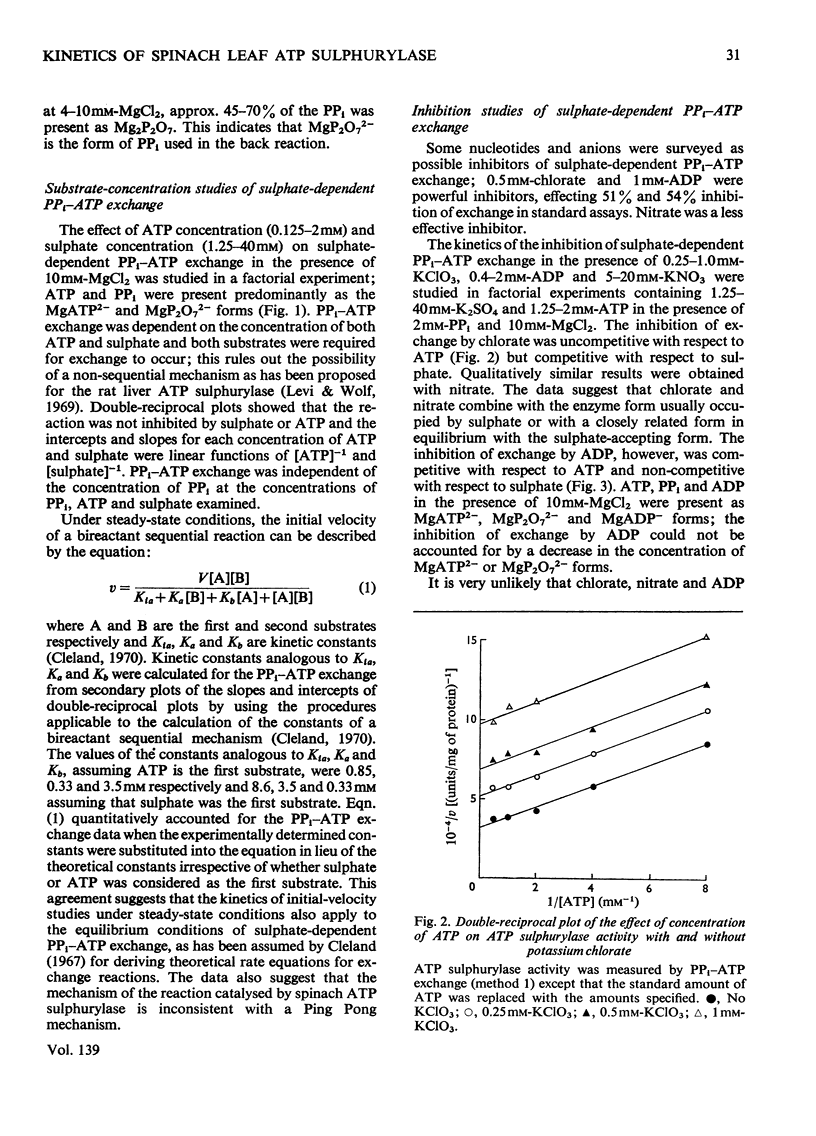
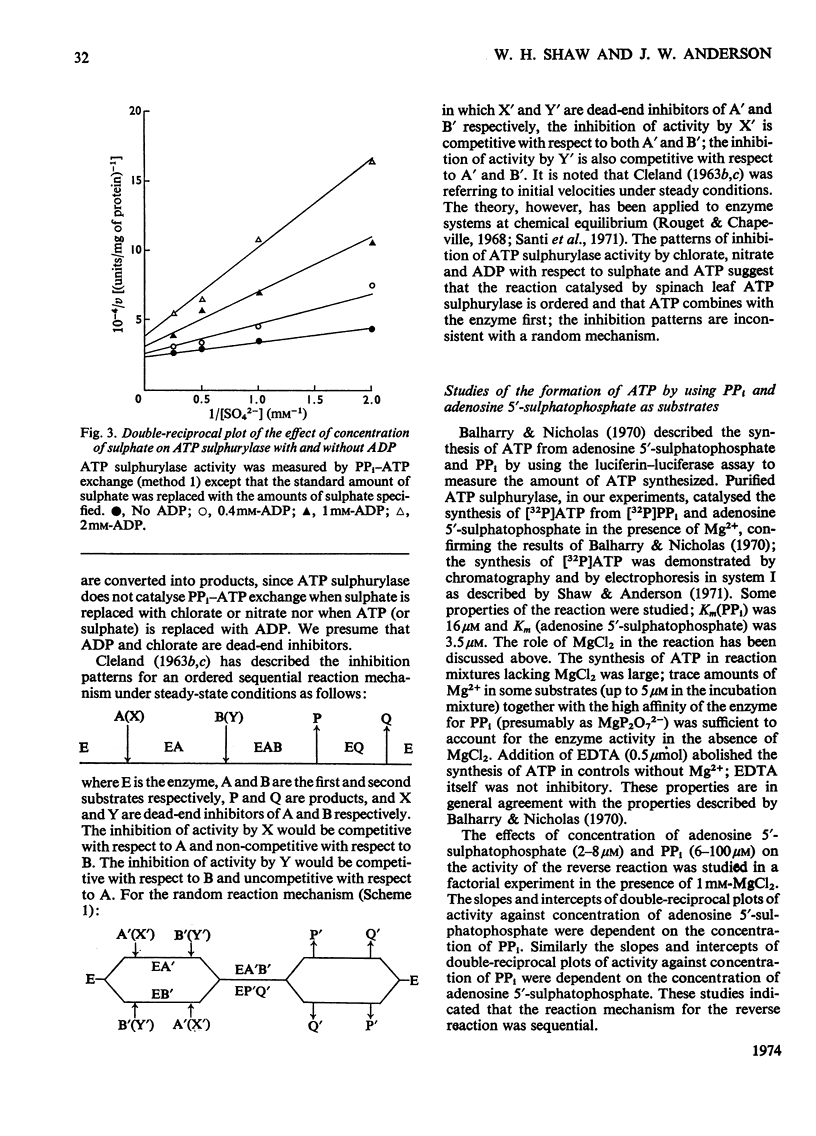
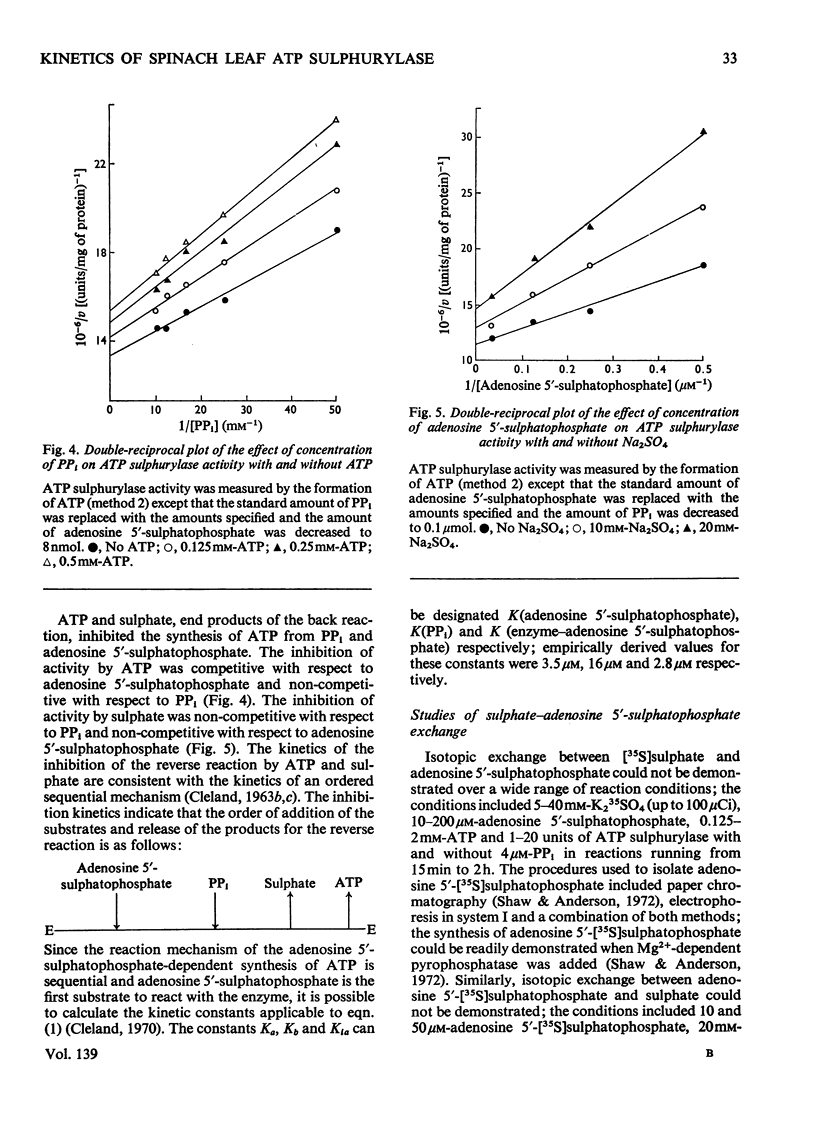
1. Sulphate-dependent PPi–ATP exchange, catalysed by purified spinach leaf ATP sulphurylase, was correlated with the concentration of MgATP2− and MgP2O72−; ATP sulphurylase activity was not correlated with the concentration of free Mg2+. 2. Sulphate-dependent PPi–ATP exchange was independent of PPi concentration, but dependent on the concentration of ATP and sulphate. The rate of sulphate-dependent PPi–ATP exchange was quantitatively defined by the rate equation applicable to the initial rate of a bireactant sequential mechanism under steady-state conditions. 3. Chlorate, nitrate and ADP inhibited the exchange reaction. The inhibition by chlorate and nitrate was uncompetitive with respect to ATP and competitive with respect to sulphate. The inhibition by ADP was competitive with respect to ATP and non-competitive with respect to sulphate. 4. ATP sulphurylase catalysed the synthesis of [32P]ATP from [32P]PPi and adenosine 5′-sulphatophosphate in the absence of sulphate; some properties of the reaction are described. Enzyme activity was dependent on the concentration of PPi and adenosine 5′-sulphatophosphate. 5. The synthesis of ATP from PPi and adenosine 5′-sulphatophosphate was inhibited by sulphate and ATP. The inhibition by sulphate was non-competitive with respect to PPi and adenosine 5′-sulphatophosphate; the inhibition by ATP was competitive with respect to adenosine 5′-sulphatophosphate and non-competitive with respect to PPi. It was concluded that the reaction catalysed by spinach leaf ATP sulphurylase was ordered; expressing the order in the forward direction, MgATP2− was the first product to react with the enzyme and MgP2O72− was the first product released. 6. The expected exchange reaction between sulphate and adenosine 5′-sulphatophosphate could not be demonstrated.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. A., Warnes G. M., Nicholas D. J. Preparation of labeled adenosine 5'-phosphosulfate using APS reductase from Thibacillus denitrificans. Anal Biochem. 1971 Jul;42(1):207–213. doi: 10.1016/0003-2697(71)90028-5. [DOI] [PubMed] [Google Scholar]
- Alberty R. A. Standard Gibbs free energy, enthalpy, and entropy changes as a function of pH and pMg for several reactions involving adenosine phosphates. J Biol Chem. 1969 Jun 25;244(12):3290–3302. [PubMed] [Google Scholar]
- Anderson J. W., Fowden L. Properties and substrate specificities of the phenylalanyl-transfer-ribonucleic acid synthetases of Aesculus species. Biochem J. 1970 Oct;119(4):677–690. doi: 10.1042/bj1190677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYER P. D. Uses and limitations of measurements of rates of isotopic exchange and incorporation in catalyzed reactions. Arch Biochem Biophys. 1959 Jun;82(2):387–410. doi: 10.1016/0003-9861(59)90136-5. [DOI] [PubMed] [Google Scholar]
- Balharry G. J., Nicholas D. J. ATP-sulphurylase in spinach leaves. Biochim Biophys Acta. 1970 Dec 16;220(3):513–524. doi: 10.1016/0005-2744(70)90282-2. [DOI] [PubMed] [Google Scholar]
- Burnell J. N., Anderson J. W. Adenosine 5'-sulphatophosphate kinase activity in spinach leaf tissue. Biochem J. 1973 Jun;134(2):565–579. doi: 10.1042/bj1340565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHERNIAK R., DAVIDSON E. A. SYNTHESIS OF ADENYLYL SULFATE AND ADENYLYL SULFATE 3'-PHOSPHATE. J Biol Chem. 1964 Sep;239:2986–2990. [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. II. Inhibition: nomenclature and theory. Biochim Biophys Acta. 1963 Feb 12;67:173–187. doi: 10.1016/0006-3002(63)91815-8. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. III. Prediction of initial velocity and inhibition patterns by inspection. Biochim Biophys Acta. 1963 Feb 12;67:188–196. doi: 10.1016/0006-3002(63)91816-x. [DOI] [PubMed] [Google Scholar]
- Cole F. X., Schimmel P. R. Isoleucyl transfer ribonucleic acid synthetase. The role of magnesium in amino acid activation. Biochemistry. 1970 Aug 4;9(16):3143–3148. doi: 10.1021/bi00818a005. [DOI] [PubMed] [Google Scholar]
- Cole F. X., Schimmel P. R. On the rate law and mechanism of the adenosine triphosphate--pyrophosphate isotope exchange reaction of amino acyl transfer ribonucleic acid synthetases. Biochemistry. 1970 Feb 3;9(3):480–489. doi: 10.1021/bi00805a005. [DOI] [PubMed] [Google Scholar]
- DAVIE E. W., KONINGSBERGER V. V., LIPMANN F. The isolation of a tryptophan-activating enzyme from pancreas. Arch Biochem Biophys. 1956 Nov;65(1):21–38. doi: 10.1016/0003-9861(56)90173-4. [DOI] [PubMed] [Google Scholar]
- Levi A. S., Wolf G. Purification and properties of the enzyme ATP-sulfurylase and its relation to vitamin A. Biochim Biophys Acta. 1969 Apr 22;178(2):262–282. doi: 10.1016/0005-2744(69)90395-7. [DOI] [PubMed] [Google Scholar]
- ROBBINS P. W., LIPMANN F. Enzymatic synthesis of adenosine-5'-phosphosulfate. J Biol Chem. 1958 Sep;233(3):686–690. [PubMed] [Google Scholar]
- Rouget P., Chapeville F. Reactions sequence of leucine activation catalysed by leucyl-RNA synthetase. 1. Kinetic studies. Eur J Biochem. 1968 Apr;4(3):305–309. doi: 10.1111/j.1432-1033.1968.tb00209.x. [DOI] [PubMed] [Google Scholar]
- Santi D. V., Danenberg P. V., Satterly P. Phenylalanyl transfer ribonucleic acid synthetase from Escherichia coli. Reaction parameters and order of substrate addition. Biochemistry. 1971 Dec 7;10(25):4804–4812. doi: 10.1021/bi00801a031. [DOI] [PubMed] [Google Scholar]
- Shaw W. H., Anderson J. W. Assay of adenosine 5-triphosphate sulfurylase by pyrophosphate exchange. Plant Physiol. 1971 Jan;47(1):114–118. doi: 10.1104/pp.47.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. H., Anderson J. W. Purification, properties and substrate specificity of adenosine triphosphate sulphurylase from spinach leaf tissue. Biochem J. 1972 Mar;127(1):237–247. doi: 10.1042/bj1270237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweedie J. W., Segel I. H. Adenosine triphosphate sulfurylase from Penicillium chrysogenum. II. Physical, kinetic, and regulatory properties. J Biol Chem. 1971 Apr 25;246(8):2438–2446. [PubMed] [Google Scholar]


