Abstract
Background
Intravenous administration of sufentanil during anesthesia induction induces cough (SIC), sometimes triggers a severe reaction. We wanted to investigate the inhibitory effect of low-dose esketamine on cough induced by sufentanil during the induction of general anesthesia, as well as its postoperative impact on mental status (MMSE score, RSS, and VAS-A).
Methods
A total of 256 adult patients were randomly allocated to receive either esketamine (Group EK) or normal saline (Group C). One minute before the administration of sufentanil, Group EK and Group C were injected with esketamine (0.05 mg/kg, diluted with normal saline to 3 ml) and normal saline (3 ml), respectively. The primary outcomes were the incidence (the basis of the presence or absence of cough) and severity (the frequency of cough episodes) of cough within 2 min after sufentanil injection.The secondary outcomes included hemodynamic variables (MAP, HR, and SPi), mental status (MMSE score, RSS, and VAS-A) and postoperative events (time to awareness, duration of orientation recovery and degree of satisfaction with anesthesia).
Results
A total of 236 adult patients were randomized into two groups (n = 236): Group EK (n = 118) and Group C (n = 118). The incidence of cough in Group EK (21.2%) was significantly lower than that in Group C (40.7%) (P < 0.05). The incidence rates for each grade were as follows: 9.3% and 16.9% for Grade 2, and 0% and 4.2% for Grade 3, respectively.The differences had statistical senses.The MAP and HR at T4 (during tracheal intubation) and T5 (1 min post-intubation) were significantly lower in Group EK (P < 0.05). There were no significant differences in MMSE score, RSS, and VAS-A, time to awareness, duration of orientation recovery or satisfaction with anesthesia.
Conclusion
Pretreatment with low-dose esketamine can reduce the incidence and severity of cough induced by sufentanil and maintain hemodynamic stability during anesthesia induction without increasing mental status (MMSE score, RSS, and VAS-A).
Trial registration
Chinese Clinical Trial Registry (ChiCTR2400084940, date of registration: 05/28/2024).
Keywords: Eketamine, Sufentanil, Pretreatment, General anesthesia, Induction, Cough, Mental system
Introduction
Opioid receptor agonists such as fentanyl, sufentanil, and remifentanil are commonly used for anesthesia induction and maintenance because of their beneficial properties, including potent pain relief, long duration, and stable hemodynamics [1]. The intravenous infusion of sufentanil during anesthesia induction is associated with a high incidence of choking or bucking reactions, which are clinically referred to as sufentanil-induced cough (SIC) [2]. Cough is generally benign, but it can occasionally lead to undesirable side effects, such as increased intra-abdominal, intracranial, or intraocular pressure [3]. Patients with traumatic brain injury, aneurysm, glaucoma, and complex respiratory diseases are at great risk [4]. Pharmacological interventions, such as lidocaine, dezocine, dexmedetomidine, ketamine, and butorphanol, have been extensively utilized to prevent the occurrence of cough. Ketamine is known to inhibit SIC through its antagonistic effect on NMDA (N-methyl-D-aspartate) receptors [5]. The right-handed version of ketamine, known as esketamine, shows a greater affinity for NMDA receptors and has a reduced impact on cognitive function and memory in the early stages following administration [2, 6]. Esketamine exhibits a more potent receptor affinity and fewer adverse effects than ketamine and exhibits shorter recovery times after brief periods of anaesthesia. As the PVT (paraventricular thalamus) plays a pivotal role in regulating wakefulness [7]. The aim of this study was to investigate the effective suppressive effect of intravenous administration of low-dose esketamine on cough triggered by sufentanil during the general anesthesia induction process, as well as its subsequent effects on postoperative mental status idcluding MMSE (Mini-Mental State Examination) score, RSS (Ramsay Sedation Scale), and VAS-A (Visual Analogue Scale for Anxiety).
Methods
Study design
This was a prospective, single-center, randomized controlled trial. The present study was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University (LunShen (2022)076 and (2022)076 − 1) and was registered in the Chinese Clinical Trial Registry (ChiCTR2400084940, date of registration: 05/28/2024).The study design adhered to the 2010 CONSORT statement. Two hundred fifty-six patients who underwent elective general anesthesia were recruited from May 2024 to July 2024, 236 of whom successfully passed the screening process. The participants (aged 18–70) were randomly divided into two groups by using a computer to generate a random number list at a 1:1 ratio to the esketamine group (Group EK) (n = 118) or the normal saline group (Group C) ( n = 118). The random group sequence number was placed in sealed envelopes by a nurse who was not involved in the anesthesia. Another anesthesiologist opened the envelope and was aware of the treatment allocation of each patient. He was responsible for drug preparation and injection. A blinded anesthesiologist recorded the indicators include the incidence (the basis of the presence or absence of cough) and severity (the frequency of cough episodes) of cough within 2 minutes after sufentanil injection, hemodynamic variables (MAP, HR, and SPi), mental status (MMSE score, RSS, and VAS-A) and postoperative events (time to awareness, duration of orientation recovery and degree of satisfaction with anesthesia). Group allocation was revealed only after data collection and analysis. Group EK received an intravenous infusion of 0.05 mg/kg (the desired amount was drawn and diluted with normal saline to 3 ml) esketamine, which was administered one minute prior to the sufentanil injection. In the Group C, a 3 ml intravenous infusion of normal saline was administered one minute prior to the injection of sufentanil. The inclusion criterias were as follows: voluntary provision of signed informed consent; between the ages of 18 and 70 (including both ends); American Society of Anesthesiologists (ASA) class I or II; and a preparation for elective tracheal intubation under general anesthesia. The exclusion criteria were as follows: patients who declined to participate; with a BMI ≤ 18 or ≥ 30 kg/m2; severe hepatic and renal dysfunction; a history of chronic cough, bronchial asthma or chronic obstructive pulmonary disease; acute upper respiratory tract infection or recent use of bronchodilators and steroid angiotensin converting enzyme inhibitors; psychiatric disorders or allergies to study drugs; andserious clinical conditions that may impact the study. The criterias for termination were as follows: incomplete clinical data and follow-up information of the subjects; noncompliance with testing and other observation diagnoses as per the study protocol; voluntary withdrawal from the study. All instances of participant withdrawal were documented for future reference, and the attrition rate was calculated after the experiment.
Study protocol
The heart rate (HR), mean artery pressure (MAP), and surgical pleth index (Spi) values were assessed for all patients upon their admission to the operating room. The upper extremity vein was punctured via an 18G catheter, and 500 ml of sodium lactate Ringer’s solution was administered. Each patient was provided with a mask delivering oxygen and air at a flow rate of 2 L/min. An intravenous infusion of 0.05 mg/kg esketamine (the desired amount was drawn and diluted with normal saline to 3 ml) was administered to patients in Group EK one minute prior to the injection of sufentanil. Patients in Group C received an intravenous infusion of normal saline (3 ml) one minute prior to the administration of sufentanil. Patients in both groups received an intravenous injection of 0.4 µg/kg sufentanil (Humanwell Healthcare Co. Ltd. batch number AB40101711) over a duration of 10 s. Two minutes later, patients in both groups received an intravenous injection of 0.02 mg/kg midazolam (Jiangsu NHWA Pharmaceutical Co. Ltd. batch number TMD23E18), 2 mg/kg propofol (AstraZeneca UK Limited, batch number X23013B), and 0.15 mg/kg cisatracurium (Jiangsu Hengrui Pharmaceuticals Co. Ltd. batch number 240408BL) during the induction of anesthesia. The decision to perform endotracheal intubation was made when the bispectral index (BIS) (Covidien IIc,15 Hampshire) value dropped below 60 after adequate muscle relaxation. Anesthesia was maintained through the inhalation of 2% sevoflurane (Maruishi Pharmaceutical Co. Ltd. batch number 3Y062), intermittent administration of sufentanil, and cisatracurium. All of the patients were ventilated with an Aspire view anesthetic machine (71034 Boeblingen, Germany). The tidal volume was maintained within the range of 8–12 ml/kg, whereas the end-respiratory carbon dioxide (PetCO2) levels were maintained between 35 and 45 mmHg. The respiratory rate (RR) was fixed at 12 breaths/min, the inspiratory-to-expiratory time ratio (I: E) was 1:2, and the inspired oxygen fraction (FiO2) was 0.5 (balanced with air) throughout the anesthesia period. During the operation, the hemodynamic parameters were maintained within 20% of the baseline values. Cisatracurium and sufentanil were intermittently injected as needed. The administration of additional cisatracurium was discontinued 30 min prior to the conclusion of the surgical procedure, whereas inhalation anesthesia was terminated 10 min before completion. The patient was transferred to the post-anaesthesia care unit (PACU) with an endotracheal tube after the surgical procedure.
Data collection
The demographic data were collected prior to the operation. The study drug was prepared, and the infusion was administered by an unblinded anesthesiologist. Another blinded anesthesiologist performed the assessment and recorded the occurrence of cough within two minutes after the administration of sufentanil. The incidence of cough was recorded on the basis of the presence or absence of cough, and the cough severity was graded according to the frequency of cough episodes as follows: no cough (0 times), mild (1 to 2 times), moderate (3 to 5 times), and severe (> 5 times) [8]. MAP, HR, and SPi were recorded at the following time points: before anesthesia induction (T0), 1 min after esketamine injection (T1), 1 min after sufentanil injection (T2), prior to tracheal intubation (T3), during tracheal intubation (T4), 1 min post-intubation (T5), and 5 min post-intubation (T6). The duration of surgery, intraoperative blood loss, and duration of PACU were recorded for both groups. The cognitive function of both groups was evaluated via the MMSE score [9](the orientation score section), RSS [10, 11], VAS-A [12], at 15 ± 2 min, 30 ± 2 min, and 60 ± 2 min after extubation. In this study, the severity of cognitive function impairment increased as the score decreased or increased. The time to awareness, duration of orientation recovery and degree of satisfaction with anesthesia were also documented in this study.
Outcomes
The primary outcomes were the incidence (the basis of the presence or absence of cough) and severity (the frequency of cough episodes) of cough within 2 min after sufentanil injection.The secondary outcomes included hemodynamic variables (MAP, HR, and SPi), mental status (MMSE score, RSS, and VAS-A) and postoperative events (time to awareness, duration of orientation recovery and degree of satisfaction with anesthesia).
Sample size calculation
Power analysis was performed using stats package in R (version 4.4.0). According to our pilot study, the incidence of cough in Group EK was 21.2%, and 40.7% in Group C. A sample size of 108 patients in each group was calculated with a type I error of 0.05 and power of 85%, with an effect size of 0.7, and considering a dropout rate of approximately 10%, we finally included 236 patients for analysis in this study.
Statistical analysis
The study included a total of 236 participants. Free Statistics software IBM SPSS Statistics 22.0 was used for statistical analysis. The numerical data were presented as numeric values or mean values with standard deviations. Comparisons between groups of continuous variables following a normal distribution were conducted via t tests or ANOVAS-A. The data for categorical variables were presented as case numbers and percentages, and were analyzed via the chi-square test. The Wilcoxon rank sum test was used for nonparametric intergroup comparisons. Statistical significance was established at P values less than 0.05.
Results
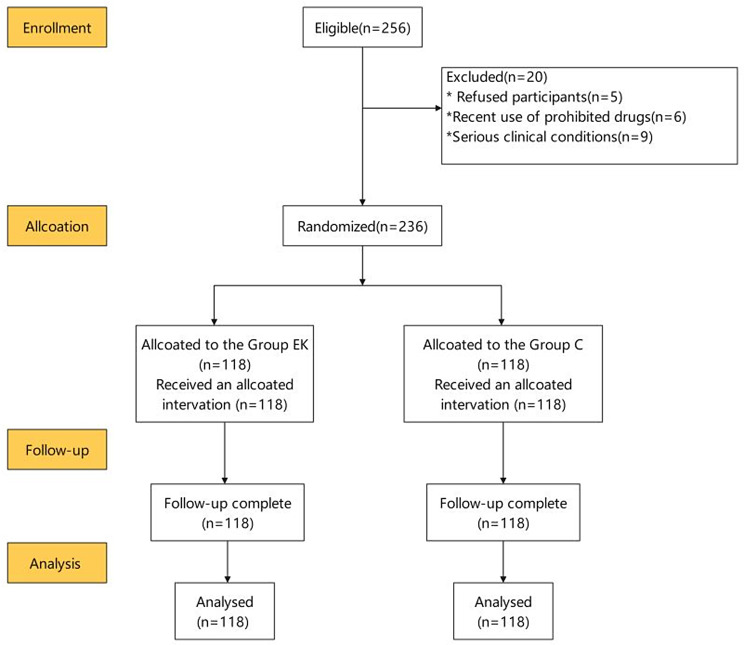
A total of 256 patients were assessed for eligibility for the study, and 236 subjects were enrolled, with 118 patients allocated to each group. Twenty subjects dropped out because of changes before anesthesia (Fig. 1). Age, BMI, sex, ASA grade, duration of surgery, volume of blood loss, and duration in the PACU were not significantly different between the two groups (P > 0.05) (Table 1). The MAP and HR at T4 (during tracheal intubation) and T5 (one minute post-intubation) were significantly lower in Group EK than in Group C (P < 0.05). The Spi values at T4 and T5 were lower than those in Group C, but the difference was not significant. There were no differences in the MAP, HR or Spi at the other time points (Table 2). The incidence of cough was significantly lower in Group EK than in Group C (21.2% vs. 40.7%, P < 0.05). The incidence of both moderate and severe cough was significantly lower in Group EK (9.3% and 0%, respectively) than in Group C (16.9% and 4.2%, respectively) (P < 0.05) (Table 3). The MMSE score, RSS, and VAS-A did not significantly differ between the two groups at the relevant time points (Table 4). The time to awareness, duration of orientation recovery, and patient satisfaction with anesthesia were assessed following surgery (Table 5). The observed data did not reveal any statistically significant disparities.
Fig. 1.
CONSORT flow diagram for patients
Table 1.
Demographic and clinical characteristics
| Group EK (n = 118) |
Group C (n = 118) |
P value | |
|---|---|---|---|
| Sex, n(%) | |||
| Male | 65(55.08) | 56(47.46) | 0.526 |
| Female | 53(44.91) | 62(52.54) | 0.752 |
| Age (yr) | 35.97 ± 8.56 | 38.85 ± 9.02 | 0.600 |
| BMI(kg/m2) | 22.15 ± 2.68 | 23.65 ± 3.02 | 0.493 |
| ASA class, n(%) | |||
| Grade I | 54(45.76) | 55(46.61) | 0.776 |
| Grade II | 64(54.24) | 63(53.39) | 0.365 |
| History, n | |||
| Smoking | 10 | 12 | 0.520 |
| Drinking | 6 | 6 | 1.000 |
| Allergy | 2 | 1 | 1.000 |
| CHD | 3 | 2 | 1.000 |
| Hypertension | 5 | 4 | 0.956 |
| Diabetes | 2 | 1 | 1.000 |
| Duration of surgery (min) | 105.25 ± 25.18 | 110 ± 30.12 | 0.524 |
| Duration of PACU(min) | 35.25 ± 2.25 | 36.17 ± 3.25 | 0.569 |
| Intraoperative blood loss(ml) | 100.35 ± 13.12 | 108.12 ± 19.15 | 0.259 |
Note: Categorical variables are expressed as the means ± standard deviations (SDs) or numbers (percentages). Group EK, iv. esketamine; Group C, iv. normal saline;Abbreviations BMI, body mass index; CHD, coronary heart disease; ASA, American Society of Anesthesiologis
Table 2.
Comparison of vital signs
| Groups (n = 118) | Variables | T0 | T1 | T2 | T3 | T4 | T5 | T6 |
|---|---|---|---|---|---|---|---|---|
| Group EK |
MAP (mmHg) |
97.25± 7.25 | 97.48± 7.38 | 98.58± 10.12 | 88.23± 7.25 | 75.20± 5.41* | 72.34± 4.23* | 85.61± 5.25 |
| Group C | 96.48± 6.49 | 96.55± 7.02 | 101.28± 10.34 | 85.12± 7.89 | 79.18± 5.25* | 78.78± 4.38* | 82.25± 4.39 | |
| t value | 0.324 | 0.578 | 0.119 | 0.575 | 4.028 | 5.889 | 0.317 | |
| P value | 0.752 | 0.568 | 0.906 | 0.586 | 0.045* | 0.025* | 0.577 | |
| Group EK |
HR (bpm) |
84.24± 8.24 | 86.54± 8.78 | 79.23± 3.25 | 78.23± 3.24 | 72.15± 8.98* | 67.34± 5.55* | 68.23± 7.23 |
| Group C | 82.18± 7.68 | 83.25± 8.01 | 84.54± 8.13 | 79.25± 5.26 | 81.24± 10.15* | 70.25± 5.23* | 67.25± 7.24 | |
| t value | 0.170 | 0.961 | 0.577 | 0.758 | 2.683 | 4.235 | 0.105 | |
| P value | 0.866 | 0.341 | 0.566 | 0.463 | 0.017* | 0.023* | 0.917 | |
| Group EK | Spi | 41.21± 4.80 | 42.02± 5.51 | 40.04± 5.84 | 39.21± 4.72 | 48.10± 5.81 | 40.22± 3.81 | 38.62± 3.13 |
| Group C | 40.02± 5.60 | 40.11± 6.12 | 41.02± 5.52 | 39.01± 4.61 | 52.02± 6.50 | 43.52± 3.61 | 38.32± 3.62 | |
| t value | 0.217 | 0.202 | 0.756 | 0.796 | 0.388 | 0.758 | 0.529 | |
| P value | 0.997 | 0.356 | 0.504 | 0.500 | 0.145 | 0.592 | 0.482 |
Note: Categorical variables were expressed as the mean ± standard deviation (SDs). Group EK, iv. esketamine; Group C, iv. normal saline; before anesthesia induction (T0), 1 min after esketamine injection (T1), 1 min after sufentanil injection (T2), prior to tracheal intubation (T3), during tracheal intubation (T4), 1 min post-intubation (T5), and 5 min post-intubation (T6);Abbreviations MAP, mean arterial pressure; HR, heart rate; Spi, surgical pleth index.*P < 0.05 vs. the Group C
Table 3.
Incidence and grade of cough
| Groups (n = 118) |
Grade 0 | Grade 1 | Grade 2 | Grade 3 | Incidence of cough, n(%) |
|---|---|---|---|---|---|
| Group EK | 93(78.8) | 14(11.9) | 11(9.3)* | 0(0)* | 25(21.2)* |
| Group C | 70(59.3) | 23(19.5) | 20(16.9)* | 5(4.2)* | 48(40.7)* |
| x2 value | 22.57 | 5.325 | 5.210 | 4.569 | 4.257 |
| P value | 0.055 | 0.075 | 0.009* | 0.027* | 0.047* |
Note: Categorical variables are expressed as numbers (proportions). Group EK, iv.esketamine; Group C, iv.normal saline; The severity of cough was evaluated within two minutes of starting sufentanil : 0 = no cough, 1 = mild (1–2 times) cough, 2 = moderate (3–5 times) cough and 3 = severe (> 5 times) cough (bucking). *P < 0.05 vs. the Group C
Table 4.
Scores between the two groups
| Variables | Groups (n = 118) |
15 min ± 2 min after extubation |
30 min ± 2 min after extubation |
60 min ± 2 min after extubation |
|---|---|---|---|---|
| MMSE score | Group EK | 8.01 ± 0.61 | 9.30 ± 0.31 | 9.43 ± 0.42 |
| Group C | 8.32 ± 0.70 | 9.40 ± 0.23 | 9.01 ± 0.45 | |
| Z value | 0.91 | 0.98 | 1.21 | |
| P value | 0.108 | 0.708 | 0.213 | |
| RSS | Group EK | 1.72 ± 0.62 | 1.81 ± 0.99 | 1.81 ± 0.47 |
| Group C | 1.93 ± 0.67 | 1.92 ± 0.62 | 1.83 ± 0.76 | |
| z value | 1.28 | 1.53 | 0.86 | |
| P value | 0.332 | 0.152 | 0.398 | |
| VAS-A | Group EK | 3.23 ± 0.67 | 2.95 ± 0.59 | 2.72 ± 0.77 |
| Group C | 3.94 ± 0.67 | 3.24 ± 0.61 | 2.31 ± 0.67 | |
| z value | 0.17 | 0.13 | 0.54 | |
| P value | 0.816 | 0.893 | 0.563 |
Note: Categorical variables are expressed as the mean ± standard deviation (SDs). Group EK, iv.esketamine; Group C, iv. normal saline; Abbreviations MMSE score, Mini-Mental State Examination (the orientation score section, adds up to 10 points); RSS, Ramsay Sedation Scale (anxiety and restlessness (1 point), orientation and quiet cooperation (2 points), response to commands (3 points), lethargy and rapid response to tapping eyebrow or loud auditory stimulation (4 points), lethargy and slow response to tapping eyebrow or loud auditory stimulation (5 points), lethargy and no response (6 points)); VAS-A, Visual Analog Scale-Anxiety (Straight lines were graded on a 10-point scale ranging from 0 (no anxiety) to 10 (extreme anxiety), a higher score indicates a higher level of current anxiety)
Table 5.
Time to awareness, duration of orientation recovery and satisfaction with Anesthesia
| Variables | Group EK (n = 118) |
Group C (n = 118) |
P value |
|---|---|---|---|
| Time to awareness(min) | 19.83 ± 9.95 | 17.81 ± 8.85 | 0.440 |
| Orientation recovery(min) | 18.84 ± 8.95 | 18.31 ± 9.95 | 0.635 |
| Satisfaction with anesthesia | 8.86 ± 0.96 | 9.01 ± 0.95 | 0.253 |
Note: Categorical variables are expressed as the mean ± standard deviation (SDs). Group EK, iv.esketamine; Group C, iv. normal saline
Discussion
In this study, the administration of esketamine at a dosage of 0.05 mg/kg resulted in a significant reduction in cough incidence and a significant lower number of patients experiencing Grade 2–3 severity than did the control group (Group C). Esketamine has about twice or triple the affinity as NMDA [13], the dextro-isomer of ketamine, these findings align with the results reported in a study about ketamine suppressed the cough reflex induced by fentanyl by Yeh CC et al. [14]. Compared with placebo therapy, the intravenous administration of 0.15 mg/kg ketamine significantly reduced fentanyl-induced coughing, demonstrating a superior effect [14].The administration of a low dose of esketamine 0.05 mg/kg can also effectively reduce the incidence of cough during the recovery period following general anesthesia, thereby serving as a preventive measure to indirectly mitigate hemodynamic instability during this phase [15, 16].
Numerous studies have documented various strategies for managing SIC, including decelerating the injection rate, diluting the drug concentrations, reducing the dosage levels, opting for peripheral injection sites, ensuring an appropriate order of drug administration, and providing patients with instructions on the huffing maneuver [17–22]. In the present study, the duration of intravenous sufentanil injection in both groups was standardized to 10 s, and the amount of sufentanil administered was identical, aiming to minimize any discrepancies between the two groups. The incidence of cough can be reduced to varying degrees by pretreatment with ketamine, propofol, and lidocaine [23, 24]. The incidence of cough in Group EK was 21.2%, which was significantly lower than that in Group C (40.7%), and the incidence of Grade 2–3 severity decreased significantly. The incidences of both moderate and severe cough were significantly lower in Group EK (9.3% and 0%, respectively) than in Group C (16.9% and 4.2%, respectively) (P < 0.05). The primary mechanism of action of esketamine involves the blockade of NMDA receptors while also exerting effects on opioid, monoaminergic, adenosine, and other receptor systems. In addition to increase the prevalence of NMDA receptors in the respiratory system, NMDA receptor is also associated with relaxation of airway smooth muscles via voltage-dependent L-type calcium channels, leading to bronchial dilation [15]. The cough reflex is elicited by activation of NMDA receptor. The administration of a low dose of ketamine was also found to be effective in reducing the incidence of remifentanil-induced cough but did not affect its severity or onset time [25]. Research has shown that pretreatment with intravenous administration of a low dose of esketamine effectively suppresses the occurrence of SIC during the induction phase of general anesthesia, thereby reducing both the frequency and severity of coughing [25, 26]. The aforementioned statement aligns with the research conducted by Li Shuying et al. [27]. In our study, pretreatment with low-dose esketamine reduced the incidence and severity of cough induced by sufentanil during the induction of general anesthesia.
In this study, the MAP and HR in Group EK were slightly higher than those in Group C at T1, but the differences were not significant. The observed outcome was attributed to stimulation of the sympathetic nerves by esketamine, resulted in increases in both blood pressure and HR [28]. The dosage administered in this study did not induce any hemodynamic instability. The MAP and HR in Group EK at the T4 and T5 time points were significantly lower than those in Group C, indicated that pretreatment with esketamine effectively attenuated the stress response induced by tracheal intubation and maintained hemodynamic stability during anesthesia induction [28–30]. Compared with HR or BP, the Spi, which was developed for quantifying intraoperative stress levels, esketamine demonstrated a superior ability to reflect noxious stimuli [31]. The Spi at T4 and T5 were lower than those in Group C in our study; however, no statistically significant difference was observed. The administration of analgesics at subtherapeutic doses or the utilization of low dosages of esketamine cannot be ruled out [32]. This study verified that pretreatment with low-dose esketamine can maintain hemodynamic stability during anesthesia induction.
The MMSE score, RSS, and VAS-A of Group EK did not significantly differ from those of Group C at any time point following extubation. Research shows it is more appropriate to use the orientation score for early postoperative cognitive dysfunction [13] which can reflect the quality of anesthesia recovery; The time to awareness, duration of orientation recovery, and patient satisfaction with anesthesia were not significantly different. These results imply that the administered dose of esketamine does not increase the occurrence of atypical psychiatric symptoms in patients [33]. Moreover, these findings are in line with the results obtained by Eberl and Susanne in a study focused on endoscopy [34]. A potential explanation is that the combination of propofol inhibits the expression of c-fos in the posterior cingulate cortex induced by ketamine [35, 36]. Alternatively, we used a small dose of esketamine, which did not delay recovery or induce postoperative mental problems. This phenomenon may also be associated with the activation of synaptic signaling pathways, the reconfiguration of synaptic connections in neural circuits, and the regeneration of damaged nerve cells [37]. Therefore, our findings indicated that pretreatment with low-dose esketamine did not increase the MMSE score, RSS, or VAS-A or prolong the time to awareness or duration of orientation recovery or reduce patient satisfaction with anesthesia.
In summary, pretreatment via intravenous administration of low-dose esketamine can effectively suppress the choking reflex induced by sufentanil during the induction phase of general anesthesia and promote hemodynamic stability. This treatment does not result in an increased occurrence of abnormal psychonervous system symptoms in patients or prolong the duration of extubation after surgery.
The study sample size, however, was limited and did not fully preclude the possibility of esketamine inducing psychiatric symptoms. Moreover, the inhibitory effect on cough was limited, possibly due to the administration of a suboptimal dose. Multiple limitations are worth considering, including potential bias from any other source, potential error, statistical uncertainty, and a lack of generalizability of the results of the present study. Further research is needed to determine the appropriate dosage for esketamine pretreatment.
Acknowledgements
Not applicable.
Abbreviations
- ASA
American Society of Anesthesiologists
- BIS
bispectral index
- BMI
body mass index
- FiO2
inspired the oxygen fraction
- HR
heart rate
- I:E
inspiratory-to-expiratory time ratio
- MAP
mean arterial pressure
- MMSE
Mini-Mental State Examination
- NMDA
N-methyl-D-aspartate
- PACU
post-anaesthesia Care Unit
- PetCO2
end-respiratory carbon dioxide
- RR
respiratory rate
- RSS
ramsay sedation scale
- SIC
sufentanil-induced cough
- Spi
surgical pleth index
- VAS-A
visual analog scale for anxiety
Author contributions
Qian contributed to the study design, drafted the manuscript, interpreted the data, and revised the manuscript. Ji revised the manuscript. Peng contributed to the study design and data collection. Shan contributed to the data analysis. Mao, Chen, Zhou contributed to data collection and follow-up. All the authors have read and approved the final manuscript.
Funding
This work was supported by the Health Talent Plan Project in Suzhou (grant number: GSWS2022007).
Data availability
The datasets generated and/or analyzed during the current study are not publicly available owing to institutional restrictions but are available from the Corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study followed the Consolidated Standards of Reporting Trials statement and the Declaration of Helsinki. This work was registered in the Chinese Clinical Trials Registry (ChiCTR2400084940) on 05/28/2024, and The Ethics Committee of the First Affiliated Hospital of Soochow University (LunShen (2022)076 and (2022)076 − 1) approved the study. All participants provided written informed consents.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Van de Donk T, Ward S, Langford R, Dahan A. Pharmacokinetics and pharmacodynamics of sublingual sufentanil for postoperative pain management. Anaesthesia. 2018;73(2):231–7. [DOI] [PubMed] [Google Scholar]
- 2.Qian Y, Huang Z, Wang G, et al. Low-dose naloxone for prophylaxis of sufentanil-induced choking and postoperative nausea and vomiting. Front Pharmacol. 2022;13:1050847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen R, Tang LH, Sun T, et al. Mechanism and management of Fentanyl-Induced Cough. Front Pharmacol. 2020;11:584177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dion GR, Teng SE, Achlatis E, Fang Y, Amin MR. Treatment of neurogenic cough with tramadol: a pilot study. Otolaryngol Head Neck Surg. 2017;157(1):77–9. [DOI] [PubMed] [Google Scholar]
- 5.Dong Y, Chang X. Comparison of five Prophylactically Intravenous drugs in preventing Opioid-Induced Cough: a bayesian network Meta-analysis of Randomized controlled trials. Front Pharmacol. 2021;12:684276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfenninger EG, Durieux ME, Himmelseher S. Cognitive impairment after small-dose ketamine isomers in comparison to equianalgesic racemic ketamine in human volunteers. Anesthesiology. 2002;96(2):357–66. [DOI] [PubMed] [Google Scholar]
- 7.Duan WY, Peng K, Qin HM, et al. Esketamine accelerates emergence from isoflurane general anaesthesia by activating the paraventricular thalamus glutamatergic neurones in mice. Br J Anaesth. 2024;132(2):334–42. [DOI] [PubMed] [Google Scholar]
- 8.Saleh AJ, Zhang L, Hadi SM, Ouyang W. A priming dose of intravenous ketamine-dexmedetomidine suppresses fentanyl-induced coughing: a double-blind, randomized, controlled study. Ups J Med Sci. 2014;119(4):333–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakurai R, Kim Y, Inagaki H, et al. MMSE Cutoff discriminates hippocampal atrophy: neural evidence for the cutoff of 24 points. J Am Geriatr Soc. 2021;69(3):839–41. [DOI] [PubMed] [Google Scholar]
- 10.Zhang C, Huang D, Zeng W, et al. Effect of additional equipotent fentanyl or sufentanil administration on recovery profiles during propofol-remifentanil-based anaesthesia in patients undergoing gynaecologic laparoscopic surgery: a randomized clinical trial. BMC Anesthesiol. 2022;22(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lang J. Appraisal of Clinical Practice Guideline: Clinical Practice Guidelines for the Prevention and Management of Pain, Sedation A. Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. J Physiother. 2022. 68(4): 282. [DOI] [PubMed]
- 12.Facco E, Stellini E, Bacci C, et al. Validation of visual analogue scale for anxiety (VAS-A) in preanesthesia evaluation. Minerva Anestesiol. 2013;79(12):1389–95. [PubMed] [Google Scholar]
- 13.Wang J, Huang J, Yang S, et al. Pharmacokinetics and safety of Esketamine in Chinese patients undergoing painless gastroscopy in comparison with ketamine: a randomized, open-label clinical study. Drug Des Devel Ther. 2019;13:4135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeh CC, Wu CT, Huh BK, et al. Premedication with intravenous low-dose ketamine suppresses fentanyl-induced cough. J Clin Anesth. 2007;19(1):53–6. [DOI] [PubMed] [Google Scholar]
- 15.Goyal S, Agrawal A. Ketamine in status asthmaticus: a review. Indian J Crit Care Med. 2013;17(3):154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JY, Kim JY, Park SY, Jung WS, Kwak HJ. Effect of low dose ketamine to prevent remifentanil-induced cough: a randomized, double-blind, placebo controlled trial. Korean J Anesthesiol. 2009;56(6):624–7. [DOI] [PubMed] [Google Scholar]
- 17.Mukherjee A, Kundu AK, Ghosh S, Choudhuri R, Bandopadhyay BK, Dasgupta S. Pre-emptive oral dexmethorphan reduces fentanyl-induced cough as well as immediate postoperative adrenocortico-tropic hormone and growth hormone level. J Anaesthesiol Clin Pharmacol. 2011;27(4):489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Said SI, Berisha HI, Pakbaz H. N-methyl-D-aspartate receptors outside the central nervous system: activation causes acute lung injury that is mediated by nitric oxide synthesis and prevented by vasoactive intestinal peptide. Neuroscience. 1995;65(4):943–6. [DOI] [PubMed] [Google Scholar]
- 19.Horng HC, Lin BF, Wang TC, et al. Priming dose of intravenous rocuronium suppresses fentanyl-induced coughing. Acta Anaesthesiol Taiwan. 2012;50(4):147–9. [DOI] [PubMed] [Google Scholar]
- 20.Weinger MB, Chen DY, Lin T, Lau C, Koob GF, Smith NT. A role for CNS alpha-2 adrenergic receptors in opiate-induced muscle rigidity in the rat. Brain Res. 1995;669(1):10–8. [DOI] [PubMed] [Google Scholar]
- 21.Lin JA, Yeh CC, Lee MS, Wu CT, Lin SL, Wong CS. Prolonged injection time and light smoking decrease the incidence of fentanyl-induced cough. Anesth Analg. 2005;101(3):670–4. [DOI] [PubMed] [Google Scholar]
- 22.Sun L, Guo R, Sun L. The impact of prophylactic intravenous lidocaine on opioid-induced cough: a meta-analysis of randomized controlled trials. J Anesth. 2014;28(3):325–33. [DOI] [PubMed] [Google Scholar]
- 23.Methods In Medicine C, Retracted. Comparison of a Small Dose of Oxycodone and Sufentanil for the Prevention of Sufentanil-Induced Cough during General Anesthesia Induction: A Prospective Randomized Controlled Trial. Comput Math Methods Med. 2023. 2023: 9798531. [DOI] [PMC free article] [PubMed]
- 24.Bahk JH, Sung J, Jang IJ. A comparison of ketamine and lidocaine spray with propofol for the insertion of laryngeal mask airway in children: a double-blinded randomized trial. Anesth Analg. 2002;95(6):1586–9. table of contents. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Zhang Q, Dai X, Xiao G, Luo H. Effect of low-dose esketamine on pain control and postpartum depression after cesarean section: a retrospective cohort study. Ann Palliat Med. 2022;11(1):45–57. [DOI] [PubMed] [Google Scholar]
- 26.Cui S, Huang P, Wei Z, Guo T, Zhang A, Huang L. Esketamine Combined with Propofol TCI versus Propofol TCI for Deep Sedation during Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration: A Prospective, Randomized, and Controlled Trial. Int J Clin Pract. 2023. 2023: 1155126. [DOI] [PMC free article] [PubMed]
- 27.Shuying L, Ping L, Juan N, Dong L. Different interventions in preventing opioid-induced cough: a meta-analysis. J Clin Anesth. 2016;34:440–7. [DOI] [PubMed] [Google Scholar]
- 28.Tu W, Yuan H, Zhang S, et al. Influence of anesthetic induction of propofol combined with esketamine on perioperative stress and inflammatory responses and postoperative cognition of elderly surgical patients. Am J Transl Res. 2021;13(3):1701–9. [PMC free article] [PubMed] [Google Scholar]
- 29.Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for Covid-19 - interim WHO Solidarity Trial results. N Engl J Med. 2021;384(6):497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarturi VZ, Teixeira LG, Coradini GP, et al. Total intravenous anesthesia with Propofol Associated or not with remifentanil, ketamine, or S-Ketamine for laparoscopic ovariectomy in female dogs. Top Companion Anim Med. 2021;45:100575. [DOI] [PubMed] [Google Scholar]
- 31.Park JH, Kim DH, Yoo SK, et al. The analgesic potency dose of remifentanil to minimize stress response induced by intubation and measurement uncertainty of Surgical Pleth Index. Minerva Anestesiol. 2018;84(5):546–55. [DOI] [PubMed] [Google Scholar]
- 32.Oh SK, Won YJ, Lim BG. Surgical pleth index monitoring in perioperative pain management: usefulness and limitations. Korean J Anesthesiol. 2024;77(1):31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu D, Wang XM, Yang JJ, et al. Effect of Intraoperative Esketamine Infusion on postoperative sleep disturbance after Gynecological Laparoscopy: a Randomized Clinical Trial. JAMA Netw Open. 2022;5(12):e2244514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eberl S, Koers L, van Hooft J, et al. The effectiveness of a low-dose esketamine versus an alfentanil adjunct to propofol sedation during endoscopic retrograde cholangiopancreatography: a randomised controlled multicentre trial. Eur J Anaesthesiol. 2020;37(5):394–401. [DOI] [PubMed] [Google Scholar]
- 35.Nagata A, Nakao S, Miyamoto E, et al. Propofol inhibits ketamine-induced c-fos expression in the rat posterior cingulate cortex. Anesth Analg. 1998;87(6):1416–20. [DOI] [PubMed] [Google Scholar]
- 36.Nishizawa N, Nakao S, Nagata A, Hirose T, Masuzawa M, Shingu K. The effect of ketamine isomers on both mice behavioral responses and c-Fos expression in the posterior cingulate and retrosplenial cortices. Brain Res. 2000. 857(1–2): 188 – 92. [DOI] [PubMed]
- 37.Khakpai F, Ebrahimi-Ghiri M, Alijanpour S, Zarrindast MR. Ketamine-induced antidepressant like effects in mice: a possible involvement of cannabinoid system. Biomed Pharmacother. 2019;112:108717. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available owing to institutional restrictions but are available from the Corresponding author on reasonable request.