Abstract
Background
Congenital heart disease (CHD) is a common birth defect. Our previous studies suggest that indoor air pollution, especially total volatile organic compounds (TVOCs), may increase fetal CHD risk, whereas vitamin and folic acid (FA) supplements in early pregnancy might offer protection against CHD. However, limited research has explored whether FA or multivitamin supplementation can mitigate the effects of TVOCs exposure on CHD.
Methods
We conducted a case-control study to investigate the association between maternal nutrient supplementation, household indoor air pollutant exposure during pregnancy, and CHD in offspring. Pregnant women with 22–30 gestational weeks were recruited from two hospitals in East China between January 2016 and March 2022. A comprehensive approach was used, incorporating questionnaires to collect nutrient supplement information, blood sample analysis to detect serum folate, vitamin B12, and homocysteine (HCY) concentrations, and field investigations to assess indoor benzene, toluene, xylene, formaldehyde, and TVOCs exposures. Logistic regression analysis was performed to identify CHD risk factors, and stratified analysis was used to evaluate the combined effects of nutrient supplementation and TVOCs on CHD.
Results
The study included 53 cases and 77 controls. Logistic regression analysis identified high maternal serum HCY (> 6.125 µmol/L) and high household TVOCs exposure (> 0.0165 mg/m³) as risk factors for CHD in offspring, with adjusted odds ratios of 2.98 (95% CI: 1.31–6.36) and 9.23 (95% CI: 3.78–22.53), respectively. Regular multivitamin supplementation mitigated the risk of high TVOCs exposure on fetal CHD, while the adverse effect of high serum HCY-related CHD risk was attenuated in the group with regular FA supplementation.
Conclusion
Exposure to high indoor TVOCs concentrations increases the risk of fetal CHD. Regular multivitamin supplementation may reduce the adverse effects of high TVOCs exposure on fetal CHD.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12940-024-01150-7.
Keywords: Total volatile organic compounds, Multivitamin supplement, Congenital heart disease, Folic acid
Introduction
Congenital heart disease (CHD) is a significant, rapidly emerging global problem in child health. The global prevalence of CHD at birth, in 2017, was estimated to be approximately 1.8 cases per 100 live births [1]. Van Mil et al. [2] estimated that 80% or more of congenital malformations are the result of interactions between subtle genetic variations and environmental exposures.
People are exposed to air pollutants on a daily basis. Previous studies revealed the relationship between environmental contaminants and CHD, but those exposure assessments were generally based on routine monitoring of pollutant concentrations at the fixed-site monitoring stations closest to maternal residence at the time of the birth [3]. Given that pregnant women, a particularly vulnerable population, spend most of their time indoors, assessing indoor pollutant exposure levels is critical. However, few studies have focused on the effects of indoor pollutant exposure on maternal and fetal health. Total volatile organic compounds (TVOCs) consist of various volatile organic compounds (VOCs), such as formaldehyde, trichloroethylene, benzene series, and hydrocarbon compounds, and are primarily attributed to indoor sources, including renovation materials, furniture, and related items [4]. Past research has shown that elevated exposure to TVOCs during the prenatal period may adversely influence early postnatal growth [5] and even lead to birth defects [6]. Our previous research has also suggested that maternal exposure to indoor TVOCs may increase the risk of giving birth to fetus with CHD [7]. Additionally, a zebrafish study provides evidence that exposure to a mixture of VOCs can induce embryonic cardiovascular development abnormalities [8].
Folate and vitamin B12 are essential water-soluble vitamins that play a significant role in one-carbon metabolism [9]. These vitamins play a significant role in cardiovascular disease development through their effects on HCY metabolism [10]. Prior studies have demonstrated that folic acid (FA) supplementation and multivitamin use have protective effects against CHD, and high maternal serum HCY levels are considered a risk factor for CHD [11–13].
However, no studies have investigated whether FA or multivitamin supplementation can protect against the cardiac developmental toxicity caused by TVOCs. To fill these knowledge gaps, our study aimed to (1) assess the effects of low and high TVOCs concentrations on CHD; (2) study the relationship between nutrient supplementation, serum biomarkers and CHD; and (3) investigate whether nutrient supplementation can moderate the effect of TVOCs on CHD.
Methods
Study population and subjects
This study utilized a case-control design with frequency matching. Participants were recruited from Shanghai Xinhua Hospital and Jiaxing Maternity and Child Health Care Hospital from January 2016 and March 2022. Inclusion criteria were: (1) a single-fetus pregnancy, (2) gestational age between 22 and 30 weeks at the time of prenatal diagnosis, and (3) completion of a comprehensive fetal echocardiography. Exclusion criteria included: (1) fetuses diagnosed with chromosomal abnormalities or other genetic syndromes, (2) pregnancies with multiple fetuses, and (3) mothers experiencing mental health conditions preventing them from providing informed consent and responding to inquiries. The study was approved in 2016 by the Ethics Committee of Xinhua Hospital (Approval No. XHEC-D-2016-401-2). The investigation followed the ethical principles set forth in the 1964 Helsinki Declaration. Only participants written informed consent was obtained from all participants in this study.
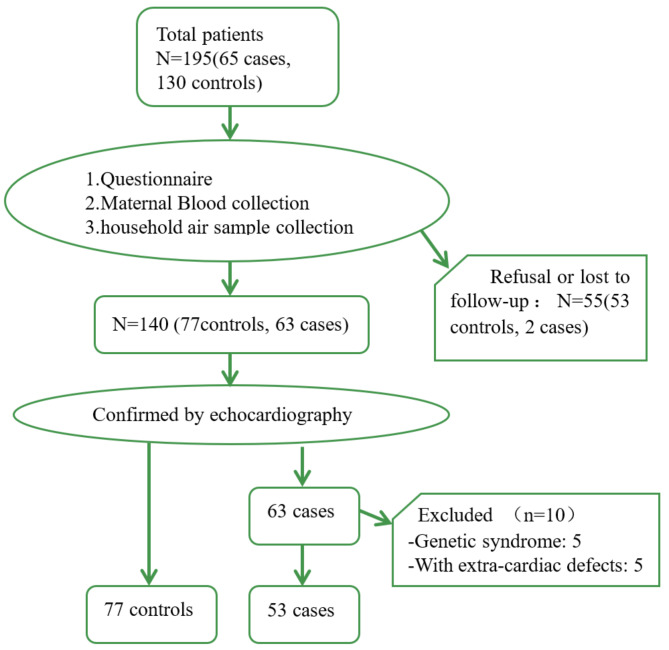
Fetuses diagnosed with CHD through fetal echocardiography or postnatal heart malformation detection were categorized as cases. Controls were defined as fetuses without CHD or other congenital malformations, were chosen from the same hospitals within the study period, maintaining a case-control ratio of 1:2. Control group gestational ages differed by no more than two weeks from those of the case group. All live births in both groups underwent comprehensive neonatal echocardiography by pediatric cardiologists post-delivery to ensure correct case or control group classification. Stillbirth and abortion cases were reviewed by a panel of four national specialists in ultrasound, pediatrics, obstetrics, and pathology to validate diagnoses. A total of 195 samples were enlisted, comprising 130 controls and 65 cases. Among these, 53 controls and 2 cases were lost to follow-up, 5 cases exhibited genetic abnormalities, and 5 cases presented with extra-cardiac malformations. Ultimately, 53 cases and 77 controls were included for analysis (Fig. 1).
Fig. 1.
The flow chart of study population recruitment
Sample size was determined using PASS v.19 (NCSS, LLC, Kaysville, UT, USA). Considering the reported indoor VOC exposure rate in China of 20–60%, we assumed a high-level TVOCs exposure rate of 20% in the control group and 60% in the case group [14]. To achieve 80% power for detecting different TVOCs exposures between the groups with an alpha error of 0.05, a total sample size of 108 (72 controls and 36 cases) was required.
Questionnaire survey data collection
Face-to-face interviews were conducted by trained researchers with the mothers of the case and control groups. A standardized, structured questionnaire was used to obtain information about (1) sociodemographic factors (including maternal and paternal age, education, occupation, ethnicity, and residence); (2) reproductive history (including gravidity and pregnancy history); (3) maternal health and lifestyle (covering basic diseases, medication use, lifestyle factors, and environmental exposures during the first trimester, such as exposure to indoor renovations, environmental pollutants around the residence, and second-hand smoke); and (4) FA and multivitamin supplement use.
Maternal FA supplementation was classified as “regularly” if mothers reported taking at least 0.4 mg of FA daily for more than 5 days per week continuously during pregnancy. It was classified as “irregularly” if they took no more than 0.4 mg of FA daily for 5 or fewer days per week or did not take FA at all. Similarly, maternal multivitamin use, defined as a dietary supplement containing a combination of essential vitamins and minerals, was considered as “regularly” if mothers reported taking one multivitamin tablet at least 5 days per week continuously during pregnancy; otherwise, it was classified as “irregular”. Multivitamin tablets primarily contained essential vitamins such as folic acid, iron, calcium, vitamin A, C, D, E, K, and B-complex vitamins (B1, B2, B3, B5, B6, B7, B9, and B12).
Maternal serum folate, vitamin b12, and hcy detection
Approximately 5 ml of peripheral blood was collected from each subject within two weeks of completing the questionnaire. Serum was separated and subsequently used to measure the concentrations of serum folate, vitamin B12 and HCY, which are commonly used as clinical indicators for assessing the nutritional status of pregnant women. Serum folate and vitamin B12 levels were determined using the chemiluminescence method, while serum HCY concentrations were measured using the circulating enzymatic method with automated biochemical techniques.
Air pollutant measurements during pregnancy
Air samples were collected from the participants’ residential environments within two weeks of completing the questionnaire and blood sample collection. The concentrations of benzene, toluene, xylene (BTX), formaldehyde and TVOCs in households were monitored as previously described in detail [7]. Briefly, the living room, where people spend the most time, was selected as the sampling area for assessing health risks in pregnant women. VOCs were collected using active samplers (TenaxTA, SUPELCO, USA) and analyzed by gas chromatography with flame ionization detection (GC-FID). Target VOCs (BTX) were quantified using multipoint external standard curves, and total quantified VOCs were included for TVOCs concentration analysis. Formaldehyde was measured and analyzed using the 3-methyl-2-benzonthiazolinone hydrazine (MBTH) method with a UV-VIS spectrophotometer. Indoor temperature and relative humidity were recorded when the samples were obtained. Additional sampling and analytical method details are provided in the Chinese National Standard GB/T 18,883–2022 [15].
Observations below the method detection limits (MDLs), 0.01 mg/m3 for formaldehyde, BTX and TVOCS concentrations, were replaced with half the detection limit for statistical analyses.
Statistical analysis
Statistical analyses were performed using IBM SPSS software, version 25.0. Data are presented as median values within interquartile ranges (IQR), and proportions, where relevant. Comparative bivariate analysis between case and control groups was conducted using t-tests for normally distributed variables, Mann-Whitney U tests for non-normally distributed variables, and chi-squared tests for categorical variables. The serum folate, vitamin B12, and HCY levels were categorized into low and high concentration groups based on the 75th percentile of the control group. Similarly, due to the skewed distribution of the indoor air pollutant concentrations, TVOCs exposure levels were categorized as “low” (below the 75th percentile) and “high” (above the 75th percentile) based on control group distribution. Benzene exposure levels were categorized as “low” (≤ the MDL) and “high” (> the MDL). Univariate logistic regression was used to evaluate associations between nutrient supplementation, serum biomarkers, TVOCs exposure levels, and CHD, with results expressed as crude odds ratios (COR) and 95% confidence intervals (CI). Adjusted odds ratios (AORs) were obtained using multivariate logistic regression, adjusting for potential confounders identified with a p-value less than 0.05 in the bivariate analysis of baseline characteristics. Correlations between the TVOCs concentrations and nutrient serum biomarkers were assessed using Spearman rank correlation coefficients. To further clarify the effect of nutrient supplementation on CHD risk in relation to serum nutrient biomarkers and TVOCs exposure, FA and multivitamin use were stratified as regular or irregular. AORs for CHD risk were then compared among mothers with different levels of serum biomarkers and TVOCs exposure. The Benjamini-Hochberg method was employed to control the false discovery rate, and adjusted p-values were calculated to account for multiple comparisons.
Results
Basic features of the study population
The demographic characteristics, reproductive history and periconceptional health status of the study population were presented in Table 1. Significant differences were observed between the case and control groups in maternal age (P = 0.009), maternal education level (P = 0.009), paternal education level (P = 0.002), and the place of residence (P = 0.043), as determined by chi-squared tests. In summary, the case group demonstrated younger maternal ages, lower parental education levels, and a higher proportion of rural residence compared to the control group.
Table 1.
Baseline Characteristics of Cases and Controls
| Control(N = 77) n(%) | Case(N = 53) n(%) | P value | |
|---|---|---|---|
| Maternal age (years) | 0.009 | ||
| <25 | 1(1.3) | 4(7.5) | |
| 25 ≤ Y<30 | 20(26.0) | 23(43.4) | |
| ≥ 30 | 56(72.4) | 26(49.1) | |
| Maternal education level | 0.009 | ||
| Below the undergraduate level | 8(10.4) | 15(28.3) | |
| College or higher | 69(89.6) | 38(71.7) | |
| Paternal education level | 0.002 | ||
| Below the undergraduate level | 8(10.4) | 17(32.1) | |
| College or higher | 69(89.6) | 36(67.9) | |
| Residence | 0.043 | ||
| Urban | 40(51.9) | 18(34.0) | |
| Rural | 37(48.1) | 35(66.0) | |
| Parity | 0.719 | ||
| Primiparous | 47(61.0) | 34(64.2) | |
| Multiparous | 30(39.0) | 19(35.8) | |
| Cold/ Fever in early pregnancy | 0.183 | ||
| no | 44(57.1) | 24(42.3) | |
| yes | 33(42.9) | 29(54.7) | |
| Maternal passive smoking | 0.613 | ||
| no | 47(61.0) | 30(56.6) | |
| yes | 30(39.0) | 23(43.4) | |
| Maternal alcohol consumption | 0.651 | ||
| never/irregularly | 76(98.7) | 52(98.1) | |
| regularly | 1(1.3) | 1(1.9) | |
| Environmental pollutants around the residence* | 0.070 | ||
| no | 40(51.9) | 19(35.8) | |
| yes | 37(48.1) | 34(64.2) | |
| House Renovation | 0.551 | ||
| no | 56(72.7) | 41(77.4) | |
| yes | 21(27.3) | 12(22.6) |
*Environmental pollutants around the residence: Within a 5-minute walking distance from home, there are farmlands/orchards, plastic greenhouses, or busy roads (served by public transportation), chemical emission sources, factories emitting smoke, and public places or large garbage disposal areas with high noise levels
Maternal nutrient supplement and serum biomarkers of the study subjects
The percentage of individuals regularly taking FA supplements was similar between the case and control groups. However, the proportion of individuals regularly supplementing with multivitamins was significantly lower in the case group (26.4% vs. 44.2%, P = 0.039).
In terms of serum nutrient biomarkers, no significant difference was observed in serum folate levels between the two groups. However, vitamin B12 levels were significantly higher in the control group [median (IQR): 284 (139.5) pmol/L] compared to the case group [253 (108.5) pmol/L] (P = 0.029). Serum HCY concentrations were notably elevated in the case group [median (IQR): 6.44 (3.3) µmol/L] compared to the control group [4.5 (3.0) µmol/L], with a highly significant difference (P < 0.001) (Table 2).
Table 2.
Maternal nutrient supplement and serum biomarkers of the study subjects
| Control (N = 77) n (%) or median (interquartile) |
Case (N = 53) n (%) or median (interquartile) |
P value | |
|---|---|---|---|
| Nutrient supplement | |||
| FA supplementation | 0.939a | ||
| Irregularly | 30(39.0) | 21(39.6) | |
| Regularly | 47(61.0) | 32(60.4) | |
| Multivitamin supplementation | 0.039a | ||
| Irregularly | 43(55.8) | 39(73.6) | |
| Regularly | 34(44.2) | 14(26.4) | |
| Nutrient serum biomarkers | |||
| Folate (nmol/L) | 29.3(13.3) | 33.9(15.8) | 0.113b |
| Vitamin B12 (pmol/L) | 284.0(139.5) | 253.0(108.5) | 0.029b |
| Homocysteine (µmol/L) | 4.5(3.0) | 6.44(3.3) | <0.001b |
a: P value was calculated by chi-squared test; b: P value was calculated by Mann–Whitney U-test
Further analysis of the effects of nutrient supplementation on serum nutrient biomarkers (Table S1) revealed that regular multivitamin supplementation was associated with significantly higher serum folate levels and lower HCY levels compared to irregular supplementation. FA supplementation did not show significant differences in these biomarkers.
Maternal exposure to indoor air pollution during pregnancy
In this study, the highest TVOCs levels were significantly higher in the case group (7.02 mg/m³) compared to the control group (1.85 mg/m³). The median (IQR) TVOCs level in cases was 0.400 (0.98) mg/m³, which is significantly higher than in controls [0.005 (0.01) mg/m³, P < 0.001] (Table 3). According to the Chinese National Standard GB/T 18,883–2022 [15], the limits are 0.60 mg/m³ for TVOCs, 0.08 mg/m³ for formaldehyde, 0.03 mg/m³ for benzene, 0.20 mg/m³ for toluene, and 0.20 mg/m³ for xylene. For TVOCs, benzene, toluene and xylenes, in a sample of 130, approximately 23%–46% of the samples exceeded the MDL. For formaldehyde, over 90% of the samples had detection values that exceeded the MDL. A significantly higher proportion of cases (37.7%) exceeded the TVOCs reference limit compared to controls (5.2%) (P < 0.001).
Table 3.
Indoor air pollutions measurements in the case and control groups
| Control(N = 77) | Case(N = 53) | P value | |
|---|---|---|---|
| TVOCsc | |||
| Median(IQR) (mg/m3) | 0.005(0.01) | 0.40(0.98) | |
| Range (mg/m3) | 0.005–1.85 | 0.005–7.02 | |
| Number at or above the MDL[n(%)] | 20[28.6%] | 41[77.4%] | |
| Number over reference limit [n(%)] | 4[5.2%] | 20[37.7%] | <0.001a |
| Benzenec | |||
| Median (IQR) (mg/m3) | 0.005(0) | 0.005(0.06) | |
| Range (mg/m3) | 0.005-1.0 | 0.005-1.0 | |
| Number at or above the MDL[n(%)] | 9[11.7%] | 22[41.5%] | |
| Number over reference limit[n(%)] | 9[11.7%] | 22[41.5%] | <0.001a |
| Toluenec | |||
| Median (IQR) (mg/m3) | 0.005(0) | 0.005(0.20) | |
| Range (mg/m3) | 0.005-0.6 | 0.005-1.0 | |
| Number at or above the MDL[n(%)] | 11[14.3%] | 24[45.3%] | |
| Number over reference limit [n(%)] | 3[3.9%] | 2[3.8%] | 1a |
| Xylenec | |||
| Median (IQR) (mg/m3) | 0.005(0) | 0.005(0.16) | |
| Range (mg/m3) | 0.005-0.6 | 0.005-1.4 | |
| Number at or above the MDL[n(%)] | 10[13.0%] | 22[41.5%] | |
| Number over reference limit [n(%)] | 5[6.5%] | 2[3.8%] | 0.7a |
| Formaldehydec | |||
| Median (IQR) (mg/m3) | 0.02(0.033) | 0.01(0.015) | |
| Range (mg/m3) | 0.005–0.79 | 0.005–0.609 | |
| Number at or above the MDL[n(%)] | 72[93.5%] | 52[98.1%] | |
| Number over reference limit [n(%)] | 11[14.3%] | 7[13.2%] | 0.86 a |
| Temperature | |||
| Median (IQR) (℃) | 28.7(5) | 25(6) | <0.001b |
| Humidness | |||
| Median (IQR) (%) | 47(15) | 38(10.5) | 0.002b |
a: P value was calculated by Chi-square analysis or Fisher exact tests. b: P value was calculated by Mann–Whitney U-test. c: TVOCs, Benzene, toluene and xylene and formaldehyde concentrations below the method of detection limit (MDL) of 0.01mg/m3 were replaced with 0.005 mg/m3 in all analyses
Formaldehyde and BTX concentrations were generally low in both groups, with median (IQR) values nearly zero. However, benzene levels above the reference limit were more common in the case group (41.5%) compared to the control group (11.7%) (P < 0.001).
Additionally, the control group had higher average temperature [28.7 °C vs. 25.0 °C, P < 0.001] and humidity [47.8% vs. 38%, P = 0.002]. Despite these conditions, which are known to increase VOCs concentrations with higher temperature and humidity, the case group still exhibited significantly higher TVOCs levels, suggesting that under similar environmental conditions, the case group’s exposure would be even more pronounced [16, 17].
Correlation analysis of serum nutrient biomarkers and TVOCs concentrations
Table S2 showed the correlation between serum folate, vitamin B12, HCY, and TVOCs concentrations. The analysis revealed a negative correlation between serum vitamin B12 and HCY, which aligns with previous research findings. Notably, there was a positive correlation (r = 0.263) between TVOCs concentration and serum HCY levels. Both TVOCs and serum HCY have been identified as risk factors for CHD in our study. This interaction further highlights the necessity of monitoring nutrient status in pregnant populations exposed to high levels of indoor pollutants.
Associations between nutrient supplementation, serum biomarkers, TVOCs exposure levels, and CHD
This study investigated the associations between nutrient supplementation, serum biomarkers, TVOCs exposure levels, and CHD (Table 4). Regular multivitamin use appeared to reduce CHD risk (COR = 0.45, 95% CI: 0.21–0.97), but this association was not statistically significant after adjusting for confounders (AOR = 0.58, 95% CI: 0.26–1.30). High serum HCY levels (> 6.125 µmol/L) were linked to an increased CHD risk (AOR = 2.98, 95% CI: 1.31–6.36). Elevated TVOCs (> 0.0165 mg/m³) and benzene (> 0.01 mg/m³) exposures significantly increased CHD risk (AOR = 9.23, 95% CI: 3.78–22.53 and AOR = 4.56, 95% CI: 1.75–11.89, respectively). Notably, high serum folate (≥ 34.55 nmol/L) was identified as a risk factor for CHD (AOR = 3.03, 95% CI: 1.33–6.88), contrary to previous studies suggesting a protective role, indicating the need for further research.
Table 4.
Associations between nutrient supplement, serum biomarkers and air pollutants exposure levels and CHD
| Controls N(%) |
Cases N(%) |
COR | AOR# | |
|---|---|---|---|---|
| Nutrient supplement | ||||
| FA | ||||
| Irregularly | 30(39.0) | 21(39.6) | Reference | Reference |
| Regularly | 47(61.0) | 32(60.4) | 0.97(0.48–1.99) | 1.13(0.52–2.44) |
| Multivitamin | ||||
| Irregularly | 43(55.8) | 39(73.6) | Reference | Reference |
| Regularly | 34(44.2) | 14(26.4) | 0.45(0.21–0.97)* | 0.58(0.26–1.30) |
| Serum biomarkers a | ||||
| Folate | ||||
| Low(≤ 34.55 nmol/L) | 58(75.3) | 21(39.6) | Reference | Reference |
| High(>34.55 nmol/L) | 19(24.7) | 32(60.4) | 2.53(1.20–5.34)* | 3.03(1.33–6.88)** |
| Vitamin B12 | ||||
| Low(≤ 370 pmol/L) | 58(75.3) | 44(83.0) | Reference | Reference |
| High(>370 pmol/L) | 19(24.7) | 9(17.0) | 0.62(0.26–1.51) | 0.79(0.31–2.07) |
| Homocysteine | ||||
| Low(≤ 6.125µmol/L) | 58(75.3) | 24(45.3) | Reference | Reference |
| High(>6.125µmol/L) | 19(24.7) | 29(54.7) | 3.69(1.70–7.80)** | 2.98(1.31–6.36)** |
| Indoor air pollution | ||||
| TVOCsa | ||||
| Low(≤ 0.0165 mg/m3) | 58(75.3) | 12(22.6) | Reference | Reference |
| High(>0.0165 mg/m3) | 19(24.7) | 41(77.4) | 10.43(4.57–23.82)*** | 9.23(3.78–22.53)*** |
| Benzeneb | ||||
| Low(≤ 0.01 mg/m3) | 68(88.3) | 31(58.5) | Reference | Reference |
| High(>0.01 mg/m3) | 9(11.7) | 22(41.5) | 5.36(2.22–12.98)*** | 4.56(1.75–11.89)** |
#Adjusted for maternal age, maternal education level, paternal education level, and residence. *Significant differences (p < 0.05) between the case and control groups are indicated. ** Significant differences (p < 0.01) between the case and control groups are indicated. *** Significant differences (p < 0.001) between the case and control groups are indicated. a: Serum folate, Vitamin B12, homocysteine and TVOCs exposure levels were categorized into low and high concentrations based on the 75th percentile of the control group. b: Benzene exposure levels were categorized into low and high concentrations based on the MDL
Given the correlations between HCY, vitamin B12, and TVOCs, as shown in Table S3, we conducted a sensitivity analysis where models were co-adjusted for these three variables. The results showed that after adjusting for confounding factors, high levels of TVOCs exposure still significantly increased the risk of CHD, but the risk associated with homocysteine was no longer statistically significant.
Effect of CHD in relation to TVOCs exposure and serum HCY levels in a stratified analysis of nutrient supplementation
Building upon the established correlation between TVOCs, serum HCY biomarkers, and CHD, we further analyzed whether the AOR of TVOCs and serum HCY in relation to CHD would be strengthened or weakened under irregular and regular nutrient supplementation.
As shown in Table 5, the adverse effect of high HCY levels on CHD was somewhat attenuated in the group with regular FA supplementation compared to the irregular supplementation group, with AOR values of 7.65 (95% CI: 1.79–32.66) and 2.90 (95% CI: 0.97–8.64), respectively. However, regular FA supplementation did not show a significant protective effect against high TVOCs exposure.
Table 5.
Effect of CHD in relation to TVOC exposure and serum HCY levels in a stratified analysis of nutrient supplementation
| Folic acid supplementation | Multivitamin supplementation | |||||||
|---|---|---|---|---|---|---|---|---|
| Irregularly(N = 51) | Regularly(N = 79) | Irregularly(N = 82) | Regularly(N = 48) | |||||
|
N, controls/cases |
AOR# |
N, controls/cases |
AOR# |
N, controls/cases |
AOR# |
N, controls/cases |
AOR# | |
| Serum Homocysteine | ||||||||
| Low(≤ 6.125µmol/L) | 21/6 | Reference | 37/18 | Reference | 31/15 | Reference | 27/9 | Reference |
| High(>6.125µmol/L) | 9/15 | 7.65(1.79–32.66)** | 10/14 | 2.90(0.97–8.64) | 12/24 | 3.63(1.33–9.93)* | 7/5 | 3.35(0.70-15.94) |
| TVOCs | ||||||||
| Low(≤ 0.0165 mg/m3) | 22/6 | Reference | 36/6 | Reference | 33/7 | Reference | 25/5 | Reference |
| High(>0.0165 mg/m3) | 8/15 | 11.95(2.68–53.39)** | 11/26 | 15.23(4.06–53.70)*** | 10/32 | 17.87(5.11–62.53)*** | 9/9 | 5.99(1.14–31.49)* |
# Adjusted for maternal age, maternal education level, paternal education level, and residence. * Significant differences (p < 0.05) between the low and high groups are indicated. ** Significant differences (p < 0.01) between the low and high groups are indicated. *** Significant differences (p < 0.001) between the low and high groups are indicate
In participants with irregular multivitamin supplementation, the AOR for high TVOCs exposure and CHD was estimated to be 17.87 (95% CI: 5.11–62.53). This risk was lower in participants with regular multivitamin supplementation, with an AOR of 5.99 (95% CI: 1.14–31.49).
In Table S4, we adjusted for relevant factors, and the results were similar to those in Table 5. Regular folic acid (FA) supplementation did not show a significant protective effect against high TVOCs exposure, while regular multivitamin supplementation reduced the risk of CHD associated with high TVOCs exposure.
These results indicate that both high serum HCY levels and high TVOCs exposure are significant risk factors for CHD, and the risks are particularly pronounced in individuals with irregular nutrient supplementation. Regular supplementation with FA and multivitamins appears to mitigate some of the risks associated with high HCY levels and TVOCs exposure, though not entirely. This underscores the importance of consistent nutrient supplementation in populations exposed to high levels of indoor air pollutants.
Discussion
Adverse effects of TVOCs on fetal CHD
Volatile organic compounds (VOCs) encompass a wide range of chemicals known for their carcinogenic, irritant, and toxic properties. Indoor environments, particularly new or recently renovated homes, often have higher concentrations of VOCs than outdoor air due to numerous sources of emissions, such as paints, adhesives, and building materials, coupled with lower ventilation rates and prolonged indoor occupancy[ [18–20]. Notably, two case-control studies in China highlighted a significant increase in the risk of cardiac defects in offspring when pregnant women moved into newly decorated homes shortly before or during the early stages of pregnancy [21, 22].
Despite the recognized sources and potential impacts of VOCs, our study did not find a significant difference in the history of home decoration between the case and control groups. This suggests that long-term exposure to products and materials commonly found in homes, ranging from building materials to daily use products like personal care and cleaning agents [23], may contribute to TVOCs levels indoors.
We reported indoor TVOCs concentrations ranging from 0.005 to 7.02 mg/m3, with a median concentration of 0.40 mg/m³ in cases and 0.005 mg/m³ in controls. For the target VOCs, such as formaldehyde and BTX, concentrations in most houses were below the MDLs in our study. A study calculated the concentrations of median benzene, toluene, and xylenes in over 17,000 residences from 2000 to 2021 using a systematic review method, with the results being 24.2 µg/m3, 40.9 µg/m3, 49.9 µg/m3, respectively [24]. According to the Chinese National Air Quality Standards GB/T 18,883 − 2022 [15], the rates of exceeding the current reference value in both groups were below 20% for most indoor air pollutants except for TVOCs and benzene in the case group (37.7% and 41.5%, respectively). Although the maternal exposure levels to indoor air pollutants in our study were generally lower than the previous study of the general population and fell below the Chinese national standard reference values, our findings suggest that the risk of CHD in offspring may still be linked to maternal exposure to high levels of TVOCs and benzene, even at low concentrations.
Prenatal exposure to high levels of TVOCs has been previously associated with adverse outcomes, including impaired mental and psychomotor development and reduced postnatal growth rates in children [5]. While research on TVOCs exposure and CHD is limited, our findings, alongside prior studies [7], suggest a strong association between high TVOCs levels during early pregnancy and an increased risk of CHD in offspring, reaffirming the need for heightened awareness and mitigation strategies to protect maternal and fetal health. Our current study further validates the link between high TVOCs exposure level and fetal CHD, revealing a more than ninefold increase in risk compared with low level (AOR: 9.23(3.78–22.53), thus highlighting the critical need for continued research and preventive measures in managing indoor air quality to safeguard against developmental risks in fetuses.
Relationship between nutrient supplementation, serum biomarkers and CHD
Folic acid, a crucial vitamin in nucleotide synthesis, amino acid conversion, and methylation, is widely recognized for its role in preventing early embryonic developmental abnormalities and birth defects [25]. In a periconceptional study conducted in the same region of China, the average serum folic acid concentration measured in pregnant women was 20.3 nmol/L [26], and the serum folic acid concentration we measured was slightly higher than that in this study(case 33.9 nmol/L, control 29.3nmol/L). However, in another study conducted in other regions of China, the average serum concentrations of folic acid for pregnant women who were supplemented and not supplemented with folic acid were 17.1 ng/ml(38.7nmol/L) and 14.7 ng/ml(33.3nmol/L) [27], respectively, which is slightly higher than in our study (regularly 31.5 nmol/L, irregularly 29.3 nmol/L). Possible reasons include regional differences, dietary habits, and varying health perceptions.
Research indicates that multivitamin supplements, particularly those enriched with vitamins D, B, and folic acid, are implicated in potentially lowering the risk of CHD in offspring, underscoring the critical nature of optimal maternal vitamin D levels during pregnancy [28, 29]. The collective evidence supports the beneficial impact of comprehensive prenatal multivitamin supplementation on mitigating CHD risk, with a specific emphasis on the importance of vitamins D and B, alongside folic acid, in prenatal nutrition strategies [11, 30, 31].
Our findings align with this perspective, demonstrating a significant reduction in CHD risk associated with regular multivitamin use (COR = 0.45, 95% CI: 0.21–0.97). Conversely, our study revealed no direct correlation between FA supplementation and CHD, suggesting that the impact of nutrient supplementation on CHD is complex. It appears that multivitamin supplementation, which includes a variety of essential vitamins and minerals, offers greater benefits in reducing CHD risk compared to folic acid alone. This highlights the superior protective effect of comprehensive multivitamins over single-nutrient supplementation in mitigating CHD risk.
The one-carbon metabolism pathway offers insight into how deficiencies in folic acid or vitamin B12 can lead to elevated HCY levels, which is a known risk factor for CHD[ [32–34]. Our research corroborates this, showing a negative correlation between serum HCY and vitamin B12 levels and identifying elevated maternal serum HCY as a significant risk factor for CHD (COR = 3.69, 95% CI: 1.70–7.80; AOR = 2.98, 95% CI: 1.31–6.36).
The relationship between nutrient supplementation and serum nutrient biomarkers is complex, involving intricate metabolic reactions in vivo. Our study revealed that regular multivitamin supplementation was associated with significantly higher serum folate levels and lower HCY levels compared to irregular supplementation. However, FA supplementation did not show significant differences in serum nutrient biomarkers. Moreover, the associations between nutrient supplementation, serum nutrient biomarkers, and CHD are influenced by various confounding factors. In our research, the adverse effect of high HCY levels on CHD was somewhat attenuated in the group with regular FA supplementation compared to the irregular supplementation group. Interestingly, our results also indicated that high levels of serum folate might increase the AOR for CHD compared to low levels. This deviation suggests that the protective effects of folic acid on CHD might be influenced by complex factors such as air pollutant exposure, maternal dietary habits, and the transient nature of serum folate concentrations.
Briefly, while our study reinforces the protective role of comprehensive multivitamin supplementation against CHD and highlights the adverse effect of high serum HCY levels on CHD, it also underscores the complex interplay between nutrient supplementation, serum biomarkers, and CHD risk. The role of FA supplementation remains less clear, emphasizing the importance of comprehensive prenatal nutrition and the need for further research to understand the underlying mechanisms and optimize strategies for preventing CHD in populations exposed to high levels of indoor air pollutants.
Nutrient supplementation modified the effect of TVOCs on CHD
Previous research has suggested that maternal exposure to indoor TVOCs may increase the risk of giving birth to a fetus with CHD. However, few studies have investigated whether nutrient supplementation can modulate the relationship between air pollution exposure and CHD, particularly with TVOCs. In other research areas, one study found that an intake of supplemental folate of ≥ 800 µg/day appeared to attenuate the adverse association between estimated NO2 exposure and the probability of live birth following assisted reproductive technology [35]. Additionally, research analyzing children’s IQ and maternal serum folate levels during mid-pregnancy suggested that maternal folate might mitigate the neurotoxic effects of prenatal air pollution [36]. The potential molecular mechanism may involve exposure to high concentrations of air pollutants leading to increased DNA methylation levels [37, 38], which can be improved with dietary modifications, including folate and vitamin B [39].
Our study found that TVOCs concentration was positively correlated with serum HCY concentration, and regular multivitamin supplementation reduced the AOR of high TVOCs for CHD risk. We hypothesize that the effect of high TVOCs on cardiac development may be moderated by increasing serum HCY concentration, and that multivitamin supplementation can reduce HCY accumulation in the body. Further studies in laboratory cells or animals are needed to confirm this hypothesis.
However, we did not find that regular FA supplementation significantly attenuated the impact of high TVOCs exposure on CHD risk. Nonetheless, the adverse effect of high HCY levels on CHD was somewhat attenuated in the group with regular FA supplementation compared to the irregular supplementation group. As previously discussed in the section on the relationship between FA supplementation, serum folate levels, and CHD, the impact of FA supplementation on TVOCs-related CHD risk must consider numerous confounding factors.
In summary, our study supports the beneficial effect of multivitamin supplementation in mitigating the cardiac developmental toxicity associated with prenatal TVOCs exposure. The role of FA supplementation in reducing the adverse effects of TVOCs on cardiac development remains less clear. Further research is needed to explore the mechanisms of nutrient supplementation on air pollution-induced developmental toxicity and their metabolic pathways.
Strengths and limitations of this study
This study has several strengths. Unlike traditional exposure assessments using urban air monitoring stations and residential addresses, our study employed a door-to-door air sample collection method, providing a more accurate reflection of individual exposure to air pollutants. Additionally, the relationship between TVOCs and CHD was explored through a comprehensive approach, incorporating questionnaires (nutrient supplementation), blood sample analysis, and air pollutant exposure assessment. This multi-faceted design allowed for a robust analysis and offered a theoretical basis for understanding the correlation between maternal nutrient supplementation, household air pollutant exposure, and CHD in offspring. The combination of questionnaires, blood sample analysis, and field investigations enhances the credibility and robustness of the findings, ensuring a well-rounded approach to data collection and analysis.
However, several limitations should be considered when interpreting our results. The door-to-door detection and blood sample collection methods required significant effort and resources, resulting in a small sample size. This limitation led to wider confidence intervals and limited statistical power, although the current sample size was deemed sufficient based on sample size calculations. Air samples were collected at 22–30 gestational weeks, after the critical cardiac development period, possibly leading to nondifferential misclassification and reduced exposure assessment accuracy. Despite potential inaccuracies due to indoor air pollution volatility, the study’s focus on unaltered lifestyles and stable indoor living environments aimed to reflect relatively quantifiable exposure. Additionally, the lack of specific dosage information for FA and multivitamin supplementation could lead to misclassification and inaccurate assessments of their effects on CHD risk. However, standardized questionnaires and data collection by trained researchers helped mitigate this limitation by accurately defining and isolating FA and multivitamin use.
Conclusions
This study highlights significant associations between household TVOCs exposure, maternal serum HCY levels, and CHD risk in offspring. Regular multivitamin supplementation was shown to reduce the risk of CHD, particularly by mitigating the adverse effects of high TVOCs exposure, while the role of FA supplementation remains less clear and warrants further investigation. These findings suggest that multivitamins, containing various essential vitamins and minerals, offer greater protective benefits against CHD compared to FA alone. Emphasizing comprehensive prenatal nutrition and targeted interventions to reduce indoor air pollution exposure during pregnancy is crucial for protecting maternal and fetal health.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by funding from a Multi-center Clinical Research Project of Shanghai Jiao Tong University School of Medicine (grant number: DLY201609) and National Natural Science Foundation of China (grant number:82300344). The authors are also grateful to all families who participated in this research.
Author contributions
X.R. contributed to the methodology, investigation, and writing of the original draft. W.S. contributed to the investigation. J.L. and Z.L. assisted with methodology, investigation, validation, and software. J.Y. was involved in the conceptualization and supervision of the study. J.C. and Y.W. participated in the investigation and project administration. K.S. and J.S. contributed to the conception and design of the study, revised the manuscript critically for important intellectual content, and provided final approval of the version to be published. All authors reviewed the manuscript.
Funding
Multi-center Clinical Research Project of Shanghai Jiao Tong University School of Medicine, grant/ award number: DLY201609. National Natural Science Foundation of China (grant/ award number: 82300344).
Data availability
The data that support the findings of this study are available on request from the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xuehua Ruan and Wenyuan Shang contributed equally to this work.
Contributor Information
Kun Sun, Email: sunkun@xinhuamed.com.cn.
Jing Sun, Email: sunjing02@xinhuamed.com.cn.
References
- 1.GBD 2017 Congenital Heart Disease Collaborators. Global, regional, and national burden of congenital heart disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Child Adolesc Health. 2020;4:185–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Mil NH, Oosterbaan AM, Steegers-Theunissen RP. Teratogenicity and underlying mechanisms of homocysteine in animal models: a review. Reprod Toxicol. 2010;30:520–31. [DOI] [PubMed] [Google Scholar]
- 3.Nicoll R. Environmental Contaminants and Congenital Heart Defects: A Re-Evaluation of the Evidence. Int J Environ Res Public Health. 2018;15:2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ji W, Wang Y, Zhao B, Liu J. Identifying high-risk volatile organic compounds in residences of Chinese megacities: A comprehensive health-risk assessment. J Hazard Mater. 2024;479:135630. [DOI] [PubMed] [Google Scholar]
- 5.Chang M, Park H, Ha M, Hong YC, Lim YH, Kim Y, et al. The effect of prenatal TVOC exposure on birth and infantile weight: the Mothers and Children’s Environmental Health study. Pediatr Res. 2017;82:423–8. [DOI] [PubMed] [Google Scholar]
- 6.Swartz MD, Cai Y, Chan W, Symanski E, Mitchell LE, Danysh HE, et al. Air toxics and birth defects: a Bayesian hierarchical approach to evaluate multiple pollutants and spina bifida. Environ Health. 2015;14:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun J, Wang J, Yang J, Shi X, Li S, Cheng J, et al. Association between maternal exposure to indoor air pollution and offspring congenital heart disease: a case-control study in East China. BMC Public Health. 2022;22:767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Z, Lu J, Ruan X, Wu Y, Zhao J, Jiao X, et al. Exposure to volatile organic compounds induces cardiovascular toxicity that may involve DNA methylation. Toxicology. 2024;501:153705. [DOI] [PubMed] [Google Scholar]
- 9.Lyon P, Strippoli V, Fang B, Cimmino L. B Vitamins and One-Carbon Metabolism: Implications in Human Health and Disease. Nutrients. 2020;12:2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ. 2002;325:1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qu Y, Lin S, Zhuang J, Bloom MS, Smith M, Nie Z, et al. First-Trimester Maternal Folic Acid Supplementation Reduced Risks of Severe and Most Congenital Heart Diseases in Offspring: A Large Case-Control Study. J Am Heart Assoc. 2020;9:e015652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D, Jin L, Zhang J, Meng W, Ren A, Jin L. Maternal Periconceptional Folic Acid Supplementation and Risk for Fetal Congenital Heart Defects. J Pediatr. 2022;240:72–8. [DOI] [PubMed] [Google Scholar]
- 13.Elizabeth KE, Praveen SL, Preethi NR, Jissa VT, Pillai MR. Folate, vitamin B12, homocysteine and polymorphisms in folate metabolizing genes in children with congenital heart disease and their mothers. Eur J Clin Nutr. 2017;71:1437–41. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Yan M, Duan H. Characteristics of Indoor Volatile Organic Compounds in Urban Residential Microenvironments in China. Res Environ Sci. 2012;25:1077–84. [Google Scholar]
- 15.Chinese National Disease Control and Prevention Administration. GB/T 18883 – 2022: Indoor Air Quality Standard. [https://www.ndcpa.gov.cn/jbkzzx/c100201/common/content/content_1666357812062392320.html] access on September 30, 2024.
- 16.Liu Z, Howard-Reed C, Cox SS, Ye W, Little JC. Diffusion-controlled reference material for VOC emissions testing: effect of temperature and humidity. Indoor Air. 2014;24:283–91. [DOI] [PubMed] [Google Scholar]
- 17.Markowicz P, Larsson L. Influence of relative humidity on VOC concentrations in indoor air. Environ Sci Pollut Res Int. 2015;22:5772–9. [DOI] [PubMed] [Google Scholar]
- 18.Park JS, Ikeda K. Variations of formaldehyde and VOC levels during 3 years in new and older homes. Indoor Air. 2006;16:129–35. [DOI] [PubMed] [Google Scholar]
- 19.Brown SK. Volatile organic pollutants in new and established buildings in Melbourne, Australia. Indoor Air. 2002;12:55–63. [DOI] [PubMed] [Google Scholar]
- 20.Mečiarová Ľ, Vilčeková S, Burdová EK, Kiselák J. Factors Effecting the Total Volatile Organic Compound (TVOC) Concentrations in Slovak Households. Int J Environ Res Public Health. 2017;14:1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qu P, Zhao D, Yan M, Liu D, Zhang R, Li S, et al. Maternal exposure to housing renovation during the periconceptional period and the risk of offspring with isolated congenital heart disease: a case-control study. Environ Health. 2023;22:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Z, Li X, Li N, Li S, Deng K, Lin Y, et al. Association between maternal exposure to housing renovation and offspring with congenital heart disease: a multi-hospital case-control study. Environ Health. 2013;12:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norris C, Fang L, Barkjohn KK, Carlson D, Zhang Y, Mo J, et al. Sources of volatile organic compounds in suburban homes in Shanghai, China, and the impact of air filtration on compound concentrations. Chemosphere. 2019;231:256–68. [DOI] [PubMed] [Google Scholar]
- 24.Liu N, Bu Z, Liu W, Kan H, Zhao Z, Deng F, et al. Indoor exposure levels and risk assessment of volatile organic compounds in residences, schools, and offices in China from 2000 to 2021: A systematic review. Indoor Air. 2022;32(9):e13091. [DOI] [PubMed] [Google Scholar]
- 25.Dong J, Yin LL, Deng XD, Ji CY, Pan Q, Yang Z, et al. Initiation and duration of folic acid supplementation in preventing congenital malformations. BMC Med. 2023;21:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li M, Chen X, Zhang Y, Chen H, Wang D, Cao C, et al. Birth Defect Prevention Group. RBC Folate and Serum Folate, Vitamin B-12, and Homocysteine in Chinese Couples Prepregnancy in the Shanghai Preconception Cohort. J Nutr. 2022;152:1496–506. [DOI] [PubMed] [Google Scholar]
- 27.Qu Y, Liu X, Lin S, Bloom MS, Wang X, Li X, et al. Maternal Serum Folate During Pregnancy and Congenital Heart Disease in Offspring. JAMA Netw Open. 2024;7:10e2438747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang M, Lee D, Park H, Ha M, Hong YC, Kim Y, et al. Prenatal TVOCs exposure negatively influences postnatal neurobehavioral development. Sci Total Environ. 2018;618:977–81. [DOI] [PubMed] [Google Scholar]
- 29.Feng Y, Wang S, Chen R, Tong X, Wu Z, Mo X. Maternal folic acid supplementation and the risk of congenital heart defects in offspring: a meta-analysis of epidemiological observational studies. Sci Rep. 2015;5:8506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koster MPH, van Duijn L, Krul-Poel YHM, Laven JS, Helbing WA, Simsek S, et al. A compromised maternal vitamin D status is associated with congenital heart defects in offspring. Early Hum Dev. 2018;117:50–6. [DOI] [PubMed] [Google Scholar]
- 31.Liu S, Joseph KS, Luo W, León JA, Lisonkova S, Van den Hof M, et al. Effect of Folic Acid Food Fortification in Canada on Congenital Heart Disease Subtypes. Circulation. 2016;134:647–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang R, Guo L, Zhao D, Qu P, Dang S, Yan H. Maternal B-vitamin intake and B-vitamin supplementation during pregnancy in relation to neonatal congenital heart defects: a case-control study with propensity score matching. Eur J Clin Nutr. 2021;75:782–91. [DOI] [PubMed] [Google Scholar]
- 33.Kemse NG, Kale AA, Joshi SR. A combined supplementation of omega-3 fatty acids and micronutrients (folic acid, vitamin B12) reduces oxidative stress markers in a rat model of pregnancy induced hypertension. PLoS ONE. 2014;9:e111902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kapusta L, Haagmans ML, Steegers EA, Cuypers MH, Blom HJ, Eskes TK. Congenital heart defects and maternal derangement of homocysteine metabolism. J Pediatr. 1999;135:773–4. [DOI] [PubMed] [Google Scholar]
- 35.Hobbs CA, Cleves MA, Melnyk S, Zhao W, James SJ. Congenital heart defects and abnormal maternal biomarkers of methionine and homocysteine metabolism. Am J Clin Nutr. 2005;81:147–53. [DOI] [PubMed] [Google Scholar]
- 36.Gaskins AJ, Mínguez-Alarcón L, Fong KC, Abu Awad Y, Di Q, Chavarro JE, et al. Supplemental Folate and the Relationship Between Traffic-Related Air Pollution and Livebirth Among Women Undergoing Assisted Reproduction. Am J Epidemiol. 2019;188:1595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loftus CT, Hazlehurst MF, Szpiro AA, Ni Y, Tylavsky FA, Bush NR, et al. Prenatal air pollution and childhood IQ: Preliminary evidence of effect modification by folate. Environ Res. 2019;176:108505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Prins S, Koppen G, Jacobs G, Dons E, Van de Mieroop E, Nelen V, et al. Influence of ambient air pollution on global DNA methylation in healthy adults: a seasonal follow-up. Environ Int. 2013;59:418–24. [DOI] [PubMed] [Google Scholar]
- 39.Yadav S, Longkumer I, Garg PR, Joshi S, Rajkumari S, Devi NK, et al. Association of air pollution and homocysteine with global DNA methylation: A population-based study from North India. PLoS ONE. 2021;16:e0260860. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.