Abstract
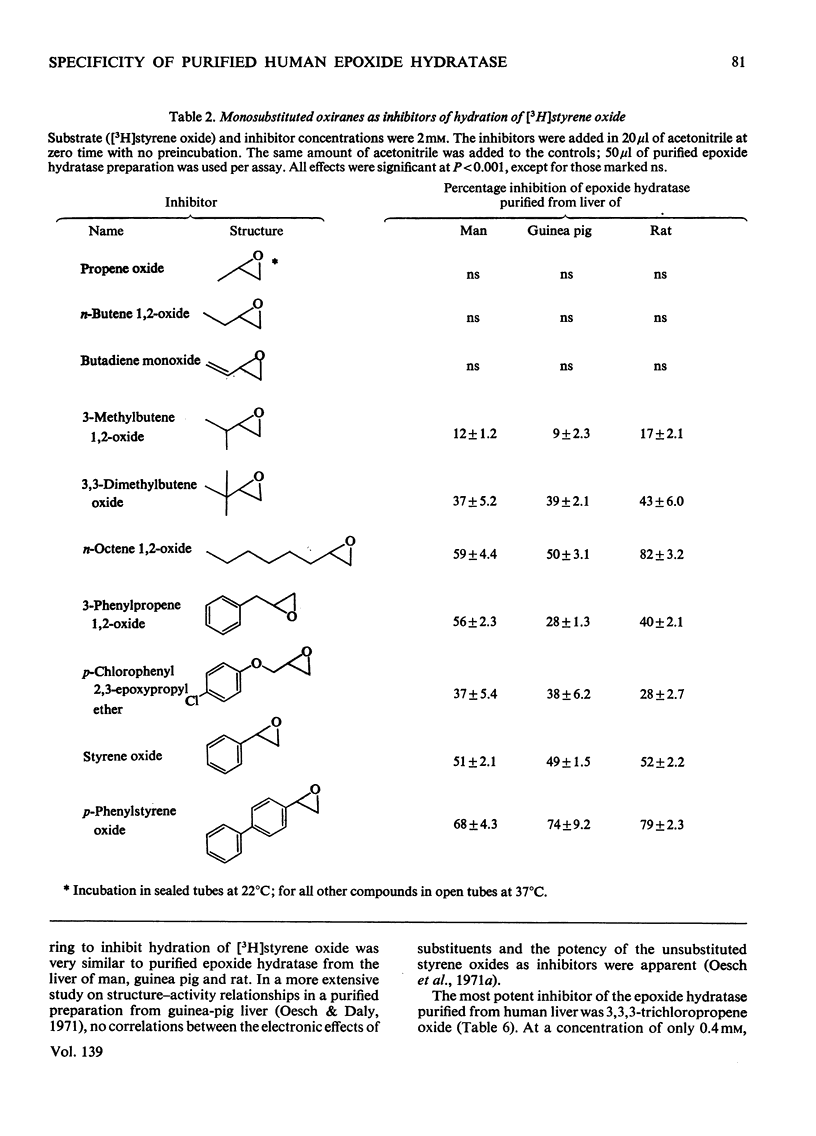
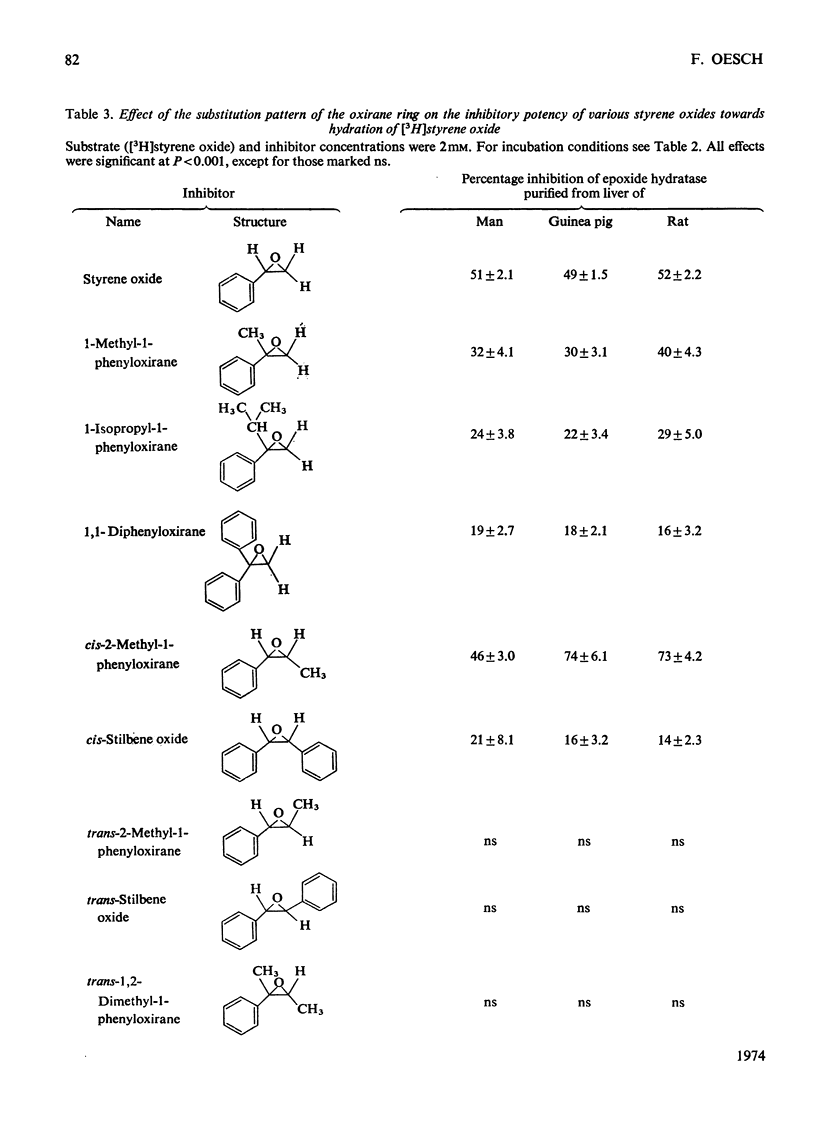
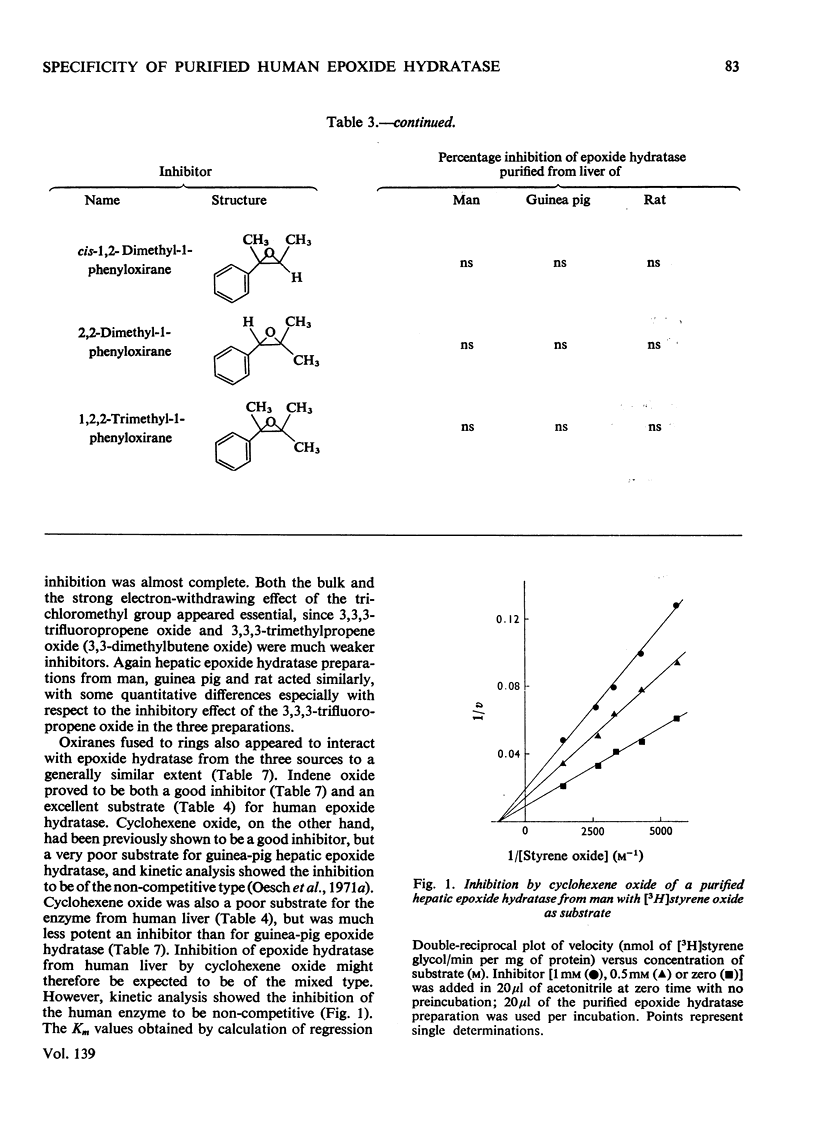
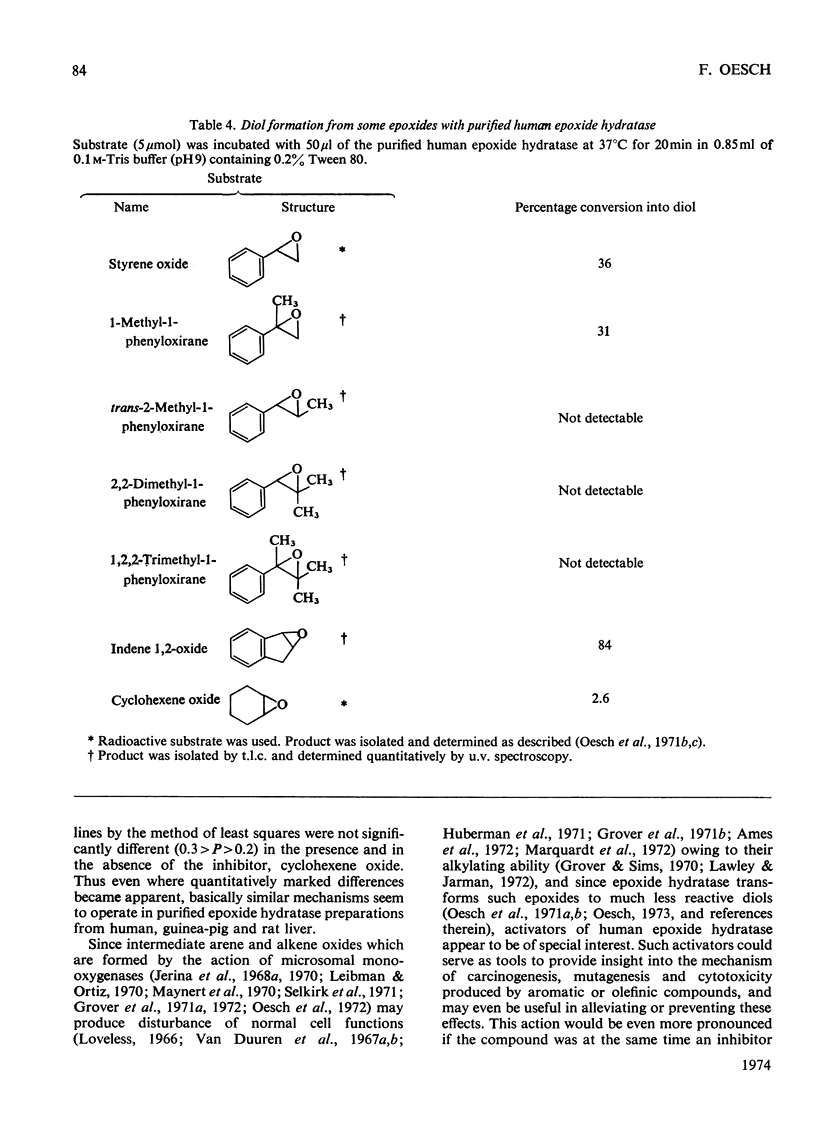
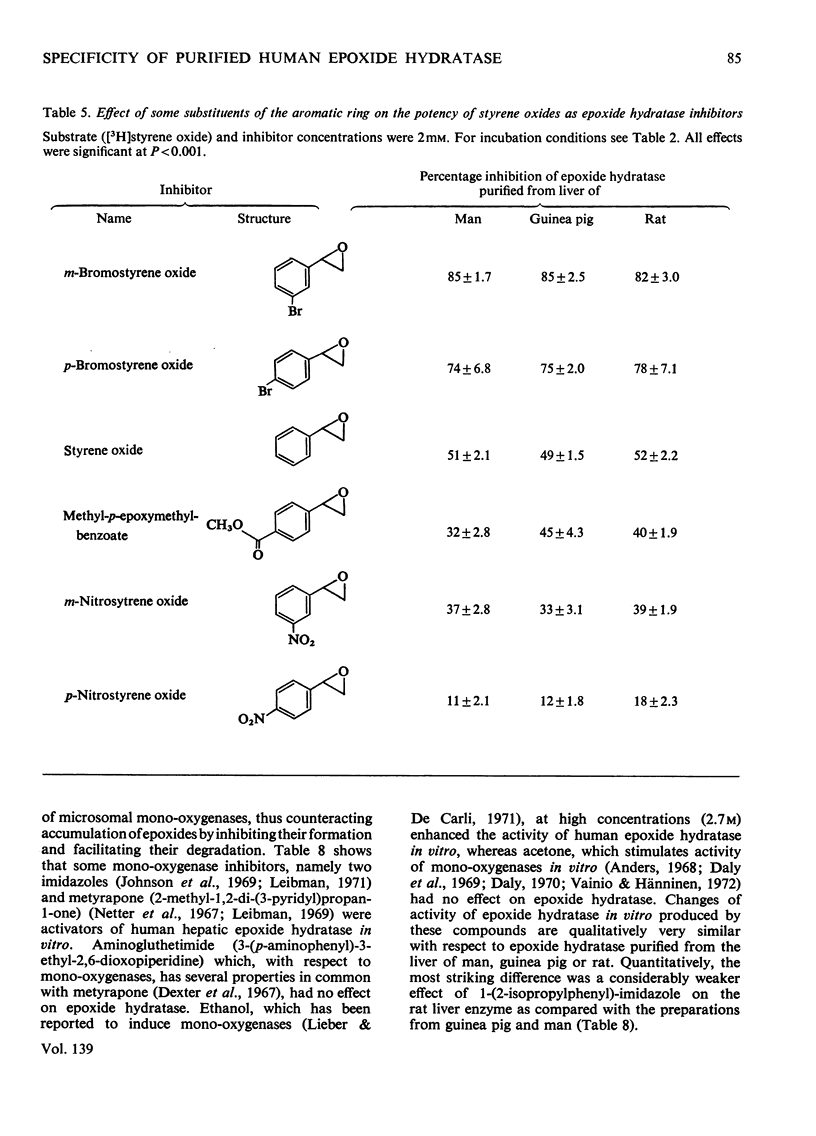
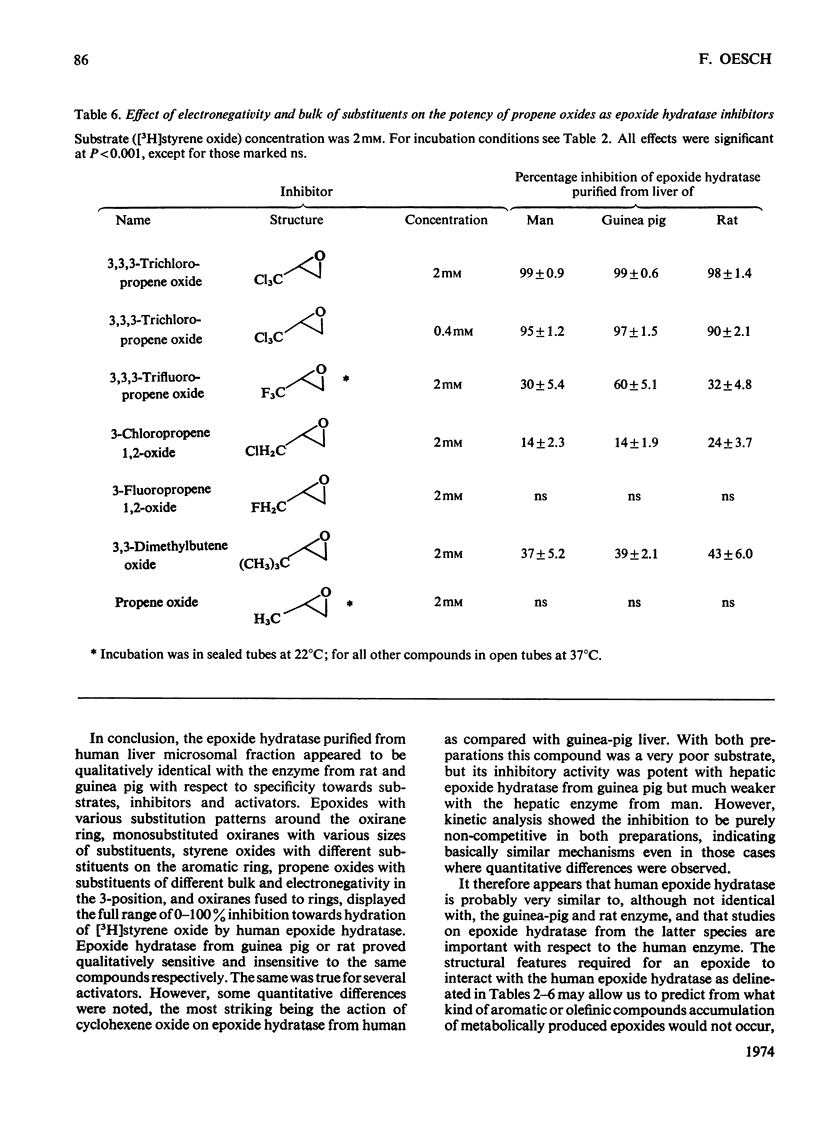
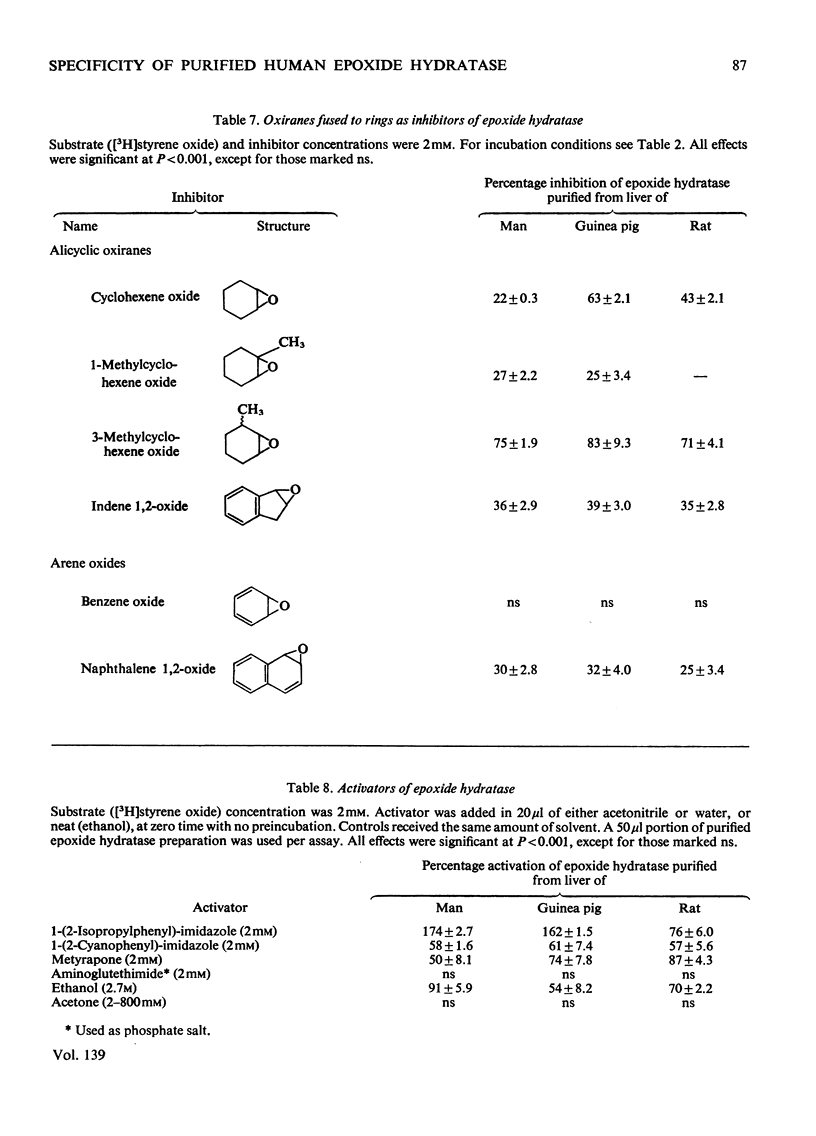
Epoxide hydratase was solubilized from human liver microsomal fractions and purified to an extent where the specific activity was 40-fold greater than that of the liver homogenate. Combination of homogenate and purified preparation showed that the increase in activity was not due to the removal of an inhibitor. Monosubstituted oxiranes with a lipophilic substituent larger than an ethyl group (isopropyl, t-butyl, n-hexyl, phenyl) readily interacted as substrates or inhibitors with this purified human epoxide hydratase, whereas those with a small substituent (methyl, ethyl, vinyl) were inactive, probably reflecting greater affinity of the former epoxides owing to lipophilic binding sites near the active site of the enzyme. In a series of oxiranes having a lipophilic substituent of sufficient size (styrene oxides), monosubstituted as well as 1,1- and cis-1,2-disubstituted oxiranes readily served as substrates or inhibitors of the enzyme, but not the trans-1,2-disubstituted, tri- or tetra-substituted oxiranes. trans-Substitution at the oxirane ring apparently prevents access of the oxirane ring to the active site by steric hindrance. Epoxide hydratase was also solubilized from microsomal fractions of rat and guinea-pig liver and purified by the same procedure. Structural requirements for effective interaction of substrates, inhibitors and activators were qualitatively identical for epoxide hydratase from the three sources. However, several quantitative differences were observed. Thus human hepatic epoxide hydratase seems to be very similar to, although not identical with, the enzyme from guinea pig or rat. Studies with epoxide hydratase from the latter two species therefore appear to be significant with respect to man. In addition, knowledge of structural requirements for epoxides to serve as substrates for human epoxide hydratase may prove useful for drug design. Compounds which need aromatic or olefinic moieties for their desired effect would not be expected to lead to accumulation of epoxides if their structure was such as to allow for a metabolically produced epoxide to be rapidly consumed by epoxide hydratase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Sims P., Grover P. L. Epoxides of carcinogenic polycyclic hydrocarbons are frameshift mutagens. Science. 1972 Apr 7;176(4030):47–49. doi: 10.1126/science.176.4030.47. [DOI] [PubMed] [Google Scholar]
- Anders M. W. Acetone enhancement of microsomal aniline para-hydroxylase activity. Arch Biochem Biophys. 1968 Jul;126(1):269–275. doi: 10.1016/0003-9861(68)90583-3. [DOI] [PubMed] [Google Scholar]
- Daly J. W. A simple radiometric assay for microsomal aryl hydroxylase activity. Anal Biochem. 1970 Feb;33(2):286–296. doi: 10.1016/0003-2697(70)90299-x. [DOI] [PubMed] [Google Scholar]
- Daly J., Jerina D., Farnsworth J., Guroff G. The migration of deuterium during aryl hydroxylation. II. Effect of induction of microsomal hydroxylases with phenobarbital or polycyclic aromatic hydrocarbons. Arch Biochem Biophys. 1969 Apr;131(1):238–244. doi: 10.1016/0003-9861(69)90127-1. [DOI] [PubMed] [Google Scholar]
- Dexter R. N., Fishman L. M., Ney R. L., Liddle G. W. An effect of adrenocorticotrophic hormone on adrenal cholesterol accumulation. Endocrinology. 1967 Nov;81(5):1185–1187. doi: 10.1210/endo-81-5-1185. [DOI] [PubMed] [Google Scholar]
- Grover P. L., Hewer A., Sims P. Epoxides as microsomal metabolites of polycyclic hydrocarbons. FEBS Lett. 1971 Oct 15;18(1):76–80. doi: 10.1016/0014-5793(71)80411-8. [DOI] [PubMed] [Google Scholar]
- Grover P. L., Hewer A., Sims P. Formation of K-region epoxides as microsomal metabolites of pyrene and benzo(a)pyrene. Biochem Pharmacol. 1972 Oct 15;21(20):2713–2726. doi: 10.1016/0006-2952(72)90020-2. [DOI] [PubMed] [Google Scholar]
- Grover P. L., Sims P., Huberman E., Marquardt H., Kuroki T., Heidelberger C. In vitro transformation of rodent cells by K-region derivatives of polycyclic hydrocarbons. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1098–1101. doi: 10.1073/pnas.68.6.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover P. L., Sims P. Interactions of the K-region epoxides of phenanthrene and dibenz (a,h)anthracene with nucleic acids and histone. Biochem Pharmacol. 1970 Jul;19(7):2251–2259. doi: 10.1016/0006-2952(70)90124-3. [DOI] [PubMed] [Google Scholar]
- Huberman E., Aspiras L., Heidelberger C., Grover P. L., Sims P. Mutagenicity to mammalian cells of epoxides and other derivatives of polycyclic hydrocarbons. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3195–3199. doi: 10.1073/pnas.68.12.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerina D. M., Daly J. W., Witkop B., Zaltzman-Nirenberg P., Udenfriend S. 1,2-naphthalene oxide as an intermediate in the microsomal hydroxylation of naphthalene. Biochemistry. 1970 Jan 6;9(1):147–156. doi: 10.1021/bi00803a019. [DOI] [PubMed] [Google Scholar]
- Jerina D. M., Daly J. W., Witkop B., Zaltzman-Nirenberg P., Udenfriend S. The role of arene oxide-oxepin systems in the metabolism of aromatic substrates. 3. Formation of 1,2-naphthalene oxide from naphthalene by liver microsomes. J Am Chem Soc. 1968 Nov 6;90(23):6525–6527. doi: 10.1021/ja01025a058. [DOI] [PubMed] [Google Scholar]
- Johnson A. L., Kauer J. C., Sharma D. C., Dorfman R. I. The synthesis of 1-arylimidazoles, a new class of steroid hydroxylation inhibitors. J Med Chem. 1969 Nov;12(6):1024–1028. doi: 10.1021/jm00306a013. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawley P. D., Jarman M. Alkylation by propylene oxide of deoxyribonucleic acid, adenine, guanosine and deoxyguanylic acid. Biochem J. 1972 Feb;126(4):893–900. doi: 10.1042/bj1260893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibman K. C. Effects of metyrapone on liver microsomal drug oxidations. Mol Pharmacol. 1969 Jan;5(1):1–9. [PubMed] [Google Scholar]
- Leibman K. C., Ortiz E. Epoxide intermediates in microsomal oxidation of olefins to glycols. J Pharmacol Exp Ther. 1970 Jun;173(2):242–246. [PubMed] [Google Scholar]
- Leibman K. C. Studies on modifiers of microsomal drug oxidation. Chem Biol Interact. 1971 Aug;3(4):289–290. doi: 10.1016/0009-2797(71)90064-0. [DOI] [PubMed] [Google Scholar]
- Lieber C. S., DeCarli L. M. Role of hepatic microsomal ethanol metabolism. Chem Biol Interact. 1971 Aug;3(4):292–293. doi: 10.1016/0009-2797(71)90066-4. [DOI] [PubMed] [Google Scholar]
- Marquardt H., Kuroki T., Huberman E., Selkirk J. K., Heidelberger C., Grover P. L., Sims P. Malignant transformation of cells derived from mouse prostate by epoxides and other derivatives of polycyclic hydrocarbons. Cancer Res. 1972 Apr;32(4):716–720. [PubMed] [Google Scholar]
- Maynert E. W., Foreman R. L., Watabe T. Epoxides as obligatory intermediates in the metabolism of olefins to glycols. J Biol Chem. 1970 Oct 25;245(20):5234–5238. [PubMed] [Google Scholar]
- Netter K. J., Jenner S., Kajuschke K. Uber die Wirkung von Metyrapon auf den mikrosomalen Arzneimittelabbau. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1967;259(1):1–16. [PubMed] [Google Scholar]
- Oesch E., Jerina D. M., Daly J. A radiometric assay for hepatic epoxide hydrase activity with [7-3H] styrene oxide. Biochim Biophys Acta. 1971 Mar 10;227(3):685–691. doi: 10.1016/0005-2744(71)90017-9. [DOI] [PubMed] [Google Scholar]
- Oesch F., Daly J. Conversion of naphthalene to trans-naphthalene dihydrodiol: evidence for the presence of a coupled aryl monooxygenase-epoxide hydrase system in hepatic microsomes. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1713–1720. doi: 10.1016/0006-291x(72)90807-8. [DOI] [PubMed] [Google Scholar]
- Oesch F., Daly J. Solubilization, purification, and properties of a hepatic epoxide hydrase. Biochim Biophys Acta. 1971 Mar 10;227(3):692–697. doi: 10.1016/0005-2744(71)90018-0. [DOI] [PubMed] [Google Scholar]
- Oesch F., Jerina D. M., Daly J. W., Lu A. Y., Kuntzman R., Conney A. H. A reconstituted microsomal enzyme system that converts naphthalene to trans-1,2-dihydroxy-1,2-dihydronaphthalene via naphthalene-1,2-oxide: presence of epoxide hydrase in cytochrome P-450 and P-448 fractions. Arch Biochem Biophys. 1972 Nov;153(1):62–67. doi: 10.1016/0003-9861(72)90420-1. [DOI] [PubMed] [Google Scholar]
- Oesch F., Jerina D. M., Daly J. W. Substrate specificity of hepatic epoxide hydrase in microsomes and in a purified preparation: evidence for homologous enzymes. Arch Biochem Biophys. 1971 May;144(1):253–261. doi: 10.1016/0003-9861(71)90476-0. [DOI] [PubMed] [Google Scholar]
- Oesch F., Kaubisch N., Jerina D. M., Daly J. W. Hepatic epoxide hydrase. Structure-activity relationships for substrates and inhibitors. Biochemistry. 1971 Dec 21;10(26):4858–4866. doi: 10.1021/bi00802a005. [DOI] [PubMed] [Google Scholar]
- Oesch F. Mammalian epoxide hydrases: inducible enzymes catalysing the inactivation of carcinogenic and cytotoxic metabolites derived from aromatic and olefinic compounds. Xenobiotica. 1973 May;3(5):305–340. doi: 10.3109/00498257309151525. [DOI] [PubMed] [Google Scholar]
- Selkirk J. K., Huberman E., Heidelberger C. An epoxide is an intermediate in the microsomal metabolism of the chemical carcinogen, dibenz(a,h)anthracene. Biochem Biophys Res Commun. 1971 Jun 4;43(5):1010–1016. doi: 10.1016/0006-291x(71)90562-6. [DOI] [PubMed] [Google Scholar]
- Vainio H., Hänninen O. Enhancement of aniline p-hydroxylation by acetone in rat liver microsomes. Xenobiotica. 1972 May;2(3):259–267. doi: 10.3109/00498257209111056. [DOI] [PubMed] [Google Scholar]
- Van Duuren B. L., Langseth L., Goldschmidt B. M., Orris L. Carcnogenicity of epoxides, lactones, and peroxy compounds. VI. Structure and carcinogenic activity. J Natl Cancer Inst. 1967 Dec;39(6):1217–1228. [PubMed] [Google Scholar]
- Van Duuren B. L., Langseth L., Orris L., Baden M., Kuschner M. Carcinogenicity of epoxides, lactones, and peroxy compounds. V. Subcutaneous injection in rats. J Natl Cancer Inst. 1967 Dec;39(6):1213–1216. [PubMed] [Google Scholar]


