Abstract
Background
Difficulties with (non-verbal) social communication, including facial expression processing, constitute a hallmark of autism. Intranasal administration of oxytocin has been considered a potential therapeutic option for improving social difficulties in autism, either by enhancing the salience of social cues or by reducing the social stress and anxiety experienced in social encounters.
Methods
We recorded fMRI brain activity while presenting neutral, fearful and scrambled faces, to compare the neural face processing signature of autistic children (n = 58) with that of matched non-autistic controls (n = 38). Next, in the autistic children group, we implemented this fMRI face processing task in a double-blind, placebo-controlled, multiple-dose oxytocin clinical trial, to evaluate the impact of four-week repeated oxytocin administration (24 IU daily dose) on brain activity in face processing regions.
Results
No significant diagnostic-group differences were identified between autistic versus non-autistic children with regard to neural face processing. Furthermore, no significant treatment effects were found in the oxytocin clinical trial. However, exploratory analyses (uncorrected for multiple comparisons) demonstrated decreases in brain activity in the left superior temporal sulcus (STS) and inferior frontal region in the oxytocin compared to the placebo group, and change-from-baseline analyses in the oxytocin group revealed significantly reduced neural activity in the core face-processing network (STS, inferior occipital, and posterior fusiform), as well as in amygdala and inferior frontal region.
Conclusion
These findings suggest an attenuating effect of multiple-dose oxytocin administration on neural face processing, potentially supporting the anxiolytic account of oxytocin.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13229-024-00635-z.
Keywords: Autism, Face processing, fMRI, Chronic oxytocin administration, Expressive faces
Introduction
Autism is an early-onset neurodevelopmental condition marked by challenges in social interaction and communication, as well as the presence of repetitive and restrictive patterns of behaviours, interests or activities [6]. Impairments in non-verbal social communication, such as inferring social meaning from a face, are included in the clinical criteria and may play a key role in the socio-communicative difficulties experienced by autistic individuals [8]. Thus far, no targeted pharmacological interventions have been established to alleviate these socio-communicative difficulties.
Functional neuroanatomy of face processing
Accurately and rapidly reading faces and facial expressions is important for navigating social interactions [73]. The human brain has an innate preference for faces compared to objects [47, 48] and relies on distinct neural face processing mechanisms [20, 30, 71]. Visual facial information enters the brain via the inferior occipital cortex, which hosts the occipital face area (OFA) and preferentially processes low-level facial features [40, 42, 68]. A ventral visual pathway connects the OFA to the fusiform face area (FFA) [51], which primarily processes static facial features (e.g. identity recognition) [10, 37]. A parallel dorsal pathway connects OFA with the posterior superior temporal sulcus (STS), a region involved in processing dynamic aspects of the face (e.g. facial expression) [40, 42, 46, 69]. Next, an extended face processing network further extracts specific emotional and semantic information from the face [41]. The amygdala attributes salience and is involved in the perception of emotional expressions (e.g. fearful expression) [1, 34, 92]. The inferior frontal gyrus plays a part in understanding dynamic features of a face such as eye-gaze [18, 31]. The anterior temporal region, eventually, hosts identity specific information of familiar faces, such as the name and biographical details [41].
Neural face processing in autism
Findings on behavioural face processing alterations in autism are generally mixed, probably due to the mobilization of compensatory strategies in the autistic population [72, 82, 83, 87]. Yet, neuroimaging studies do reveal atypical processing strategies in autism, with reviews mainly emphasizing reduced face processing activity in the inferior occipital, fusiform, superior temporal and inferior frontal regions, as well as in the amygdala [25, 53, 66, 67]. In particular, when examining expressive face processing, reduced activity has been observed in the amygdala, fusiform and superior temporal regions in autistic children, adolescents and adults [8, 25, 53, 66, 67]. Conversely, some studies have also demonstrated increased activity in the amygdala in autism, both towards neutral and expressive faces [63, 81, 88], which has been interpreted as increased emotional arousal in response to an aversively experienced social stimulus. In this regard, Kleinhans et al. [54] found that higher amygdala activity within the autism group was related to increased social anxiety.
Effect of intranasal oxytocin administration on fMRI face processing responses
Oxytocin is an endogenous neuropeptide synthesized in the hypothalamus. It plays an important role in human social behaviour [43, 75] and acts as a neuromodulator in several brain regions, including the amygdala and other face processing regions [11, 12, 49, 55]. Oxytocin can be delivered intranasally and has been shown to improve facial identity and expression recognition and prosocial behaviour, both in neurotypicals and in various clinical populations [11, 13, 84]. At a mechanistic level, two leading (not mutually exclusive) accounts propose that oxytocin can mediate social behaviour either by enhancing the salience of social stimuli or by reducing social stress.
First, the social salience hypothesis argues that oxytocin primarily enhances attention to and perception of social cues (e.g. faces) by selectively increasing neural activity in the corresponding brain regions [76]. Specifically, research has demonstrated that a single-dose of intranasal oxytocin administration can increase activity in the amygdala, fusiform, superior temporal and inferior frontal regions during expressive face processing in non-autistic adults [29, 35]. Likewise, in autistic adults, single-dose oxytocin studies have linked improved facial expression recognition with increased amygdala reactivity [27, 28]. Similarly, a single-dose of oxytocin induced enhanced STS activity in autistic adults, while processing emotionally charged point-light displays expressing body language [15]. Importantly, however, no differences in STS activity were evident in this study after a four-week multiple-dose oxytocin regime, indicating differential effects of single- versus multiple-dose oxytocin administration [15]. In children with autism, single-dose intranasal oxytocin administration increased neural activity in brain regions related to social attention and perception (i.e. STS, posterior cingulate and premotor cortex) [38].
Secondly, the anxiolytic account of oxytocin highlights its regulating function on (autonomic) stress and (social) anxiety, which may thereby promote social approach behaviour and reduce social avoidance behaviour [16, 61, 70, 79]. In contrast with the social salience account, previous research has also demonstrated that a single-dose of intranasal oxytocin administration can attenuate amygdala activity during expressive face processing in non-autistic adults [26, 35, 50, 52]. This oxytocin-induced dampening of amygdala reactivity has been related to decreased social stress and facilitated social interaction behaviour [61]. Likewise, multiple-dose administration of oxytocin in autistic adults has been shown to dampen amygdala activity and amygdala-frontal functional connectivity while viewing social stimuli [3, 15]. This decreased amygdala-frontal functional connectivity was interpreted as a reduced need for top-down frontal control once amygdala activity has been attenuated [3]. Together, these studies provide evidence for the attenuating effect of oxytocin on neural activity in response to socially relevant cues (e.g. faces), likely reflecting the neuromodulatory effect of oxytocin in reducing emotional arousal and stress.
In an attempt to reconcile these conflicting findings and theories, it has been suggested that the contrasting neural effects of oxytocin may be due to variability in person-dependent characteristics (such as anxiety levels, diagnosis, gender, personal relevance, etc.[5, 61]) or variability in context-dependent aspects (e.g. oxytocin enhances cooperation within a trusting context, but decreases prosocial behaviour in a threatening context [24, 77]). In a similar vein, it has been put forward that oxytocin by itself may not induce any therapeutic effect, yet, it may open up a window of opportunity to facilitate social learning, depending on the social context during which it is administered [33, 36].
The current study
Using a classical functional magnetic resonance imaging (fMRI) paradigm [17, 74] and a double-blind placebo-controlled randomized clinical trial design, we assessed the effect of four weeks of daily intranasal oxytocin administration on neural face processing in school-aged children with autism. In line with previous multiple-dose oxytocin studies [3, 15], it can be hypothesized that oxytocin administration may induce a general attenuation of arousal and neural responsivity towards faces, thus a dampening of neural activity. However, in line with the social salience account of oxytocin (and with several single-dose oxytocin studies, cf. supra), increased neural activity could also be anticipated, possibly induced by the increased salience of the presented faces. In addition to the group of autistic boys and girls, an age and gender matched group of non-autistic children also performed the fMRI face processing task once (pre-treatment; non-autistic children did not receive any oxytocin administration), in order to examine baseline diagnosis-related differences in neural face processing. Here, similar to prior findings, we generally expected to find reduced neural activity along the face processing network in the autistic as compared to the non-autistic children [66], even though enhanced neural activity in amygdala could also be anticipated in autism [54, 63, 81, 88].
Materials & methods
Clinical trial design
We conducted a single-centre, two-arm, double-blind, randomized, placebo-controlled parallel study at the Leuven University Hospital (Belgium) to assess the effects of four weeks intranasal oxytocin administration on the face processing circuitry using fMRI (see Fig. 1 for the CONSORT flow diagram). Children with autism performed a classical face processing block design fMRI task at baseline (T0) and post-treatment (T1) (24 h after the last nasal spray administration). Additionally, at baseline (T0), the face processing fMRI task was also administered in a sample of non-autistic controls to compare neural face processing correlates with those in the autism group. Study procedures and informed consent forms were approved by the Ethics Committee for Biomedical Research at the University of Leuven, KU Leuven (S61358) in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). The trial was registered on 06/07/2018 at the European Clinical Trial Registry (EudraCT 2018–000769-35; https://www.clinicaltrialsregister.eu/ctr-search/trial/2018-000769-35/BE). The current task-based fMRI recordings were part of a larger assessment protocol, which aimed at evaluating clinical efficacy of oxytocin treatment on several autism symptom questionnaires and on neural sensitivity towards expressive faces as assessed with EEG. In short, these parallel reports indicate a general but no treatment-specific improvement in autism characteristics [23] and a significant oxytocin-induced dampening of the neural sensitivity (EEG) towards subtle socio-communicative facial cues [62]. Notably, at baseline, the autistic children displayed highly significantly reduced neural sensitivity for discriminating fearful and happy facial expressions as compared to their non-autistic peers, as demonstrated by frequency-tagging EEG [62].
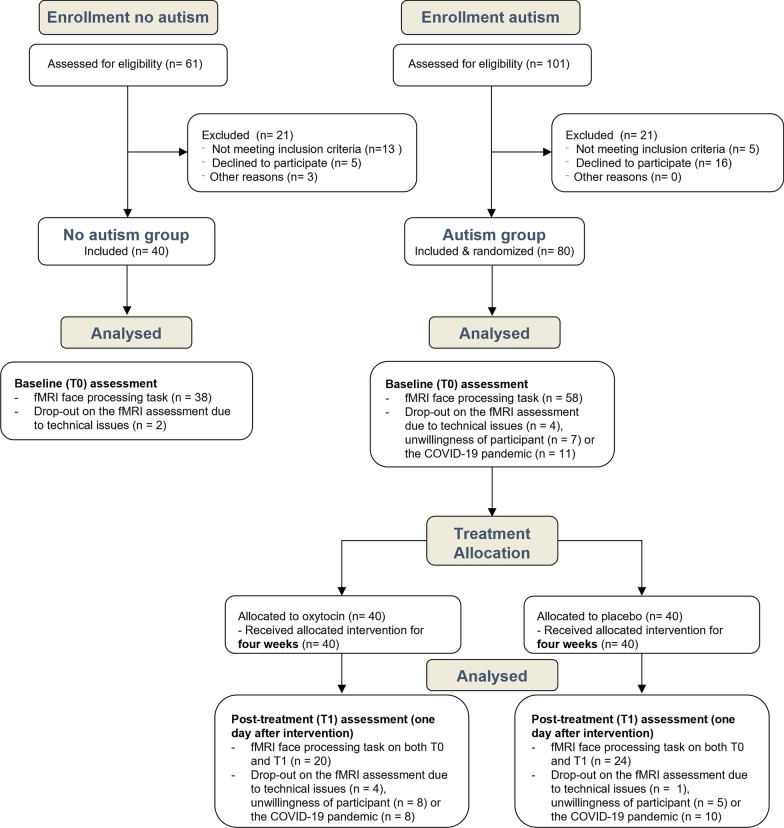
Fig. 1.
CONSORT flow diagram. Participants of the non-autistic and autism groups were recruited and assessed at baseline (T0). Next, the autism group was allocated to receive either oxytocin or placebo (four weeks of twice daily intranasal administrations), followed by a post-treatment (T1) assessment. As outlined, for some participants, fMRI recordings were not acquired at one or both assessment sessions due to physical contact restrictions and closing down of hospital facilities during the COVID-19 pandemic or due to technical issues
Participants
Autistic participants were recruited between July 2019 and January 2021 through the Leuven Autism Expertise Centre, KU Leuven. Non-autistic children were recruited through elementary schools. For all children the parent-rated Social Responsiveness Scale-Children (SRS-2 [21] and verbal and performance intelligence quotients (IQ; WISC-NL [86]) were acquired. In the autistic children, the Autism Diagnostic Observation Schedule (ADOS-2 [60]) was also administered. Both groups were matched on age, gender and performance IQ, although verbal IQ was significantly higher in the no-autism compared to the autism group (Table 1). Before the treatment, the autistic children were randomly allocated to receive oxytocin or placebo. There were no statistically significant differences between randomized treatment groups in terms of age, gender, IQ and autism symptomatology (Table 1). See Suppl. Mat., for more participant information.
Table 1.
Demographic characteristics of the trial participants at baseline
| Measures | Autism (n = 58) |
No autism (n = 38) |
P-valuea | Autism-oxytocin (n = 20) |
Autism-placebo (n = 24) |
P-valuea |
|---|---|---|---|---|---|---|
| ♂:♀ | 46:12 | 30:8 | 0.99 | 18:2 | 21:3 | 0.95 |
| Ageb (mean ± SD) | 9.93 ± 1.26 | 9.79 ± 1.28 | 0.59 | 10.20 ± 1.36 | 9.88 ± 1.26 | 0.47 |
| VIQc (mean ± SD) | 108.78 ± 12.24 | 117.76 ± 15.22 | < 0.01* | 104.25 ± 16.05 | 111.61 ± 16.61 | 0.15 |
| PIQd (mean ± SD) | 104.40 ± 13.77 | 107.76 ± 12.46 | 0.22 | 101.60 ± 13.47 | 103.43 ± 12.01 | 0.64 |
| SRS-2e (mean ± SD) | 88.66 ± 20.97 | 21.03 ± 12.27 | < 0.001* | 88.65 ± 22.53 | 85.63 ± 20.78 | 0.65 |
| ADOS-2f (mean ± SD) | – | – | – | 9.22 ± 3.41 | 9.16 ± 4.13 | 0.96 |
aP-values based on independent, two-sample t-tests or Chi-square tests
bAge expressed in years
cVerbal Intelligence Quotient (IQ) was derived from the subtests Similarities and Vocabulary, Wechsler Intelligence Scale for Children, Fifth Edition, Dutch version (WISC-V-NL [86])
dPerformance IQ was derived from the subtests Block Design and Figure Puzzles (WISC-V-NL [86])
eSocial Responsiveness Scale-Children, 2nd edition (SRS-2 [21])
fPrior to randomization, the Autism Diagnostic Observation Schedule, 2nd edition (ADOS-2 [60]) was administered in the autism group
*Significant difference at p < 0.05 statistical threshold
Oxytocin administration
Autistic participants received oxytocin (Syntocinon®, Sigma-tau) or placebo nasal sprays. Placebo sprays contained all identical ingredients as the active solution, except the oxytocin compound. Sprays were prepared by the University Hospital of Heidelberg (Germany) in identical 10 ml brown glass bottles with a white nasal pump (0.05 ml or 2 IU /puff). Before the start of the study, participants and their parents received clear instructions on how to administer the nasal spray [39].
Participants administered the nasal spray twice daily—six puffs (three per nostril) or 12 IU in the morning and six puffs in the afternoon (after school)—resulting in a daily dose of 24 IU, following a conservative dosing scheme used in children with autism [91].
On day 28, i.e. the last day before the post-treatment assessment (T1), participants withheld the afternoon spray, to avoid single-dose oxytocin effects during testing the next day. Potential adverse events were recorded through weekly parent reports and daily parent and child diaries, and revealed minimal and non-treatment specific side effects (see [23]).
Face processing fMRI
Block-design fMRI task
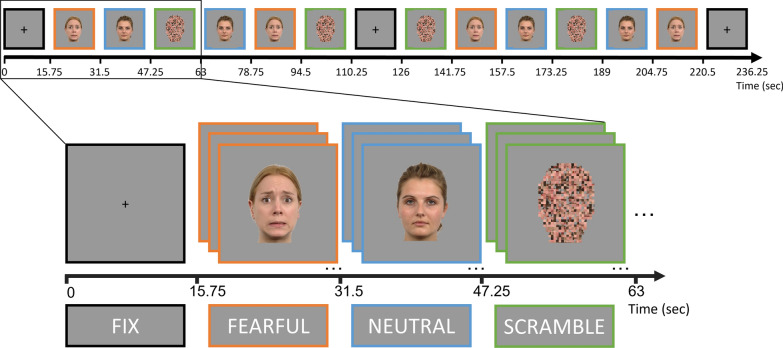
During scanning, blocks of neutral (N), fearful (F) and scrambled (S) faces were alternately projected on a screen behind the MRI scanner, which could be seen via a mirror (Fig. 2). Each block lasted 15.75 s, a whole run lasted 236.25 s and each participant completed two runs. Twenty-one faces (randomly sampled from seven different identities) were presented for 0.75 s each in one block. A fixation cross (fix), was shown for 15.75 s at the beginning of the run, after six face blocks, and at the end of the run. Within each run, faces were semi-randomly presented using MATLAB R2018b (MATLAB and Statistics Toolbox Release 2018b, The MathWorks, Inc., Natick, Massachusetts, United States). Male and female faces were shown in separate runs, counterbalanced across runs. During the run with female faces, a male face appeared randomly 2 or 3 times per block, and vice versa for the male run. To ensure focus on the screen and attention to the face characteristics, participants were instructed to press a button with the thumb of their dominant hand whenever this gender switch occurred. Likewise, during the scrambled condition the scrambled faces randomly (2 or 3 times per block) changed to a plain face-silhouette, and participants were instructed to also press the button on this occasion. Average performance accuracy on this orthogonal task was 80.81%, with no diagnostic-group (autism = 76.41%, no-autism = 81.45%; t(87) = 1.27, p = 0.21) or nasal spray-related group differences (autism-oxytocin = 83.33%, autism-placebo = 82,04%; t(35) = 0.28, p = 0.78). Neither were there diagnostic-group (autism = 0.78 s, no-autism = 0.78 s; t(81) = -0.30, p = 0.76) or treatment-related group differences (autism-oxytocin = 0.79 s, autism-placebo = 0.78 s; t(32) = 0.18, p = 0.86) in reaction time.
Fig. 2.
The face processing fMRI paradigm. Blocks of neutral, fearful and scrambled faces were alternately projected. A fixation cross (fix) was shown for 15.75 s at the beginning of the run, after six face blocks, and at the end of the run. Each block lasted 15.75 s, a whole run lasted 236.25 s. Twenty-one faces (from seven different identities) were presented for 0.75 s each in one block. Male and female faces were shown in separate runs, counterbalanced across runs
Stimuli
Seven male and seven female identities were chosen from the Radboud Faces Database (RaFD) [57]. For each identity, a neutral and fearful face was included. All fearful faces were reliably rated as “fearful” (91.4% agreement[57]). The scrambled faces were made from three fearful and four neutral faces with different identities with a grid size of ten, using webmorph.org. All images were 5.5 × 8 cm and placed on an 8 × 12 cm grey background. The visual angle of the face stimuli during MRI acquisition was 17.15 degrees.
fMRI data acquisition
Structural and functional MRI images were obtained using a 3 Tesla Philips Ingenia CX MR scanner (Best, The Netherlands) with a 32-channel head coil. fMRI series were acquired using BOLD sensitive echo planar imaging sequence (TR/TE 1500/30 ms, 80° flip angle, 228 × 228 mm2 field of view, 48 axial slices, multiband slice order (factor of 2), 2.75 mm tick, in plane voxel size 2.75 mm2). Structural scans were collected using a standard T1-weighted pulse sequence (TR/TE 9.6/4.6 ms, 8° flip angle, 250 × 250 mm2 field of view and voxel size 0.97 × 0.97 × 1.2 mm3).
fMRI data analysis
Preprocessing
fMRI data were preprocessed using the CONN SPM toolbox 2017 [89], within MATLAB 2022b (MATLAB and Statistics Toolbox Release 2022b, The MathWorks, Inc., Natick, Massachusetts, United States). First, structural images were manually positioned according to the anterior commissure—posterior commissure line. Second, functional images were slice timing corrected and realigned to the first functional image. The six head motion parameters (three translational and three rotational), obtained during this process were used as confounds in the general linear model. Third, outliers due to excessive head motion (framewise displacement (FD) exceeding 0.9 mm) were scrubbed using Artifact Detection Toolbox (https://www.nitrc.org/projects/artifact_detect/). Average FD head motion did not differ between diagnostic-groups (t(94) = 1.2, p = 0.23) nor between treatment-groups at baseline or after spray administration period (t(42) = 0.42, p = 0.68). Fourth, structural images were co-registered to the functional images. Fifth, structural and functional images were normalized to MNI space, resampling the functional scans to a voxel size of 2 × 2 × 2 mm. Lastly, functional images were smoothed with a Gaussian kernel with a full-width half maximum (FWHM) of 8 mm to increase the signal to noise ratio.
General analysis approach
At subject level, a first-level general linear model (GLM) was built, based on the onset and duration of each stimulus block (condition). The GLM contained the following variables: two runs with each four conditions (fearful, neutral, scramble and fixation) and additionally six head-motion parameters. Estimation of the GLM resulted in beta-values for each condition, which were used in the subsequent second-level analyses. We first performed a series of whole-brain univariate analyses, aimed at pinpointing the (expressive) face processing brain circuitry with an increasing level of specificity (by making the consecutive contrasts more specific, i.e. NEUTRALvsFIX, NEUTRALvsSCRAMBLE, FEARFULvsFIX, FEARFULvsSCRAMBLE, FEARFULvsNEUTRAL). For each of the contrasts, we investigated T0 baseline activity for the no-autism and the autism group separately, as well as for the diagnostic group comparison (all Family Wise Error (FWE) corrected at a threshold of p < 0.05). Here, we can already mention that -contrary to our expectation- the most specific contrasts and particularly the FEARFULvsNEUTRAL contrast did not reveal any significant activation, imposing us to focus on the more general face processing network with the use of the FACES(neutral + fearful)vsFIX contrast. Second, we performed a more targeted region of interest (ROI) based analysis within multiple regions of a predefined face processing network (cf. [44] see infra). In these ROIs, we performed the same analysis approach, comparing univariate brain activity for several contrasts for the no-autism and the autism groups separately, as well as for the diagnostic group comparison, controlling for multiple comparisons by calculating False Discovery Rate (FDR) corrected p-values. Again, here, we were obliged to proceed with the most general FACESvsFIX contrast that yielded significant activity. Third, we explored whether a multivariate representational similarity analysis might be more sensitive to reveal expression-specific activity patterns (i.e. FEARFULvsNEUTRAL) in particular brain regions in any of the groups. Yet, again, this approach did not yield any selective activity patterns. Finally, based on all these analyses at T0 baseline, we determined to investigate the treatment-related effects (i.e. T1 versus T0) based on the univariate FACESvsFIX contrast throughout the 13 ROIs.
Definition of ROIs
Based on the face processing neuroimaging literature, we performed more targeted ROI analyses. Specifically, we used the ROIs delineated by Hendriks et al. [44] which were applied in a multi-method fMRI study on neural face processing in adults with and without autism. Seven brain regions involved in the core and extended face processing network were delineated: the inferior occipital cortex (including the OFA), the posterior fusiform cortex (including the FFA), and the superior temporal cortex (including the STS), the amygdala, the anterior temporal cortex, the inferior frontal cortex, and primary visual cortex V1, see Fig. S1. As all regions were subdivided in left and right hemisphere ROIs, except V1, we were left with 13 ROIs in total. These ROIs were created by calculating the intersection of anatomical masks from the WFU PickAtlas’ ‘aal’ (Wake Forrest University PickAtlas, http://fmri.wfubmc.edu/cms/software) and face-responsive voxels from a whole-brain second level “faces versus fixation” contrast across all their participants (n = 52) (see Hendriks et al., [44], for details).
Univariate ROI analyses
We conducted an ROI-based univariate analysis (using custom code in MATLAB and R (RStudio Team (2022)) to examine diagnosis- and treatment-related differences in the processing of faces. Per participant, the average beta-values were computed for every contrast and every ROI. Values exceeding two standard deviations from the mean were excluded from the analysis to enhance the accuracy and reliability by mitigating their disproportionate influence. First, per group, these were entered in a one-sample t-test to pinpoint condition-specific activity. Second, these beta-values were used in two-sample t-tests to evaluate group differences. For the diagnosis-related group differences, this was performed across all the predefined contrasts (from more general to more specific, i.e. FACESvsFIX, NEUTRALvsFIX, FEARFULvsFIX, NEUTRALvsSCRAMBLED, FEARFULvsSCRAMBLED, and FEARFULvsNEUTRAL). To examine treatment-related group differences, this was only performed for the FACESvsFIX contrast. In particular, change-from-baseline scores were calculated, i.e. we subtracted the beta-values for the activity per ROI at T0 from the beta-values for the activity per ROI at T1. Across all these analyses, we corrected for multiple comparisons by controlling the false discovery rate (FDR) with q < 0.05 [14].
Multivariate ROI analyses
To further pinpoint selective brain activity for the fearful versus neutral faces and explore possible group differences in expressive face processing, we performed a multivariate analysis in each of the predefined ROIs by comparing neural dissimilarity in the multivoxel patterns elicited by the fearful versus neutral face condition. Specifically, representational similarity analysis (RSA), resulting in representational dissimilarity matrices (RDM), was applied to quantify the quality of the neural representation and to examine potential diagnosis-related group differences in expressive vs. neutral face processing. For each ROI, neural RDMs comprised the pairwise correlation distance between activity patterns (beta values) for the different conditions (i.e. fearful and neutral faces) [90]. We then tested the significance of differences between these distance values per ROI, both within and between diagnosis-groups, using paired t-tests. Then, p-values were corrected for multiple comparisons across all ROIs using false discovery rate (FDR) with q < 0.05 [14].
Results
No diagnostic-group differences in whole-brain face processing activity
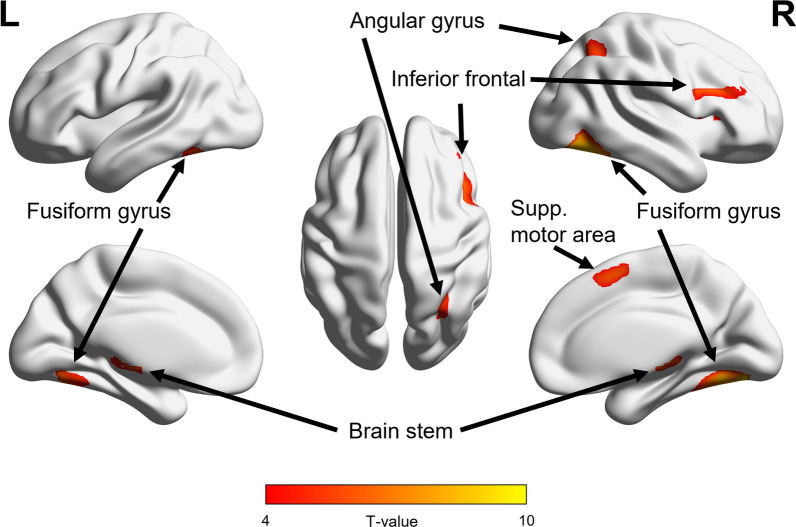
Contrary to our expectation, the most specific FEARFULvsNEUTRAL contrast did not yield any selective activity for the whole-brain analysis, not for the separate groups, nor for the diagnostic group comparison (all pFWE > 0.05). Likewise, inclusion of the SCRAMBLE control condition already appeared to largely evoke the face processing network, thereby abolishing any specific activity for the NEUTRALvsSCRAMBLE and FEARFULvsSCRAMBLE contrasts (all pFWE > 0.05). Against this background, we thus proceeded with the more general FACESvsFIX contrast. Face processing related brain activity was evident across all participants (n = 96) from the FACESvsFIX whole-brain contrast, with peak activity in clusters in the right fusiform gyrus (t(95) = 9.64, pFWE < 0.001, 1711 voxels), left fusiform gyrus(t(95) = 7.57, pFWE < 0.001, 430 voxels), brain stem (t(95) = 6.74, pFWE < 0.001, 644 voxels), right inferior frontal gyrus (t(95) = 6.74, pFWE < 0.001, 390 voxels), right supplementary motor area (t(95) = 6.40, pFWE < 0.001, 255 voxels) and right angular gyrus (towards the end of the STS) (t(95) = 5.61, pFWE = 0.001, 205 voxels), see Fig. 3. Notably, a diagnostic group comparison did not yield any significant diagnosis-related differences in activity for this FACESvsFIX whole-brain contrast (pFWE > 0.05).
Fig. 3.
fMRI face processing responses across all participants. As there were no diagnostic-group differences, the figure shows the whole-brain face processing activity (FACESvsFIX contrast) across all autistic and non-autistic participants (n = 96, pFWE < 0.05). Face processing related brain activity was evident in the right and left fusiform gyrus, brain stem, right inferior frontal gyrus, right supplementary motor area and right angular gyrus (wraps the end of the STS)
No diagnostic-group differences in ROI-based face processing activity
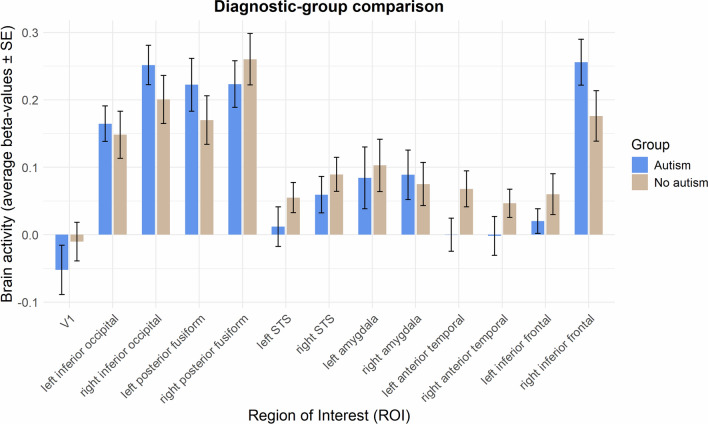
Table 2 and Fig. 4 display average beta-values for the univariate FACESvsFIX contrast in each of the ROIs for each group, as well as statistics for the group comparison. One-sample t-tests per group confirm that the brain activity is centred around the key regions of the extended face processing network, i.e. inferior occipital (OFA), posterior fusiform (FFA), inferior frontal, STS and amygdala. Comparison of the average ROI activity between autism and no-autism yielded no significant effect of diagnostic-group (all pFDR > 0.14; see Table 2 and Fig. 4).
Table 2.
Diagnostic-group comparison of the average face processing activity in each of the ROIs
| ROI activity FACES vs. FIX contrast |
Autism beta-value |
No autism beta-value |
Group comparison | ||
|---|---|---|---|---|---|
| t value | Punc | PFDR | |||
| V1 | − 0.05 | − 0.01 | − 0.91 | 0.37 | 0.55 |
| Left inferior occipital | 0.16* | 0.15* | 0.37 | 0.71 | 0.78 |
| Right inferior occipital | 0.25* | 0.20* | 1.11 | 0.27 | 0.55 |
| Left posterior fusiform | 0.22* | 0.17* | 0.98 | 0.33 | 0.55 |
| Right posterior fusiform | 0.22* | 0.26* | − 0.72 | 0.48 | 0.60 |
| Left STS | 0.01 | 0.06* | − 1.17 | 0.25 | 0.55 |
| Right STS | 0.06° | 0.09* | − 0.81 | 0.42 | 0.57 |
| Left amygdala | 0.08 | 0.10* | − 0.31 | 0.76 | 0.78 |
| Right amygdala | 0.09* | 0.08* | 0.28 | 0.78 | 0.78 |
| Left anterior temporal | 0.00 | 0.07* | − 1.87 | 0.07 | 0.55 |
| Right anterior temporal | 0.00 | 0.05* | − 1.36 | 0.18 | 0.55 |
| Left inferior frontal | 0.02 | 0.06 | − 1.13 | 0.26 | 0.55 |
| Right inferior frontal | 0.26* | 0.18* | 1.58 | 0.12 | 0.55 |
Beta-values with an asterisks (° p < 0.05 uncorrected, * q < 0.05 FDR corrected) indicate whether the FACESvsFIX contrast yields significant brain activity within a particular ROI within a particular group, based on a one-sample t-test with H0: Mean X = 0. P-values in bold (p < 0.05) indicate whether the FACESvsFIX contrast yields significant brain activity within a particular ROI between both groups, based on a two-sample t-test. However, no significant group-differences were observed
Fig. 4.
Diagnostic-group comparison of average ROI activity during face processing. fMRI face processing responses (average beta-values for the FACESvsFIX contrast) across all voxels within each of the ROIs are shown in bar graphs, for the autism and no-autism groups. Error bars denote standard errors of the mean
Given the specific interest in expressive face processing for the diagnostic-group comparison, we also investigated the ROI-based average activity for the more specific contrasts (see Supplementary Table S1A-E). Contrary to our expectation, however, the FEARFULvsNEUTRAL contrast did not reveal any significant activation (all pFDR > 0.78; see Table S1E), nor did the other contrasts across any of the ROIs (all pFDR > 0.11; see Table S1A-D). To further pinpoint selective brain activity for the fearful versus neutral faces and explore possible diagnostic-group differences in expressive face processing, we performed a multivariate representational similarity analysis. However, here again, this analysis did not allow to significantly differentiate the neural representations of fearful versus neutral facial expressions (all pFDR > 0.89, see Suppl. Mat., Table S2), thereby making any further group comparisons invalid. Accordingly, based on this series of analyses, the focus of the subsequent treatment-specific group comparisons was on the FACESvsFIX contrast, similar to Hendriks et al., [44].
No specific treatment-related effects on fMRI face processing responses in autism
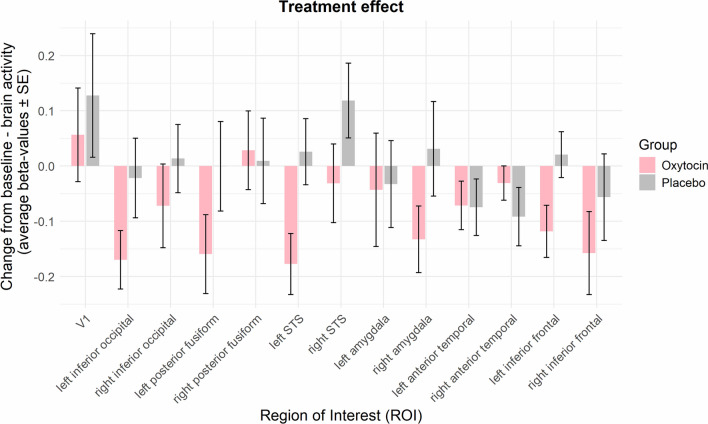
A whole-brain analysis comparing the oxytocin versus placebo group for the FACESvsFIX contrast did not reveal any significant group differences in activity at pFWE < 0.05. Next, for each of the ROIs we calculated change-from-baseline scores by subtracting the average beta-values at T0 from the average beta-values at T1. Only in the oxytocin and not in the placebo group did the neural activity decrease from T0 to T1, in the left inferior occipital (t(19) = − 3.21, pFDR = 0.04) and left STS (t(18) = − 3.20, pFDR = 0.04) regions and a similar trend, at an uncorrected level, was found for the right amygdala (t(19) = − 2.20, punc = 0.04), the left posterior fusiform (t(18) = − 2.23, punc = 0.04) and the left inferior frontal (t(18) = − 2.51, punc = 0.02) regions.
While these change-from-baseline effects were only significant within the oxytocin group, no significant treatment-effect was found when contrasting the oxytocin with the placebo group corrected for multiple comparisons (all pFDR > 0.25; see Table 3 and Fig. 5). Note that at an uncorrected level, activity in the left STS (t(38) = -2.49, punc = 0.02) and the left inferior frontal (t(37) = -2.21, punc = 0.03) were decreased in the oxytocin compared to the placebo.
Table 3.
Oxytocin versus placebo treatment effect on the change-from-baseline (CFB) activity per ROI during face processing
| ROI activity FACES vs. FIX contrast |
Oxytocin CFB-value |
Placebo CFB-value |
Group comparison | ||
|---|---|---|---|---|---|
| t value | Punc | PFDR | |||
| V1 | 0.06 | 0.13 | − 0.51 | 0.61 | 0.82 |
| Left inferior occipital | − 0.17* | − 0.02 | − 1.65 | 0.11 | 0.32 |
| Right inferior occipital | − 0.07 | 0.01 | − 0.87 | 0.39 | 0.58 |
| Left posterior fusiform | − 0.16° | 0.00 | − 1.47 | 0.15 | 0.32 |
| Right posterior fusiform | 0.03 | 0.01 | 0.18 | 0.86 | 0.96 |
| Left STS | − 0.18* | 0.03 | − 2.49 | 0.02 | 0.25 |
| Right STS | − 0.03 | 0.12 | − 1.52 | 0.14 | 0.32 |
| Left amygdala | − 0.04 | − 0.03 | − 0.08 | 0.94 | 0.96 |
| Right amygdala | − 0.13° | 0.03 | − 1.57 | 0.13 | 0.32 |
| Left anterior temporal | − 0.07 | − 0.07 | 0.05 | 0.96 | 0.96 |
| Right anterior temporal | − 0.03 | − 0.09 | 0.99 | 0.33 | 0.58 |
| Left inferior frontal | − 0.12° | 0.02 | − 2.21 | 0.03 | 0.25 |
| Right inferior frontal | − 0.16 | − 0.06 | − 0.93 | 0.36 | 0.58 |
CFB-values with an asterisks (° p < 0.05 uncorrected. * q < 0.05 FDR corrected) indicate whether the T1 versus T0 change-from-baseline contrasts yield significant brain activity within a particular ROI within a particular treatment group based on a one-sample t-test with H0: Mean X = 0. P-values in bold (p < 0.05) indicate whether the FACESvsFIX contrast yields significant brain activity within a particular ROI between both treatment-groups, based on a two-sample t-test
Fig. 5.
Treatment-specific effect on change-from-baseline average ROI activity during face processing. fMRI face processing responses (average change-from-baseline beta-values for the FACESvsFIX contrast) across all voxels within each of the ROIs are shown in bar graphs, for the oxytocin and placebo groups. Note that, even though not strictly significant, the oxytocin group generally displays a reduction in brain activity across the face processing network at T1 compared to T0. Error bars denote standard errors of the mean
Discussion
The present study compared fMRI face processing neural activity of autistic children versus matched non-autistic controls, and subsequently investigated the impact of a four-week course of repeated oxytocin administration on these neural responses in the autism cohort. No significant diagnostic-group differences were identified in the children, possibly due to large inter-individual variability. Crucially, repeated oxytocin administration did not significantly alter the neural activity in the face processing circuitry, compared to placebo, at a stringent statistical threshold. Only at a more lenient threshold, a pattern of oxytocin-induced decreased neural activity was identified in STS and inferior frontal regions, compared to placebo.
No diagnostic-group differences in the mobilisation of the face processing circuitry
Even though previous research generally reported reduced activation of face processing brain regions in individuals with autism [8, 22, 67], we did not observe any significant diagnosis-related group differences, not via the whole-brain analysis nor via the focused ROI analysis, despite our representative neuroimaging dataset from 58 autistic and 38 non-autistic children. Our results do align with a prior study by Hendriks et al., [44], similarly showing no diagnosis-related differences, using an identical set of face processing regions-of-interest in autistic adults. Likewise, a recent study of Langenbach et al., [56], compared amygdala activity between a large cohort of autistic vs. non-autistic individuals during emotional face processing and found no differences [56].
Notably, in a prior study from our lab, a significant diagnostic group difference was revealed in the same cohort of autistic/non-autistic children, using an EEG-based frequency-tagging facial expression discrimination paradigm, i.e., indicating reduced implicit facial discrimination processing in autistic children [62]. These results sharply contrast with the lack of a significant group difference in facial expression processing as assessed in the current study, using fMRI. The observation of facial processing difficulties as shown using EEG [62], but not using the current fMRI paradigm, may therefore indicate the superiority of this frequency-tagging EEG approach in revealing more subtle difficulties in implicit facial processing. Possibly due to the fact that MRI can be perceived as stressful (loud noises, supine position, etc.) compared EEG, which may interfere with the detection of subtle neural activations related to emotional face processing. It is also possible that our results are task-dependent and other tasks (e.g. including faces in a more naturalistic settings) are more sensitive to discriminate between the diagnostic groups.
Oxytocin treatment effects on fMRI face processing responses in autism
Next, we implemented the fMRI face processing task in a randomized, double-blind, placebo-controlled clinical trial, to monitor the effect of oxytocin administration for the autistic children. Crucially, no robust oxytocin-specific differences were observed. However, at a more lenient exploratory threshold we did observe lower neural activity in left STS and left inferior frontal region in the oxytocin group compared to the placebo group. Examining the change-from-baseline in the oxytocin group separately, indeed showed that in the oxytocin group, not in the placebo group, neural activity in the STS and inferior occipital regions significantly reduced from the pre (T0) to the post (T1), neuroimaging assessment. Similar pre-to-post changes were identified in the posterior fusiform region, inferior frontal region and amygdala, indicating significant pre-to-post reductions in neural activity in these regions in the oxytocin group, but not in the placebo group.
At a functional level, changes in STS activity might relate to changes in facial expression processing ability, especially since STS has classically been designated as the core face processing region involved in processing the dynamic aspects of faces, such as expressions [41, 65]. The observation of oxytocin-induced reduction in STS activity is in line with prior studies investigating the effects of oxytocin in individuals with autism. For instance, Aoki et al., [9] demonstrated reduced STS activity, although not significant (p = 0.075), after a single-dose of oxytocin in autistic adults when they had to infer others’ emotions. Likewise, Andari et al., [7] reported diminished activity in the middle temporal cortex during a social ball-tossing game, also upon single-dose oxytocin administration in autistic adults [7]. In contrast, other single-dose oxytocin studies have reported increased oxytocin-induced activity in STS in autism [15, 38]. For example, Bernaerts et al., [15] found increased activity in STS in autistic adults while processing point-light biological motion, after a single dose of oxytocin, but no consistent long-term changes in STS activity were induced after a four-week multiple-dose administration. Note, however, that this same study did observe consistent and long-term reductions in amygdala activity after a four-week oxytocin treatment [15], similar to the current trend of reduced amygdala activity upon chronic oxytocin administration.
The inferior frontal region is believed to play a role in semantic knowledge about faces [18, 45]. It shows stronger activation when viewing familiar faces compared to newly learned faces [59] and responds more to the face of one’s partner than one’s own face [80], suggesting that the region is involved in monitoring social information rather than familiarity alone [44]. Given these roles, the observed reduction in inferior frontal activity induced by oxytocin compared to placebo (although uncorrected for multiple testing), might suggest reduced recruitment of cognitive control resources as the orthogonal face processing task required selective attention for interpreting the gender of the face stimuli. Of note, in our study, no oxytocin-related performance differences were found on the orthogonal task, indicating and equal level of overt attention to the face stimuli.
While we did not administer any behavioural face processing tasks throughout the clinical trial, in a parallel report on this same participant cohort we describe oxytocin-induced changes in neural sensitivity for subtle facial expression cues as assessed by frequency-tagging EEG [62]. Interestingly, in the EEG data, the selective neural sensitivity for facial expression discrimination, as indexed by occipito-temporal responses, significantly increased after receiving placebo, but this effect was dampened after receiving the four-week oxytocin treatment [62]. Strikingly, the current change-from-baseline neural activity reveals a similar decrease after oxytocin treatment in the inferior occipital, posterior fusiform and STS core face processing regions. Thus, together, the EEG and fMRI findings converge on demonstrating evidence for a generally attenuating effect of oxytocin on face processing activity. This is additionally supported by the observed (uncorrected) trend of oxytocin-induced lowered activity in the amygdala and the inferior frontal gyrus, regions known to play a crucial role in emotion processing [2, 19], [42], [78].
While this conclusion conflicts with the social salience account of oxytocin, it may corroborate the anxiolytic and social stress reduction theory of oxytocin, as possibly aversive facial stimuli may be processed in a more attenuated manner. To further explore this theory, the association between oxytocin-induced reductions in neural activity and self-reported anxiety, measured as the change-from-baseline using the Screen for Child Anxiety Related Disorders (SCARED) questionnaire [64] was exploratively investigated. Improved self-reported anxiety was significantly associated with attenuated left inferior occipital (Pearson correlation r = 0.61; p < 0.01) and left inferior frontal (r = 0.61; p < 0.01) activity, and marginally significant with reduced left STS activity (r = 0.45; p = 0.06). Note, however, that no treatment-specific improvements were demonstrated on the SCARED questionnaire (for more information on the behavioural data see Daniels et al., [23]).
Further evidence corroborating this social stress reduction account is offered by Alaerts and colleagues [4], showing that this four-week oxytocin course reduced cardiac autonomic arousal in this same sample of autistic children, as evidenced by increased high-frequency heart rate variability. Yet, despite these beneficial anxiolytic effects of chronic oxytocin administration, caution is warranted about the possible functional impact of the reduced fine-grained face processing capacities [62].
A recent systematic review summarized the impact of intranasal oxytocin administration on fMRI responses in autism [32]. Note that thus far only two studies (beside the current study, i.e. Bernaerts et al., [15] and Watanabe et al., [85]) assessed the neural effect of multiple-dose oxytocin on the processing of socio-emotional stimuli. While the authors acknowledge that oxytocin administration can alter brain activity in individuals with autism, they also emphasize that this largely depends on the type of task and thus the actual context of (single-dose) oxytocin administration [32, 33]. More specifically, performing a social task within the time window of actively circulating oxytocin administration might boost the circuitry involved in this task, whereas these circuitries might not be affected by oxytocin alone (in isolation). This might explain why some single-dose studies demonstrate increased neural activity in response to faces (in line with the social salience hypothesis), as the brain is concurrently primed by a social task. On the other hand, the current and previous multiple-dose oxytocin study [15], did not specifically prime the brain with either a social task or a positive social context while exogenous oxytocin was administered and circulating, and therefore they may not have boosted the related neural circuitry, thus not resulting in enhanced neural activity. A unique recent multiple-dose clinical trial in children with autism did explicitly combine oxytocin administration with psychosocial stimulation, and did demonstrate enhanced social attention (increased looking at the eye region of a face) and consistent clinical improvements in autism symptoms [58]. Yet, this latter study did not incorporate neuroimaging, leaving the neural mechanisms of social improvements after oxytocin treatment uncertain. This calls for further exploration of the impact of combining multiple-dose oxytocin treatment with targeted socially-stimulating tasks in order to uncover the underlying neural mechanisms of the resulting behavioural/clinical effects.
Limitations
Although the current study reveals important insights on the effects of repeated oxytocin administration on fMRI face processing responses in children with autism, some limitations need to be addressed. First considering the possible lack of sensitivity of the block-design during fMRI neuroimaging, it is plausible that subtle group differences in neural activity during face processing are not detected. This may explain why results from a related investigation, adopting a similar face processing task assessed during EEG (with higher temporal resolution) in the same cohort of autistic and non-autistic children, did convincingly show diagnostic group differences and oxytocin-related effects [62]. Secondly, we implemented anatomically defined ROIs, which were functionally restricted to ensure that only face-selective voxels were included. Yet, these anatomical ROIs are based on fMRI scans of adults with and without autism, which may be divergent from children with and without autism. Third, the higher prevalence of medication use (e.g., methylphenidate) and comorbidities (e.g., attention-deficit/hyperactivity disorder) in the autism group compared to the non-autistic group may have influenced the observed fMRI activity. Importantly, a stable medication-use regimen was required for participation in the study, potentially mitigating its impact on the results. Furthermore, the inclusion of children on medication and with comorbidities reflects the real-world population of autistic children, enhancing the ecological validity of the findings and ensuring that the sample is representative of the broader clinical population. Fourth, considering the study design, we did not include a single-dose oxytocin assessment, so no direct comparison between single and multiple-dose oxytocin effects on neural face processing could be made. Lastly, a more consistent standardization or monitoring of the social context during heightened exogenous oxytocin availability throughout the long-term trial would have been informative, also to understand possible interindividual variability in neural response patterns.
Conclusion
Reading someone’s face is crucial for social interaction, which is often altered in individuals with autism. The underlying neural correlates of face processing have been extensively examined using fMRI, allowing to quantify brain activity with great spatial resolution. We used an fMRI task to compare the neural face processing signature of children with autism with that of matched non-autistic controls, but no robust diagnostic-group differences were identified.
Next, we implemented this fMRI face processing task in a double-blind, placebo-controlled, multiple-dose oxytocin clinical trial in children with autism, to evaluate the impact of oxytocin on face processing brain activity. No significant treatment effects were found. Yet, uncorrected for multiple-comparisons, we did observe lower left STS and inferior frontal activity in the oxytocin group, compared to the placebo group. Furthermore, looking at the change-from-baseline in the oxytocin group separately, reduced neural activity was found in core face-processing regions (STS, inferior occipital and posterior fusiform regions) as well in the amygdala and the inferior frontal region. These findings suggest an attenuating effect of multiple-dose oxytocin administration on face processing, possibly supportive of the anxiolytic account of oxytocin.
Supplementary Information
Acknowledgements
We are grateful to Prof. Patrick Dupont and Prof. Hans Op de Beeck for the support on the fMRI analyses. We thank all the participants and their families who generously participated in this study.
Abbreviations
- ADOS
Autism diagnostic observation schedule, second edition
- CFB
Change from T0 baseline
- IQ
Intelligence quotient
- MRI
Magnetic resonance imaging
- ROI
Region of interest
- SRS
Parent-rated social responsiveness scale-children
- WISC
Wechsler intelligence scale for children
Author contributions
M.M.: conceptualization. methodology. investigation. data curation. validation. writing—original draft. writing—review and editing. visualization. project administration. N.D.: conceptualization. methodology. investigation. data curation. validation. writing—review and editing. project administration. S.VdD.: methodology. investigation. data curation. validation. writing—review and editing. visualization. T.T.: methodology. investigation. data curation. validation. writing—review and editing. J.P.: investigation. data curation. validation. writing—review and editing. E.Y.: methodology. validation. writing—review and editing. J.S.: supervision. validation. writing—review and editing. funding acquisition. K.A.: supervision. methodology. validation. writing—review and editing. funding acquisition. B.B.: supervision. conceptualization. methodology. validation. writing—review and editing. funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a KU Leuven grant (C14/17/102), a Doctor Gustave Delport fund of the King Baudouin Foundation (2019-J1811190-212989) and a TBM grant of the Flanders Fund for Scientific Re-search (FWO-TBM T001821N) granted to K.A. and B.B., as well as by the Branco Weiss fellowship of the Society in Science – ETH Zurich granted to K.A. and the Excellence of Science grant (EOS; G0E8718N; HUMVISCAT) and Flanders Fund for Scientific Research grant (FWO; G023923N) granted to B.B.. M.M. was supported by a KU Leuven Postdoctoral Mandate (PDM/22/065). J.P. was supported by the Marguerite-Marie Delacroix foundation and a postdoctoral fellowship of the Flanders Fund for Scientific Research (FWO; 1257621N). T.T. was supported by the Fund Child Hospital UZ Leuven. S.VdD. was supported by a KU Leuven Postdoctoral Mandate (PDM/20/170) and a postdoctoral fellowship of the Flanders Fund for Scientific Research (FWO; 12C9723N).
Data availability
Data is available upon reasonable request.
Declarations
Competing interests
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Matthijs Moerkerke and Nicky Daniels have contributed as shared first authors.
Kaat Alaerts and Bart Boets have contributed as shared last authors.
References
- 1.Adolphs R. Fear, faces, and the human amygdala. Curr Opin Neurobiol. 2008;18(2):166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adolphs R, Tranel D, Hamann S, Young AW, Calder AJ, Phelps EA, Anderson A, Lee GP, Damasio AR. Recognition of facial emotion in nine individuals with bilateral amygdala damage. Neuropsychologia. 1999;37(10):1111–7. [DOI] [PubMed] [Google Scholar]
- 3.Alaerts K, Bernaerts S, Prinsen J, Dillen C, Steyaert J, Wenderoth N. Oxytocin induces long-lasting adaptations within amygdala circuitry in autism: a treatment-mechanism study with randomized placebo-controlled design. Neuropsychopharmacology. 2020;45(7):1141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alaerts K, Daniels N, Moerkerke M, Evenepoel M, Tang T, Van Der Donck S, Chubar V, Claes S, Steyaert J, Boets B, Prinsen J. At the head and heart of oxytocin’s stress-regulatory neural and cardiac effects: a chronic administration RCT in children with autism. Psychother Psychosom. 2023;92:315–28. [DOI] [PubMed] [Google Scholar]
- 5.Alaerts K, Taillieu A, Daniels N, Soriano JR, Prinsen J. Oxytocin enhances neural approach towards social and non-social stimuli of high personal relevance. Sci Rep. 2021. 10.1038/s41598-021-02914-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 7.Andari E, Richard N, Leboyer M, Sirigu A. Adaptive coding of the value of social cues with oxytocin, an fMRI study in autism spectrum disorder. Cortex. 2016;76:79–88. [DOI] [PubMed] [Google Scholar]
- 8.Aoki Y, Cortese S, Tansella M. Neural bases of atypical emotional face processing in autism: a meta-analysis of fMRI studies. World J Biol Psychiatry. 2015;16(5):291–300. [DOI] [PubMed] [Google Scholar]
- 9.Aoki Y, Yahata N, Watanabe T, Takano Y, Kawakubo Y, Kuwabara H, Iwashiro N, Natsubori T, Inoue H, Suga M, Takao H, Sasaki H, Gonoi W, Kunimatsu A, Kasai K, Yamasue H. Oxytocin improves behavioural and neural deficits in inferring others’ social emotions in autism. Brain. 2014;137(11):3073–86. [DOI] [PubMed] [Google Scholar]
- 10.Axelrod V, Yovel G. Successful decoding of famous faces in the fusiform face area. PLoS ONE. 2015;10(2): e0117126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bakermans-Kranenburg M, Van Ijzendoorn M. Sniffing around oxytocin: review and meta-analyses of trials in healthy and clinical groups with implications for pharmacotherapy. Transl Psychiatry. 2013;3(5):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends Cognit Sci. 2011;15(7):301–9. [DOI] [PubMed] [Google Scholar]
- 13.Bate S, Bennetts R, Parris BA, Bindemann M, Udale R, Bussunt A. Oxytocin increases bias, but not accuracy, in face recognition line-ups. Soc Cognit Affect Neurosci. 2015;10(7):1010–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc: Ser B (Methodol). 1995;57(1):289–300. [Google Scholar]
- 15.Bernaerts S, Boets B, Steyaert J, Wenderoth N, Alaerts K. Oxytocin treatment attenuates amygdala activity in autism: a treatment-mechanism study with long-term follow-up. Transl Psychiatry. 2020;10(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bethlehem RAI, Baron-Cohen S, van Honk J, Auyeung B, Bos PA. The oxytocin paradox. Front Behav Neurosci. 2014;8(48):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bölte S, Hubl D, Feineis-Matthews S, Prvulovic D, Dierks T, Poustka F. Facial affect recognition training in autism: can we animate the fusiform gyrus? Behav Neurosci. 2006;120(1):211–6. [DOI] [PubMed] [Google Scholar]
- 18.Brambati SM, Benoit S, Monetta L, Belleville S, Joubert S. The role of the left anterior temporal lobe in the semantic processing of famous faces. Neuroimage. 2010;53(2):674–81. [DOI] [PubMed] [Google Scholar]
- 19.Brothers L. The neural basis of primate social communication. Motiv Emot. 1990;14(2):81–91. [Google Scholar]
- 20.Busigny T, Joubert S, Felician O, Ceccaldi M, Rossion B. Holistic perception of the individual face is specific and necessary: evidence from an extensive case study of acquired prosopagnosia. Neuropsychologia. 2010;48(14):4057–92. [DOI] [PubMed] [Google Scholar]
- 21.Constantino J, Gruber C. Social responsiveness scale 2nd. ed: SRS-2. Manual. Western Psychological Services. 2012.
- 22.Costa C, Cristea IA, Dal Bò E, Melloni C, Gentili C. Brain activity during facial processing in autism spectrum disorder: an activation likelihood estimation (ALE) meta-analysis of neuroimaging studies. J Child Psychol Psychiatry. 2021;62(12):1412–24. [DOI] [PubMed] [Google Scholar]
- 23.Daniels N, Moerkerke M, Steyaert J, Bamps A, Debbaut E, Prinsen J, Tang T, Van Der Donck S, Boets B, Alaerts K. Effects of multiple-dose intranasal oxytocin administration on social responsiveness in children with autism: a randomized, placebo-controlled trial. Mol Autism. 2023;14:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Declerck CH, Boone C, Kiyonari T. Oxytocin and cooperation under conditions of uncertainty: the modulating role of incentives and social information. Horm Behav. 2010;57(3):368–74. [DOI] [PubMed] [Google Scholar]
- 25.Di Martino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, Milham MP. Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation meta-analysis. Biol Psychiat. 2009;65(1):63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Domes G, Heinrichs M, Gläscher J, Büchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiat. 2007;62(10):1187–90. [DOI] [PubMed] [Google Scholar]
- 27.Domes G, Heinrichs M, Kumbier E, Grossmann A, Hauenstein K, Herpertz SC. Effects of intranasal oxytocin on the neural basis of face processing in autism spectrum disorder. Biol Psychiat. 2013;74(3):164–71. [DOI] [PubMed] [Google Scholar]
- 28.Domes G, Kumbier E, Heinrichs M, Herpertz SC. Oxytocin promotes facial emotion recognition and amygdala reactivity in adults with Asperger syndrome. Neuropsychopharmacology. 2014;39(3):698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35(1):83–93. [DOI] [PubMed] [Google Scholar]
- 30.Duchaine B, Nakayama K. The Cambridge Face Memory Test: Results for neurologically intact individuals and an investigation of its validity using inverted face stimuli and prosopagnosic participants. Neuropsychologia. 2006;44(4):576–85. [DOI] [PubMed] [Google Scholar]
- 31.Duchaine B, Yovel G. A Revised neural framework for face processing. Ann Rev Vision Sci. 2015;1(1):393–416. [DOI] [PubMed] [Google Scholar]
- 32.Fathabadipour S, Mohammadi Z, Roshani F, Goharbakhsh N, Alizadeh H, Palizgar F, Cumming P, Michel TM, Vafaee MS. The neural effects of oxytocin administration in autism spectrum disorders studied by fMRI: a systematic review. J Psychiatr Res. 2022;154(August):80–90. [DOI] [PubMed] [Google Scholar]
- 33.Ford CL, Young LJ. Refining oxytocin therapy for autism: context is key. Nat Rev Neurol. 2021;18(2):67–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, Benedetti F, Abbamonte M, Gasparotti R, Barale F, Perez J, McGuire P, Politi P. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34(6):418–32. [PMC free article] [PubMed] [Google Scholar]
- 35.Gamer M, Zurowski B, Büchel C. Different amygdala subregions mediate valence- related and attentional effects of oxytocin in humans. Proc Natl Acad Sci. 2010;108(7):3092–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geschwind DH. Oxytocin for autism spectrum disorder — down, but not out. N Engl J Med. 2021;385(16):1524–5. [DOI] [PubMed] [Google Scholar]
- 37.Goesaert E, Op de Beeck HP. Representations of facial identity information in the ventral visual stream investigated with multivoxel pattern analyses. J Neurosci. 2013;33(19):8549–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gordon I, Vander Wyk BC, Bennett RH, Cordeaux C, Lucas MV, Eilbott JA, Zagoory-Sharon O, Leckman JF, Feldman R, Pelphrey KA. Oxytocin enhances brain function in children with autism. Proc Natl Acad Sci. 2013;110(52):20953–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guastella AJ, Hickie IB, McGuinness MM, Otis M, Woods EA, Disinger HM, Chan HK, Chen TF, Banati RB. Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinology. 2013;38(5):612–25. [DOI] [PubMed] [Google Scholar]
- 40.Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4(6):223–33. [DOI] [PubMed] [Google Scholar]
- 41.Haxby JV, Gobbini MI. Distributed neural systems for face perception. In The Oxford Handbook of Face Perception. Oxford: Oxford University Press; 2011. [Google Scholar]
- 42.Haxby JV, Petit L, Ungerleider LG, Courtney SM. Distinguishing the functional roles of multiple regions in distributed neural systems for visual working memory. Neuroimage. 2000;11:380. [DOI] [PubMed] [Google Scholar]
- 43.Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol. 2009;30(4):548–57. [DOI] [PubMed] [Google Scholar]
- 44.Hendriks MHA, Dillen C, Vettori S, Vercammen L, Daniels N, Steyaert J, Op de Beeck H, Boets B. Neural processing of facial identity and expression in adults with and without autism: a multi-method approach. NeuroImage: Clin. 2021;29:102520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ishai A. Let’s face it: it’s a cortical network. Neuroimage. 2008;40(2):415–9. [DOI] [PubMed] [Google Scholar]
- 46.Ishai A, Schmidt CF, Boesiger P. Face perception is mediated by a distributed cortical network. Brain Res Bull. 2005;67(1–2):87–93. [DOI] [PubMed] [Google Scholar]
- 47.Johnson MH, Dziurawiec S, Ellis H, Morton J. Newborns’ preferential tracking of face-like stimuli and its subsequent decline. Cognition. 1991;40(1–2):1–19. [DOI] [PubMed] [Google Scholar]
- 48.Johnson MH, Senju A, Tomalski P. The two-process theory of face processing: modifications based on two decades of data from infants and adults. Neurosci Biobehav Rev. 2015;50:169–79. [DOI] [PubMed] [Google Scholar]
- 49.Jurek B, Neumann ID. The oxytocin receptor: from intracellular signaling to behavior. Physiol Rev. 2018;98(3):1805–908. [DOI] [PubMed] [Google Scholar]
- 50.Kanat M, Heinrichs M, Mader I, Van Elst LT, Domes G. Oxytocin modulates amygdala reactivity to masked fearful eyes. Neuropsychopharmacology. 2015;40(11):2632–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17(11):4302–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kirsch P. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25(49):11489–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kleinhans NM, Richards T, Johnson LC, Weaver KE, Greenson J, Dawson G, Aylward E. fMRI evidence of neural abnormalities in the subcortical face processing system in ASD. Neuroimage. 2011;54(1):697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kleinhans NM, Richards T, Weaver K, Johnson LC, Greenson J, Dawson G, Aylward E. Association between amygdala response to emotional faces and social anxiety in autism spectrum disorders. Neuropsychologia. 2010;48(12):3665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25(3–4):150–76. [DOI] [PubMed] [Google Scholar]
- 56.Langenbach BP, Grotegerd D, Mulders PCR, Tendolkar I, van Oort J, Duyser F, van Eijndhoven P, Vrijsen JN, Dannlowski U, Kampmann Z, Koelkebeck K. Autistic and non-autistic individuals show the same amygdala activity during emotional face processing. Mol Autism. 2024;15(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Langner O, Dotsch R, Bijlstra G, Wigboldus DHJ, Hawk ST, van Knippenberg A. Presentation and validation of the radboud faces database. Cogn Emot. 2010;24(8):1377–88. [Google Scholar]
- 58.Le J, Zhang L, Zhao W, Zhu S, Lan C, Kou J, Zhang Q, Zhang Y, Li Q, Chen Z, Fu M, Montag C, Zhang R, Yang W, Becker B, Kendrick KM. Infrequent intranasal oxytocin followed by positive social interaction improves symptoms in autistic children: a pilot randomized clinical trial. Psychother Psychosom. 2022;91:335. [DOI] [PubMed] [Google Scholar]
- 59.Leveroni CL, Seidenberg M, Mayer AR, Mead LA, Binder JR, Rao SM, Bellgowan P, Cabeza R, Cox R, Cunningham J, Fuller S, Hammeke T, Hyde J, Paller K, Parsons M, Prieto T, Rosen A, Rowe K, Stein E, Woodley S. Neural systems underlying the recognition of familiar and newly learned faces. J Neurosci. 2000;20(2):878–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lord C, Rutter M, Dilavore PC, Risi S, Gotham K, Bishop SL, Luyster RJ, Guthrie W. ADOS-Autisme diagnostisch observatieschema Handleiding. Amsterdam: Hogrefe; 2012. [Google Scholar]
- 61.Ma Y, Shamay-Tsoory S, Han S, Zink CF. Oxytocin and social adaptation: insights from neuroimaging studies of healthy and clinical populations. Trends Cogn Sci. 2016;20(2):133–45. [DOI] [PubMed] [Google Scholar]
- 62.Moerkerke M, Daniels N, Van der Donck S, Tibermont L, Tang T, Debbaut E, Bamps A, Prinsen J, Steyaert J, Alaerts K, Boets B. Can repeated intranasal oxytocin administration affect reduced neural sensitivity towards expressive faces in autism? A randomized controlled trial. J Child Psychol Psychiatry. 2023;64(11):1583–95. [DOI] [PubMed] [Google Scholar]
- 63.Monk CS, Weng S, Wiggins JL, Kurapati N, Louro HMC, Carrasco M, Maslowsky J, Risi S, Lord C. Neural circuitry of emotional face processing in autism spectrum disorders. J Psychiatry Neurosci. 2010;35(2):105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muris P, Bodden D, Hale W, Birmaher B, Mayer B. SCARED-NL. Vragenlijst over angst en bang-zijn bij kinderen en adolescenten Handleiding bij de gereviseerde Nederlandse versie van de Screen for Child Anxiety Related Emotional Disorders. Amsterdam: Boom Uitgevers; 2007. [Google Scholar]
- 65.Muukkonen I, Salmela VR. Representational structure of fMRI/EEG responses to dynamic facial expressions. Neuroimage. 2022;263(September): 119631. [DOI] [PubMed] [Google Scholar]
- 66.Nomi JS, Uddin LQ. Face processing in autism spectrum disorders: from brain regions to brain networks. Neuropsychologia. 2015;71:201–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Philip RCM, Dauvermann MR, Whalley HC, Baynham K, Lawrie SM, Stanfield AC. A systematic review and meta-analysis of the fMRI investigation of autism spectrum disorders. Neurosci Biobehav Rev. 2012;36(2):901–42. [DOI] [PubMed] [Google Scholar]
- 68.Pitcher D, Walsh V, Duchaine B. The role of the occipital face area in the cortical face perception network. Exp Brain Res. 2011;209(4):481–93. [DOI] [PubMed] [Google Scholar]
- 69.Puce A, Allison T, Bentin S, Gore JC, McCarthy G. Temporal cortex activation in humans viewing eye and mouth movements. J Neurosci. 1998;18(6):2188–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Quintana DS, Westlye LT, Rustan OG, Tesli N, Poppy CL, Smevik H, Tesli M, Røine M, Mahmoud RA, Smerud KT, Djupesland PG, Andreassen OA. Low-dose oxytocin delivered intranasally with Breath Powered device affects social-cognitive behavior: a randomized four-way crossover trial with nasal cavity dimension assessment. Transl Psychiatry. 2015;5(7):e602–e602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Riddoch MJ, Johnston RA, Bracewell RM, Boutsen L, Humphreys GW. Are faces special? A case of pure prosopagnosia. Cognit Neuropsychol. 2008;25(1):3–26. 10.1080/02643290801920113. [DOI] [PubMed] [Google Scholar]
- 72.Rosset DB, Rondan C, Da Fonseca D, Santos A, Assouline B, Deruelle C. Typical emotion processing for cartoon but not for real faces in children with autistic spectrum disorders. J Autism Dev Disord. 2008;38(5):919–25. [DOI] [PubMed] [Google Scholar]
- 73.Rossion B. Understanding individual face discrimination by means of fast periodic visual stimulation. Exp Brain Res. 2014;232(6):1599–621. [DOI] [PubMed] [Google Scholar]
- 74.Schobert AK, Corradi-Dell’Acqua C, Frühholz S, van der Zwaag W, Vuilleumier P. Functional organization of face processing in the human superior temporal sulcus: a 7T high-resolution fMRI study. Soc Cognit Affect Neurosci. 2018;13(1):102–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schulze L, Lischke A, Greif J, Herpertz SC, Heinrichs M, Domes G. Oxytocin increases recognition of masked emotional faces. Psychoneuroendocrinology. 2011;36(9):1378–82. [DOI] [PubMed] [Google Scholar]
- 76.Shamay-Tsoory SG, Abu-Akel A. The social salience hypothesis of oxytocin. Biol Psychiat. 2016;79(3):194–202. [DOI] [PubMed] [Google Scholar]
- 77.Shamay-Tsoory SG, Fischer M, Dvash J, Harari H, Perach-Bloom N, Levkovitz Y. Intranasal administration of oxytocin increases envy and schadenfreude (Gloating). Biol Psychiat. 2009;66(9):864–70. [DOI] [PubMed] [Google Scholar]
- 78.Sprengelmeyer R, Rausch M, Eysel UT, Przuntek H. Neural structures associated with recognition of facial expressions of basic emotions. Proc Royal Soc London Series: B Biol Sci. 1998;265:1927–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stoop R. Neuromodulation by oxytocin and vasopressin in the central nervous system as a basis for their rapid behavioral effects. Curr Opin Neurobiol. 2014;29:187–93. [DOI] [PubMed] [Google Scholar]
- 80.Taylor MJ, Arsalidou M, Bayless SJ, Morris D, Evans JW, Barbeau EJ. Neural correlates of personally familiar faces: parents, partner and own faces. Human Brain Mapp. 2009;30(7):2008–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tottenham N, Hertzig ME, Gillespie-Lynch K, Gilhooly T, Millner AJ, Casey BJ. Elevated amygdala response to faces and gaze aversion in autism spectrum disorder. Soc Cognit Affect Neurosci. 2014;9(1):106–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Uljarevic M, Hamilton A. Recognition of emotions in autism: a formal meta-analysis. J Autism Dev Disord. 2013;43(7):1517–26. [DOI] [PubMed] [Google Scholar]
- 83.Van Der Geest JN, Kemner C, Verbaten MN, Van Engeland H. Gaze behavior of children with pervasive developmental disorder toward human faces: a fixation time study. J Child Psychol Psychiatry. 2002;43(5):669–78. [DOI] [PubMed] [Google Scholar]
- 84.Van IJzendoorn MH, Bakermans-Kranenburg MJ. A sniff of trust: meta-analysis of the effects of intranasal oxytocin administration on face recognition, trust to in-group, and trust to out-group. Psychoneuroendocrinology. 2012;37(3):438–43. [DOI] [PubMed] [Google Scholar]
- 85.Watanabe T, Kuroda M, Kuwabara H, Aoki Y, Iwashiro N, Tatsunobu N, Takao H, Nippashi Y, Kawakubo Y, Kunimatsu A, Kasai K, Yamasue H. Clinical and neural effects of six-week administration of oxytocin on core symptoms of autism. Brain. 2015;138(11):3400–12. [DOI] [PubMed] [Google Scholar]
- 86.Wechsler D. WISC-V-NL. Wechsler Intelligence Scale for Children. 5th ed. Amsterdam: Pearson Benelux B.V; 2018. [Google Scholar]
- 87.Weigelt S, Koldewyn K, Kanwisher N. Face identity recognition in autism spectrum disorders: a review of behavioral studies. Neurosci Biobehav Rev. 2012;36(3):1060–84. [DOI] [PubMed] [Google Scholar]
- 88.Weng S-J, Carrasco M, Swartz JR, Wiggins JL, Kurapati N, Liberzon I, Risi S, Lord C, Monk CS. Neural activation to emotional faces in adolescents with autism spectrum disorders. J Child Psychol Psychiatry. 2011;52(3):296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–41. [DOI] [PubMed] [Google Scholar]
- 90.Yargholi E, de Beeck HO. Category trumps shape as an organizational principle of object space in the human Occipitotemporal cortex. J Neurosci. 2023;43(16):2960–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yatawara CJ, Einfeld SL, Hickie IB, Davenport TA, Guastella AJ. The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: a randomized clinical crossover trial. Mol Psychiatry. 2016;21:1225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zalla T, Sperduti M. The amygdala and the relevance detection theory of autism: an evolutionary perspective. Front Human Neurosci. 2013;7:894. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available upon reasonable request.