Abstract
Background
Bovine mastitis significantly impacts the dairy industry, causing economic losses through reduced milk production, lower milk quality, and increased health risks, and early detection is critical for effective treatment. This study analyzed milk electrical conductivity data from 9,846 Chinese Holstein cows over a two-year period, collected during three daily milking sessions, alongside smart collar data and dairy herd improvement test results. The aim was to conduct a comprehensive genetic analysis and assess the potential of milk electrical conductivity as a biomarker for the early detection of bovine subclinical mastitis.
Results
The results revealed significant phenotypic and strong genetic correlations (-0.286 to 0.457) between milk electrical conductivity, somatic cell score, milk yield, activity quantity, and milking speed. Logistic regression models yielded area under the curve values ranging from 0.636 to 0.697 and odds ratio values from 9.70 to 10.69, demonstrating a certain predictive capability of milk electrical conductivity for identifying subclinical mastitis. Various factors influencing milk electrical conductivity, including lactation stage, environmental conditions, age at first calving, parity, and body condition score, were identified. The random regression model demonstrated moderate to high heritability of milk electrical conductivity (0.458 to 0.487), particularly during the early to mid-lactation periods, with all estimates exceeding 0.35 However, after day 275 of lactation, the heritability decreased to below 0.2. Notably, shifts in genetic factors affecting milk components were observed around 60 and 270 days into lactation, with increased environmental sensitivity to milk electrical conductivity during these periods.
Conclusions
This study demonstrates that milk electrical conductivity is influenced by multiple factors, such as age at first calving, parity, and body condition score, and exhibits significant phenotypic associations with somatic cell score, milk yield, activity quantity, and milking speed. Although milk electrical conductivity showed moderate to high heritability and potential as a predictor for subclinical mastitis, its low genetic correlations with SCS limit its effectiveness as a standalone indicator. Future research should focus on combining EC with other indicators to improve the accuracy of mastitis detection.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12864-024-11157-6.
Keywords: Milk electrical conductivity, Mastitis predictive ability, Random regression model, Genetic characteristics
Introduction
Bovine mastitis, an inflammation of the mammary gland, is among the most common and economically detrimental diseases affecting dairy cows. Its occurrence is strongly influenced by both environmental factors—such as pathogens, environmental conditions, and management practices—and genetic factors [1–4]. The most commonly utilized indicators for selecting mastitis resistance are clinical mastitis incidents for direct selection and somatic cell count (SCC) for indirect selection [5, 6]. However, the increasing scale of dairy farms complicates the management of mastitis detection due to greater workloads for milking staff and frequent turnover of personnel [2, 7]. In many large-scale Chinese dairy farms, SCC measurements are typically performed monthly as part of the dairy herd improvement (DHI) program, which can result in delays in mastitis detection [8, 9]. Consequently, farmers are exploring more direct and effective methods for detecting mastitis.
Milk electrical conductivity (EC) has emerged as a promising tool for early mastitis detection, as bacterial infections in the udder alter the concentrations of anions and cations (K + , Na + , Cl-), leading to an increase in EC [10, 11]. With the advancement of digital and mechanized farm management tools, EC can now be automatically measured during milking sessions using sensors integrated into automatic milking systems, providing multiple daily readings. This capability has made EC a widely accepted method for early mastitis detection in modern dairy management practices [12]. Despite numerous studies highlighting a strong positive correlation between EC and clinical mastitis incidence [13–15], previous research on the heritability and genetic parameters of EC as an indicator for mastitis has been constrained by small sample sizes and models that inadequately capture genetic variability across lactation stages. Moreover, there is limited research on the EC characteristics of Chinese Holstein cows [16, 17]. Random regression test-day model, which offers a more precise analysis of genetic and environmental influences across different lactation stages, has been extensively applied for genetic evaluation of dairy production traits, resulting in more reliable genetic evaluations [18–20]. The model requires repeated measurements of multiple traits over time for each cow and a large dataset to accurately model individual and population-level variances [20]. As a result, the random regression model is particularly well-suited for analyzing the genetic characteristics of daily measurement data collected through automated milking systems in dairy cows.
This study aims to achieve three objectives: first, to quantify the association between EC and milk production traits in Chinese Holstein cow; second, to explore the genetic characteristics of the EC trait using a random regression model; and third, to evaluate the feasibility of using EC as an indicator for diagnosing bovine mastitis. This research is expected to help reduce the incidence of mastitis and support the high-quality development of the dairy industry.
Materials and methods
Animals, diets, and feeding
The data used in this study were derived from 9,846 Holstein cows, housed in three medium- to large-scale contemporary dairy farms located in Jiangsu Province, China. These cows were accommodated in free-stall housing within a double-row barns, with bedding made of dried bovine manure. They were fed a total mixed ration (TMR) and were milked and fed three times daily.
Phenotype collections and preparations
This study collected data from 9,846 Holstein dairy cows using milking machines and smart collars, recording data across three daily intervals (6:00, 14:00, 21:00) throughout 2021–2022. The milking machine recorded various parameters, including the EC at each milking session—first (EC_1), second (EC_2), third (EC_3)—as well as the daily average EC (EC_ave) and average milking speed (MS). The MS was defined as the ratio of milk production to the time spent per milking session. The smart collars recorded the activity quantity (AQ) of cows in the barn before each milking session, capturing a total of 1,889,318 data records, with AQ defined as the number of steps a cow taken per hour. The system could gather multiple parameters such as EC, milk yield (MY), and MS for each milking session, while cow activity data was recorded bihourly via the smart collars. Additionally, SCC data from DHI over the two-year period contributed 76,554 records. Both the milking information system and smart collars were products of Afimilk company. Furthermore, body condition scores (BCS) for all 9,846 cows were assessed following the method of Wildman et al. [21], focusing on the degree of flesh over the lumbar, pelvic, and tailhead regions. The BCS ranged from 1 (extremely thin) to 5 (extremely fat), with increments of 0.25 points.
After data collection, rigorous quality control measures were implemented based on specified criteria: 1) first calving age between 22 and 32 months, with lactation days between 5 and 305; 2) daily milk production ranging from 5 to 80 kg, and somatic cell score (SCS) values between 0 and 9, calculated using the formula SCS = log₂(SCC/100,000) + 3, where SCC is the somatic cell count; 3) for activity metrics, only data recorded within the barn were considered, with averages calculated on a daily basis; and 4) for each trait, values exceeding the mean by more than three standard deviations were removed. Following these quality control steps, a dataset was compiled for 8,455 cows, containing 1,048,575 entries each for EC_1, EC_2, EC_3, EC_ave, MY, MS, and AQ, along with 67,560 entries for SCS. For genetic evaluation, cows with distinct phenotypes were traced back through three generations (parents, grandparents, maternal grandparents), resulting in an average pedigree depth of 3.25 generations and a comprehensive pedigree file encompassing 16,423 Holsteins (195 bulls and 16,228 cows).
Analysis of variance for EC
A General Linear Model (GLM) procedure was used to perform an analysis of variance (ANOVA) on the EC in R statistical software (v4.2.1). This analysis considered various factors including the season of measurement, age at first calving, measurement parity, and BCS. To control for Type I errors in post hoc multiple comparisons, the Duncan test was employed. The fixed effects model used to identify factors influencing EC is specified as follows:
| 1 |
where denotes the individual phenotypic value of EC at each milking session, is the overall mean. The is the -th farm effect; is classified into two discrete categories corresponding to the -th year of measurement: 2021 and 2022; Seasonal effects () are segmented into four periods: spring (March to May), summer (June to August), autumn (September to November), and winter (December to February). The reflects the -th month of first calving age effect, categorized into six levels, with each group spanning a two-month range from 22 to 34 months (e.g., 22 < age ≤ 24 months, 24 < age ≤ 26 months, etc.). The denotes the -th parity effect. The is the BCS for the -th individual ranges from 2.5 to 4.0 in 0.25-point increments, and the day in milk () is categorized into three intervals, covering days 5 to 305 of lactation. Lastly, the represents the residual effect.
Phenotypic correlation analysis
The correlation analysis was performed using R statistical software (v4.2.1) to quantify the relationships between EC measurements from different milking sessions and various milk production traits. Pearson’s correlation coefficient was calculated using the cor function. For correlations involving SCS, only records from test days where SCS measurements were available and coincided with the daily records of EC and milk production traits were included in the analysis. To enhance the interpretability of the statistical findings, the corrplot package in R software (v4.2.1) was employed to generate a color-coded graphical representation of the correlation matrix.
Logistic regression analysis
To better understand the predictive capacity of EC traits for identifying subclinical mastitis in dairy cows, we established a threshold aligned with international standards: 200,000 somatic cells per milliliter in milk [22, 23]. This benchmark serves as a critical indicator for assessing bovine health and determining the potential onset of mastitis. A SCC exceeding this threshold classifies a cow as having subclinical mastitis, while a count below it indicates a healthy status. This criterion enables a more precise analysis of mastitis prevalence within the dairy cow population. Following this classification, logistic regression modeling was employed to evaluate the impact of EC and other factors, such as the season of measurement, age at first calving, parity, and BCS, on the occurrence of subclinical mastitis in dairy cows. To determine the overall significance of the variables, the Wald test was conducted before proceeding with group comparisons. The logistic regression model used is outlined as follows:
| 2 |
where represents the probability of the cow has subclinical mastitis; is the constant term; The variables , , , , and are defined as in Eq. 1. Additionally, represents the BCS for each cow, which ranges from 2.5 to 4.0, with increments of 0.25. In this model, and are included as a covariate; encompasses and , with only one EC trait evaluated in the model at a time. Here, , , and referred to the EC at the first, second, and third milking sessions of the day, respectively, and represents the daily average EC. The regression coefficients and correspond to the effects of , while represents the residual error.
Genetic characteristics analysis
Genetic parameters for EC and milk production traits were estimated using a random regression model. Variance components were calculated through the average information restricted maximum likelihood (AI-REML) algorithm, as implemented in DMU software (v5.6) [24]. The expectation–maximization (EM) algorithm was used when the AI-REML algorithm did not converge. The model is defined as follows:
| 3 |
where represents the phenotypic observation recorded on the measurement day, with denoting the effect of the -th year, season, and herd. The effects , , and align with those specified in Eq. 1 and are included as fixed effects. The term represents the fixed regression coefficient of the -th order polynomial, while indicates the random regression coefficient of the -th order polynomial for the additive genetic effect of the -th cow. Similarly, is the random regression coefficient for the permanent environmental effect of the -th cow. The function corresponds to the covariate of the -th order polynomial on the lactation day, is the standardized value for the -th lactation day, calculated as . The polynomial degree λ represents the highest order of polynomial included in the model: λ is set to 3 for EC_1, EC_2, EC_3, SCS, MY, MS, and AQ, and 4 for EC_ave. The random residual is assumed to have homogeneous variance throughout the lactation stage. Model convergence is determined by two criteria: the norm of the parameter update vector being less than 1.0 × 10−7 or the norm of the gradient vector (AI) being less than1.0 × 10−6. Pedigree information is utilized within the model to construct the relationship matrix, ensuring accurate representation of genetic kinships.
After removing all fixed effects, the phenotype of each individual was adjusted to the 305-day performance value according to the following formula:
| 4 |
where is the adjusted phenotypic value corresponding to the electrical conductivity of the cow at each milking session, and denotes the random residual corresponding to the -th lactation day of each parity. The remaining elements in this formula are consistent with those defined in formula 3.
The additive genetic variance, permanent environmental effect variance, heritability, and genetic correlation, as well as the correlation of permanent environmental effect at different time points within the lactation stage were calculated according to the following formulas:
| 5 |
| 6 |
| 7 |
| 8 |
| 9 |
| 10 |
| 11 |
where denotes the variance of additive genetic effects on the -th lactation day, while represents the variance of permanent environmental effects specific to the same lactation day. The covariance between additive genetic effects across two distinct lactation days, and , is represented by , and the covariance of permanent environmental effects between these days is indicated by . The vector corresponds to the Legendre polynomial associated with the -th lactation day. The additive genetic correlation between two lactation days, and , is designated as , while the correlation of permanent environmental effects for these days is denoted as . The matrices and are the estimated (co)variance matrices for the stochastic regression terms, based on the order of the Legendre polynomial, and represent the additive genetic and permanent environmental effects, respectively. Heritability on the -th lactation day is indicated by , whereas refers to the overall heritability of the trait adjusted to 305-day performance. Lastly, specifies the residual variance within the model.
In addition, to explore the genetic relationships between EC traits and milk production traits (milk production, milking speed, activity level and SCS), a multi-trait animal model was developed using the corrected phenotypes of these eight traits.
| 12 |
where represents the adjusted phenotypic value of EC_1, EC_2, EC_3, EC_ave, SCS, MY, MS, and AQ, with the adjustment method following the approach outlined in a previous study [19]. The term is a unit vector, and is the mean of the evaluation population. The variable is the additive genetic effects of all individuals, assumed to follow a anormal distribution with a mean value of 0 and variance , where is the kinship matrix constructed from pedigree data, and is the design matrix associated with . The term is the random residual of the model, which follow a normal distribution with a mean of 0 and variance , where is the unit vector.
Results
The statistical description of phenotype
In the analysis of 104,857 records from 8,455 Chinese Holstein cows, the mean EC values across different sessions showed slight variations, ranging from 9.314 to 9.372, with the smallest variation observed in the daily average (Table 1). The SCS had a low mean but exhibited high variability, while MY exhibited a broad range from 9.5 to 64.6, reflecting diverse production levels. Similar variability was noted for MS and AQ (Table S1). Frequency observations and cumulative density results indicated that all traits approximate a normal distribution, as depicted in Figure S1.
Table 1.
Descriptive statistical analysis of EC in Chinese Holstein dairy cattle
| EC (mS/cm) | RN | Mean | SD | Min | Max | Skew | Kurtosis |
|---|---|---|---|---|---|---|---|
| EC_1 | 1048575 | 9.372 | 0.674 | 7.100 | 11.412 | 0.010 | 0.006 |
| EC_2 | 1048575 | 9.314 | 0.672 | 6.900 | 11.124 | 0.045 | 0.002 |
| EC_3 | 1048575 | 9.321 | 0.672 | 7.400 | 11.100 | 0.040 | 0.006 |
| EC_ave | 1048575 | 9.343 | 0.591 | 7.200 | 11.252 | 0.024 | −0.001 |
RN Record number, SD Standard deviation, Min the minimum value, Max the maximum value, EC_1 the EC at the first milking session in a day, EC_2 the EC at the second milking session in a day, EC_3 the EC at the third milking session in a day, EC_ave the daily average EC
Factors affecting the variation of EC
The ANOVA test revealed the impact of various factors on the EC traits from Chinese Holstein dairy cows, with particular emphasis on the seasonal variation, age at first delivery, parity, and BCS (Table 2). Seasonal changes had a consistent influence on electrical conductivity measurements across the first three milking sessions (EC_1, EC_2, EC_3) and the daily average (EC_ave), with the highest conductivity observed in the second season. The cows with a first calving age of 25–27 months exhibited lower EC values, whereas those with a first calving age of 34–36 months presented marginally higher conductivity values. Additionally, EC displayed a subtle yet steady increase in conductivity with an increasing number of parities. The relationship between BCS and EC was more complex, as neither the leanest nor the fattest cows showed a high electrical conductivity, indicating an optimal range for conductivity that correlates with cow health. These factors, each interacting differently with electrical conductivity, underscore the multifaceted nature of milk composition traits.
Table 2.
The ANOVA test analysis of various factors influencing the EC
| Factor | Level | Number | EC_1 | EC_2 | EC_3 | EC_ave |
|---|---|---|---|---|---|---|
| Farm | 1 | 346649 | 9.371 ± 0.673A | 9.378 ± 0.671A | 9.376 ± 0.671A | 9.371 ± 0.610A |
| 2 | 332941 | 9.342 ± 0.674A | 9.373 ± 0.673A | 9.373 ± 0.673A | 9.376 ± 0.611A | |
| 3 | 369546 | 9.382 ± 0.675A | 9.373 ± 0.672A | 9.378 ± 0.673A | 9.374 ± 0.615A | |
| Year | 2021 | 513862 | 9.474 ± 0.672B | 9.474 ± 0.675B | 9.474 ± 0.672B | 9.474 ± 0.607B |
| 2022 | 534713 | 9.274 ± 0.684A | 9.274 ± 0.661A | 9.274 ± 0.657A | 9.274 ± 0.601A | |
| Season | Spring | 337040 | 9.287 ± 0.668A | 9.248 ± 0.667B | 9.254 ± 0.671B | 9.263 ± 0.609B |
| Summer | 263005 | 9.477 ± 0.679B | 9.413 ± 0.674C | 9.402 ± 0.675C | 9.430 ± 0.606C | |
| Autumn | 202002 | 9.483 ± 0.662C | 9.414 ± 0.667C | 9.423 ± 0.664D | 9.440 ± 0.601D | |
| Winter | 246528 | 9.285 ± 0.657A | 9.218 ± 0.654A | 9.246 ± 0.657A | 9.250 ± 0.598A | |
| First calving age | 22–24 | 104845 | 9.305 ± 0.622B | 9.250 ± 0.626B | 9.248 ± 0.626B | 9.268 ± 0.562B |
| 24–26 | 74790 | 9.532 ± 0.479E | 9.466 ± 0.611E | 9.486 ± 0.464E | 9.495 ± 0.274E | |
| 26–28 | 533015 | 9.286 ± 0.675A | 9.231 ± 0.679A | 9.237 ± 0.675A | 9.251 ± 0.607A | |
| 28–30 | 197600 | 9.414 ± 0.675C | 9.354 ± 0.671C | 9.362 ± 0.672C | 9.377 ± 0.611C | |
| 30–32 | 62739 | 9.515 ± 0.687D | 9.456 ± 0.692D | 9.465 ± 0.686D | 9.479 ± 0.617D | |
| 32–34 | 39251 | 9.710 ± 0.682F | 9.654 ± 0.692F | 9.664 ± 0.679F | 9.676 ± 0.612F | |
| Parity | 1 | 376314 | 9.040 ± 0.553A | 8.999 ± 0.550A | 8.992 ± 0.545A | 9.010 ± 0.488A |
| 2 | 285091 | 9.443 ± 0.656B | 9.370 ± 0.653B | 9.383 ± 0.653B | 9.399 ± 0.586B | |
| 3 | 143330 | 9.605 ± 0.667C | 9.535 ± 0.676C | 9.552 ± 0.670C | 9.564 ± 0.599C | |
| 4 | 101731 | 9.602 ± 0.640C | 9.541 ± 0.650D | 9.558 ± 0.642D | 9.567 ± 0.572C | |
| 5 | 142109 | 9.708 ± 0.654D | 9.654 ± 0.665E | 9.669 ± 0.658E | 9.677 ± 0.582D | |
| DIM | 5–100 | 364183 | 9.375 ± 0.651 | 9.375 ± 0.649 | 9.375 ± 0.649 | 9.375 ± 0.589 |
| 101–200 | 483725 | 9.355 ± 0.674 | 9.355 ± 0.673 | 9.355 ± 0.673 | 9.355 ± 0.612 | |
| 201–305 | 200667 | 9.407 ± 0.712 | 9.407 ± 0.707 | 9.407 ± 0.708 | 9.407 ± 0.642 | |
| BCS | 2.25 | 5983 | 9.683 ± 0.667G | 9.627 ± 0.689G | 9.639 ± 0.680G | 9.650 ± 0.610G |
| 2.50 | 27549 | 9.643 ± 0.652F | 9.578 ± 0.661F | 9.591 ± 0.658F | 9.604 ± 0.588F | |
| 2.75 | 164788 | 9.526 ± 0.662D | 9.462 ± 0.669D | 9.475 ± 0.666D | 9.488 ± 0.599D | |
| 3.00 | 268385 | 9.376 ± 0.666C | 9.319 ± 0.664C | 9.328 ± 0.666C | 9.341 ± 0.603C | |
| 3.25 | 391975 | 9.325 ± 0.670B | 9.267 ± 0.665B | 9.271 ± 0.665B | 9.288 ± 0.605B | |
| 3.50 | 155857 | 9.258 ± 0.663A | 9.207 ± 0.659A | 9.211 ± 0.658A | 9.226 ± 0.598A | |
| 3.75 | 31765 | 9.369 ± 0.697C | 9.315 ± 0.696C | 9.316 ± 0.0694C | 9.333 ± 0.630C | |
| 4.00 | 2273 | 9.608 ± 0.750E | 9.554 ± 0.748E | 9.544 ± 0.735E | 9.569 ± 0.670E |
The mean and standard deviation of EC
EC_1 the EC at the first milking session in a day, EC_2 the EC at the second milking session in a day, EC_3 the EC at the third milking session in a day, EC_ave the daily average EC
The averages with the same letter between indicate that the difference is not significant, and those without the same letter indicate that the difference is significant (p < 0.05)
The phenotypic correlation between electrical conductivity and milk production-related traits
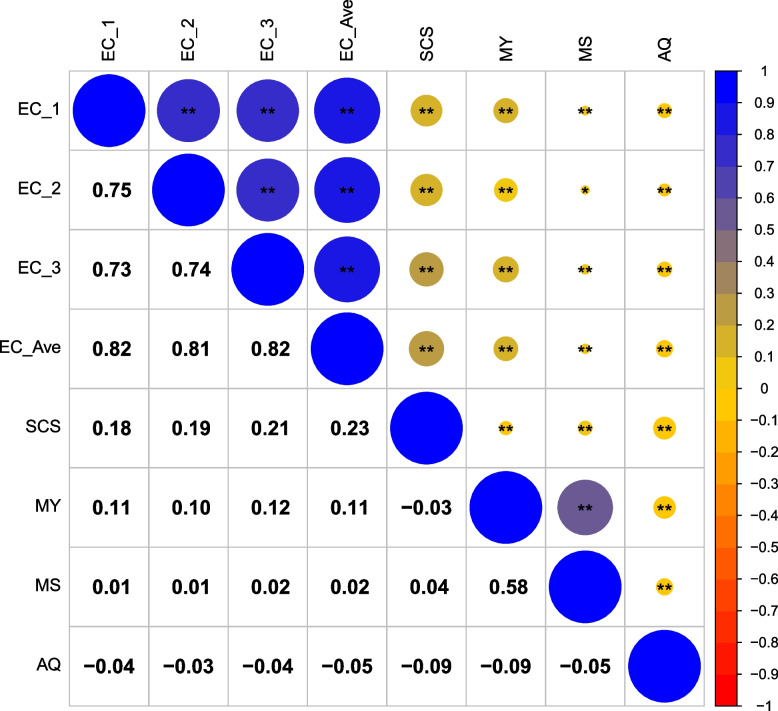
The phenotypic correlation between EC traits and various milk production-related traits in Chinese Holstein dairy cows was examined in the study, with the results visualized using a correlation matrix heatmap. As shown in Fig. 1, significant positive correlations, with coefficients between 0.73 to 0.82, were observed between the EC measurements of the first (EC_1), second (EC_2), and third (EC_3) milking sessions, as well as with the daily average EC (EC_ave). The SCS showed moderate positive correlations with EC traits, with coefficients between 0.18 to 0.23, while MY and average MS displayed weaker correlations. Interestingly, the AQ of cows demonstrated a slightly negative relationship with other traits.
Fig. 1.
The phenotype correlation between electrical conductivity and milk production-related traits of Chinese Holstein dairy cows. Note: EC_1 = the EC at the first milking session in a day; EC_2 = the EC at the second milking session in a day; EC_3 = the EC at the third milking session in a day; EC_ave = the daily average EC; SCS = somatic cell score of the milk; MY = the milk production yield; MS = the average milk speed in a day; AQ = the activity quantity of each cow in a day. The lower left corner is the relevant value, the upper right corner is the corresponding color expression, and the asterisks indicate significant correlation between two traits (*. p < 0.05 and **. p < 0.01)
The genetic analysis of electrical conductivity and milk production-related traits
The heritability estimates for EC traits exhibited a slight upward trend from the first to the third daily milking sessions (EC_1, EC_2, EC_3), with values of 0.458, 0.472, 0.471, respectively, and 0.487 for the average daily EC (EC_ave). Standard errors for these heritability values ranged from 0.101 to 0.105 (Table 3). Genetic analysis revealed strong genetic correlations within the EC measurements across the different milking sessions: the first (EC_1), second (EC_2), and third (EC_3), with values exceedingly close to unity (0.992 to 0.998, Table 4). The genetic correlations between daily EC measurements (EC_1, EC_2, EC_3, and EC_ave) and SCS were slightly positive (−0.098 to 0.135), suggesting that an increase in EC may be genetically linked to a higher propensity for elevated SCC. The correlations between EC traits and MY as well as MS were low and negative, while AQ exhibited a substantial negative genetic correlation (−0.286 to −0.111). Notably, AQ demonstrated a significant positive genetic correlation (0.475) with SCS.
Table 3.
The heritability of EC in Chinese Holstein dairy cattle
| EC (mS/cm) | se | ||||
|---|---|---|---|---|---|
| EC_1 | 40.435 | 47.703 | 0.148 | 0.458 | 0.103 |
| EC_2 | 43.675 | 48.708 | 0.149 | 0.472 | 0.101 |
| EC_3 | 43.357 | 48.547 | 0.149 | 0.471 | 0.105 |
| EC_ave | 41.441 | 43.524 | 0.067 | 0.487 | 0.102 |
= the sum of additive genetic (co)variance and covariance for EC from 5 to 305 days, = the sum of permanent environmental (co)variance and covariance for EC from 5 to 305 days, = the sum of residual variance for EC from 5 to 305 days, = the overall heritability of the EC traits adjusted to 305-day performance, se = standard error of the heritability
Table 4.
The genetic correlation between electrical conductivity and milk production-related traits of Chinese Holstein dairy cows
| EC_1 | EC_2 | EC_3 | EC_ave | SCS | MY | MS | AQ | |
|---|---|---|---|---|---|---|---|---|
| EC_1 | 1.000 | 0.014 | 0.011 | 0.021 | 0.050 | 0.050 | 0.067 | 0.112 |
| EC_2 | 0.994 | 1.000 | 0.009 | 0.002 | 0.042 | 0.044 | 0.060 | 0.104 |
| EC_3 | 0.992 | 0.998 | 1.000 | 0.004 | 0.055 | 0.045 | 0.062 | 0.114 |
| EC_ave | 0.998 | 0.999 | 0.997 | 1.000 | 0.048 | 0.041 | 0.056 | 0.109 |
| SCS | 0.098 | 0.113 | 0.122 | 0.135 | 1.000 | 0.089 | 0.130 | 0.166 |
| MY | −0.113 | −0.113 | −0.060 | −0.090 | −0.009 | 1.000 | 0.029 | 0.074 |
| MS | −0.057 | −0.084 | −0.009 | −0.035 | 0.162 | 0.457 | 1.000 | 0.098 |
| AQ | −0.286 | −0.200 | −0.118 | −0.111 | 0.475 | 0.422 | 0.027 | 1.000 |
EC_1 the EC at the first milking session in a day, EC_2 the EC at the second milking session in a day, EC_3 the EC at the third milking session in a day, EC_ave the daily average EC, SCS somatic cell score of the milk, MY the daily milk yield, MS the average milk speed in a day, AQ the activity quantity of each cow in a day
The bold fonts are genetic correlation values, and the non-bold fonts are the corresponding standard errors
Logistic regression analysis of mastitis using SCS
The results of logistic regression analysis for predicting subclinical mastitis using EC traits in dairy cows are summarized in Tables 5 and 6. Notably, EC_1, EC_2, EC_3 and EC_ave were significantly positively associated with an increased likelihood of subclinical mastitis, with odds ratios (OR) ranging from 10.23 to 10.96. The area under the curve (AUC) values for these EC traits were ranged from 0.636 to 0.697 (Table 6, Fig. S2). The asymptotic significance of each parameter was less than 0.05 (Table 6), indicating that their diagnostic effectiveness was statistically significant.
Table 5.
The logistic regression analysis of EC and mastitis in dairy cows diagnosed by SCS
| Factor | Level | Beta | SE | Wald X2 | P Value | OR Value | 95% Confidence Interval | |
|---|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | |||||||
| EC_1 | 0.37 | 0.37 | 0.03 | 138.56 | 0.02 | 10.74 | 9.70 | 9.94 |
| EC_2 | 0.38 | 0.38 | 0.02 | 187.22 | 0.01 | 10.23 | 9.88 | 10.39 |
| EC_3 | 0.42 | 0.42 | 0.05 | 244.47 | 0.00 | 10.38 | 10.22 | 10.58 |
| EC_ave | 0.58 | 0.58 | 0.07 | 354.52 | 0.00 | 10.96 | 10.69 | 11.47 |
Beta Slope value of regression analysis, SE standard error, Wald wald test value, OR Value Odds Ratio value, EC_1 the EC at the first milking session in a day, EC_2 the EC at the second milking session in a day, EC_3 the EC at the third milking session in a day, EC_ave the daily average EC
Table 6.
The binary classifier performance evaluation
| Variable | AUC | SEa | ASb | Asymptotic 95% Confidence Interval | |
|---|---|---|---|---|---|
| Lower Limit | Upper Limit | ||||
| EC_1 | 0.636 | 0.004 | 0.002 | 0.624 | 0.645 |
| EC_2 | 0.638 | 0.004 | 0.005 | 0.632 | 0.643 |
| EC_3 | 0.641 | 0.003 | 0.000 | 0.637 | 0.655 |
| EC_ave | 0.697 | 0.006 | 0.000 | 0.664 | 0.707 |
EC_1 the EC at the first milking session in a day, EC_2 the EC at the second milking session in a day, EC_3 the EC at the third milking session in a day, EC_ave the daily average EC, AUC area under the curve, AS asymptotic significance
aaccording to non-parametric assumptions
bnull hypothesis
True region = 0.5
The phenotypic and genetic changes of EC within the lactation of dairy cows
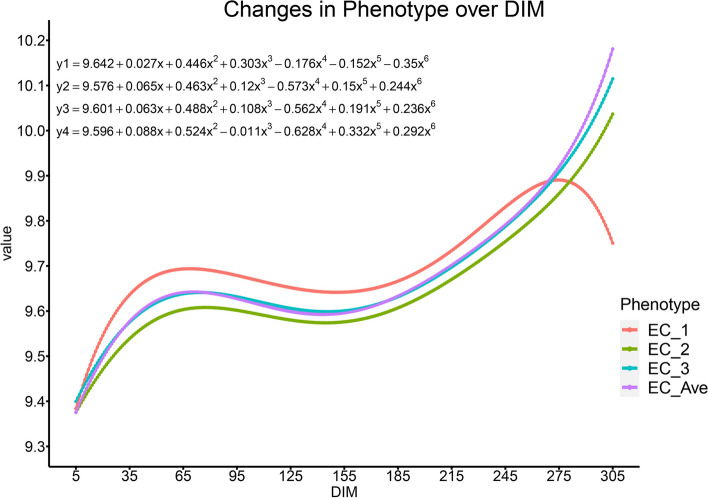
The trends in EC traits throughout lactation periods in dairy cows were analyzed in this study (Fig. 2). All the curves displayed a similar pattern: a rise during the early lactation period (5–100 days), followed by a leveling off between approximately 50 to 80 days, which continued through to the end of mid-lactation (200–305 days). After day 200 of lactation (DIM200), a rapid increase in EC was observed. The mid-lactation period (100–200 days) showed relative stability across all EC traits, indicating a homeostatic phase in milk production. In contrast, from around DIM270 to the end of the lactation period, the red curve deviated with a downward trend, opposite to the other curves. Prior to this divergence, the red curve maintained higher EC values compared to the other curves, while the v green curve consistently exhibited lower values.
Fig. 2.
The phenotypic curves of EC within the lactation of dairy cows. Note: The red line represents the EC at the first milking session in a day (EC_1); the green line represents the EC at the second milking session in a day (EC_2); the blue line represents the EC at the third milking session in a day; the purple line represents the daily average EC (EC_ave). The upper left corner represents the equation of the curve, where , , and are the value of EC_1, EC_2, EC_3, and EC_ave, respectively, and is Standardized DIM ranging from 5 to 305, where ; the abscissa represents days in milk (DIM) and the ordinate represents the size of the value
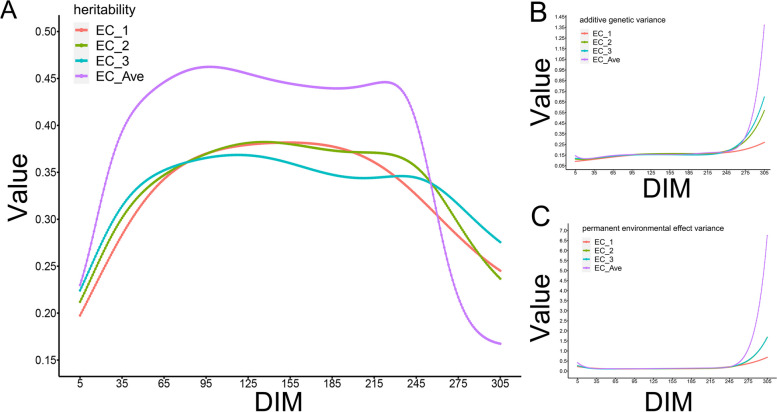
The heritability patterns for EC across different lactation stages in dairy cows, delineated by distinct periods, exhibited marked variation. During early lactation (DIM5- DIM100), the heritability of EC_1 exhibited considerable fluctuation, which transitioned into a phase of relative genetic stability during mid-lactation (DIM100- DIM200). As cows entered late lactation (DIM200- DIM305), especially at DIM270–DIM305, the heritability trends for the EC measurements began to show a downward trend. Before DIM270, the heritability of EC_ave was higher than that of electrical conductivity detected at different sessions (EC_1, EC_2, and EC_3) (Fig. 3A). The additive genetic effect curves for EC in dairy cows remained consistent and stable across all the EC traits (EC_1, EC_2, EC_3, and EC_ave) up until approximately 270 days, after which a noticeable upward shift occurred in all curves, with EC_ave experiencing a more pronounced increase compared to the others (Fig. 3B). The trends for the permanent environmental effects of EC mirrored those of the additive genetic effects throughout the entire lactation period (DIM5–DIM305, Fig. 3C).
Fig. 3.
The genetic parameter curves of EC within the lactation of dairy cows. Note: A = the heritability of EC within the lactation of dairy cows; B = the additive genetic effects of EC within the lactation of dairy cows; C = the permanent environmental effects of EC within the lactation of dairy cows. The red line represents the EC at the first milking session in a day (EC_1); the green line represents the EC at the second milking session in a day (EC_2); the blue line represents the EC at the third milking session in a day (EC_3); the purple line represents the daily average EC (EC_ave); the abscissa represents days in milk (DIM) and the ordinate represents the size of the value
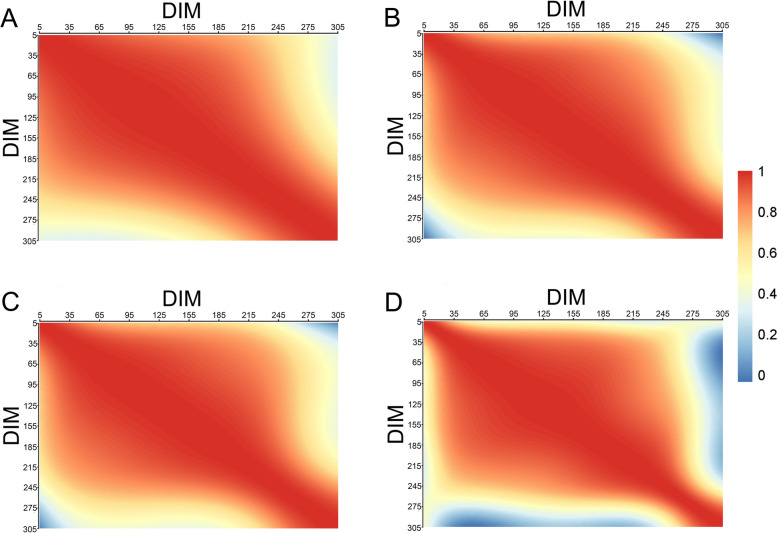
Additive genetic and permanent environmental correlations of EC during lactation in dairy cows
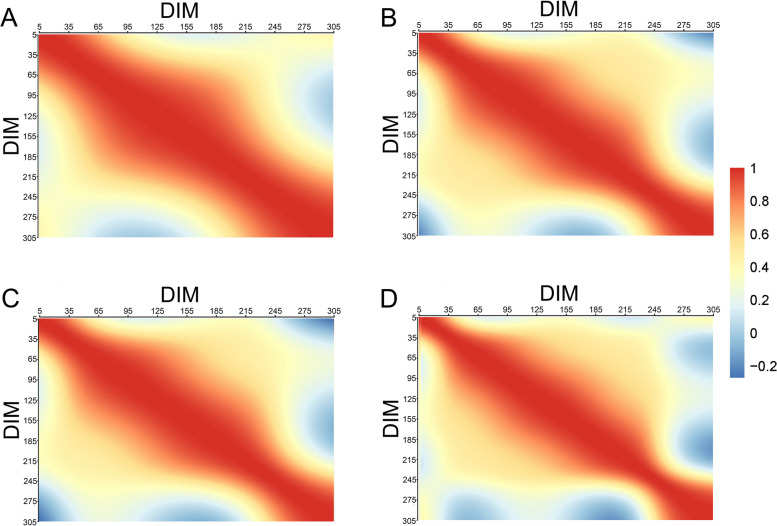
As shown in Fig. 4, the genetic correlations of EC throughout the lactation period in dairy cows were all positive but tended to decrease as lactation progressed. The correlation were particularly weak between the early and late stages of lactation. Before DIM270, the correlation between the additive genetic effects of EC and EC_ave were generally high (> 0.8) at most time points. However, EC_ave exhibited low correlations with additive genetic effects both before and after DIM270 at most time points. In contrast, for EC_1, EC_2, and EC_3 exhibited low correlations with additive genetic factors only during the periods from DIM5 to DIM35 and DIM270 to DIM305.
Fig. 4.
The additive genetic correlations of EC within the lactation of dairy cows. Note: A = the genetic correlations of EC at the first milking session in a day (EC_1); B = the genetic correlations of EC at the second milking session in a day (EC_2); C = the genetic correlations of EC at the third milking session in a day (EC_3); D = the genetic correlations of the daily average EC (EC_ave). The red and blue color gradients represent the magnitude of the correlation
As illustrated in Fig. 5, the permanent environmental effect correlations of EC in dairy cows showed similar patterns for EC_1, EC_2, EC_3, and EC_ave across lactation stages, generally exhibiting a downward trend over time, with occasional negative correlations (highlighted by the darkest blue in Fig. 5). Specifically, during DIM5-DIM35 and DIM275-DIM305, the permanent environmental effects for EC_1 and EC_ave demonstrated strong correlations. In contrast, EC_2 and EC_3 showed closer correlations in their permanent environmental effects during the periods of DIM35-DIM95 and DIM275-DIM305.
Fig. 5.
The permanent environmental correlations of EC within the lactation of dairy. Note: A = the permanent environmental correlations of EC at the first milking session in a day (EC_1); B = the permanent environmental correlations of EC at the second milking session in a day (EC_2); C = the permanent environmental correlations of EC at the third milking session in a day (EC_3); D = the permanent environmental correlations of the daily average EC (EC_ave). The red and blue color gradients represent the magnitude of the correlation
Discussion
Feasibility of electrical conductivity as an indicator of mastitis in dairy cows
Early identification of mastitis is considered the most effective strategy to ensure dairy cows have the optimal chance for a swift recovery [25, 26]. Traditionally, mastitis detection has relied on the observation of clinical symptoms, such as udder swelling or the presence of clots in the milk [27, 28]. EC has emerged as an indicator of the mammary gland health in cows. Under normal conditions, the concentrations of ions such as Na + , Cl-, K + , and Mg2 + in milk remain stable. However, udder infections can disrupt this balance, increasing the concentration of conductive particles and thereby elevating milk's EC. This occurs primarily due to bacterial invasions that trigger an immune response, resulting in an influx of white blood cells and enhanced permeability of the milk-blood barrier. This process significantly increases the concentrations of ions, such as Na + and Cl-, reflecting changes in the udder health [29–31]. As a result, EC levels rise above normal values, correlating with increased SCC. Research has shown that variations in milk EC can reflect the severity of mastitis, making EC measurements useful for diagnosing subclinical mastitis [29, 31]. Our study identified significant phenotypic correlations (0.18 to 0.23, Fig. 1) and demonstrated that EC_ave had a significant detection rate for subclinical mastitis in dairy cows in the regression analysis (Tables 5 and 6). However, the low genetic correlations between EC_1, EC_2, EC_3, and EC_ave with SCS were observed (0.098 to 0.135, Table 4) suggest challenges in using EC as a consistent genetic indicator for subclinical mastitis in cows.
We observed that EC_ave was more closely associated with SCS than individual milking session values (EC_1, EC_2, EC_3, Fig. 1 and Table 4). This suggests that milk composition varies throughout the day, potentially due to fluctuations in the efficiency of milk synthesis and secretion by mammary cells at different times. Previous studies have shown that the milk quality, in terms of protein (3.26%) and fat (3.37%) contents, is lower in the morning compared to the afternoon, where protein levels rise to 4.03% and fat to 3.79%, with changes also observed in lactose content [32]. Additionally, shorter milking intervals of 12:12 h produce higher milk fat content than longer intervals of 16:8 h [32, 33]. Thus, using the average daily EC helps to smooth out these variations in milk composition across different milking sessions, providing a more accurate detection of mastitis occurrences.
Non-genetic factors affecting EC
In this study, we demonstrated that the EC is influenced by a variety of factors, including the measurement year, the measurement season, age at first calving, parity, and BCS (Table 2). This finding is consistent with previous research of Pyörälä and Woolford et al., who reported that parity and different lactation stages significantly affect the milk EC levels [34, 35]. Specifically, we observed significant variations in EC during the early and late stages of lactation, which may be related to changes in the solid component content of milk throughout lactation [36]. Seasonal factors, particularly temperature changes, also significantly influence EC. Our research suggests that EC is generally lower in winter and spring compared to summer and autumn, likely because higher temperatures decrease milk viscosity. This reduction in viscosity impacts the binding of dissolved calcium and phosphate with casein micelles and affects the formation of solid calcium phosphate Calcium phosphate, leading to an increase in EC [37]. These seasonal changes in milk composition and properties underscore the influence of temperature variations on EC.
Our findings further highlight the impact of age at first calving and parity on EC. Cows with a first calving age of 26–28 months exhibited the lowest EC values, whereas those with a first calving age of 32–34 months showed the highest. Additionally, EC levels increased significantly with rising parity, suggesting that the risk of mastitis in dairy cows gradually increases with age [4, 8, 38]. This trend may result from cumulative exposure to pathogens, changes in mammary tissue, and a potential decline in immune system efficiency over time [39]. Studies have shown that cows in their second or later lactations have a higher risk of developing mastitis compared to those in their first lactation, a vulnerability that may be influenced by management practices, including milking hygiene, nutrition, and stress management [8, 40]. Furthermore, the prevalence of specific mastitis-causing pathogens may vary with age, with older cows being more susceptible to infections from environmental pathogens [40].
The BCS has a significant impact on the milk EC traits in this study, with the lowest EC values observed at a BCS of 3.5. EC values gradually increased as BCS either rose or fell from this point (Table 2). BCS is an assessment of the fatness level of dairy cows, and research indicates that cows with higher fat levels are more susceptible to mastitis and reproductive diseases [41]. Conversely, cows with a low BCS may not achieve their expected milk production levels, leading to a decrease in milk fat content [42, 43]. These cows may also exhibit signs of anestrus or have lower conception rates, which can alter milk composition and consequently increase EC. For cows in early lactation stage, a BCS above 4 poses numerous health risks, such as retained placenta, uterine atony, mastitis, metabolic diseases, and puerperal metritis [44]. The ideal BCS during mid-lactation is approximately 3.25, while for late lactation stage, it should range between 3 and 3.5. A BCS below 2.75 during this lactation may indicate long-term malnutrition or illness [45, 46]. A BCS of 3.5 is considered optimal, as it minimizes the risk of mastitis and metabolic diseases, correlating with lower EC level. Looking ahead, beyond mastitis detection, EC could also serve as a potential indicator trait for reproductive diseases in dairy cows, suggesting promising avenues for research.
Correlation between EC and milk production-related traits
In this study, we discovered that the phenotypic expression of EC significantly correlates with traits including MY, MS, AQ, and SCS (Fig. 1). It is commonly believed that cows with mastitis, due to discomfort in the udder, exhibit more frequent lying down and standing up, which reduces rest time and milk production [47, 48]. However, our findings reveal a relatively low negative correlation between AQ and both EC as well as other milk production traits. From a genetic perspective, activity level exhibited a stronger negative correlation with EC (−0.111 to −0.286) and a higher positive correlation with MY (0.422, Table 4). This could be because the dairy cows in our study were generally healthy and did not suffer from clinical mastitis, allowing increased activity to promote blood circulation and metabolism, which in turn supports udder health and lowers EC value in the milk [48]. Additionally, increased activity can enhance the cow's appetite and feed intake, indirectly boosting milk production [47, 49].
Our research also indicates a positive correlation between MY and EC, though the correlations differ at the phenotypic and genetic levels (Table 4). This discrepancy may be due to the fact that increased milk yield is often associated with higher metabolic activity and salt concentration in the mammary gland, leading to elevated EC levels [50, 51]. However, the negative genetic correlation suggests that genetic factors may promote both higher MY and improved mammary health and inflammation resistance, resulting in lower EC. This implies that genetic selection can independently affect these traits, causing their genetic relationships to diverge from their phenotypic expressions. Furthermore, average daily EC (EC_ave) demonstrated higher phenotypic and genetic correlations with milk production traits compared to EC_1, EC_2, and EC_3 (Fig. 1, Table 4). This suggests that using average daily EC data accounts for physiological fluctuations and environmental changes throughout the day, offering a more accurately and comprehensively reflection of udder health and milk production performance than data from a single milking session.
The phenotypic changes in EC during lactation
Our study revealed consistent patterns in EC changes throughout the lactation cycle, characterized by a sharp increase during the early lactation phase, a stable plateau in mid-lactation, and a rise again in the late lactation phase (Fig. 2). The initial increase in EC can be attributed to the reorganization and repair processes of mammary cells [52]. During this transitions from a rest phase to active milk production, the mammary tissue undergoes changes that may include mild inflammatory responses and increased intercellular space, leading to changes in electrolyte concentrations, such as sodium and chloride [53, 54], thereby elevating EC levels. In the mid-lactation phase, as mammary function stabilizes, milk composition becomes more consistent, resulting in a more stable EC [55, 56]. The increase in EC observed during late lactation phase may be linked to physiological aging of mammary tissue, a decline in milk yield, and an elevated risk of inflammation. As the lactation cycle nears its end, the mammary gland begins to reduce milk production in preparation for the dry period, potentially increasing intercellular space and making the udder more susceptible to pathogen invasion, leading to cellular damage and inflammation [57, 58], which may in turn affects EC.
Additionally, our findings indicate that morning EC values are higher than those measured at other times of the day, while afternoon EC values tend to be lower (Fig. 2). This difference may result from the overnight accumulation of electrolytes in the udder, leading to higher morning EC, whereas the shorter interval before afternoon milking allows less time for electrolyte build-up, resulting in lower EC values. Furthermore, we observed a rapid decline in morning EC as the lactation period nears its end, likely due to reduced milk yield and diminished mammary activity, which leads to lower electrolyte concentrations [53, 54].
The genetic pattern of EC
In this study, the heritability estimates for electrical conductivity (EC_1, EC_2, EC_3, and EC_ave) ranged from 0.458 to 0.487, indicating medium to high heritability (Table 3). Previous studies have reported a wide range of heritability estimates for EC, varying from 0.12 to 0.56 [59–63]. For example, one study on Holstein cows reported heritability estimates for EC between 0.12 and 0.36 [63], while research on 1,899 primiparous Polish Holstein–Friesian cows found estimates ranging from 0.269 to 0.466 [64]. Another study involving 922 Holstein cows across three German farms found heritability values between 0.37 and 0.46 [60], and a study on 421 cows from three dairy farms in Lithuania estimated a heritability of 0.51 [61]. Additionally, research on Hungarian Holstein-Friesians reported a heritability of 0.56 [62]. These differences may result from variations in the definition of EC, lactation stage, statistical models used, population size, and the genetic diversity of the cattle breeds studied, highlighting the importance of considering these factors when interpreting the variability in EC heritability estimates across studies.
The heritability of EC traits showed a rapid increase in the early lactation period, stabilized during mid-lactation, and then declined in late lactation (Fig. 3A). Specifically, the heritability and additive genetic effects of EC traits during the first milking session (EC_1) were higher than at other milking sessions before 250 days of lactation but became lower after DIM250 (Fig. 3A, B). The initial rise in heritability during early lactation period may be due to the active state of mammary cells, with genetic differences becoming more apparent during mammary development and the onset of milk production [65]. The stability observed in the mid-phase corresponds to the mammary gland reaching a steady state of milk production, where genetic influence remains relatively consistent [56]. The decline in heritability towards late lactation period may be associated with the physiological aging of the mammary gland and a decrease in milk yield (Fig. 3C), during which environmental factors play a more significant role [57]. The pronounced genetic influence on EC, especially for EC_1, can be attributed to the long intervals without milking overnight, allowing genetic factors to more strongly impact milk composition during the first morning milking.
Genetic correlations of EC remained very high (> 0.5) throughout the lactation period until 250 days, but they decreased significantly after 275 days, especially between 275–305 days (Fig. 4). This suggests that physiological changes in the mammary gland occur after 250 days, with environmental, nutritional, and management factors playing a more influential role in udder health and EC during this period. The high genetic correlations before 250 days indicate minimal variation in the expression of genes regulating milk component during this phase [65], while significant shifts in gene expression likely occur after DIM250. These findings underscore the importance of recording performance data throughout the entire lactation period for accurate evaluation of EC traits in milk [66, 67]. However, missing records for individuals before DIM250 may not significantly affect the overall accuracy of genetic evaluations.
Additionally, we observed that the correlation of permanent environmental effects on EC gradually decreases over time, although they remained high on consecutive days within the lactation period. This pattern allows the lactation period to be categorized into three distinct phases: 5–60 days, 61–250 days, and 251–305 days. The correlations of permanent environmental effects between these phases were lower or even negative (Fig. 5), indicating that the impact of environmental and management conditions on mammary health and EC varies across different stages. The high correlation on consecutive days suggests a consistent impact from environmental and management measures over the short term. Notably, the physiological shifts around DIM60 and DIM250, which correspond to peak milk production and preparation for the dry period, increase the impact of environmental factors such as feeding management and nutritional status on udder health and EC [31, 68]. Thus, ensuring proper feeding and management practices during these critical periods is essential for maintaining optimal milk production and udder health.
Conclusion
This study offers a comprehensive genetic analysis of EC in Chinese Holstein cows and evaluates its potential as a biomarker for early detection of subclinical mastitis. The findings reveal that milk EC traits are influenced by several factors, including age at first calving, parity, and BCS, and show significant phenotypic associations with SCS, MY, AQ, and MS. The milk EC traits demonstrated moderate to high heritability, particularly during early to mid-lactation, suggesting that genetic improvement of these traits could be feasible. While logistic regression models demonstrated the predictive capability of milk EC for identifying subclinical mastitis, with AUC values ranging from 0.636 to 0.697, the relatively low genetic correlations between milk EC and SCS indicate limitations to its effectiveness as a standalone genetic indicator for subclinical mastitis. Future research should focus on integrating milk EC with other indicators to enhance the accuracy of subclinical mastitis detection and to further investigate environmental factors that influence EC during critical lactation stages.
Supplementary Information
Acknowledgements
We are grateful to Guosheng Su from the Center for Quantitative Genetics and Genomics at Aarhus University for his expert guidance on genetic evaluation and statistical analysis in this study.
Authors’ contributions
Z.Y., Z.L., and Y.M. designed the work; Z.Z., H.Z., and F.Z. analyzed the data; X.L. and M.L. wrote the manuscript.
Funding
This work is funded by the Biological Breeding-Major Projects in National Science and Technology (2023ZD0404902-02), the Biological Breeding-National Science and Technology Major Project (2023ZD0406805), the National Natural Science Foundation of China (32402712), the Ningxia Hui Autonomous Region Key Research and Development Project (2023BCF01004), the Jiangsu Province Key Research and Development Project (BE2023329), and the Jiangsu Province Seed Industry Revitalization Project (JBGS[2021]115).
Data availability
The datasets analyzed during the current study are available in the Mendeley Data, V4, 10.17632/32hkvvbcgf.4.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cheng WN, Han SG. Bovine mastitis: Risk factors, therapeutic strategies, and alternative treatments—A review. Asian Australas J Anim Sci. 2020;33(11):1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruegg PL. A 100-Year Review: Mastitis detection, management, and prevention. J Dairy Sci. 2017;100(12):10381–97. [DOI] [PubMed] [Google Scholar]
- 3.Abebe R, Hatiya H, Abera M, Megersa B, Asmare K. Bovine mastitis: prevalence, risk factors and isolation of Staphylococcus aureus in dairy herds at Hawassa milk shed. South Ethiopia BMC veterinary research. 2016;12(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma N, Singh SG, Sharma S, Gupta S, Hussain K. Mastitis occurrence pattern in dairy cows and importance of related risk factors in the occurrence of mastitis. J Animal Res. 2018;8(2):315–26. [Google Scholar]
- 5.Miles AM. Understanding the genetics underlying mastitis using a multi-pronged approach. 2019.
- 6.Heringstad B, Gianola D, Chang YM, Odegard J, Klemetsdal G. Genetic associations between clinical mastitis and somatic cell score in early first-lactation cows. J Dairy Sci. 2006;89(6):2236–44. [DOI] [PubMed] [Google Scholar]
- 7.Simitzis P, Tzanidakis C, Tzamaloukas O, Sossidou E. Contribution of precision livestock farming systems to the improvement of welfare status and productivity of dairy animals. Dairy. 2021;3(1):12–28. [Google Scholar]
- 8.Litwińczuk Z, Król J, Brodziak A. Factors determining the susceptibility of cows to mastitis and losses incurred by producers due to the disease–a review. Annals of Animal Science. 2015;15(4):819–31. [Google Scholar]
- 9.Dufour S, Dohoo I. Monitoring herd incidence of intramammary infection in lactating cows using repeated longitudinal somatic cell count measurements. J Dairy Sci. 2013;96(3):1568–80. [DOI] [PubMed] [Google Scholar]
- 10.Paulauskas A, Juozaitiene V, Dzermeikaite K, Baceninaite D, Urbonavicius G, Tusas S, Slyzius E, Baumgartner W, Rutkauskas A, Antanaitis R. Association between milk electrical conductivity biomarkers with lameness in dairy cows. Vet Sci. 2023;10(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juozaitiene V, Anskiene L, Cereskiene E, Juozaitis A, Zymantiene J, Zilaitis V, Bobiniene R. Electrical conductivity of milk in different milking phases and relationship with subclinical mastitis and mastitis pathogens of cows. J Animal Plant Sci. 2017;27(6):1829–35. [Google Scholar]
- 12.Simitzis P, Tzanidakis C, Tzamaloukas O, Sossidou E. Contribution of precision livestock farming systems to the improvement of welfare status and productivity of dairy animals. Dairy. 2022;3(1):12–28. [Google Scholar]
- 13.Norberg E, Rogers GW, Ødegård J, Cooper JB, Madsen P. Short communication: genetic correlation between test-day electrical conductivity of milk and mastitis. J Dairy Sci. 2006;89(2):779–81. [DOI] [PubMed] [Google Scholar]
- 14.Norberg E, Ødegård J, Madsen P. Comparison of variance components for test-day electrical conductivity of milk and test-day somatic cell score for first lactation cows in an experimental herd. Acta Agriculturae Scandinavica, Section A — Animal Science. 2004;54(4):181–6. [Google Scholar]
- 15.Samaraweera AM, Boerner V, Disnaka S, van der Werf JJH, Hermesch S. Genetic associations between mastitis, milk electrical conductivity, and milk flow rate in temperate dairy cows in tropics. Livest Sci. 2022;264:105064. [Google Scholar]
- 16.O’Sullivan M, Butler S, Pierce K, Crowe M, O’Sullivan K, Fitzgerald R, Buckley F. Reproductive efficiency and survival of Holstein-Friesian cows of divergent Economic Breeding Index, evaluated under seasonal calving pasture-based management. J Dairy Sci. 2020;103(2):1685–700. [DOI] [PubMed] [Google Scholar]
- 17.Brito L, Bedere N, Douhard F, Oliveira H, Arnal M, Peñagaricano F, Schinckel A, Baes CF, Miglior F. Genetic selection of high-yielding dairy cattle toward sustainable farming systems in a rapidly changing world. Animal. 2021;15:100292. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Gao H, Madsen P, Li R, Liu W, Bao P, Xue G, Gao Y, Di X, Su G. Impact of the order of legendre polynomials in random regression model on genetic evaluation for milk yield in dairy cattle population. Front Genet. 2020;11:586155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu X, Arbab AAI, Abdalla IM, Liu D, Zhang Z, Xu T, Su G, Yang Z. Genetic parameter estimation and genome-wide association study-based loci identification of milk-related traits in Chinese Holstein. Front Genet. 2022;12:799664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaeffer LR. Application of random regression models in animal breeding. Livest Prod Sci. 2004;86(1):35–45. [Google Scholar]
- 21.Wildman EE, Jones GM, Wagner PE, Boman R, Troutt HF. Lesch TNJJoDS: a dairy cow body condition scoring system and its relationship to selected production characteristics. 1982;65:495–501. [Google Scholar]
- 22.Van den Borne B, Vernooij J, Lupindu A, Van Schaik G, Frankena K, Lam T, Nielen M. Relationship between somatic cell count status and subsequent clinical mastitis in Dutch dairy cows. Prev Vet Med. 2011;102(4):265–73. [DOI] [PubMed] [Google Scholar]
- 23.Sumon SMR, Parvin MS, Ehsan MA, Islam MT. Relationship between somatic cell counts and subclinical mastitis in lactating dairy cows. Veterinary World. 2020;13(8):1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madsen P, Jensen J: A User’s Guide to DMU. Version 6, Release 5.2. Center for Quantitative Genetics and Genomics. Dept. of Molecular Biology and Genetics. 2013.
- 25.Algharib SA, Dawood AS, Huang L, Guo A, Zhao G, Zhou K, Li C, Liu J, Gao X, Luo W. Basic concepts, recent advances, and future perspectives in the diagnosis of bovine mastitis. J Vet Sci. 2024;25(1):e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rainard P, Gilbert FB, Germon P, Foucras G. Invited review: a critical appraisal of mastitis vaccines for dairy cows. J Dairy Sci. 2021;104(10):10427–48. [DOI] [PubMed] [Google Scholar]
- 27.de Jong E, McCubbin KD, Uyama T, Brummelhuis C, Bodaneze J, Kelton DF, Dufour S, Sanchez J, Roy J-P, Heider LC. Adoption and decision factors regarding selective treatment of clinical mastitis on Canadian dairy farms. J Dairy Sci. 2024;107(1):463–75. [DOI] [PubMed] [Google Scholar]
- 28.Hogeveen H, Klaas IC, Dalen G, Honig H, Zecconi A, Kelton DF, Mainar MS. Novel ways to use sensor data to improve mastitis management. J Dairy Sci. 2021;104(10):11317–32. [DOI] [PubMed] [Google Scholar]
- 29.Bonestroo J, van der Voort M, Fall N, Emanuelson U, Klaas IC, Hogeveen H. Estimating the nonlinear association of online somatic cell count, lactate dehydrogenase, and electrical conductivity with milk yield. J Dairy Sci. 2022;105(4):3518–29. [DOI] [PubMed] [Google Scholar]
- 30.Nielen M, Deluyker H, Schukken Y, Brand A. Electrical conductivity of milk: measurement, modifiers, and meta analysis of mastitis detection performance. J Dairy Sci. 1992;75(2):606–14. [DOI] [PubMed] [Google Scholar]
- 31.Norberg E. Electrical conductivity of milk as a phenotypic and genetic indicator of bovine mastitis: A review. Livest Prod Sci. 2005;96(2–3):129–39. [Google Scholar]
- 32.Garantjang S, Rusdy M, Hatta M, Nohong B. Sema: Effect of milking time on milk production and milk quality of dairy cow fed with fermented corn cob. IOP Conference Series: Earth Environ Sci. 2020;492(1):012054. [Google Scholar]
- 33.Vergi MD, Suprayogi TH, Sayuthi SM. Kandungan lemak, total bahan kering dan bahan kering tanpa lemak susu sapi perah akibat interval pemerahan berbeda. Animal Agriculture Journal. 2016;4:195–9. [Google Scholar]
- 34.Woolford MW, Williamson JH, Henderson HV. Changes in electrical conductivity and somatic cell count between milk fractions from quarters subclinically infected with particular mastitis pathogens. J Dairy Res. 1998;65(2):187–98. [DOI] [PubMed] [Google Scholar]
- 35.Pyörälä S. Indicators of inflammation in the diagnosis of mastitis. Vet Res. 2003;34(5):565–78. [DOI] [PubMed] [Google Scholar]
- 36.Auldist M, Coats S, Rogers G, McDowell G. Changes in the composition of milk from healthy and mastitic dairy cows during the lactation cycle. Aust J Exp Agric. 1995;35(4):427–36. [Google Scholar]
- 37.Henningsson M, Östergren K, Dejmek P. The electrical conductivity of milk—the effect of dilution and temperature. Int J Food Prop. 2005;8(1):15–22. [Google Scholar]
- 38.Tiwari J, Babra C, Tiwari H, Williams V, De Wet S, Gibson J, Paxman A, Morgan E, Costantino P, Sunagar R. Trends in therapeutic and prevention strategies for management of bovine mastitis: an overview. Journal of Vaccines & Vaccination. 2013;4(1):1–11. [Google Scholar]
- 39.Burvenich C, Bannerman DD, Lippolis J, Peelman L, Nonnecke B, Kehrli M Jr, Paape M. Cumulative physiological events influence the inflammatory response of the bovine udder to Escherichia coli infections during the transition period. J Dairy Sci. 2007;90:E39–54. [DOI] [PubMed] [Google Scholar]
- 40.Wilson E, Woodd SL, Benova L. Incidence of and risk factors for lactational mastitis: a systematic review. J Hum Lact. 2020;36(4):673–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berry DP, Lee JM, Macdonald KA, Stafford K, Matthews L, Roche JR. Associations among body condition score, body weight, somatic cell count, and clinical mastitis in seasonally calving dairy cattle. J Dairy Sci. 2007;90(2):637–48. [DOI] [PubMed] [Google Scholar]
- 42.Glatz-Hoppe J, Boldt A, Spiekers H, Mohr E, Losand B. Relationship between milk constituents from milk testing and health, feeding, and metabolic data of dairy cows. J Dairy Sci. 2020;103(11):10175–94. [DOI] [PubMed] [Google Scholar]
- 43.Singh A, Bhakat C. The relationship between body condition score and milk production, udder health and reduced negative energy balance during initial lactation period: A review. Iranian Journal of Applied Animal Science. 2022;12(1):1–9. [Google Scholar]
- 44.Bisla A, Yadav V, Dutt R, Singh G, Gahalot SC. Fertility augmentation approaches in dairy animals-a review. Int J Curr Microbiol App Sci. 2018;7(2):2995–3007. [Google Scholar]
- 45.Vailati-Riboni M, Farina G, Batistel F, Heiser A, Mitchell M, Crookenden M, Walker C, Kay J, Meier S, Roche J. Far-off and close-up dry matter intake modulate indicators of immunometabolic adaptations to lactation in subcutaneous adipose tissue of pasture-based transition dairy cows. J Dairy Sci. 2017;100(3):2334–50. [DOI] [PubMed] [Google Scholar]
- 46.Lake S, Scholljegerdes E, Atkinson R, Nayigihugu V, Paisley S, Rule D, Moss G, Robinson T, Hess B. Body condition score at parturition and postpartum supplemental fat effects on cow and calf performance. J Anim Sci. 2005;83(12):2908–17. [DOI] [PubMed] [Google Scholar]
- 47.Medrano-Galarza C, Gibbons J, Wagner S, De Passillé A, Rushen J. Behavioral changes in dairy cows with mastitis. J Dairy Sci. 2012;95(12):6994–7002. [DOI] [PubMed] [Google Scholar]
- 48.Fogsgaard KK, Bennedsgaard TW, Herskin M. Behavioral changes in freestall-housed dairy cows with naturally occurring clinical mastitis. J Dairy Sci. 2015;98(3):1730–8. [DOI] [PubMed] [Google Scholar]
- 49.Leduc A, Souchet S, Gelé M, Le Provost F, Boutinaud M. Effect of feed restriction on dairy cow milk production: a review. J Animal Sci. 2021;99(7):skab130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herve L, Quesnel H, Lollivier V, Portanguen J, Bruckmaier R, Boutinaud M. Mammary epithelium disruption and mammary epithelial cell exfoliation during milking in dairy cows. J Dairy Sci. 2017;100(12):9824–34. [DOI] [PubMed] [Google Scholar]
- 51.Pulina G, Nudda A: Milk production. In: Dairy sheep nutrition. CABI Publishing Wallingford UK; 2004: 1–12.
- 52.Dai W-t, Zou Y-x. White RR, Liu J-x, Liu H-y: Transcriptomic profiles of the bovine mammary gland during lactation and the dry period. Funct Integr Genomics. 2018;18:125–40. [DOI] [PubMed] [Google Scholar]
- 53.Singh K, Vetharaniam I, Dobson J, Prewitz M, Oden K, Murney R, Swanson K, McDonald R, Henderson H, Stelwagen K. Cell survival signaling in the bovine mammary gland during the transition from lactation to involution. J Dairy Sci. 2016;99(9):7523–43. [DOI] [PubMed] [Google Scholar]
- 54.Kaur TP, Verma R, Choudhary RK: Introduction to mammary gland and its cell types. In: Stem Cells in Veterinary Science. Springer; 2022: 25–37.=
- 55.Gellrich K, Meyer H, Wiedemann S. Composition of major proteins in cow milk differing in mean protein concentration during the first 155 days of lactation and the influence of season as well as short-term restricted feeding in early and mid-lactation. 2014.
- 56.Lérias JR, Hernández-Castellano LE, Suárez-Trujillo A, Castro N, Pourlis A, Almeida AM. The mammary gland in small ruminants: major morphological and functional events underlying milk production–a review. J Dairy Res. 2014;81(3):304–18. [DOI] [PubMed] [Google Scholar]
- 57.Nitz J, Wente N, Zhang Y, Klocke D, Tho Seeth M, Krömker V. Dry period or early lactation-time of onset and associated risk factors for intramammary infections in dairy cows. Pathogens. 2021;10(2):224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vilar MJ, Rajala-Schultz PJ. Dry-off and dairy cow udder health and welfare: Effects of different milk cessation methods. Vet J. 2020;262:105503. [DOI] [PubMed] [Google Scholar]
- 59.Norberg EJLPS. Electrical conductivity of milk as a phenotypic and genetic indicator of bovine mastitis: A review. Livestock Prod Sci. 2005;96(2):129–39. [Google Scholar]
- 60.Santos LV, Brügemann K, Ebinghaus A, König S. Genetic parameters for longitudinal behavior and health indicator traits generated in automatic milking systems. Arch Anim Breed. 2018;61(2):161–71. [Google Scholar]
- 61.Juozaitienė V, Juozaitis A, Brazauskas A, Žymantienė J, Žilaitis V, Antanaitis R, Stankevičius R. Bobinienė RJJoMiE: Investigation of electrical conductivity of milk in robotic milking system and its relationship with milk somatic cell count and other quality traits. 2015;3(3):63–70. [Google Scholar]
- 62.Gáspárdy A, Ismach G, Bajcsy AC, Veress G, Márkus S, Komlósi I. Evaluation of the on-line electrical conductivity of milk in mastitic dairy cows. Acta Vet Hung. 2012;60(1):145–55. [DOI] [PubMed] [Google Scholar]
- 63.Norberg E, Rogers GW, Goodling RC, Cooper JB, Madsen P. Genetic Parameters for Test-Day Electrical Conductivity of Milk for First-Lactation Cows from Random Regression Models. J Dairy Sci. 2004;87(6):1917–24. [DOI] [PubMed] [Google Scholar]
- 64.Piwczyński D, Sitkowska B, Ptak EJa. Genetic relationship among somatic cell score and some milking traits in Holstein-Friesian primiparous cows milked by an automated milking system. Animal. 2021;15(2):100094. [DOI] [PubMed] [Google Scholar]
- 65.Wall EH, Bond JP, McFadden TB. Milk yield responses to changes in milking frequency during early lactation are associated with coordinated and persistent changes in mammary gene expression. BMC Genomics. 2013;14:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ojango JM, Mrode R, Rege J, Mujibi D, Strucken E, Gibson J, Mwai O. Genetic evaluation of test-day milk yields from smallholder dairy production systems in Kenya using genomic relationships. J Dairy Sci. 2019;102(6):5266–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bignardi A, El Faro L, Cardoso V, Machado PF, Albuquerque LGd. Parametric correlation functions to model the structure of permanent environmental (co) variances in milk yield random regression models. J Dairy Sci. 2009;92(9):4634–40. [DOI] [PubMed] [Google Scholar]
- 68.Zigo F, Vasil’ M, Ondrašovičová S, Výrostková J, Bujok J, Pecka-Kielb E. Maintaining optimal mammary gland health and prevention of mastitis. Front Vet Sci. 2021;8:607311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are available in the Mendeley Data, V4, 10.17632/32hkvvbcgf.4.