Abstract
Background
CD19 CAR T-cell therapy is a novel anti-cancer treatment that has produced remarkable responses in relapsed or refractory B-cell hematological malignancies. Cytokine Release Syndrome (CRS) is a dysregulated immune response that frequently occurs after CAR T-cell infusion. It can cause cardiac dysfunction and circulatory collapse negatively impacting outcomes and survival. To endure the insults of CRS, patients are typically screened for adequate cardiac reserve before treatment. The relationship between baseline cardiac function by echocardiography and the development of moderate to severe presentations of CRS is unclear.
Methods
This study aimed to identify baseline echocardiographic variables that can predict the development of hemodynamically significant CRS (CRS ≥ 2), evaluate their behavior at follow-up, and investigate the incidence of cancer therapy-related cardiac dysfunction (CTRCD). An observational retrospective cohort study of patients treated with CD19 CAR T-cell therapy with a baseline echocardiogram was performed. Demographic, clinical and echocardiographic variables were abstracted from the electronic health record. Patients were grouped and compared by the occurrence of CRS < 2 and ≥ 2. Adjusted logistic regression analysis was used to evaluate the association between echocardiographic variables and the development of CRS ≥ 2.
Results
291 patients were included in the study. Median age was 60 (IQR: 51, 67 years), 73% were male, and 71% had diffuse large B-cell lymphoma. Logistic regression analysis did not reveal any significant baseline echocardiographic predictors of CRS ≥ 2, including left ventricular ejection fraction and global longitudinal strain. Systolic and diastolic echocardiographic variables remained within normal limits at follow-up overall and in both CRS groups. The incidence of CTRCD was 4.5% and occurred mostly in the setting of CRS ≥ 2.
Conclusion
No specific echocardiographic variables predicted the development of CRS ≥ 2, and therefore the mechanism leading to hemodynamic decompensation and producing worsening hypoxia and hypotension could be multifactorial and not directly cardiac mediated.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40959-024-00290-6.
Keywords: Echocardiography, Chimeric antigen T-cell therapy, B-cell lymphoma, Cytokine release syndrome, Cancer therapy-related cardiac dysfunction, Cardiotoxicity
Introduction
CD19-directed Chimeric Antigen Receptor (CAR) T-cell therapy has revolutionized the treatment of B-cell derived hematological malignancies in recent years [1–3]. This innovative therapy involves genetically engineering the patient’s own T-cells to express receptors targeting cancer antigens, thereby enhancing their ability to identify and eliminate tumor cells. Upon activation, CAR T-cells trigger a systemic increase in inflammatory cytokines and chemokines, such as IL-6, IL-10, interferon gamma, among others, which recruit and activate local immune cells like monocytes and macrophages to aid in tumor cell lysis [4, 5]. However, this immune response can lead to severe adverse effects, most notably Cytokine Release Syndrome (CRS), characterized by fever, hypotension, and hypoxia (Table 1), which can result in transfer to specialized intensive care units [6, 7].
Table 1.
ASTCT 2019 Consensus Grading for Cytokine Release Syndrome
| CRS Parameter | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Fever | Temperature ≥ 38 °C | Temperature ≥ 38 °C | Temperature ≥ 38 °C | Temperature ≥ 38 °C |
| With | ||||
| Hypotension | None | Not requiring vasopressors | Requiring a vasopresor with or without vasopressin | Requiring multiple vasopressors (excluding vasopressin) |
| And/or | ||||
| Hypoxia | None | Requiring low-Flow nasal canula or blow-by | Requiring high-flow nasal cannula, face mask, non-rebreather mask, or Venturi mask | Requiring positive pressure (eg. CPAP, BiPAP, intubation and mechanical ventilation) |
Adapted from: D.W. Lee et al. / Biol Blood Marrow Transplant 25 (2019) 625–638
The incidence of CRS varies widely depending on factors such as the specific CAR T-cell therapy used and patient characteristics, with rates ranging from 37 to 93% in various studies [2, 6, 8–11]. Risk factors for moderate to severe forms of CRS (CRS ≥ 2) include high tumor burden, high CAR T-cell dose, thrombocytopenia, prior lymphodepleting chemotherapy regimens, and older patient age [2, 5, 12]. Importantly, CRS can also precipitate significant cardiovascular complications, particularly in patients with pre-existing cardiac conditions such as hyperlipidemia, coronary artery disease, aortic stenosis and hypertension [6, 8, 11, 13–17].
The relationship between the heart and CRS is complex and an active area of research. The mechanism of cardiotoxicity in CRS primarily involves IL-6-mediated endothelial activation, which can lead to capillary leakage, complement activation, coagulation disturbances, hypotension, and myocardial dysfunction [4, 18–20]. Cardiovascular complications associated with CRS include hypotension, sinus tachycardia, elevated troponin levels, cardiomyopathy or cancer therapy-related cardiac dysfunction (CTRCD), decompensated heart failure, arrhythmias (most commonly atrial fibrillation), and in severe cases, cardiovascular death [6, 16, 17, 21–23]. To endure this wide range of potential cardiovascular complications brought on by CRS, assessment of cardiac function has become a pillar for state-of-the-art management of patients treated with CAR T-cell therapy.
Transthoracic echocardiography (TTE) plays a crucial role in assessing cardiac function before, during, and after CAR T-cell therapy due to its noninvasive nature and widespread availability [24, 25]. Recent guidelines emphasize the use for TTE for baseline cardiac evaluation in all patients undergoing CAR T-cell therapy, particularly those with known cardiovascular risk factors, as well as for monitoring patients who develop moderate to severe CRS (CRS ≥ 2) [25].
Currently, we have a limited understanding of the echocardiographic profiles and cardiac dynamics of patients treated with CAR T-cell therapy. While myocardial dysfunction associated with CRS may revert to baseline in many cases, suggesting a self-limiting process, severe deterioration leading to fatal outcomes can occur [12, 15]. Although cardiotoxicity usually occurs in the context of CRS, it is unclear if baseline cardiac function plays a role in mitigating or propagating the hemodynamic effects of CRS.
The aim of this study was to identify baseline echocardiographic variables that predict the development of CRS ≥ 2, evaluate their evolution over time, and assess the incidence of CTRCD based on updated guideline definitions in adult patients treated with CD19 CAR T-cell therapy. Characterizing these parameters and understanding their predictive power could guide the development of tailored approaches to enhance patient safety and treatment efficacy in the era of CAR T-cell therapy.
Materials and methods
Study design
This is a retrospective observational longitudinal cohort clinical study. We included adult patients treated between April 2018 and December 2022 with commercial CD19 CAR-T products for refractory or relapsed aggressive B-cell hematological malignancies at Mayo Clinic that had a baseline transthoracic echocardiogram (TTE). Patients were extracted from the Mayo Clinic database using the Current Procedural Terminology (CPT) codes for inpatient and outpatient administration of autologous CAR T-cell products. Patients < 18 years of age, without baseline TTE performed at our institution, treated for other malignancies, or with investigational CAR T-cell therapies were excluded.
CTRCD was defined as per the ESC 2022 Guidelines on Cardio-Oncology during the index hospitalization or readmission in the following thirty days [25]. CTRCD is classified as: Severe (new LVEF reduction to < 40%), Moderate (new LVEF reduction by ≥ 10% to 40–49% or reduction of < 10% to 40–49% and new relative decline in GLS by > 15% or new rise in cardiac biomarkers) or Mild (LVEF ≥ 50% and new relative decline in GLS by ≥ 15% and/or new rise in cardiac biomarkers) [25]. Baseline demographic, oncologic, CV characteristics and in-hospital complications were systematically abstracted from the electronic medical record (EMR) via detailed chart review by dedicated research personnel.
Baseline characteristics
Variables included age (in years at the time of CAR-T treatment), sex assigned at birth, race, patient reported ethnicity and type of cancer. Exposure to prior cardiotoxic treatments such as anthracycline containing chemotherapy regimens, chest and/or neck radiation, and history of stem cell transplantation was recorded. Established cardiovascular disease and risk factors included for analysis were prior diagnosis of hypertension, hyperlipidemia, diabetes, smoking status, history of cardiomyopathy, coronary artery disease, arrhythmias and body mass index at the day of CAR T-cell infusion.
Transthoracic echocardiography studies
Baseline echocardiograms were defined as the first available TTE performed at our institution before CAR T-cell infusion. The following TTEs after CAR T-cell infusion with LVEF measurement were included for comparison when available. TTEs where image quality was not adequate were excluded. All echocardiograms were performed by a registered diagnostic cardiac sonographer following the recommendations of the American Society of Echocardiography using commercially available echocardiography machines at Mayo Clinic [26]. Echocardiographic measurements included were LVEF, left ventricular end systolic dimension (LVESD), left ventricular end diastolic dimension (LVEDD), left atrial volume index (LAVI), right ventricular systolic pressure (RVSP), diastolic transmitral flow velocity (E/A), ratio of early mitral inflow to tissue velocity of the medial and lateral mitral annulus (E/e’), tricuspid regurgitant velocity, cardiac index (CI), stroke volume index (SVI) and GLS by 2D STE. When LVEF was reported in a range the averaged value rounded up to the nearest unit was retrieved for analysis.
Cytokine release syndrome
CRS was retrospectively graded for all patients within 30 days of CAR T-cell infusion according to the ASTCT 2019 consensus criteria [7] (Table 1) with chart history and vital signs reported in the EMR. Days to peak CRS, neurotoxicity, hypoxemia, hypotension, use of immune modulatory drugs, Intensive Care Unit (ICU) admission, sepsis, and use of vasopressors were abstracted and described. For CRS grading, fever was defined as a temperature of ≥ 38 degrees Celsius, hypotension was defined as a systolic blood pressure < 90 mmHg, and hypoxemia was defined as any respiratory imbalance requiring oxygen supplementation regardless of a particular cutoff value by pulse oximetry. The absence of fever was considered as no CRS.
Statistical analysis
Continuous variables are presented as mean ± standard deviation (SD) or median (interquartile range [IQR]) according to data distribution. Categorical variables are presented as frequencies and percentages. Patients were grouped by the occurrence of CRS ≥ 2 and CRS < 2. Paired and unpaired Student’s t-tests or Wilcoxon rank-sum tests were used to compare continuous variables, and Chi-squared or Fisher’s exact tests were used to compare categorical variables as appropriate. Univariate logistic regression analysis adjusted by age, sex and cardiovascular risk factors was used to evaluate the association between echocardiographic variables and the development of CRS ≥ 2. Patients with a follow-up TTE were grouped into a sub-cohort for baseline and follow-up TTE variable comparison. A p value of < 0.05 was considered statistically significant. All statistical analyses were performed using BlueSky Statistics® software version 7.40.
Results
We identified 291 patients treated with commercial CAR T-cell therapies for B-cell malignancies who were eligible for this study (Fig. 1). The median age at the time of CAR T-cell infusion was 60 years (IQR: 51 to 67 years) and most patients were male (197 [67%]) and white (255 [88%]). 120 (41%) developed CRS ≥ 2, 123 (42%) developed CRS 1 (only fever) and 48 (17%) had no CRS. Diffuse large B-cell lymphoma was the predominant cancer diagnosis (208 [71%]), and the primary CAR T-cell therapy product administered was axicabtagene ciloleucel (215 [74%]) (Supplemental Table 1). The most prevalent cardiovascular comorbidities observed were prior smoking history (102 [35%]), hypertension (97 [33%]), obesity (91 [31%], and dyslipidemia (75 [26%]). Post-infusion immunomodulation with tocilizumab and steroids was observed in 58% and 52% of all patients respectively. Patients that developed CRS ≥ 2 had statistically significant higher rates of tocilizumab and steroid administration when compared to patients with CRS < 2 (p < 0.001) (Supplemental Table 6). No statistically significant association was observed between baseline cardiovascular comorbidities and the development of CRS ≥ 2 (Table 2).
Fig. 1.
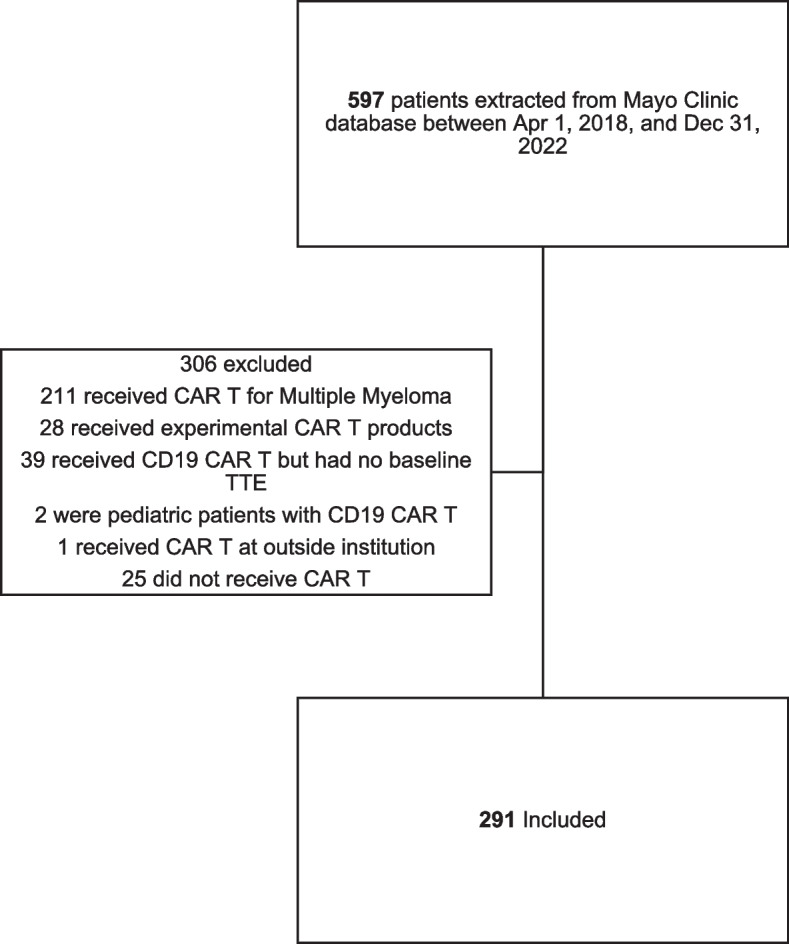
Cohort assembly. CAR T = Chimeric Antigen Receptor T-cell. TTE = transthoracic echocardiogram
Table 2.
Baseline characteristics of patients treated with CD19 CAR T-cell therapy
| Baseline characteristics of patients treated with CD19 CAR T-cell therapy | Entire cohort (n = 291) | Post-CAR T CRS status | ||
|---|---|---|---|---|
| CRS < 2 (n = 171). | CRS ≥ 2 (n = 120). | P value | ||
| Age at CAR T-cell infusion | 60 (51, 67) | 60 (51, 66.5) | 60 (51, 67) | 0.879 |
| Males | 194 (67%) | 109 (64%) | 85 (71%) | 0.256 |
| White | 255 (88%) | 151 (88%) | 104 (87%) | 0.256 |
| Hispanic | 17 (6%) | 9 (5%) | 8 (7%) | 0.575 |
| BMI, kg/m2 | 27.8 (6.2) | 27.8 (6.5) | 27.8 (5.9) | 0.690 |
| Obesity | 91 (31%) | 54 (32%) | 37 (31%) | 1.000 |
| Hypertension | 97 (33%) | 63 (37%) | 34 (28%) | 0.165 |
| Dyslipidemia | 75 (26%) | 42 (25%) | 33 (28%) | 0.588 |
| Diabetes | 47 (16%) | 33 (19%) | 14 (12%) | 0.105 |
| Smoking history | 102 (35%) | 58 (34%) | 44 (37%) | 0.708 |
| Atrial fibrillation | 30 (10%) | 17 (10%) | 13 (11%) | 0.440 |
| History of cardiomyopathy | 18 (6%) | 9 (5%) | 9 (8%) | 0.466 |
| Coronary Artery Disease | 26 (9%) | 17 (10%) | 9 (8%) | 0.536 |
Data are n (%), mean (SD), or median (Q1, Q3). CAR T-cell Chimeric Antigen T-cell, CRS Cytokine Release Syndrome
BMI Body mass index
All patients underwent baseline echocardiography, which occurred at a median 40 days before CAR T-cell infusion (IQR: 33–50 days). The mean LVEF was 60% (SD 6) and GLS was −18.7% (SD 2.6) (Table 3). The remaining systolic and diastolic parameters evaluated were also within normal range. No significant differences in baseline echocardiographic variables were observed between patients who developed CRS ≥ 2 and those who did not (Table 3). Univariate logistic regression models evaluating the association for each of the diastolic and systolic baseline echocardiographic variables and the occurrence of CRS ≥ 2 were not statistically significant even after adjustment by baseline comorbidities and immunomodulator administration (Supplemental Table 2).
Table 3.
Comparison of baseline pre-CAR T-cell infusion echocardiographic characteristics
| Echocardiographic variable | Total cohort (n = 291). | Post-CAR T-cell CRS status | ||
|---|---|---|---|---|
| CRS < 2 (n = 171). | CRS ≥ 2 (n = 120) | P value | ||
| Left ventricular global longitudinal strain | −18.7% (2.6) | −18.6% (2.6) | −18.9% (2.5) | 0.487 |
| Left ventricular ejection fraction | 60.1% (6) | 60% (5.7) | 60.3% (6.5) | 0.481 |
| Left ventricular end diastolic dimension, mm | 48.6 (5.6) | 48.7 (5.7) | 48.5 (5.4) | 0.908 |
| Left ventricular end systolic dimension, mm | 31.9 (4.7) | 32.1 (4.9) | 31.6 (4.3) | 0.503 |
| Left atrial volume index, mL/m2 | 29.0 (9.3) | 29.3 (10.1) | 28.5 (8) | 0.810 |
| Right ventricular systolic pressure, mmHg | 28.1 (6.3) | 27.8 (6.1) | 28.5 (6.5) | 0.677 |
| Cardiac Index, l/min/m2 | 3.2 (0.7) | 3.2 (0.6) | 3.2 (0.9) | 0.646 |
| Stroke Volume Index, mL/m2 | 42.3 (8.0) | 42.0 (7.4) | 42.6 (8.7) | 0.615 |
| Mitral E/e’ ratio (medial) | 8.7 (3.2) | 8.7 (3.1) | 8.7 (3.3) | 0.842 |
| Mitral E/e’ ratio (lateral) | 6.4 (2.6) | 6.4 (2.9) | 6.5 (2.1) | 0.248 |
| E/A ratio | 1.0 (0.4) | 1.0 (0.4) | 1.0 (0.3) | 0.907 |
| Tricuspid regurgitant velocity, m/sec | 2.4 (0.7) | 2.4 (0.8) | 2.5 (0.6) | 0.506 |
| Date of echocardiogram pre-CAR T-cell infusion | 40 (33, 50) | 41 (34, 49.5) | 38 (32, 50.5) | 0.107 |
Data are mean (SD) or days (Q1, Q3). CAR T-cell Chimeric Antigen T-cell, CRS Cytokine Release Syndrome
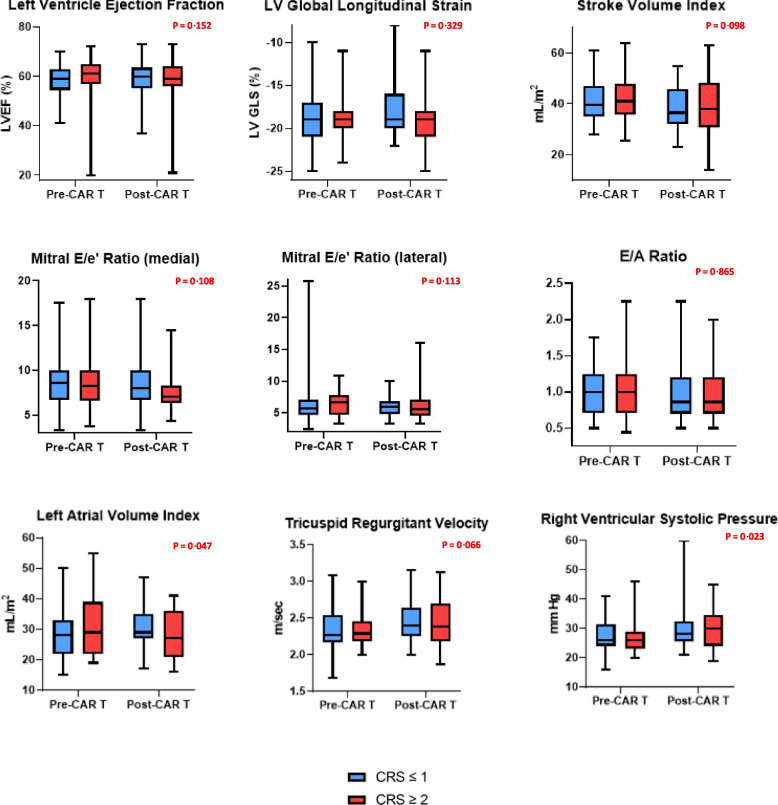
A total of 114 patients had one or more post-CAR T-cell therapy follow-up TTE at a median time of 5.7 weeks (IQR: 1.3, 28) after infusion. We observed statistically significant differences in RVSP, Stroke Volume Index and tricuspid regurgitant velocity at follow-up for the entire sub-cohort, but the ranges for these variables were found to be within normal limits (Table 4). When comparing by CRS groups, time to follow-up TTE differed and patients with CRS ≥ 2 were more likely to have had an echocardiogram performed sooner than patients with CRS < 2 (2.1 weeks [IQR:1, 10.8] vs 16.5 weeks [IQR:4.3, 48], p < 0.001) (Supplemental Table 3). We observed a similar distribution of baseline characteristics and clinical outcomes among CRS groups (Supplemental Table 3). Among patients with CRS ≥ 2 (54%), no significant differences were observed in systolic echocardiographic parameters at follow up (Supplemental Table 4). We observed a modest increase in RVSP and a decrease in LAVI with statistically significant differences when compared to baseline (Fig. 2). However, these differences were within normal limits. In patients with CRS < 2 (46%), there was a modest reduction in Stroke Volume Index that was statistically significant, but this was within normal range and like the pattern observed for the sub-cohort (Supplemental Table 5).
Table 4.
Comparison of pre-CAR T-cell and post CAR T-cell echocardiographic variables
| Echographic parameter | Pre-CAR T TTE (n = 114) | Post-CAR T TTE(n = 114) | P value |
|---|---|---|---|
| Left ventricular global longitudinal strain, %, mean (SD) | −18.7 (2.99) | −18.6 (2.94) | 0.811 |
| Left ventricular ejection fraction, %, mean (SD) | 59.4 (6.8) | 58.6 (8.4) | 0.246 |
| Left ventricular end diastolic dimension, mm, mean (SD) | 48.3 (5.6) | 48.0 (6.0) | 0.485 |
| Left ventricular end systolic dimension, mm, mean (SD) | 32.2 (5.0) | 31.5 (5.2) | 0.129 |
| Left atrial volume index, mL/m2, mean (SD) | 29.9 (9.3) | 28.5 (9.5) | 0.393 |
| Right ventricular systolic pressure, mmHg, mean (SD) | 27.4 (6.1) | 30.1 (7.6) | 0.007 |
| Cardiac Index, mean (SD) | 3.2 (0.8) | 3.2 (0.7) | 0.561 |
| Stroke Volume Index, mean (SD) | 41.7 (8.1) | 38.7 (10.8) | 0.008 |
| Mitral E/e’ ratio (medial), mean (SD) | 8.6 (2.9) | 8.1 (2.9) | 0.210 |
| Mitral E/e’ ratio (lateral), mean (SD) | 6.5 (1.9) | 6 (1.6) | 0.194 |
| E/A ratio, mean (SD) | 1 (0.4) | 1 (0.4) | 0.834 |
| Tricuspid regurgitant velocity, m/sec, mean (SD) | 2.34 (0.29) | 2.44 (0.31) | 0.024 |
Data are mean (SD). CAR T-cell Chimeric Antigen T-cell, TTE transthoracic echocardiogram
Fig. 2.
Echocardiographic systolic and diastolic parameters for patients with CRS ≥ 2 and CRS < 2. CAR T = Chimeric Antigen Receptor T-cell. CRS = Cytokine Release Syndrome. P values for paired Student’s t-tests comparing echocardiographic parameters in patients that developed CRS ≥ 2
A total of 13 patients (4.5%) developed CTRCD. Five patients exhibited Mild CTRCD, six patients developed Moderate CTRCD, and two patients developed Severe CTRCD. Patients with Mild CTRCD were characterized primarily by a relative decrease in GLS with one patient exhibiting both a decrease in GLS and elevation in troponin. 5/6 patients with Moderate CTRCD were characterized by a decline in LVEF ≥ 10% (two patients in this group also had troponin elevation) and one patient by a decline in LVEF < 10% and a decline in GLS. Two patients with Severe CTRCD had a decline in LVEF to less than 40%, and one patient also had an elevation of cardiac biomarkers. Baseline and follow-up cardiac biomarkers were not routinely performed for all patients and were obtained at the discretion of the treating team. Tocilizumab administration was common among patients with CTRCD (77%, n = 10/13) with only one patient with Moderate CTRCD and two patients with Mild CTCRD not receiving it. Nine of the 13 patients (69%) who developed CTRCD also developed CRS ≥ 2 (p = 0.036, Supplemental Table 6). Patients with CRS ≥ 2 had a higher incidence of Moderate and Severe forms of CTRCD compared to patients who developed CRS < 2 (Supplemental Table 6). No baseline echocardiographic variables, including strain, or comorbidities were found to be associated with the development of CTRCD. One patient who developed Moderate CTRCD returned to baseline LVEF by day 10, and another patient with Severe CTRCD showed an improvement in LVEF to less than 50%. No additional follow-up echocardiographic data was available for the remaining patients.
Discussion
To our knowledge, this is the first and largest study of patients treated with CD19 CAR T-cell therapies to evaluate the relationship between echocardiographic variables and the development of hemodynamically significant CRS. The main findings were that no baseline echocardiographic variables were predictive for the occurrence of CRS ≥ 2, no overall clinically significant differences were observed in systolic and diastolic variables after CAR T-cell therapy, and the incidence of CTRCD was 4.5% for the cohort during the study period which mostly occurred in the setting of CRS ≥ 2.
Adult patients treated with CAR T-cells represent a complex clinical scenario from a Cardio-Oncology standpoint as they are typically older, have overlapping risk factors for both cancer and cardiovascular disease, would have probably received previous cardiotoxic anti-cancer treatments, and could be severely compromised by the extent of their disease. Additionally, the mechanism leading to CAR T-cell activation is the same that produces toxicities, which makes efficacy and toxicity mechanistically intertwined [27, 28].
It is generally accepted that the main mediators of the clinical manifestations observed in CRS-related cardiotoxicity are capillary leak syndrome, endothelial injury, and IL-6 mediated myocardial depression produced by abnormal cytokine release by CAR T-cells and surrounding immune cells upon antigen engagement [4, 5, 27, 29, 30]. IL-6 alters Ca2+ pathways that can reduce myocardial contractility and potentially result in diastolic dysfunction and arrhythmias [4, 20]. Tocilizumab, an anti-IL6 receptor monoclonal antibody, is commonly used to curb the inflammatory response and recent studies support earlier administration without loss of CAR T-cell therapeutic efficacy [31].
Patients with greater CRS and toxicities resulting from increased CAR T-cell expansion were expected to have better survival and response rates. Yet, it has been demonstrated that toxicity is not related to clinical responses and long-term progression free survival [11, 27, 32–34]. Theoretically, adequate cardiac reserve appears to be crucial to mitigate the hemodynamic effects of CRS, but this has not been reliably proven in clinical practice. We chose to address this gap in knowledge and study the inverse relationship, evaluating if any objective functional alteration by echocardiography at baseline could influence the degree of hemodynamic effects produced by CRS.
Studies evaluating echocardiographic variables in adult patients treated with CD19 CAR T-cells and their relationship to adverse cardiovascular outcomes have been scarce, small, and descriptive in nature. Recently, two prospective studies have evaluated the cardiovascular effects of CAR T-cell therapy and their relationship to inflammation by CRS. Lefebvre and colleagues described a cohort of 44 patients who had both echocardiograms and serum cardiac biomarkers performed before CAR T-cell infusion and repeated at 4 timepoints (2 days, 1 week, 1 month, and 6 months after infusion). 25% of patients developed CRS ≥ 2 and there were no baseline clinical or echocardiographic differences between patients that developed CRS and those who did not. Longitudinal echocardiographic follow-up showed only subtle cardiac dysfunction demonstrated by modest declines of GLS with a pattern of reversibility. No changes in LVEF were observed, however the authors noted that in 14 (32%) patients there was an increase in levels of NT-proBNP measured during CRS episodes when compared to baseline. Building on this study, Camilli et al. recently published the CARdio-TOX study in this journal where they recruited and followed 27 adult patients receiving CD19 CAR T-cell therapy. [35] Patients also had echocardiograms and serum cardiac and inflammatory biomarkers performed before and 7 days after infusion. The primary outcome was the incidence of CTRCD using the updated ESC Cardio-Oncology definitions. The authors noted statistically significant increases in NT-proBNP and high-sensitivity troponin at 7 days post infusion. 59% of patients were diagnosed with CTRCD and had a higher prevalence of CRS ≥ 2 compared to those who did not develop CTRCD. This high incidence of CTRCD in the acute period was largely due to the novel integration of serum cardiac biomarkers and echocardiographic measurements which reflects the full spectrum of clinical and sub-clinical myocardial dysfunction and the time-dependent nature of the observable effects; this is a strength of this study. A limitation of this study is its short follow-up; however, further surveillance of this cohort will likely contribute greatly to the understanding of potential long-term complications of the acute CRS-mediated adverse cardiac effects. Interestingly, no correlation was observed between CTRCD and serum levels of IL-6. Previous retrospective studies have also aimed to elucidate the adverse cardiac dynamics after CAR T-cell therapy.
Patel and colleagues evaluated the association of baseline echocardiographic variables and adverse cardiac events (CE) in 75 patients treated with CAR T-cell therapy for B-cell malignancies and multiple myeloma [36]. 10 CEs were reported in nine patients, however, their association to CRS grades was not described [36]. Lower GLS and higher MV E/e’ were associated with a higher risk of cardiac events. Higher grades of CRS (2–4) were underrepresented in this cohort, and no association was found between baseline echocardiographic variables and CRS grades (defined as no CRS [Grade 0] vs Grade 1–2). This limits comparison with our study, which had a better representation of the clinical spectrum of CRS. Lefebvre et al. described a retrospective cohort of 145 patients (78 had comprehensive baseline echocardiograms) evaluating clinical, laboratory, and echocardiographic parameters associated with major adverse cardiovascular events (MACE). [11] They observed a borderline significant association between MACE and higher mitral E/e’ ratio (HR: 1.15 [1.00, 1.31], p = 0.046), and CRS > 2 was significantly associated with the development of MACE [11]. Alvi and colleagues in an earlier study described the cardiac profiles of 137 patients who received CAR T-cell therapy. In the 129 patients with a pre–CAR T-cell TTE no significant differences in baseline echocardiographic variables were found when comparing patients that developed CRS ≥ 2 with those who did not. 29 patients had echocardiographic data pre and post CAR T-cell infusion, and CTRCD was reported in eight of these patients (5.8% of the cohort) who all developed CRS ≥ 2 and had elevated troponin levels [17]. Finally, in a study evaluating the development of CTRCD after CAR T-cell therapy, Ganatra et al. described a cohort of 187 patients. CTRCD was reported in 12 patients (10.3%), and they were found to be older, with a higher prevalence of hyperlipidemia, hypertension and coronary artery disease, and a higher incidence of CRS ≥ 2 (11/12) [15]. In both the Alvi and Ganatra studies, CTRCD (or cardiomyopathy) was described as a new drop in LVEF by > 10% to an absolute value of < 50% which is why the prevalence is significantly lower than that observed by Camilli et al. All the cases of CTRCD observed in retrospective studies so far, including pediatric patients, have generally demonstrated a self-limiting and reversible pattern, with most patients returning to baseline LVEF levels. [12, 15] In summary, evidence suggests that there is a strong correlation between CRS ≥ 2 and the development of adverse cardiovascular outcomes. However, these findings have been described in relatively small patient populations, and the role of baseline cardiac function in relation to CRS has not been adequately established. In our study, we included a significantly larger patient cohort increasing statistical power. Although we did not observe a statistically significant association between baseline echocardiographic variables and the development of CRS ≥ 2, our results are in line with those previously reported. We observed a relatively low incidence of CTRCD which more strongly correlated to the development of CRS ≥ 2, and in patients with serial TTEs there was a pattern of reversibility. An additional strength of our study was that as the CARdio-ToX study, CTRCD was defined as per the latest 2022 ESC Cardio-Oncology Guidelines which include GLS in addition to LVEF and represents a wider range of myocardial systolic dysfunction [37]. However, because this study was retrospective in nature and echocardiograms and cardiac biomarkers were not performed uniformly and at set intervals, the true incidence of CTRCD was most likely higher in our cohort as most patients were classified by variations in LVEF and GLS and did not primarily rely in cardiac serum biomarkers.
Our results suggest that baseline cardiac function by echocardiography is not directly associated with the severity of CRS in this specific patient population, and thus the subsequent risk of cardiotoxicity may be independently mediated by the other previously established predictive risk factors for CRS such as high disease burden, high CAR T-cell dose and high intensity lymphodepleting regimen [5, 16, 38]. This is also supported by the fact that in our study baseline cardiovascular comorbidities and prior cardiotoxic treatments did not predict worsening CRS levels, suggesting the involvement of multifactorial pathways beyond baseline cardiac function. Moving forward, pooled data analysis and further research is warranted to better understand the interplay between tumor burden, CAR T-cell dose, cardiac comorbidities, and baseline cardiac function in the development of CRS. More prospective studies exploring whether post-CRS cardiotoxicity is non-cardiac mediated and rather driven by systemic inflammation, in a process akin to sepsis or COVID-19 [39, 40], will have important implications in increasing the eligibility of patients with cardiac disease.
Limitations to this study, as discussed, are its retrospective scope and the fact that the study population was derived from an integrated hospital network and was disproportionately white and male. Adequate cardiac function is part of the selection criteria for CAR T-cell infusion which leads to exclusion of patients with known clinical cardiac dysfunction. Additionally, because follow-up echocardiograms were not routinely performed, the prevalence of CTRCD was likely underestimated, especially mild and moderate forms. However, given the rarity of the treatment, this study provides important insights and highlights the need to evaluate more inclusive and diverse patient populations. Another challenge arises from the presence of missing data in some echocardiographic variables due to technical limitations during image acquisition which was duly reported. We chose to evaluate real-world data to ensure representativeness of this rare treatment scenario.
Conclusions
In conclusion, our study contributes significant findings to the field of CAR T-cell therapy by examining the largest cohort of echocardiographic profiles to date. No specific echocardiographic variables, including LV GLS, predicted the development of CRS ≥ 2, and therefore the mechanism leading to hemodynamic impairment producing worsening hypoxia and hypotension could be multifactorial and independent from the baseline echocardiographic profile. Additionally, no overall systolic and diastolic variable compromise was observed after CRS ≥ 2 suggesting that there is no underlying or residual cardiac dysfunction after CAR T-cell therapy in this group of patients. We observed low incidence of CTRCD in this cohort, which primarily occurred in the setting of CRS ≥ 2, and therefore warrant closer monitoring. Considering the full clinical and subclinical spectrum of myocardial dysfunction observed thus far, we propose a comprehensive pretreatment cardiovascular evaluation that includes cardiac risk factor management, echocardiographic and serum cardiac biomarker measurement, and tailored clinical surveillance and follow-up. This approach would most likely allow early identification of patients at risk and allow for prompt intervention to avoid adverse long-term cardiac outcomes.
Supplementary Information
Additional file 1: Supplementary appendix. Table of Contents: Supplemental Table 1. Oncologic characteristics of patients treated with CD19 CAR T-cell therapy. Supplemental Table 2. Univariable logistic regression models for baseline echocardiographic parameters predictive of CRS ≥2 after CAR T-cell therapy. Supplemental Table 3. Comparison of baseline characteristics, cardiovascular disease, risk factors and clinical outcomes in patients with post-CAR T therapy echocardiogram. Supplemental Table 4. Comparison of Pre-CAR T and Post-CAR T echocardiographic variables in patients who developed CRS ≥2. Supplemental Table 5. Comparison of pre-CAR T and post-CAR T echocardiographic variables in patients who developed CRS <2. Supplemental Table 6. Comparison of clinical outcomes in patients who underwent CD19 CAR T-cell therapy.
Acknowledgements
We thank the Mayo Clinic Center for Clinical and Translational Science (CCaTS) for their advisory and assistance in statistical planning and execution. We also thank patients with cancer and their families who bravely fight this disease.
Abbreviations
- 2D
2 Dimensional
- 2D STE
2-Dimensional speckle tracking echocardiography
- CRS
Cytokine Release Syndrome
- CAR
Chimeric antigen receptor
- CD19
Cluster of differentiation 19
- CE
Cardiac events
- CV
Cardiovascular
- CTRCD
Cancer treatment related cardiac dysfunction
- LV
Left ventricle
- EF
Ejection fraction
- CI
Confidence interval
- SD
Standard deviation
- BMI
Body Mass Index
- SVI
Stroke volume index
- GLS
Global longitudinal strain
- LVEDD
Left ventricular end diastolic dimension
- LVESD
Left ventricular end systolic dimension
- LAVI
Left atrial volume index
- ASE
American Society of Echocardiography
- TTE
Transthoracic echocardiogram
Authors’ contributions
A.D., H.V., and M.A. were responsible for the conceptualization of the study. Methodology was developed by A.D. and H.V., who also handled data curation. The original draft of the manuscript was written by A.D., H.V., M.A., and J.P. All authors contributed to the review and editing of the manuscript.
Funding
This study was conducted without external funding.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request and IRB approval. Raw data with deidentified patient information are stored securely and can be made available in compliance with ethical and privacy regulations.
Declarations
Ethics approval and consent to participate
The Mayo Clinic Institutional Review Board approved this study and waived the requirement for consent.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–52. [DOI] [PubMed] [Google Scholar]
- 2.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med. 2019;380(1):45–56. [DOI] [PubMed] [Google Scholar]
- 3.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017;377(26):2531–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ganatra S, Dani SS, Yang EH, Zaha VG, Nohria A. Cardiotoxicity of T-Cell Antineoplastic Therapies: JACC: CardioOncology Primer. JACC CardioOncol. 2022;4(5):616–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hay KA, Hanafi LA, Li D, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130(21):2295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marar RI, Abbasi MA, Prathivadhi-Bhayankaram S, et al. Cardiotoxicities of Novel Therapies in Hematologic Malignancies: Chimeric Antigen Receptor T-Cell Therapy and Bispecific T-Cell Engager Therapy. JCO Oncol Pract 2023: OP2200713. [DOI] [PubMed]
- 7.Lee DW, Santomasso BD, Locke FL, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transplant. 2019;25(4):625–38. [DOI] [PubMed] [Google Scholar]
- 8.Stein-Merlob AF, Ganatra S, Yang EH. T-cell Immunotherapy and Cardiovascular Disease: Chimeric Antigen Receptor T-cell and Bispecific T-cell Engager Therapies. Heart Fail Clin. 2022;18(3):443–54. [DOI] [PubMed] [Google Scholar]
- 9.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20(1):31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lefebvre B, Kang Y, Smith AM, Frey NV, Carver JR, Scherrer-Crosbie M. Cardiovascular Effects of CAR T Cell Therapy: A Retrospective Study. JACC CardioOncol. 2020;2(2):193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shalabi H, Sachdev V, Kulshreshtha A, Cohen JW, Yates B, Rosing DR, Sidenko S, Delbrook C, Mackall C, Wiley B, Lee DW, Shah NN. Impact of cytokine release syndrome on cardiac function following CD19 CAR-T cell therapy in children and young adults with hematological malignancies. J Immunother of Cancer. 2020;8(2):e001159. 10.1136/jitc-2020-001159. [DOI] [PMC free article] [PubMed]
- 13.Prathivadhi-Bhayankaram S, Abbasi MA, Ismayl M, et al. Cardiotoxicities of Novel Therapies in Hematological Malignancies: Monoclonal Antibodies and Enzyme Inhibitors. Curr Probl Cardiol. 2023;48(8): 101757. [DOI] [PubMed] [Google Scholar]
- 14.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganatra S, Redd R, Hayek SS, et al. Chimeric Antigen Receptor T-Cell Therapy-Associated Cardiomyopathy in Patients With Refractory or Relapsed Non-Hodgkin Lymphoma. Circulation. 2020;142(17):1687–90. [DOI] [PubMed] [Google Scholar]
- 16.Burns EA, Gentille C, Trachtenberg B, Pingali SR, Anand K. Cardiotoxicity Associated with Anti-CD19 Chimeric Antigen Receptor T-Cell (CAR-T) Therapy: Recognition, Risk Factors, and Management. Diseases (Basel, Switzerland). 2021;9(1):20. 10.3390/diseases9010020. [DOI] [PMC free article] [PubMed]
- 17.Alvi RM, Frigault MJ, Fradley MG, et al. Cardiovascular Events Among Adults Treated With Chimeric Antigen Receptor T-Cells (CAR-T). J Am Coll Cardiol. 2019;74(25):3099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murthy H, Iqbal M, Chavez JC, Kharfan-Dabaja MA. Cytokine Release Syndrome: Current Perspectives. Immunotargets Ther. 2019;8:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ganatra S, Carver JR, Hayek SS, et al. Chimeric Antigen Receptor T-Cell Therapy for Cancer and Heart: JACC Council Perspectives. J Am Coll Cardiol. 2019;74(25):3153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ali A, Boutjdir M, Aromolaran AS. Cardiolipotoxicity, Inflammation, and Arrhythmias: Role for Interleukin-6 Molecular Mechanisms. Front Physiol. 2018;9:1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nenna A, Carpenito M, Chello C, Nappi P, Annibali O, Vincenzi B, Grigioni F, Chello M, Nappi F. Cardiotoxicity of Chimeric Antigen Receptor T-Cell (CAR-T) Therapy: Pathophysiology, Clinical Implications, and Echocardiographic Assessment. Int J Mol Sci. 2022;23(15):8242. 10.3390/ijms23158242. [DOI] [PMC free article] [PubMed]
- 22.Goldman A, Maor E, Bomze D, et al. Adverse Cardiovascular and Pulmonary Events Associated With Chimeric Antigen Receptor T-Cell Therapy. J Am Coll Cardiol. 2021;78(18):1800–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel NP, Doukas PG, Gordon LI, Akhter N. Cardiovascular Toxicities of CAR T-cell Therapy. Curr Oncol Rep. 2021;23(7):78. [DOI] [PubMed] [Google Scholar]
- 24.Baldassarre LA, Ganatra S, Lopez-Mattei J, et al. Advances in Multimodality Imaging in Cardio-Oncology: JACC State-of-the-Art Review. J Am Coll Cardiol. 2022;80(16):1560–78. [DOI] [PubMed] [Google Scholar]
- 25.Lyon AR, Lopez-Fernandez T, Couch LS, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022;43(41):4229–361. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell C, Rahko PS, Blauwet LA, et al. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2019;32(1):1–64. [DOI] [PubMed] [Google Scholar]
- 27.Chohan KL, Bansal R, Hathcock MA, Paludo J, Bennani NN, Johnston PB, Khurana A, Durani U, Wang Y, Ruff MW, Villasboas Bisneto JC, Ansell SM, Lin Y, Kenderian SS. Real-world associations of cytokine release syndrome and neurotoxicity with efficacy in patients receiving anti-CD-19 chimeric antigen receptor T-cell therapy for large B-cell lymphoma: the Mayo Clinic experience. Leukemia & lymphoma. 2024;65(3):389–93. 10.1080/10428194.2023.2285236. [DOI] [PubMed]
- 28.Chohan KL, Siegler EL, Kenderian SS. CAR-T Cell Therapy: the Efficacy and Toxicity Balance. Curr Hematol Malig Rep. 2023;18(2):9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Totzeck M, Michel L, Lin Y, Herrmann J, Rassaf T. Cardiotoxicity from chimeric antigen receptor-T cell therapy for advanced malignancies. Eur Heart J. 2022;43(20):1928–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghosh AK, Chen DH, Guha A, Mackenzie S, Walker JM, Roddie C. CAR T Cell Therapy-Related Cardiovascular Outcomes and Management: Systemic Disease or Direct Cardiotoxicity? JACC CardioOncol. 2020;2(1):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banerjee R, Marsal J, Huang CY, et al. Early Time-to-Tocilizumab after B Cell Maturation Antigen-Directed Chimeric Antigen Receptor T Cell Therapy in Myeloma. Transplant Cell Ther 2021; 27(6): 477 e1- e7. [DOI] [PubMed]
- 32.Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N Engl J Med. 2020;382(14):1331–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobs MT, Jain MD, Gao F, et al. Severity of Cytokine Release Syndrome Influences Outcome After Axicabtagene Ciloleucel for Large B cell Lymphoma: Results from the US Lymphoma CAR-T Consortium. Cl Lymph Myelom Leuk. 2022;22(10):753–9. [DOI] [PubMed] [Google Scholar]
- 34.Bhaskar ST, Patel VG, Porter DL, et al. Chimeric antigen receptor T-cell therapy yields similar outcomes in patients with and without cytokine release syndrome. Blood Adv. 2023;7(17):4765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camilli M, Viscovo M, Felici T, et al. Inflammation and acute cardiotoxicity in adult hematological patients treated with CAR-T cells: results from a pilot proof-of-concept study. Cardiooncology. 2024;10(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel NP, Dalal PJ, Meng Z, Baldridge AS, Cascino GJ, Sunderraj A, Sinha A, Karmali R, Feinstein MJ, Akhter N. Myocardial strain is associated with adverse cardiac events in patients treated with chimeric antigen receptor (CAR) T-cell therapy. Euro J Haematol. 2024;112(1):102–10. 10.1111/ejh.14088. [DOI] [PubMed]
- 37.Temporal patterns of left ventricular systolic and diastolic metrics changes in adult patients with haematological malignancies treated with chimeric antigen receptor (CAR)-T cells: results from the CARdio-Tox prospective study European Heart Journal - Cardiovascular Imaging. 2024;25(3):e101–3. 10.1093/ehjci/jead317. [DOI] [PubMed]
- 38.Yan Z, Zhang H, Cao J, et al. Characteristics and Risk Factors of Cytokine Release Syndrome in Chimeric Antigen Receptor T Cell Treatment. Front Immunol. 2021;12:611366. [DOI] [PMC free article] [PubMed]
- 39.Pathan N, Hemingway CA, Alizadeh AA, et al. Role of interleukin 6 in myocardial dysfunction of meningococcal septic shock. Lancet. 2004;363(9404):203–9. [DOI] [PubMed] [Google Scholar]
- 40.Young KA, Krishna H, Jain V, et al. Serial Left and Right Ventricular Strain Analysis in Patients Recovered from COVID-19. J Am Soc Echocardiogr. 2022;35(10):1055–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary appendix. Table of Contents: Supplemental Table 1. Oncologic characteristics of patients treated with CD19 CAR T-cell therapy. Supplemental Table 2. Univariable logistic regression models for baseline echocardiographic parameters predictive of CRS ≥2 after CAR T-cell therapy. Supplemental Table 3. Comparison of baseline characteristics, cardiovascular disease, risk factors and clinical outcomes in patients with post-CAR T therapy echocardiogram. Supplemental Table 4. Comparison of Pre-CAR T and Post-CAR T echocardiographic variables in patients who developed CRS ≥2. Supplemental Table 5. Comparison of pre-CAR T and post-CAR T echocardiographic variables in patients who developed CRS <2. Supplemental Table 6. Comparison of clinical outcomes in patients who underwent CD19 CAR T-cell therapy.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request and IRB approval. Raw data with deidentified patient information are stored securely and can be made available in compliance with ethical and privacy regulations.