Abstract
Background
Spinal schwannomas presenting with an intraspinal hematoma or subarachnoid hemorrhage are extremely rare, and patients often have severe spinal cord compression symptoms. However, the mechanism underlying the bleeding remains unclear.
Case presentation
We present the case of a 53-year-old Chinese female diagnosed with a T12 schwannoma accompanied by an intratumoral hematoma. The patient suddenly experienced unbearable pain in the lower limbs. An emergency operation was necessary, and during surgery, we resected a tumor and evacuated a hematoma. We found a spinal nerve root fracture, intratumoral congestion, tumor capsule rupture, and bleeding. Pathological analysis indicated a schwannoma.
Conclusion
Injury to the nerve roots and vessels during motion, nerve root torsion, twisting, venous obstruction, tumor congestion, swelling, and capsule rupture are important processes in spinal schwannoma hemorrhage. Early diagnosis and proactive surgery are key points for treatment.
Keywords: Schwannoma, Spinal tumor, Subarachnoid hemorrhage, Intratumoral hematoma
Background
Schwannomas are common benign tumors that usually occur in the posterolateral region of the spinal canal [1]. Intraspinal hematoma or subarachnoid hemorrhage due to a schwannoma stroke is extremely rare. However, patients often have severe spinal cord compression symptoms that require emergency surgery. However, the mechanism underlying the bleeding remains unclear. This case describes a patient with an intraspinal schwannoma that presented with breakage and bleeding of the spinal nerve root, intratumoral congestion, tumor envelope rupture and bleeding, and intraspinal hematoma. In addition, we review studies on spinal schwannoma hemorrhage and explore the mechanisms of bleeding.
Case presentation
Informed consent was obtained from the patient. We present the case of a 53-year-old Chinese female who had no chronic diseases. The patient was hospitalized for surgical treatment of a lumbar disc herniation in November 2022. Magnetic resonance imaging (MRI) revealed a space-occupying lesion in the spinal canal at T12 measuring 1 cm × 1 cm × 1 cm (Fig. 1). The lesion was not treated further at that time. Fifty days before admission, the patient suddenly experienced pain in both lower limbs after long-distance running. The pain was mild, tolerable, and relieved with painkillers. On 13 April 2023, the pain suddenly increased and became unbearable. Lumbar MRI showed that the spinal canal at T12–L1 was occupied, with a 2 cm × 2 cm × 3 cm T11–L1 intraspinal hematoma (Fig. 2). The patient was transferred to our hospital for treatment. Physical examination revealed decreased sensation in both lower limbs, and no obvious positive signs were detected in the rest of the body. The pain was unbearable, and the patient underwent emergency surgery.
Fig. 1.
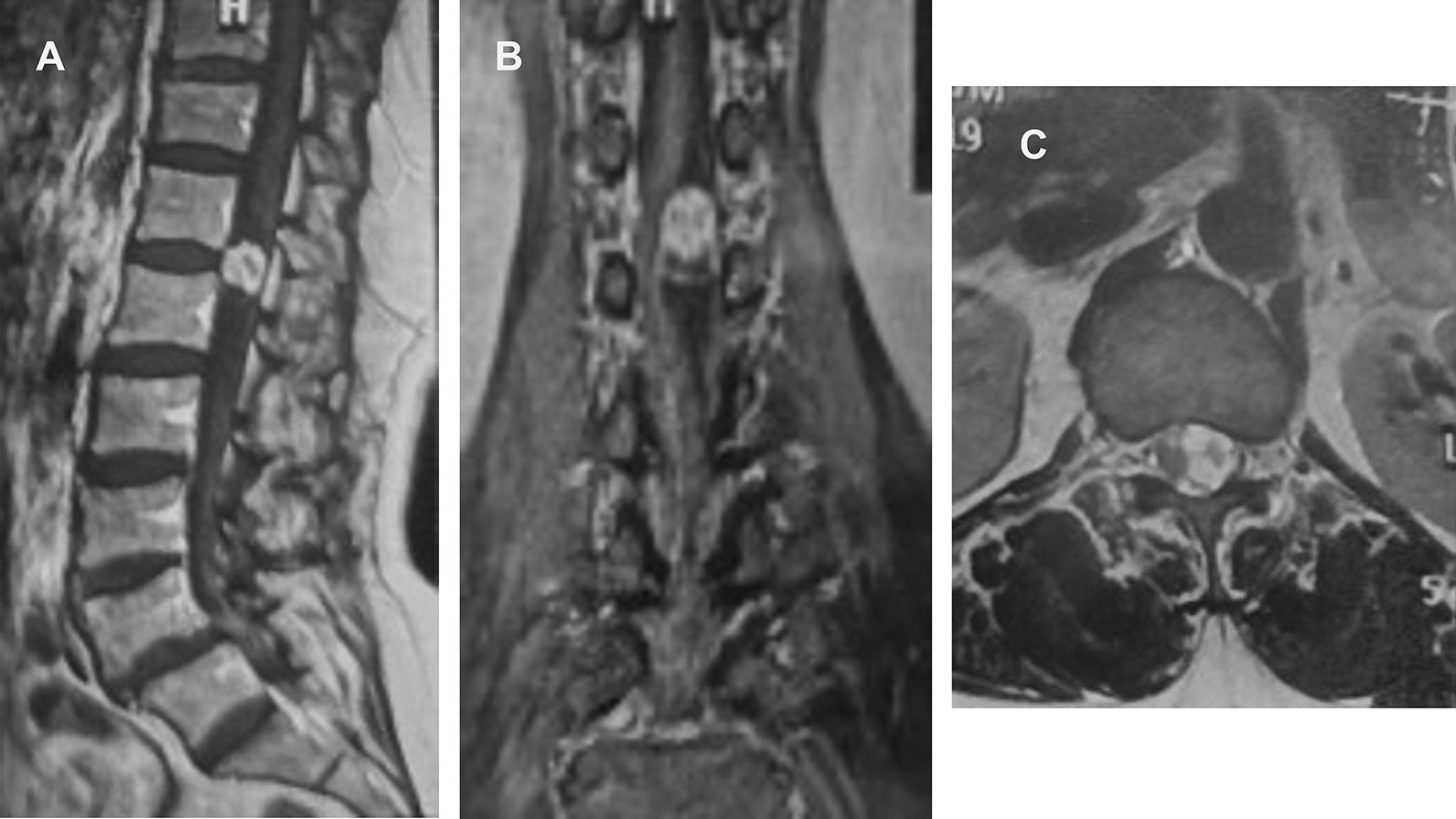
Three months before admission, magnetic resonance imaging revealed a 1 cm × 1 cm × 1 cm space-occupying lesion of the spinal canal at T12. The tumor is enhanced and considered a schwannoma. A Sagittal position. B Coronal position, C Axial position
Fig. 2.
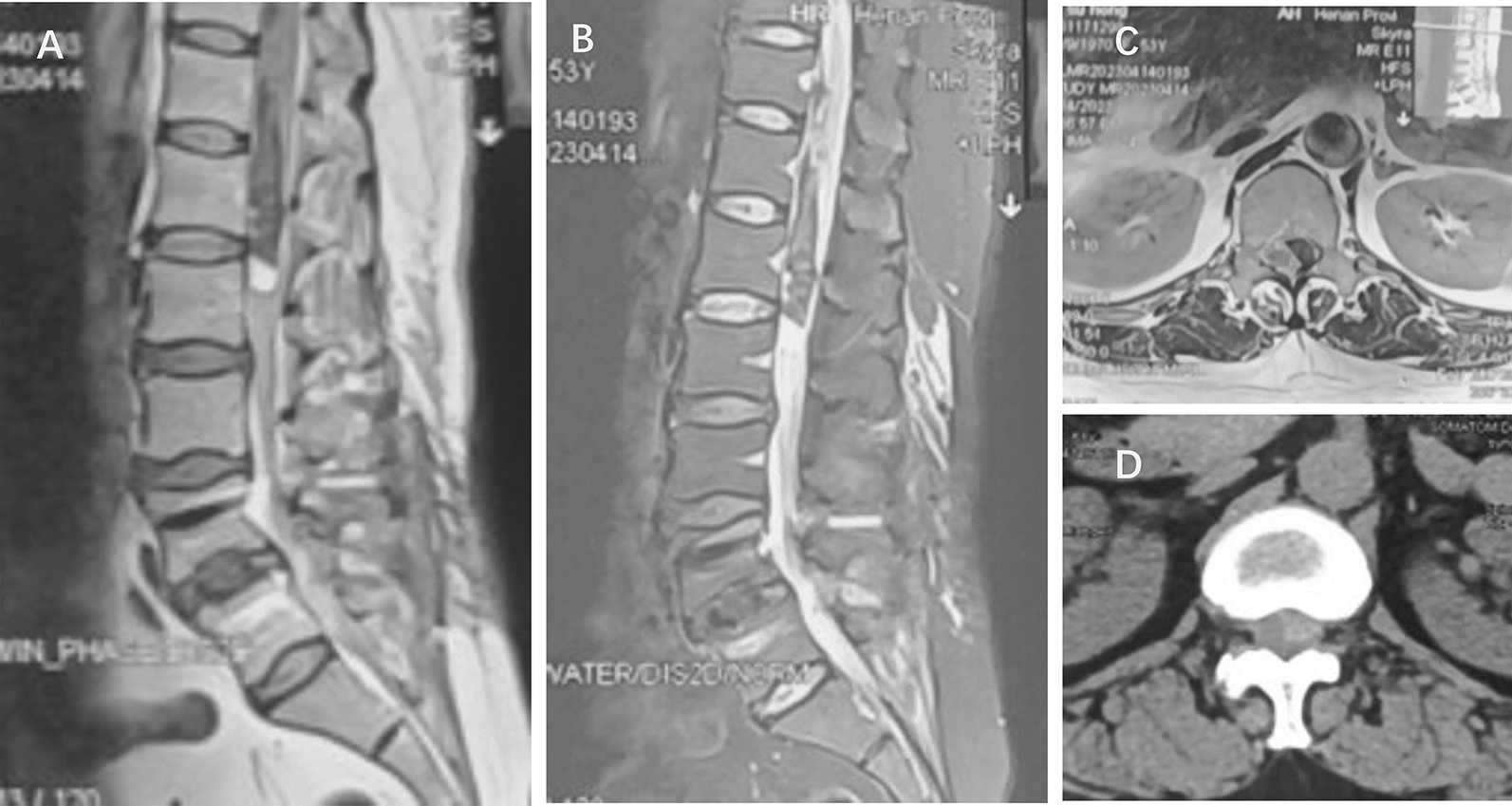
Two days before admission, the patient experienced increased pain. Magnetic resonance imaging showed the spinal canal at T12-L1 was occupied, with a 2 cm × 2 cm × 3 cm T11–L1 intraspinal hematoma. A T1 weighted. B T2 weighted. C Magnetic resonance imaging axial position showed the lesion on the left side. D Computed tomography axial position showed a highly dense lesion
A T11–L1 laminectomy was performed using an ultrasonic bone scalpel to expose the spinal canal. After a longitudinal incision, the dura showed that the arachnoid membrane was intact (Fig. 3), and many subarachnoid hematomas (approximately 5 ml) were found. The main body of the tumor was located at the T12–L1 vertebral body level. The tumor was tough, reddish brown, and a rupture of the tumor envelope was visible (Figs. 4, 5, 6). The rostral nerve root was broken and fractured (Fig. 4), and the tumor was tightly connected to the caudal nerve root (Fig. 6). The hematoma was completely removed under the microscope, the carrier nerve was cut off, and the tumor was completely resected. Longitudinal sectioning of the tumor revealed diffuse congestion and swelling (Fig. 7A).
Fig. 3.
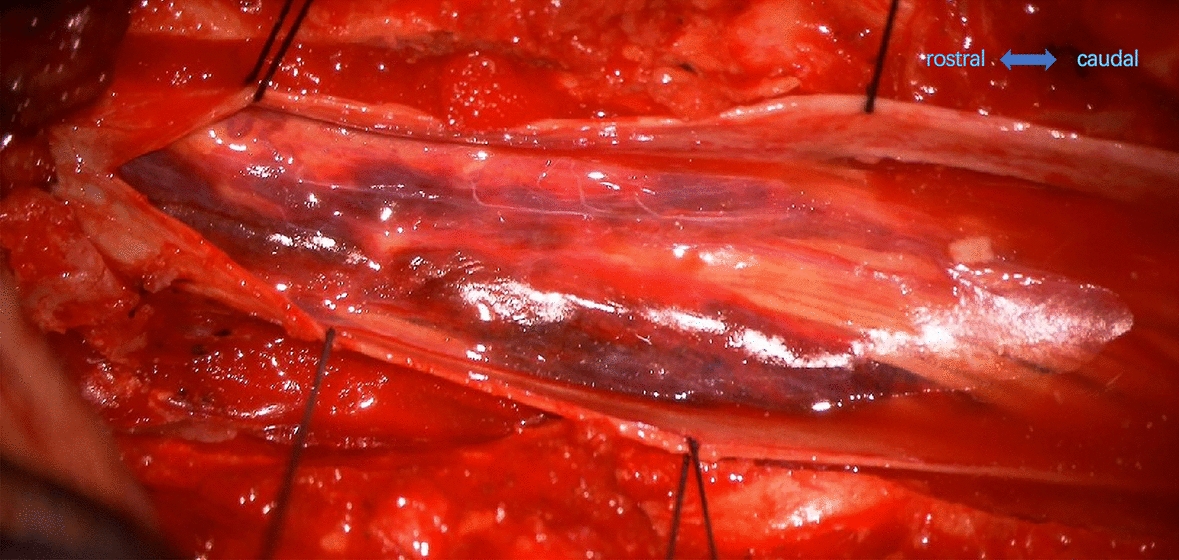
After opening the dura, the arachnoid membrane was visibly intact
Fig. 4.
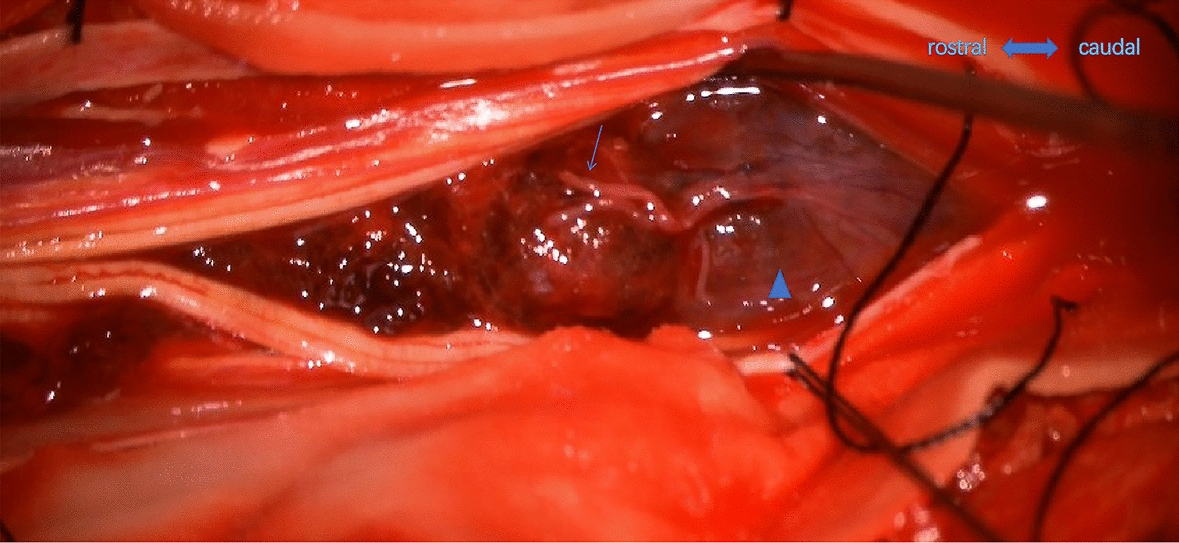
The tumor (triangle) is reddish brown. The rostral nerve root was broken and fractured (arrow)
Fig. 5.
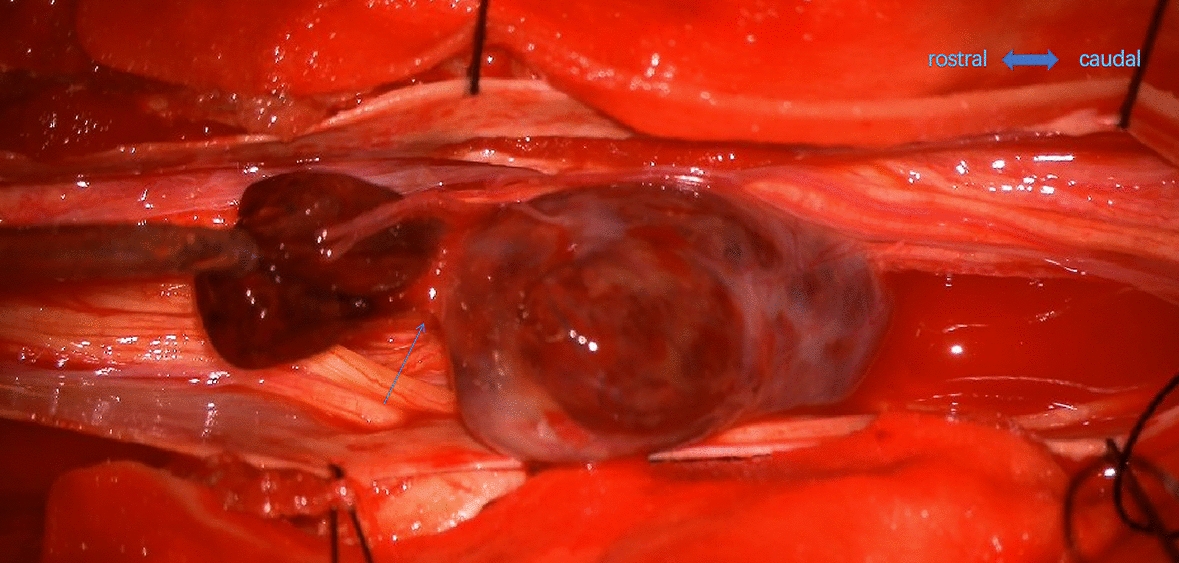
The breakage of the tumor capsule (arrow) is on the rostral side, with a hematoma connected with the intratumor
Fig. 6.
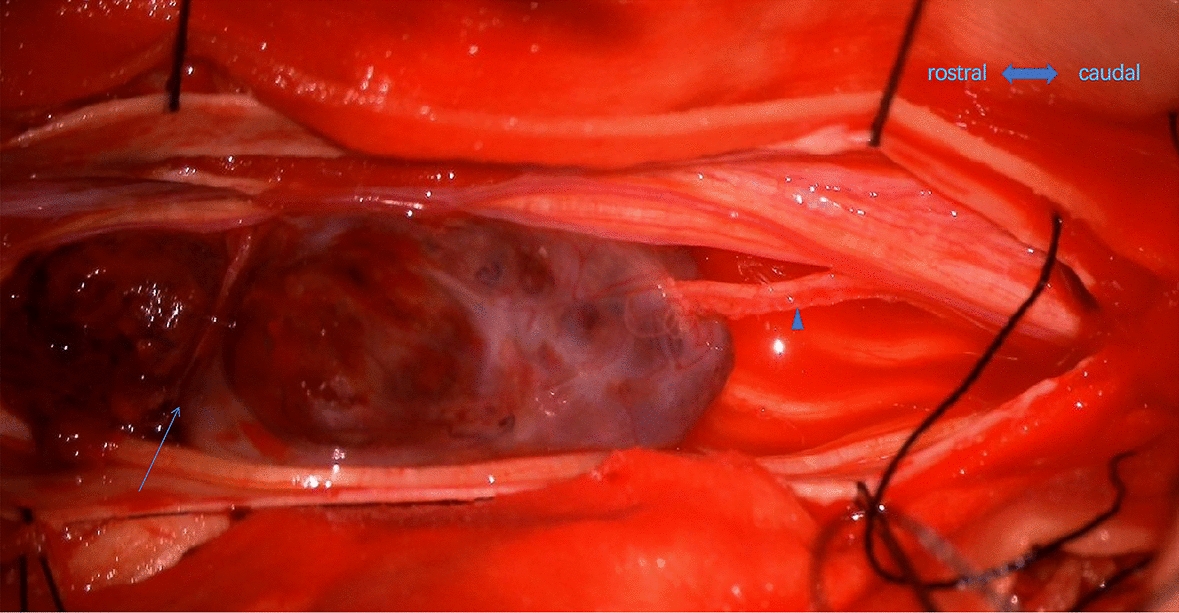
The tail of the tumor is tightly connected to the nerve root (triangle). On the rostral part, the breakage of the tumor capsule was visualized (arrow)
Fig. 7.
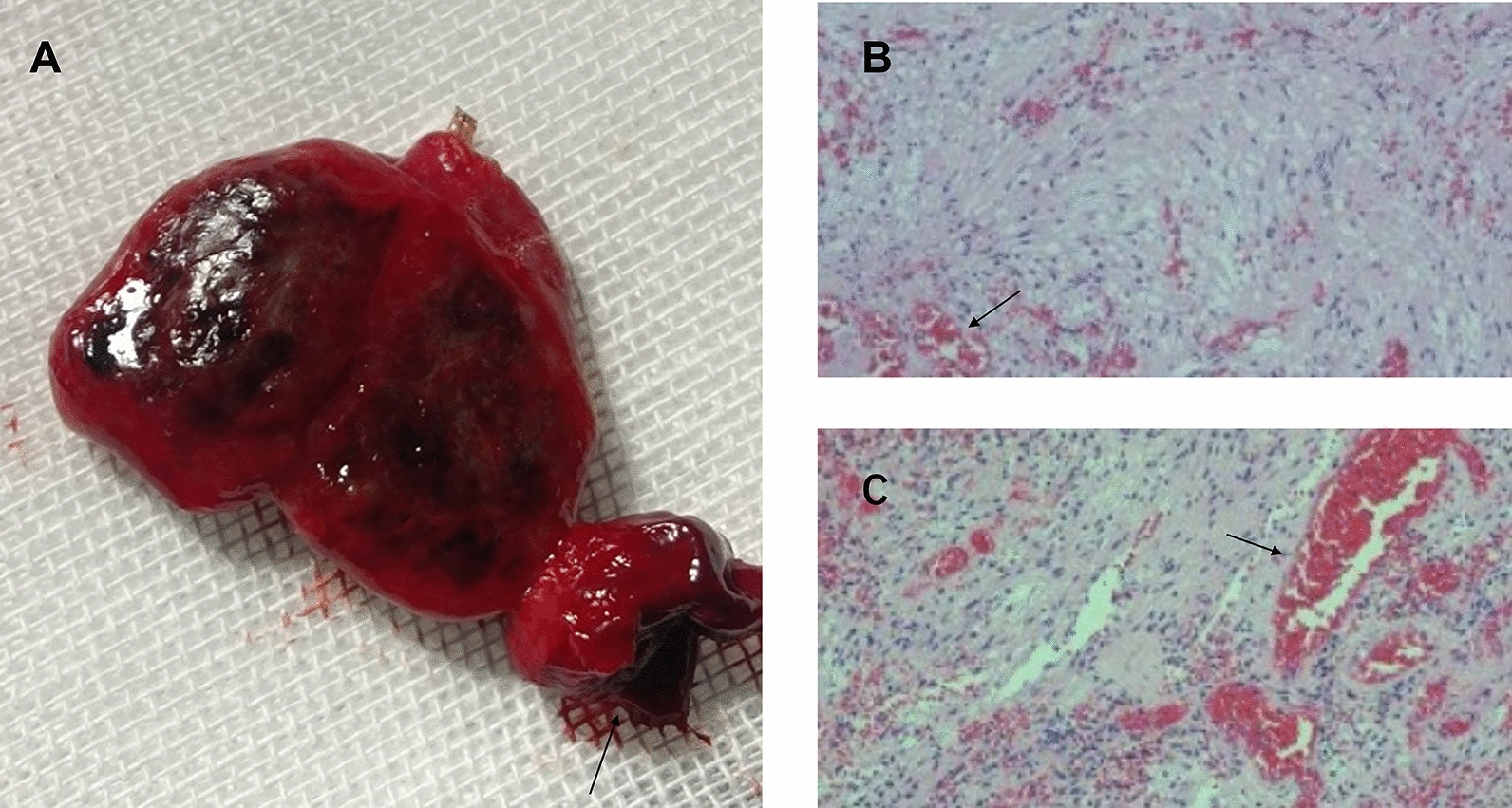
A The tumor is reddish brown in the whole body. The breakage of the tumor capsule (arrow) was apparent. After longitudinal sectioning, diffuse congestion and edema were seen in the tumor. B, C Hematoxylin and eosin staining at 100 × magnification showed a tumor dominated by loose cells, many dilated blood vessels (arrow), and many intravascular red blood cells (arrow). No necrotic tissue was found
After formalin treatment, the specimens were yellowish brown and 2 cm × 2 cm × 3 cm in diameter. Hematoxylin and eosin-stained sections showed a tumor dominated by a loose matrix with uniformly distributed round to oval regular nuclei. Many dilated blood vessels and numerous intravascular red blood cells were observed on a clear mucoid background. No necrotic tissue, signs of nuclear atypia, or mitotic activity were found (Fig. 7B, C). Immunohistochemical results revealed: EMA (−), GFAP (−), S-100 (+), SOX-10 (+), DES (−), STAT-6 (−), CD34 (blood vessels +), Ki67 (2% +), and SMA (−). The pathological analysis indicated a schwannoma.
The patient recovered well, with no complications. After surgery, the pain in the right lower limb was significantly relieved, and urine and bowel movements were normal. The patient was satisfied with the treatment she received. Two weeks postoperatively, the patient underwent rehabilitation exercises and gradually began to walk. Three months after surgery, the patient had returned to her normal life and work but still had mild numbness and pain in the left lower limb.
Discussion
Intraspinal schwannoma hemorrhage is rare and may present as an intratumoral hemorrhage, subarachnoid hemorrhage, or both. To explore the mechanism underlying the bleeding, we reviewed the literature published over the past 30 years and found 23 cases of intraspinal schwannoma hemorrhage, summarized in Table 1 [2–23]. Among all patients, 9 (39.1%) occurred in the cervical spine, 8 (34.8%) in the cauda equina, and 17 (73.9%) in the craniocervical, cervicothoracic, or thoracolumbar joints. Nine patients (39.1%) had definite induction of spinal movement before bleeding. Five patients had only subarachnoid hemorrhage (SAH). Four patients experienced only intratumoral bleeding. Seven patients experienced both SAH and intratumoral bleeding. Among the patients, 3 had clear tumor capsule rupture and bleeding, and 18 had intact and unbroken tumor capsules. In addition, three patients experienced nerve root injury, including torsion, rupture, and drainage vein bleeding.
Table 1.
Summary of the cases of spinal schwannomas with intraspinal hemorrhage in the last 30 years
| Author, year | Country | Age | Gender | Site of tumor | SAH | Intratumoral hemorrhage | Inducing factor |
|---|---|---|---|---|---|---|---|
| Chalif et al. 1990 | America | 56 | F | C1–2 | Yes | NR | NR |
| Mills et al. 1993 | New Zealand | 53 | M | C7-T1 | Yes | Yes | NR |
| Barquero et al. 1994 | Spain | 68 | M | C5–7 | Yes | NR | NR |
| Corriero et al. 1996 | Italy | 37 | M | C7–T1 | Yes | NR | Physical stress |
| Uemura et al. 1998 | Japan | 58 | F | T11–L2 | No | Yes | NR |
| Cordan et al. 1999 | Turkey | 28 | F | L1–2 | Yes | NR | NR |
| Cohen et al. 2000 | Israel | 52 | M | L1 | Yes | Yes (rupture of capsule) | Fall from a ladder |
| Ng.2001 | Singapore | 43 | NR | C6–7 | Yes | Yes (rupture of capsule) | NR |
| Parmar et al. 2004 | Singapore | 56 | M | T11–L1 | Yes | No | NR |
| Ciappetta et al. 2008 | Italy | 44 | F | C2 | Yes | Yes | NR |
| Ji et al. 2010 | Korea | 44 | M | C1–2 | Yes | No | Sneezing |
| Sun et al. 2010 | China | 32 | M | T4–5 | Yes | NR | Sneezing |
| Kukreja et al. 2014 | America | 47 | M | L1–2 | Yes | Yes | Seizure episode |
| Bennett et al. 2015 | America | 66 | F | L4 | Yes | No | NR |
| Jenkins et al. 2015 | America | 62 | M | L2–3 | No | Yes (nerve root torsion) | NR |
| Zhang et al. 2016 | China | 47 | F | T9 | Yes | No | Cough |
| Tanki et al. 2018 | India | 11 | F | T11–L1 | Yes | NR | NR |
| Gandhoke et al. 2018 | India | 38 | F | C2–4 | No | Yes | NR |
| Dobran et al. 2019 | Italy | 38 | M | T11 | Yes | Yes | Car accident |
| Singh et al. 2020 | India | 35 | F | C3–T3 | No | Yes | NR |
| Chen et al. 2021 | China | 45 | M | T2 | Yes | NR (nerve root bleeding) | Hard physical work |
| Zhang et al. 2022 | China | 40 | M | L3 | Yes | No | No |
| This case 2023 | China | 53 | F | T12–L1 | Yes | Yes (rupture of capsule and nerve root rupture) | Long-distance running |
M male, F female, C cervical, T thoracic, L lumber, NR not reported, SAH subarachnoid hemorrhage
Two main theories have been proposed to explain the mechanisms of spinal schwannoma hemorrhage. First is the vascular theory, where the proliferative and dilated blood vessels of tumors may cause spontaneous thrombus formation. The distal end of the thrombus can cause tumor necrosis and bleeding [3]. No histological signs of necrosis were found in our case. Although bleeding may be a common finding in schwannoma histology, necrosis has not been observed in any other case report [2–23]. Moreover, the vascular theory does not explain the pathogenesis in patients with SAH and without intratumoral hemorrhage. In the longitudinal section of the gross specimen, we saw that the tumor was dark red throughout, with diffuse congestion and swelling rather than local necrosis and bleeding. Similar pathological specimens have been previously reported [14, 16, 19]. Furthermore, on pathological examination, the tumor cells were relatively sparse but rich in dilated blood vessels and intravascular red blood cells. Similar histology has been reported in other cases [3, 5, 8, 19, 20, 22]. Therefore, we believe that the pathological type of bleeding within a schwannoma is congestion caused by venous obstruction rather than bleeding.
The other proposed theory to explain spinal schwannoma hemorrhage is the mechanical theory. The location of the tumor is vital for hemorrhage, especially in the cauda equina and cervical vertebra [11]. Excessive movement between the tumor and spinal cord attachment produces traction, destroying the fragile vascular structure on the surface, leading to subarachnoid hemorrhage. We believe this explanation is reasonable. We observed a rupture of the tumor-bearing nerve root during surgery, and other cases have reported torsion of the nerve root [16] and venous bleeding drainage [22]. This theory also supports our statistical results, in which 39.1% of the patients had a clear inducement of spinal movement before bleeding. The rate of bleeding in males is slightly higher than in females. The proportion of bleeding occurring in the cervical vertebrae, cauda equina, and spinal junction area is relatively higher, possibly related to increased movement. Owing to the effect of gravity on the tumor, repeated pulling and dragging from the tumor during movement may damage the carrier nerve root and its blood vessels, even causing a rupture of the nerve root, which leads to SAH. Degenerative changes in the spine can exacerbate the injury [14]. Similarly, tumor movement may cause distortion or rotation of the nerve roots. Blood supply to the tumor comes entirely from the bidirectional supply of vessels that run through the tumor carrier nerve roots. The rotation of a schwannoma can cause twisting of blood vessels. Because of the higher pressure of the arterial blood vessels, the impact of the vascular kink is relatively small, resulting in the occlusion of drainage veins, leading to diffuse congestion and swelling of the tumor. This is similar to the effect of drainage vein blockage during arteriovenous malformation resection. In severe cases, tumor capsule rupture and bleeding may occur.
In summary, we conclude that three important processes are involved in the occurrence of hemorrhage in spinal schwannoma: (1) injury and bleeding of the carrier nerve root and its vessels during tumor motion, (2) tumor carrier nerve root torsion, twisting, and venous obstruction, and (3) tumor congestion and swelling with capsule rupture and bleeding in severe cases (Fig. 8).
Fig. 8.
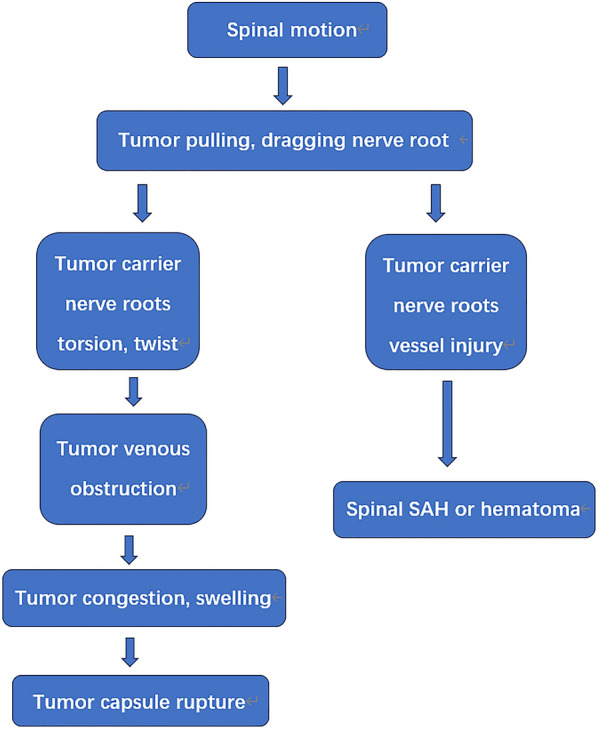
The hemorrhaging processes in a spinal schwannoma
We believe that early surgery should be performed to treat schwannoma hemorrhages. Surgical treatment should be performed once a diagnosis is made. Hematomas should be removed as soon as possible, and tumors should be completely resected. This course of action will relieve the compression of the spinal cord and nerves caused by tumors and hematomas, minimizing the dysfunction and nerve root stimulation symptoms caused by compression. For an accurate diagnosis and corresponding treatment, it is necessary to fully understand the pathogenesis and pathological processes of schwannoma hemorrhage.
Conclusion
Injury to tumor carrier nerve roots and vessels during motion, nerve root torsion, twisting, venous obstruction, tumor congestion and swelling, and capsule rupture are important in spinal schwannoma hemorrhage processes. Early diagnosis and proactive surgery are critical for treatment.
Acknowledgements
We acknowledge all of the clinical staff at Department of Neurosurgery II of the First Affiliated Hospital of Henan University of Science and Technology, China, for the successful management of this patient; without the efforts of the whole team, a good outcome would not have been achieved.
Author contributions
Zhe She: clinical data collection, writing—draft preparation, literature search. Haoyang Chen: literature search and organization. Haosheng Wang: writing—polishing. Yaoqi Wang: clinical data collection. Tao Li: supervision, writing, review and editing. All authors read and approved the final manuscript.
Funding
This research did not receive any external funding.
Availability of data and materials
Available on demand.
Declarations
Ethics approval and consent to participate
No ethical approval was sought for this report as this is not a research study. As this case report involved private information disclosure, informed consent was obtained from the reported patient.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhai XD, Chen HW, Tang QF, Cui ZM, Yao Y, Yin QH. Differentiation between intraspinal schwannoma and meningioma by MR characteristics and clinic features. Radiol Med. 2019;124(6):510–21. [DOI] [PubMed] [Google Scholar]
- 2.Chalif DJ, Black K, Rosenstein D. Intradural spinal cord tumor presenting as a subarachnoid hemorrhage: magnetic resonance imaging diagnosis. Neurosurgery. 1990;27(4):631–4. [DOI] [PubMed] [Google Scholar]
- 3.Mills B, Marks PV, Nixon JM. Spinal subarachnoid haemorrhage from an “ancient” schwannoma of the cervical spine. Br J Neurosurg. 1993;7:557–9. [DOI] [PubMed] [Google Scholar]
- 4.Vázquez-Barquero A, Pascual J, Quintana F, Figols J, Izquierdo JM. Cervical schwannoma presenting as a spinal subdural haematoma. Br J Neurosurg. 1994;8(6):739–41. [DOI] [PubMed] [Google Scholar]
- 5.Corriero G, Lacopino DG, Valentini S, Lanza PL. Cervical neuroma presenting as a subarachnoid hemorrhage: case report. Neurosurgery. 1996;39(5):1046–9. [DOI] [PubMed] [Google Scholar]
- 6.Uemura K, Matsumura A, Kobayashi E, Tomono Y, Nose T. CT and MR presentation of acute hemorrhage in a spinal schwannoma. Surg Neurol. 1998;50:210–20. [DOI] [PubMed] [Google Scholar]
- 7.Cordan T, Bekar A, Yaman O, Tolunay S. Spinal Subarachnoid Hemorrhage attributable to Schwannoma of the cauda equina. Surg Neurol. 1999;51:373–5. [DOI] [PubMed] [Google Scholar]
- 8.Cohen ZR, Knoller N, Hadani M, Davidson B, Nass D, Ram Z. Traumatic intratumoral hemorrhage as the presenting symptom of a spinal neurinoma. J Neurosurg (Spine 2). 2000;93:327–9. [DOI] [PubMed] [Google Scholar]
- 9.Ng PY. Schwannoma of the cervical spine presenting with acute haemorrhage. J Clin Neurosci. 2001;8(3):277–8. [DOI] [PubMed] [Google Scholar]
- 10.Parmar H, Pang BC, Lim CCT, Chng SM, Tan KK. Subarachnoid hemorrhage: a diagnostic spinal schwannoma with acute challenge. AJNR Am J Neuroradiol. 2004;25(5):846–50. [PMC free article] [PubMed] [Google Scholar]
- 11.Ciappetta P, D’Urso PI, Colamaria A. Giant craniovertebral junction hemorrhagic schwannoma. Neurosurgery. 2008;62(5):e1166. [DOI] [PubMed] [Google Scholar]
- 12.Ji C, Ahn JG, Huh HY, Park CK. Cervical schwannoma presenting with acute intracranial subarachnoid hemorrhage. J Korean Neurosurg Soc. 2010;47(2):137–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun LY, Chen Z, Jian FZ, Ling F. Spinal schwannoma: an unusual cause of acute subarachnoid hemorrhage. Neurol India. 2010;58(1):155–6. [DOI] [PubMed] [Google Scholar]
- 14.Kukreja S, Ambekar S, Sharma M, Nanda A. Cauda equina schwannoma presenting with intratumoral hemorrhage and intracranial subarachnoid hemorrhage. J Neurosurg Spine. 2014;21(3):357–60. [DOI] [PubMed] [Google Scholar]
- 15.Bennett SJ, Katzman GL, Mehta AS, Ali S. Hemorrhagic schwannoma presenting with subarachnoid hemorrhage and resulting cauda equina syndrome. Spine J. 2015;15(12):17–8. [DOI] [PubMed] [Google Scholar]
- 16.Jenkins AL, Ahuja A, Oliff AH, Sobotka S. Spinal Schwannoma presenting due to torsion and hemorrhage: case report and review of literature. Spine J. 2015;15(8):1–4. [DOI] [PubMed] [Google Scholar]
- 17.Zhang HM, Zhang YX, Zhang Q, Song SJ, Liu ZR. Subarachnoid hemorrhage due to spinal cord schwannoma presenting findings mimicking meningitis. J Stroke Cerebrovasc Dis. 2016;25(8):123–5. [DOI] [PubMed] [Google Scholar]
- 18.Tanki H, Singh H, Raswan US, Bhat AR, Saija Y, Kirmani AR, Javaid I. A rare case of spinal schwannoma in a child presenting with subarachnoid hemorrhage: a case report with review of literature. J Pediatr Neurosci. 2018;13(4):503–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gandhoke CS, Syal SK, Singh D, Batra V, Nallacheruvu Y. Cervical C2 to C4 schwannoma with intratumoral hemorrhage presenting as acute spastic quadriparesis: a rare case report. Surg Neurol Int. 2018;9:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mauro D, Davide N, Martina DC, Francesco F. Intralesional and subarachnoid bleeding of a spinal schwannoma presenting with acute cauda equina syndrome. BMJ Case Reports. 2019;12(7):e229251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raj SP, Nitish N, Kumar GS, Kumar SR, Anju S, Suresh NL. Hemorrhage in long segment cervical schwannoma; case report and literature review. Surg Neurol Int. 2020;11:476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen P, Guo Y, Huang R, Xiao J, Cheng Z. Spinal schwannoma causes acute subarachnoid haemorrhage: a case report and literature review. Neurochirurgie. 2021;67(5):495–9. [DOI] [PubMed] [Google Scholar]
- 23.Zhang D, Fan T, Fan W, Wang Y. Spinal intradural schwannoma presenting with acute subarachnoid hemorrhage: a case report and review of published reports. J Int Med Res. 2022;50(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available on demand.


