Abstract
Background
Men who have sex with men (MSM) globally face a high risk of HIV infection. Previous studies indicate that customized short message service (SMS) interventions could reduce high-risk behaviors that associated with HIV transmission. This study aims to evaluate the health and economic impacts of such interventions among MSM in China.
Methods
A decision tree-Markov model was developed for a simulated cohort of 100,000 MSM of 20 years old. We assessed three intervention strategies: (1) routine strategy with standard health information; (2) SMS strategy with customized messages based on individual high-risk behaviors, with 50.1% efficacy and 50% coverage; (3) LEN-LA (lenacapavir long-acting) strategy as pre-exposure prophylaxis (PrEP), with 100% efficacy lasting for 0.5-year and 50% coverage. The study period was 45 years. Primary outcomes included the number of HIV infections and HIV-related deaths. The cost-effectiveness, cost-utility and cost-benefit analyses were conducted along with sensitivity analyses from the healthcare sector perspective.
Results
The SMS strategy was more effective, averting 6,191 (22.0%) HIV infections and 2,100 (38.5%) HIV-related deaths when compared with routine strategy. The average cost-effectiveness ratios (ACERs) were US$6,361 (95% CI: 5,959-6,613) per HIV infection averted and US$18,752 (95% CI: 17,274 − 20,530) per HIV-related death averted. It had incremental cost-effectiveness ratios (ICERs) of US$1,743 (95% CI: 1,673-1,799) per QALY, with a benefit cost ratio (BCR) of 1.98 (95% CI: 1.94–2.02), compared with routine strategy. While the LEN-LA strategy may be the most effective, its high cost, coupled with the highest ICER, currently presents a considerable obstacle to its widespread adoption. The ICERs were most affected by the probability of HIV infection, intervention cost and coverage.
Conclusions
SMS strategy for preventing HIV among MSM in China is cost-effective and could be a promising strategy for HIV prevention. These findings may have implications for public health policy and resource allocation in HIV prevention efforts targeting high-risk populations.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-024-20857-3.
Keywords: HIV, MSM, Text messages, Cost-effectiveness, Decision tree-Markov model
Introduction
Globally, HIV remains a major public health issue. In 2023, an estimated 630,000 people died from HIV-related causes and an estimated 1.3 million people acquired HIV [1]. Men who have sex with men (MSM) are particularly vulnerable to HIV infection. They are affected by HIV at a notably high rate. In 2020, China reported approximately 81,000 newly diagnosed HIV-infected individuals, with MSM comprising 23.3% of these cases [2, 3]. Moreover, young MSM are also significantly more likely to engage in high-risk sexual behaviors [4]. Between 2006 and 2015, 47.0% of MSM diagnosed with HIV were aged 20 to 29 years old [5]. The number of HIV infections among Chinese university students increased by 35% annually from 2011 to 2015. In 2015, 82% of those diagnosed were infected through male-to-male sexual intercourse [6]. Therefore, intervention methods among MSM are urgently needed to prevent the transmission of HIV.
In response to these vulnerabilities, recent interventions utilizing cell phone and internet platforms to reduce risk behaviors have emerged [7]. There have been explorations around the world of HIV interventions targeting MSM. For example, in the US, one study concluded that a video-based outpatient intervention effectively reduced the incidence of HIV and other sexually transmitted infections [8]; another study conducted a web-based randomized controlled trial among 474 black MSM and found that the intervention could reduce the risk of condomless anal intercourse (CAI) compared with the control group [9]. Among other strategies, short message service (SMS) have shown advantages in HIV prevention, including convenience of delivering information directly and better reach individuals with sexual stigma [10]. Yun et al. conducted a 3-month mobile phone-based intervention with 192 MSM, which raised the condom use rate and lowered the number of sex partners in the intervention group [11]. Reback et al. designed a 9-month lasting SMS system that tailored to MSM and significantly reduced their risk behaviors [12]. Furthermore, a study applying tailored messages conducted in India was able to statistically decrease CAI acts [13].
Pre-exposure prophylaxis (PrEP) is also an important biomedical tool for preventing HIV transmission. On June 20, 2024, Gilead Sciences announced interim analysis results from a pivotal Phase 3 PURPOSE 1 trial, demonstrating that the company’s semiannual injectable HIV-1 capsid inhibitor, lenacapavir long-acting (LEN-LA), showed 100% efficacy in preventing HIV among women [14]. Many interventions have been developed to evaluate the health and economic value in HIV prevention. However, these interventions differ in their costs and effectiveness. Moreover, the health and economic benefits of long duration under the Chinese context has not been investigated previously.
In our previous study, a behavioral intervention based on customized SMS was developed. It was shown that the intervention was able to reduce the HIV-related high-risk behaviors among MSM in China during the intervention period, especially CAI [15]. Therefore, building on these results, this study aims at investigating the long-term value of the previous customized SMS strategy among a larger number of MSM in China. Findings on cost-effectiveness can be useful in guiding policymakers’ allocation decisions.
Methods
Data source
The study utilized data from a previous study [15], literature and expert opinions. The research team conducted a randomized controlled trial with assistance from Kunshan Rainbow, a non-government organization in Kunshan, Jiangsu province. Participants were recruited through online, on-site, and outreach methods popular among MSM in China. Between May 2020 and March 2021, 631 HIV-negative MSM were randomly assigned to SMS and control groups. They received four follow-up visits in two years, completing questionnaires, rapid HIV, syphilis tests and sexually transmitted infections (STI) clinical examinations. High-risk behaviors were documented, and biweekly personalized interventions, including texts and pictures, were sent to the SMS group, adjusted based on behavior changes. Our modelling study was informed by data from this trial.
Model structure
The cohort size of 100,000 individuals is commonly applied in health economic modelling studies [16]. Thus, decision tree-Markov model was developed for a hypothetical population of 100,000 HIV-negative MSM in China, using TreeAge Pro 2022 (TreeAge Inc, MA, USA). All participants were assumed to be 20 years old. HIV prevalence was not treated as a fixed value. Instead, we set an initial prevalence, which changed based on the strategies and the natural progression of the disease. Due to the chronic condition of HIV/AIDS, a lifetime horizon was applied, assuming a total lifetime of 78 years [17]. The cycle period was defined as 1 year.
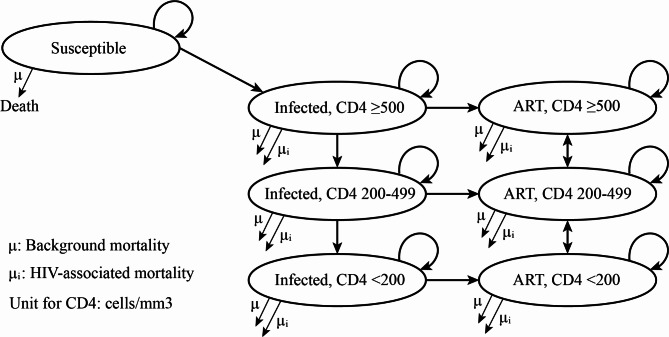
One study in China reported that 91.1% of the newly diagnosed HIV-infected MSM initiated antiretroviral therapy (ART) within 1 month and 97.3% of the diagnosed MSM within 6 months [18]. Thus, we assumed that the infected participants initiated ART shortly following diagnosis of HIV infections. HIV-infected individuals were classified as “infected” and “on ART”. Infections were diagnosed through self-testing and partitioned according to CD4 cell count (CD4 ≥ 500 cells/mm3, 200–499, <200). It was assumed that during one cycle, the CD4 states could only remain in original state, transfer to an adjacent state, or directly lead to death. Transitional probabilities among states were collected from literature. Transitional relationship between Markov states were shown in Fig. 1 and model parameters were described in Table 1.
Fig. 1.
The transitional relationship between Markov states. Abbreviation: ART, antiretroviral treatment
Table 1.
Key model parameters and assumptions
| Parameter | Value | Range | Data Source |
|---|---|---|---|
| Population simulated | |||
| Cohort size of MSM | 100,000 | - | - |
| Cohort age, mean | 20 | - | [40] |
| Transmission probabilities | |||
| Probability of HIV infection, % | 0.60 | 0.20–1.80 | [41] |
| Protection of SMS strategy against HIV, % | 50.10 | 26.40–66.20 | [15] |
| Coverage of SMS strategy, % | 50.00 | 30.00–70.00 | [11] |
| Base HIV testing rate, % | 38.00 | 33.00–46.00 | [42] |
| Improvement of HIV testing rate due to SMS strategy | 1.66 | 1.34–2.02 | Meta-analysis (Table S3) |
| Efficacy of LEN-LA strategy against HIV, % | 100.00 | 90.00-100 | [43] |
| Coverage of LEN-LA strategy, % | 50.00 | 40.00–70.00 | [43] |
| CD4 decrease, undiagnosed | |||
| CD4 ≥ 500, % per year | 24.50 | 19.60–29.40 | [44] |
| CD4 200–499, % per year | 55.00 | 44.00–55.00 | [44] |
| CD4 decrease, on ART | |||
| CD4 ≥ 500, % per year | 16.64 | 16.27–20.02 | [45] |
| CD4 200–499, % per year | 17.80 | 17.45–22.10 | [45] |
| CD4 increase on ART | |||
| CD4 200–499, % per year | 61.13 | 44.25-63.00 | [45] |
| CD4 < 200, % per year | 57.65 | 41.45–58.60 | [45] |
| HIV-related mortality, % per year | |||
| Infected, CD4 ≥ 500 | 0.10 | 0.08–0.12 | [46] |
| Infected, CD4 200–499 | 0.70 | 0.69–0.73 | [46] |
| Infected, CD4 < 200 | 39.00 | 37.00-53.70 | [16] |
| On ART, CD4 ≥ 500 | 0.10 | 0.08–0.12 | [46] |
| On ART, CD4 200–499 | 0.50 | 0.43–0.55 | [46] |
| On ART, CD4 < 200 | 9.00 | 7.50–22.00 | [16] |
| Background mortality by age | China population and employment statistics yearbook | - | [29] |
| Annual cost | |||
| SMS per MSM, $ | 33.8 | 5.0–75.0 | [15] |
| LEN-LA per MSM, $ | 42,250.0 | 21,125.0-63,375.0 | [43] |
| HIV self-testing, $ | 9.8 | 4.9–19.6 | [30] |
| CD4 and viral load testing, $ | 304.0 | 152.0-456.0 | [30] |
| Direct medical costs, $ | |||
| ART cost | |||
| Infected | 0 | - | - |
| On ART | 1,053.3 | (Table S2) | [47, 48] (Table S2) |
| Non-ART medical costs, $ | |||
| Infected, CD4 < 200 | 3,199.2 | 2,169.8-5,239.7 | [29] |
| On ART, CD4 < 200 | 1,599.6 | 477.2-2,619.9 | [29] |
| Utilities | |||
| Infected, CD4 ≥ 500 | 0.910 | 0.888-1 | [49] |
| Infected, CD4 200–499 | 0.790 | 0.760–0.815 | [49] |
| Infected, CD4 < 200 | 0.720 | 0.680–0.760 | [49] |
| On ART, CD4 ≥ 500 | 0.865 | 0.845–0.888 | [49] |
| On ART, CD4 200–499 | 0.830 | 0.825–0.845 | [49] |
| On ART, CD4 < 200 | 0.820 | 0.815–0.825 | [49] |
Abbreviations: MSM, men who have sex with men; SMS, short message service; ART, antiretroviral therapy
Intervention strategies
Three different strategies were assessed: S1, Routine strategy: The routine strategy group (the control group) received routine health information, which refers to the health education provided to all MSM who came to Kunshan Rainbow for HIV testing and MSM who received health information from other public media; S2, SMS strategy: The SMS strategy group received tailored SMS information, with an coverage of 50% and protection rate of 50.1%. We assumed that CAI was the sole route of HIV infection. Therefore, the efficacy of the SMS strategy in protecting against HIV infection was based on the efficacy in guarding against CAI as reported in the previous study [15]. The impact of SMS interventions often diminishes after half a year [7]. Accordingly, the SMS strategy was provided annually to deliver consistent effects. In addition, in our previous study, customized short messages also included texts and pictures designed to promote HIV self-testing after CAI. Plenty studies have also confirmed that SMS intervention is effective in raising the HIV testing rate [19–21]. As a result, we assumed that the SMS strategy also increased the HIV testing rate of MSM; S3, LEN-LA strategy: This group received LEN-LA strategy as PrEP. Based on the trial results of Gilead Sciences, we assumed the LEN-LA would have an efficacy of 100% reduction in risk of HIV infection. In China, only less than 1% of the MSM have started taking PrEP [22]. To evaluate the impacts of LEN-LA, we consulted a modelling study focusing on MSM in China, and campaigns it every year with moderate coverage of 50% in our model [23]; the structure of the decision tree-Markov model was shown in Figure S1. The strategies were assumed to have no disutility. The Chinese Center for Disease Control and Prevention suggested that HIV surveillance age range for adult MSM should be 18–65 years [24]. For this reason, the duration of the SMS and LEN-LA strategy was set to be 45 years, covering the sexually active age of MSM to 65 years old [25]. After the intervention period, the probability of HIV infection was set to be 0 for all groups.
The main health outcomes investigated were HIV infections and HIV-related deaths prevented. Quality-adjusted life year (QALY) was used as the health utility. QALY represents the life year obtained by the model, multiplied by a specific utility value, expressed as QALY = ∑WY, where W is the utility value and Y is the length of life. The utilities are associated with CD4 states. A higher CD4 cell count corresponds to a lower likelihood of opportunistic infections or HIV-related diseases, thus a higher CD4 state has a higher utility value. However, when an HIV-positive individual with a high CD4 count (≥ 500) begins antiretroviral therapy, there is a decrease in utility. This decline can be attributed to the demanding nature of the treatment, which incurs additional time and financial costs and involves social and psychological challenges that can diminish quality of life. For patients with high CD4 counts, these adverse effects may outweigh the benefits of the treatment. The utility value of 1 was assigned to represent health, while a value of 0 was assigned to death.
Costs
The analysis was performed from the healthcare sector perspective, covering only direct medical costs. The annual cost of LEN-LA was US$42,250 [26]. The SMS strategy costs included texts sending fees, facility costs, incentives, office supplies, costs for HIV testing kits and postage fees, which were collected from administrative records of previous study [15]. Labor costs for text platform operators were estimated according to Jiangsu Statistical Yearbook, 2023 [27]. The annual cost of offering the SMS strategy to one MSM was estimated to be $33.8 (Table S1). We assumed all MSM use tenofovir disoproxil + lamivudine + efavirenz (TDF + 3TC + EFV) or bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF) as first line regimen and use zidovudine + lamivudine + lopinavir/ritonavir (AZT + 3TC + LPV/r) as second line regimen for MSM experience first line treatment failure [28]. The annual cost of ART was calculated by:
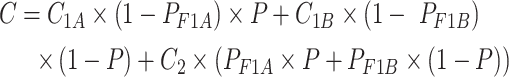 |
where C represents the annual cost of ART; C1A represents the annual cost of first line ART regimen (B/F/TAF); C1B represents the annual cost of another first line ART regimen (TDF + 3TC + EFV) (Table S2); P represents the usage proportion of B/F/TAF regimen. We assumed that 50% of MSM would use B/F/TAF and the remaining 50% use TDF + 3TC + EFV as their first line ART regimen; PF1A represents the prevalence of HIV virological failure of TDF + 3TC + EFV regimen; PF1B represents the prevalence of HIV virological failure of B/F/TAF regimen; C2 represents the annual cost of second line ART regimen (AZT + 3TC + LPV/r) (Table S2). Optimistic infections were considered for MSM whose CD4 < 200, resulting in extra treatment costs [29]. The diagnostic cost was US$9.8 [30]. All costs were converted to 2023 USD using the Chinese consumer price index (CPI) [31]. Costs and utilities were discounted at a 3% annual rate.
Cost-effectiveness and cost-benefit analyses
In our cost-utility analysis (CUA), we calculated average cost-effectiveness ratios (ACERs) which quantify the mean additional costs of screening for each additional HIV infection and HIV-related death. effective case. Incremental cost-effectiveness ratios (ICERs) were also calculated in cost-effectiveness analysis (CEA), which determined the extra expenditure per QALY gained. These measurements were used to assess whether the intervention was cost-effective. According to the World Health Organization’s criteria, interventions with an ICER less than the average Gross Domestic Product (GDP) per capita for a given country or region are deemed cost-effective. In this study, the willingness-to-pay (WTP) threshold for determining cost-effectiveness was set at US$12,675, reflecting the GDP of China in 2023.
We also conducted a cost-benefit analysis (CBA) to evaluate the economic outcomes and attractiveness of the proposed strategy. This involved calculating key financial metrics such as the benefit, net monetary benefit (NMB), and benefit-to-cost ratio (BCR). Concrete valuations were assigned to these benefits, reflecting both treatment cost reductions and the monetized value of averted QALYs. The NMB was determined by subtracting the increased intervention costs from the accrued benefits. To calculate the BCR, the projected benefits were divided by the projected costs.
Sensitivity analysis
The one-way sensitivity analyses were conducted to capture the uncertainties in input variables and verify the robustness of the results. Tornado diagrams were presented to illustrate the 10 variables with the most impact. A probabilistic sensitivity analysis (PSA) using Monte Carlo simulations was also conducted to test model uncertainty and calculate 95% confidence interval (CI). We ran 1,000 iterations using Monte Carlo simulations, in which each parameter was randomly selected from its distribution, and the results were presented in the form of the cost-effectiveness acceptability curve (CEAC) and scatter plot.
Results
Health impact
During the lifetime period of 78 years, our model estimated that 28,124 (95% CI: 27,845 − 28,403) of the 100,000 MSM were infected in the control group, 5,459 (95% CI: 5,319-5,602) of which died from HIV. In the SMS strategy group, the number of HIV infections was 21,933 (95% CI: 21,677 − 22,191), and 3,359 (95% CI: 3,248-3,473) died from HIV. Further, the estimated infections and HIV-related deaths in LEN-LA group were 12,339 (95% CI: 12,136 − 12,544) and 2,892 (95% CI: 2,789-2,998), respectively (Table 2). Accordingly, compared with routine strategy, the SMS strategy was able to prevent 6,191 (95% CI: 6,042 − 6,342) HIV infections (25.0% reduction) and 2,100 (95% CI: 2,012 − 2,191) HIV-related deaths (38.5% reduction). The LEN-LA strategy achieved the best health outcomes, which could prevent 15,785 (95% CI: 15,560 − 16,012) HIV infections (56.1%) and 2,567 (95% CI: 2,470-2,667) HIV-related deaths (47.02%). The number needed to treat (NNT) represents the average number of individuals who need to be intervened to prevent 1 HIV infection or HIV-related death. Compared with routine strategy, the NNT for the SMS strategy to prevent 1 additional HIV infection was 17, and to prevent 1 additional HIV-related death was 48.
Table 2.
Cost and economic analyses of the SMS and LEN-LA strategy
| Routine | SMS | LEN-LA | |
|---|---|---|---|
| Total HIV infection | 28,124 (27,845–28,403) | 21,933 (21,677–22,191) | 12,339 (12,136–12,544) |
| HIV-related deaths | 5,459 (5,319–5,602) | 3,359 (3,248–3,473) | 2,892 (2,789–2,998) |
| QALYs | 2,569,209 (2,567,706–2,570,693) | 2,591,805 (2,591,464–2,592,278) | 2,600,954 (2,600,315–2,601,724) |
| C (total cost), US$ million | 367.08 (362.26-371.86) | 406.46 (402.11-411.41) | 109,886.88 (108,040.45-111,402.01) |
| CIntervention | 0 | 109.15 (109.10-109.19) | 109,694.93 (107,814.46-111,081.73) |
| CART−treatment | 367.08 (362.26-371.86) | 297.31 (292.87-302.73) | 191.95 (184.90-199.73) |
| SMS vs. Routine | LEN-LA vs. Routine | LEN-LA vs. SMS | |
| Intermediate outcomes | |||
| ΔC (incremental cost), US$ million | 39.38 (36.89–40.93) | 109,499.80 (107,704.81-111,133.83) | 109,480.42 (108,139.42-110,995.77) |
| ΔCIntervention | 109.15 (106.19-111.02) | 109,694.93 (107,759.96-111,227.80) | 109,585.78 (107,801.03-111,108.61) |
| ΔCART−treatment | 69.77 (67.63–71.47) | 175.13 (170.59-178.98) | 105.36 (76.94-138.81) |
| ΔE (QALYs averted) | 22,596 (22,217–22,939) | 31,745 (31,104–32,476) | 9,149 (8,804–9,511) |
| ΔInfection | 6,191 (6,042–6,342) | 15,785 (15,560–16,012) | 9,594 (9,412–9,778) |
| ΔDeaths | 2,100 (2,012–2,191) | 2,567 (2,470–2,667) | 467 (426–511) |
| Cost-effectiveness analysis (CEA) | |||
| ACER, US$/infection |
6,361 (5,959–6,613) |
6.95 million (6.82 million-7.04 million) |
11.41 million (11.28 million-11.56 million) |
| ACER, US$/death |
18,752 (17,274–20,530) |
42.62 million (41.67 million-43.61 million) |
234.43 million (231.56 million-237.73 million) |
| Cost-utility analysis (CUA) | |||
| ICER (ΔC/ΔE), US$/QALY | 1,743 (1,673–1,799) | 3,449,987 (3,363,757–3,557,755) | 11,967,321 (11,434,873–12,608,953) |
| Cost-benefit analysis (CBA) | |||
| ΔB (ΔCART−treatment + λΔEa), US$ million | 216.63 (211.83-220.99) | 577.50 (562.76-586.13) | 226.96 (222.54-231.55) |
| NMB (ΔB –ΔCIntervention), US$ million | 107.48 (102.68-111.61) | -108,922.34 (-108,931.47 to -108,911.40) | -109,812.74 (-109,817.19 to -109,806.76) |
| BCR (ΔB/ ΔCIntervention) | 1.98 (1.94–2.02) | 0.0053 (0.0052–0.0054) | 0.0021 (0.0019–0.0022) |
Note: 95% confidence intervals are in parentheses
aThe λΔE represented the monetized averted QALY benefit, with λ meaning the threshold of willingness-to-pay (WTP) set at 1 × national per capita gross domestic product (pGDP) of US$12,675
Abbreviations: QALYs, quality-adjusted life-years; ACER, average cost-effectiveness ratio; ICER, incremental cost effectiveness ratio; NMB, net monetary benefit; BCR, benefit-cost ratio
Cost-effectiveness and cost-benefit
The SMS strategy was identified as the most cost-effective strategy. The ACERs indicated that the SMS strategy cost US$6,361 (95% CI: 5,959-6,613) to prevent an additional HIV infection and US$18,752 (95% CI: 17,274 − 20,530) to avert an additional HIV-related death compared with routine strategy. The LEN-LA strategy, compared with routine strategy, saved 31,745 (95% CI: 31,104 − 32,476) more QALYs at an additional cost of US$109,499.80 million (95% CI: 107,704.81 million-111,133.83 million), resulting in an ICER of US$3,449,987 (95% CI: 3,363,757-3,557,755) per QALY gained, which exceeded the WTP threshold of $12,675, the GDP per capita in 2023, suggesting that the strategy was not cost-effective. The notably high cost of LEN-LA was the reason for this outcome (Table 2). In contrast, the SMS strategy led to an ICER of US$1,743 (95% CI: 1,673-1,799) per QALY compared with routine strategy, indicating that it was the most cost-effective strategy (Table 2).
CBA was performed to enable more comprehensive decision making. Compared with routine strategy, the SMS strategy gained 22,596 (95% CI: 22,217 − 22,939) additional QALYs, with benefits and net benefits of US$216.63 million (95% CI: 211.83-220.99 million) and US$107.48 million (95% CI: 102.68-111.61 million), respectively. The BCR for the SMS strategy was 1.98 (95% CI: 1.94–2.02) compared with routine strategy, at a WTP threshold of $12,675 (Table 2).
Sensitivity analyses
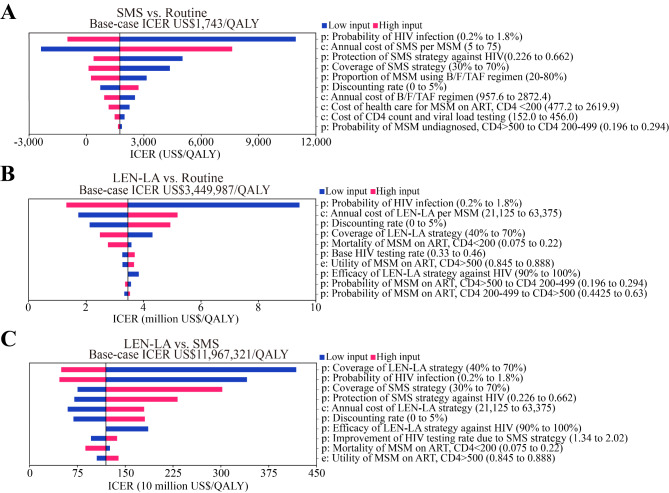
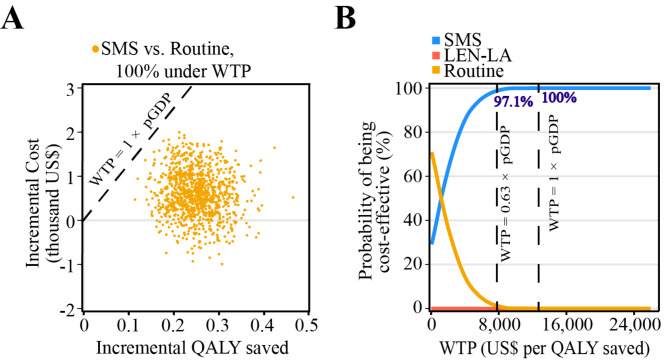
To evaluate how variations in the input variables might influence the projected results, sensitivity analyses were performed. Model uncertainty from the one-way sensitivity analysis was summarized in tornado diagrams, applying 10 variables that influence ICERs the most (Fig. 2). The one-way sensitivity analysis revealed that the variables most significantly associated with the ICER (SMS vs. routine) included the probability of HIV infection, the per capita price of the SMS strategy and proportion of MSM using B/F/TAF regimen. During the one-way sensitivity analysis, the ICER remained lower than GDP per capita of China. Compared with routine strategy, LEN-LA was not cost-effective. While the coverage of LEN-LA changed from 40 to 70%, the ICERs associated with this increase were US$4,304,983 per QALY and US$2,473,116 per QALY gained. In the probabilistic sensitivity analysis, the results with 1,000 iterations were presented in Fig. 3. The cost-effectiveness acceptability curves showed how preferable a strategy is as the WTP increased. Considering a WTP of China’s GDP per capita (US$ 12,675), the SMS strategy had a 100% probability of being cost-effective. We also adopted a reduced WTP threshold, setting the WTP threshold at 0.63 times the national GDP per capita, considering the trade-offs with other health needs [32]. When this threshold was applied, the SMS strategy also remained the best strategy with 97.1% probability of being cost-effective. Therefore, we could consider the SMS strategy to be the most cost-effective option.
Fig. 2.
Tornado diagrams of one-way sensitivity analyses applying 10 most influential factors. (A) One-way sensitivity analysis (SMS vs. routine); (B) One-way sensitivity analysis (LEN-LA vs. routine); (C) One-way sensitivity analysis (SMS vs. LEN-LA). Abbreviations: SMS, short message service; MSM, men who have sex with men; B/F/TAF, bictegravir/emtricitabine/tenofovir alafenamide; ART, antiretroviral treatment; LEN-LA, lenacapavir long-acting
Fig. 3.
(A) Incremental cost-effectiveness scatterplot (SMS vs. routine). (B) Cost-effectiveness acceptability curve of 1,000 iterations of Monte Carlo simulation. The dashed lines show the willingness-to-pay threshold of 1 × and 0.63 × national per capita gross domestic product. Abbreviations: SMS, short message service; WTP, willingness-to-pay; QALY, quality-adjusted life-years; GDP, gross domestic product
Discussion
The high risk of infection suggests MSM is a key target population for HIV prevention. Various interventions for MSM have demonstrated their effectiveness and ability to prevent HIV. Mobile phone-based customized messages, which can be personalized to meet the specific needs of different individuals, are more effective than non-tailored messages and more acceptable than facility-based interventions. We hope that the study’s results will benefit research and policy-making efforts on HIV prevention.
Principal findings
The principal findings of this study indicated that the SMS strategy could prevent a similar number of HIV infections and deaths as LEN-LA compared with routine strategy, but at a significantly lower cost. Consequently, the SMS strategy demonstrated the most favorable health outcomes. Cost-effectiveness analyses suggested that the SMS strategy was more effective and cost-effective than LEN-LA and the routine strategy. Notably, the SMS strategy was considered cost-effective compared with the LEN-LA strategy. The high price of the LEN-LA limits its use. According to one-way sensitivity analysis, the only condition under which the LEN-LA strategy is cost-effective is that the annual cost per MSM is below US$155.64. The CBA further demonstrated that the SMS strategy yielded more QALYs without a substantial increase in cost, resulting in a higher BCR, underscoring its economic advantage over LEN-LA. Therefore, the customized SMS strategy emerges as the most effective and economical method, offering health benefits and saving costs.
Comparison with other studies
Some other primary prevention strategies for HIV were cost-effective, including a daily used PrEP prevention (US$ 12,176) [33], a community-level HIV risk reduction intervention for women (US$ 65,000) [34], an HIV screening strategy (US$ 22,382) [35] and. Some app-based intervention methods achieved results close to ours. A pre-post trial provided a smartphone app called “HealthMindr” for MSM, who used the app for comprehensive HIV prevention services, including HIV testing and condom use, lead to a high rate of preventive commodities usage [36]. Zhu et al. built a WeChat group to provide app-based messages and referrals to health services related to HIV. The HIV testing rate was significantly increased in the intervention group compared to the control group [21]. The reason why the results of our customized SMS strategy showed certain effectiveness and cost-effectiveness may be attributed to significant differences in HIV risk behavior across subgroups of MSM population, and the emphasis on the use of health resources. In our study, participants were recruited by volunteers through methods including online publicity and peer promotion, which made it easier to reach the “hidden” MSM populations, resulting in more accurate use of health resources. Compared with facility-based interventions, internet and mobile phone-based interventions are much less costly and easier to deliver health information, which might contribute to the economic results observed in our study. On the other hand, one study performed a one-year cost-effectiveness analysis of text message intervention in China, which indicated that the intervention was cost-effective [37]; another hypothetical modelling study in Brazil showed that a 20-year text strategy to improve adherence of HIV patients to ART was cost-saving compared with current strategy [38]. Our study further explored the effect of long-term SMS strategy in HIV primary prevention within a broader context and over a lifetime horizon.
Strength and limitations
The assessment boasts several notable strengths. Firstly, it utilizes real-world data derived from a local RCT study to model the health impact of the SMS strategy, resulting in more grounded simulations for the long-term health outcomes. Additionally, it employs reliable result evaluation methods to verify the advantages of the proposed screening approach. Secondly, the study’s use of a decision-tree Markov model to project health outcomes, such as HIV infections, deaths, and QALYs, over a lifetime horizon is a significant strength. To the best of our knowledge, this is the first simulated modeling study to assess the lifetime health and economic impacts of a customized SMS strategy among MSM in China. Secondly, the study utilizes extensive sensitivity analyses with varying distributions rather than fixed figures to ensure the reliability of its conclusions. It also makes comparisons with other studies to verify the rationality of the model.
Nonetheless, a few limitations should be noted. First, as with all mathematical models, the risk-equation model was a simplification of reality. The assumption that MSM would complete the 45-year-long SMS strategy or LEN-LA was optimistic. The adherence may change over time due to reasons including strategy fatigue. Second, our model faces difficulties in capturing real-world complexities, such as the actual effectiveness of lenacapavir used as PrEP and varying mortality rates among MSM. Third, price discrepancies within China’s national health insurance system limit the broader applicability of our findings. Last, the differentiation within the MSM population based on detectable and undetectable viral loads was not acknowledged due to the lack of relevant data. Implementation of treatment and prevention strategies, including progress toward the 95-95-95 targets, can significantly influence the HIV epidemic, which also aligns with the “Undetectable = Untransmittable” (U = U) concept.
Policy implications
Based on the findings of the study, several policy implications can be identified. Given that the SMS strategy has been demonstrated to be both cost-effective and highly beneficial in preventing HIV transmission, it could be a valuable addition to national HIV prevention programs. Policymakers should consider integrating SMS-based interventions into existing HIV prevention frameworks, especially as they are cost-efficient and scalable. The fact that the SMS strategy is more cost-saving compared with LEN-LA suggests that behavioral interventions should be prioritized in resource-limited settings, where the cost of biomedical interventions like PrEP can be prohibitive. Despite its efficacy, the high cost of LEN-LA highlights the need for a more balanced approach to HIV prevention, incorporating both biomedical and behavioral strategies. Furthermore, the latest machine learning techniques have been used to assess HIV infection risk [39]. Expanding access to HIV testing, as facilitated by the SMS strategy, could play a key role in HIV early detection, treatment and risk prediction, which are crucial for improving health outcomes among MSM. Given the rapid rise in HIV cases among young MSM in China [6], these findings underscore the importance of early, targeted interventions to curb the spread of HIV within this high-risk group.
Conclusions
Our study demonstrates that the customized short message service-based intervention to prevent HIV infection among MSM is cost-effective. In China, the customized SMS strategy could be a promising strategy for HIV prevention, which informs both public health policymakers in their decisions about selecting and recommending intervention measures, especially given the currently low coverage of PrEP in China. These findings may also help in the exploration of innovative HIV intervention strategies to more effectively allocate resources to the prevention of HIV among MSM.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Ms. Yu-Xin Cao and Dr. Meng-Zhao Yin for valuable advice on model development.
Abbreviations
- 3TC
Lamivudine
- ACER
Average cost-effectiveness ratio
- ART
Antiretroviral treatment
- AZT
Zidovudine
- BCR
Benefit cost ratio
- B/F/TAF
Bictegravir/Emtricitabine/Tenofovir alafenamide
- CAI
Condomless anal intercourse
- CBA
Cost-benefit analysis
- CEA
Cost-effectiveness analysis
- CI
Confidence interval
- CPI
Chinese consumer price index
- EFV
Efavirenz
- GDP
Gross domestic product
- ICER
Incremental cost-effectiveness ratio
- LEN-LA
Lenacapavir long-acting
- LPV/r
Lopinavir/ritonavir
- MSM
Men who have sex with men
- NMB
Net monetary benefit
- PrEP
Pre-exposure prophylaxis
- QALY
Quality-adjusted life-year
- SMS
Short message service
- STI
Sexually transmitted infections
- TDF
Tenofovir disoproxil
- WTP
Willingness-to-pay
Author contributions
GQ, M-YZ and XZ conceived and designed the research. R-QF, HH, Q-WG, M-YZ and XZ collected the data for analysis. R-QF, J-TS and L-YS carried out the statistical analysis. R-QF and L-YS wrote the first draft of the manuscript. GQ and XZ made the key revision. All authors contributed to the scientific discussions and approved the final draft.
Funding
This work was supported in part by the Ministry of Science and Technology of China (grant 2022YFC2304901 to XZ), Nantong Municipal Natural Science Foundation (grant MSZ2023129 to M-YZ), Jiangsu Provincial Research Hospital (grants YJXYY202204-YSA03 to GQ).
Data availability
The datasets used during the current study available from the corresponding author on reasonable request (tonygqin@ntu.edu.cn).
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Gang Qin is an editorial board member of BMC Public Health. The remaining authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rui-Qi Fan, Jun-Tao Shu, Hao Huang and Ling-Yi Shi contributed equally to this work.
Contributor Information
Xun Zhuang, Email: xzhuang@ntu.edu.cn.
Mei-Yin Zou, Email: zoumeiyin@126.com.
Gang Qin, Email: tonygqin@ntu.edu.cn.
References
- 1.World Health Organization. HIV and AIDS [https://www.who.int/news-room/fact-sheets/detail/hiv-aids]
- 2.Han MJCQ, Xu P, Ying S. Make great efforts to make AIDS prevention and control in the 13th five-year plan a new journey-review and prospect of AIDS prevention and control in China. China AIDS and STD; 2021.
- 3.Jiao K, Wang C, Liao M, Ma J, Kang D, Tang W, Tucker JD, Ma W. A differentiated digital intervention to improve antiretroviral therapy adherence among men who have sex with men living with HIV in China: a randomized controlled trial. BMC Med. 2022;20(1):341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beyrer C, Baral SD, van Griensven F, Goodreau SM, Chariyalertsak S, Wirtz AL, Brookmeyer R. Global epidemiology of HIV infection in men who have sex with men. Lancet. 2012;380(9839):367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin Q, Guo W, Tang W, Mahapatra T, Wang L, Zhang N, Ding Z, Cai C, Cui Y, Sun J. Spatial analysis of the human immunodeficiency Virus Epidemic among men who have sex with men in China, 2006–2015. Clin Infect Dis. 2017;64(7):956–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.In the past five years, the new reported number of HIV/AIDS cases in China university students has increased by 35% annually [https://zqb.cyol.com/html/2015-11/26/nw.D110000zgqnb_20151126_6-01.htm]
- 7.Nguyen LH, Tran BX, Rocha LEC, Nguyen HLT, Yang C, Latkin CA, Thorson A, Stromdahl S. A systematic review of eHealth interventions addressing HIV/STI Prevention among men who have sex with men. AIDS Behav. 2019;23(9):2253–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sweat M, O’Donnell C, O’Donnell L. Cost-effectiveness of a brief video-based HIV intervention for African American and latino sexually transmitted disease clinic clients. AIDS. 2001;15(6):781–7. [DOI] [PubMed] [Google Scholar]
- 9.Hightow-Weidman LB, LeGrand S, Muessig KE, Simmons RA, Soni K, Choi SK, Kirschke-Schwartz H, Egger JR. A Randomized Trial of an Online Risk Reduction Intervention for Young Black MSM. AIDS Behav. 2019;23(5):1166–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noar SM, Willoughby JF. eHealth interventions for HIV prevention. AIDS Care. 2012;24(8):945–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yun K, Chu Z, Zhang J, Geng W, Jiang Y, Dong W, Shang H, Xu J. Mobile phone intervention based on an HIV Risk Prediction Tool for HIV Prevention among men who have sex with men in China: Randomized Controlled Trial. JMIR Mhealth Uhealth. 2021;9(4):e19511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reback CJ, Grant DL, Fletcher JB, Branson CM, Shoptaw S, Bowers JR, Charania M, Mansergh G. Text messaging reduces HIV risk behaviors among methamphetamine-using men who have sex with men. AIDS Behav. 2012;16(7):1993–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mimiaga MJ, Thomas B, Biello K, Johnson BE, Swaminathan S, Navakodi P, Balaguru S, Dhanalakshmi A, Closson EF, Menon S, et al. A pilot randomized controlled trial of an Integrated In-person and mobile phone delivered counseling and text messaging intervention to reduce HIV Transmission Risk among male sex workers in Chennai, India. AIDS Behav. 2017;21(11):3172–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilead’s Twice-Yearly Lenacapavir Demonstrated. 100% Efficacy and Superiority to Daily Truvada® for HIV Prevention [https://www.gilead.com/news-and-press/press-room/press-releases/2024/6/gileads-twiceyearly-lenacapavir-demonstrated-100-efficacy-and-superiority-to-daily-truvada-for-hiv-prevention]
- 15.Huang H, Xu Z, Ge Q, Zhou X, Zou M, Qin G, Cao Y, Duan X, Chu M, Zhuang X. The Impact of Customized Short Message Service on high-risk behaviors among MSM in China, a Randomized Controlled Trial Study. AIDS Behav. 2023;27(8):2720–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao R, Fairley CK, Cook AR, Phanuphak N, He S, Tieosapjaroen W, Chow EPF, Phillips TR, Jin Tan RK, Wei Y, et al. Optimising HIV pre-exposure prophylaxis and testing strategies in men who have sex with men in Australia, Thailand, and China: a modelling study and cost-effectiveness analysis. Lancet Glob Health. 2024;12(2):e243–56. [DOI] [PubMed] [Google Scholar]
- 17.Statistical Bulletin of National Economic and Social Development of the. People’s Republic of China in 2022. [http://www.nhc.gov.cn/cms-search/xxgk/getManuscriptXxgk.htm?id=51b55216c2154332a660157abf28b09d].
- 18.Li Jumei GX, Yin Xinjie, et al. The timing of antiretroviral therapy initiation and influencing factors among HIV-infected individuals in Sichuan Province from 2018 to 2022. Chin J AIDS STD. 2024;30:820–5. [Google Scholar]
- 19.Nyatsanza F, McSorley J, Murphy S, Brook G. It’s all in the message’: the utility of personalised short message service (SMS) texts to remind patients at higher risk of STIs and HIV to reattend for testing-a repeat before and after study. Sex Transm Infect. 2016;92(5):393–5. [DOI] [PubMed] [Google Scholar]
- 20.Tang W, Wei C, Cao B, Wu D, Li KT, Lu H, Ma W, Kang D, Li H, Liao M, et al. Crowdsourcing to expand HIV testing among men who have sex with men in China: a closed cohort stepped wedge cluster randomized controlled trial. PLoS Med. 2018;15(8):e1002645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu X, Zhang W, Operario D, Zhao Y, Shi A, Zhang Z, Gao P, Perez A, Wang J, Zaller N, et al. Effects of a Mobile Health intervention to promote HIV Self-testing with MSM in China: a Randomized Controlled Trial. AIDS Behav. 2019;23(11):3129–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Tang W, Shang H. Expansion of PrEP and PEP services in China. Lancet HIV. 2022;9(7):e455–7. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Peng P, Wu Y, Ma X, Soe NN, Huang X, Wu H, Markowitz M, Meyers K. Modelling the epidemiological impact and cost-effectiveness of PrEP for HIV Transmission in MSM in China. AIDS Behav. 2019;23(2):523–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prevention CCfDCa: National AIDS Sentinel Surveillance Implementation Plan. Operator’s Manual http://www.jygcdc.com/userfiles/files/%E5%85%A8%E5%9B%BD%E8%89%BE%E6%BB%8B%E7%97%85%E5%93%A8%E7%82%B9%E7%9B%91%E6%B5%8B%E5%AE%9E%E6%96%BD%E6%96%B9%E6%A1%88(2017%E7%89%88).pdf. In.; 2017: 96.
- 25.Zhang XTMK, Ling Q, et al. Development on methods for HIV surveillance among target populations and its implication to practice in China. Chin J AIDS STD. 2021;27:1010–45. [Google Scholar]
- 26.New drug provides total. protection from HIV in trial of young African women [https://www.nytimes.com/2024/06/21/health/lenacapavir-hiv-prevention-africa.html]
- 27.Jiangsu Statistical Yearbook. 2023 [http://tj.jiangsu.gov.cn/2022/nj03/nj0322.html]
- 28.Acquired Immunodeficiency Syndrome Professional Group SoID, Chinese Medical Association;, Prevention CCfDCa. Chinese guidelines for diagnosis and treatment of human immunodeficiency virus infection/acquired immunodeficiency syndrome (2024 edition). Chin J Infect Dis. 2024;42(05):257–84. [Google Scholar]
- 29.Jin X, Shi L, Wang C, Qiu T, Yin Y, Shen M, Fu G, Peng Z. Cost-effectiveness of oral pre-exposure prophylaxis and expanded antiretroviral therapy for preventing HIV infections in the presence of drug resistance among men who have sex with men in China: a mathematical modelling study. Lancet Reg Health West Pac. 2022;23:100462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X. Economic Evaluation for Prevention of Mother To Child Transmission of Human Immunodeficiency Virus (HIV) in China. Doctoral thesis 2016.
- 31.China Statistical Yearbook. 2022 [https://www.stats.gov.cn/sj/ndsj/2022/indexch.htm.]
- 32.Ochalek J, Wang H, Gu Y, Lomas J, Cutler H, Jin C. Informing a cost-effectiveness threshold for Health Technology Assessment in China: a marginal Productivity Approach. PharmacoEconomics. 2020;38(12):1319–31. [DOI] [PubMed] [Google Scholar]
- 33.Nichols BE, Boucher CAB, van der Valk M, Rijnders BJA, van de Vijver D. Cost-effectiveness analysis of pre-exposure prophylaxis for HIV-1 prevention in the Netherlands: a mathematical modelling study. Lancet Infect Dis. 2016;16(12):1423–9. [DOI] [PubMed] [Google Scholar]
- 34.Johnson-Masotti AP, Pinkerton SD, Sikkema KJ, Kelly JA, Wagstaff DA. Cost-effectiveness of a community-level HIV risk reduction intervention for women living in low-income housing developments. J Prim Prev. 2005;26(4):345–62. [DOI] [PubMed] [Google Scholar]
- 35.Long EF, Brandeau ML, Owens DK. The cost-effectiveness and population outcomes of expanded HIV screening and antiretroviral treatment in the United States. Ann Intern Med. 2010;153(12):778–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sullivan PS, Driggers R, Stekler JD, Siegler A, Goldenberg T, McDougal SJ, Caucutt J, Jones J, Stephenson R. Usability and acceptability of a Mobile Comprehensive HIV Prevention App for men who have sex with men: a pilot study. JMIR Mhealth Uhealth. 2017;5(3):e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yun K, Yu J, Liu C, Zhang X. A cost-effectiveness analysis of a Mobile phone-based Integrated HIV-Prevention intervention among men who have sex with men in China: economic evaluation. J Med Internet Res. 2022;24(11):e38855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santos WMD, Primeira MR, Paiva LG, Padoin SMM. Economic and epidemiological evaluation of text message-based interventions in patients with the human immunodeficiency virus. Rev Lat Am Enfermagem. 2020;28:e3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ge Q, Lu X, Jiang R, Zhang Y, Zhuang X. Data mining and machine learning in HIV infection risk research: an overview and recommendations. Artif Intell Med. 2024;153:102887. [DOI] [PubMed] [Google Scholar]
- 40.Zou H, Xu J, Hu Q, Yu Y, Fu G, Wang Z, Lu L, Zhuang M, Chen X, Fu J, et al. Decreasing age at first anal intercourse among men who have sex with men in China: a multicentre cross-sectional survey. J Int AIDS Soc. 2016;19(1):20792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang L, Chow EP, Jing J, Zhuang X, Li X, He M, Sun H, Li X, Gorgens M, Wilson D, et al. HIV prevalence in China: integration of surveillance data and a systematic review. Lancet Infect Dis. 2013;13(11):955–63. [DOI] [PubMed] [Google Scholar]
- 42.Zou H, Hu N, Xin Q, Beck J. HIV testing among men who have sex with men in China: a systematic review and meta-analysis. AIDS Behav. 2012;16(7):1717–28. [DOI] [PubMed] [Google Scholar]
- 43.Adamson B, Dimitrov D, Devine B, Barnabas R. The potential cost-effectiveness of HIV vaccines: a systematic review. Pharmacoecon Open. 2017;1(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nosyk B, Min J, Lima VD, Yip B, Hogg RS, Montaner JS, Group SHAS. HIV-1 disease progression during highly active antiretroviral therapy: an application using population-level data in British Columbia: 1996–2011. J Acquir Immune Defic Syndr. 2013;63(5):653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cohen C, Davis K, Meyers J. Abstracts of the Eleventh International Congress on Drug Therapy in HIV infection, 11–15 November 2012, Glasgow, UK. J Int AIDS Soc. 2012;15(Suppl 4):18060–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao Y, Wei L, Dou Z, Zhao D, Gan X, Wu Y, Han M. Changing mortality and patterns of death causes in HIV infected patients — China, 2013–2022. China CDC Wkly. 2023;5(48):1073–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Centre of China Government Procurement. Drug Prices. [https://www.ccgp.gov.cn/]
- 48.Acquired Immunodeficiency Syndrome Professional Group SoID, Chinese Medical Association. Chinese Center for Disease Control and Prevention: Chinese guidelines for diagnosis and treatment of human immunodeficiency virus infection/acquired immunodeficiency syndrome (2024 edition). Chin J Infect Dis 2024, 42(5).
- 49.Nosyk B, Min JE, Lima VD, Hogg RS, Montaner JS, group SHAs. Cost-effectiveness of population-level expansion of highly active antiretroviral treatment for HIV in British Columbia, Canada: a modelling study. Lancet HIV. 2015;2(9):e393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used during the current study available from the corresponding author on reasonable request (tonygqin@ntu.edu.cn).