Abstract
Background
Section 1262 Consolidated Appropriations Act of 2023 eliminates the federal DATA waiver registration requirement to prescribe buprenorphine for opioid use disorder (OUD), along with patient limits, perhaps as a way to increase provider capacity to prescribe buprenorphine. Understanding the factors that influence provider capacity, patient access, and whether community need for MAT is met could inform how to capitalize on DATA waiver eliminations in the United States.
Methods
This observational study utilized required reporting from two cohorts of the Rural Communities Opioid Response Program (RCORP). Consortia (N = 80) provided data on OUD/SUD-related services, service area information, consortium membership, and grant progress, including barriers to and facilitators of achievements. These data were combined with National Survey of Drug Use and Health (NSDUH) and U.S. Census Bureau’s 2016−2020 American Community Survey (ACS) 5-Year Estimates Data to examine MAT capacity, access, and service area need.
Results
A 79% increase in potential buprenorphine prescribers from 2019 to 2022 resulted in 1,060 rural providers with the ability to prescribe buprenorphine. The number of individuals who received MAT increased by 42% over the same three years, with over 20,000 individuals receiving MAT by the end of the funding period. While both capacity and access did increase, an additional 11,454 individuals could have potentially received buprenorphine if all waivered providers prescribed to a conservative patient limit of thirty patients. 70% of consortia provided MAT to at least 11.5% of their estimated service area need (national rate of MAT provision among individuals 18 years and older with an OUD), indicating unused MAT capacity was not related to lack of service area need. Provider (e.g., concerns of clinical complexity), patient (e.g., mistrust of the healthcare system), pharmacy (e.g., cost concerns), and pharmacist (e.g., stigma) barriers impacted MAT provision and availability.
Conclusion
MAT treatment capacity is a necessary but not exclusive requirement for increasing access to MAT. Addressing the multi-faceted barriers to prescribing MAT, particularly buprenorphine, will be critical to ensure the Consolidated Appropriations Act of 2023 does in fact result in a larger workforce that actually prescribes buprenorphine and a pharmacy system that stocks these medications.
Keywords: Rural, Buprenorphine, Opioids, Treatment capacity, MAT
Background
As opioid overdose deaths (alone or in combination with other drugs such as fentanyl and methamphetamine) show few signs of abatement [1, 2], federal agencies have funded and continue to fund initiatives to increase access to medication-assisted treatments (MAT)1 for opioid use disorder [e.g., SAMSHA’s State Targeted Response to the Opioid Epidemic (STR), State Opioid Response Grants (SOR), Medication-Assisted Treatment – Prescription Drug and Opioid Addiction (MAT-PDOA), HRSA Rural Communities Opioid Response Program (RCORP)]. The opioid agonist buprenorphine tends to be the focus of medication expansion efforts due to its robust evidence base [3–6], low overdose risk [6], and induction without the need for a week-long period of abstinence [7]. Additionally, buprenorphine can be prescribed in non-specialty settings such as primary care facilities, which is advantageous in areas with limited substance use disorder (SUD) treatment and MAT access such as within rural communities [3, 8, 9].
Key to increasing MAT access, specifically buprenorphine access, is having a workforce able to prescribe MAT. This condition is particularly critical in rural communities, given that nearly 30% of rural Americans lived in a county without a buprenorphine provider in 2017 [10]. Importantly, the number of providers able to prescribe buprenorphine, generally called waivered providers,2 has increased over the years, with some of the largest increases seen in rural counties [11]. However, many waivered providers either do not prescribe at all or do not prescribe to the maximum limit allowed [12, 13]. For example, Jones and colleagues [12] found that 36–44% of waivered providers had not prescribed buprenorphine at all, and among those who did prescribe, the most common caseload was only 1 to 4 patients per month. Regulatory, training, and caseload limit requirements were often seen as barriers to developing a buprenorphine workforce [6, 9, 14, 15]. Hence, Sect. 1262 of the Consolidated Appropriations Act of 2023 [16] eliminates the federal DATA waiver registration requirement to prescribe buprenorphine for opioid use disorder (OUD), along with eliminating patient limits, perhaps as a way to increase provider capacity to prescribe. Now, any practitioner with a current Drug Enforcement Administration (DEA) registration number and Schedule III authority who meets Sect. 1263’s statutory training requirements can prescribe buprenorphine.
As the impact of the waiver elimination unfolds, understanding the factors that historically influenced provider capacity and prescribing, patient access, and whether community need for MAT is met could inform how to capitalize on DATA waiver eliminations particularly in rural communities. Consequently, the purpose of this paper is to: 1) explore the relationship between MAT capacity and access (i.e., buprenorphine) in rural communities over time within the first two cohorts of a federal initiative designed to reduce the morbidity and mortality of OUD through increases in MAT provision3; 2) examine whether rural consortia meet the national minimum rate of MAT provision (i.e., MAT benchmark); and 3) identify barriers that impact the results. This focus on MAT capacity and access in rural areas as well as the examination of whether unused MAT slots is a result of limited treatment demand contributes to the scientific conversation of this topic.
Methods
Study design and participant cohorts
This observational study occurred as a part of an evaluation of the Health Resources and Services Administration’s (HRSA) Rural Communities Opioid Response Program (RCORP). The rural consortia (i.e., a lead entity with member organizations) included in this analysis received a three-year grant in Fiscal Year (FY) 2019 (9/1/2019–8/31/2022) to reduce the morbidity and mortality of OUD in their communities. As part of required reporting, consortia provided data on OUD/SUD-related services, service area information, and consortium membership to the funding agency on behalf of their consortium member organizations every six months during the award period through HRSA’s Performance Improvement and Measurement System (PIMS). To facilitate reporting, consortium members received and were trained in a data collection workbook that included the measures to be reported, guidance on what did and did not count, and a data collection checklist to flag potential inaccuracies that should be addressed prior to submission. Automated fields aggregated consortium member information for reporting by the lead agency to reduce aggregation errors. Additionally, monthly data coordinator meetings reviewed guidance and addressed areas of confusion to facilitate data accuracy. Of the 92 FY 2019 consortia, 12 were excluded from PIMS analyses due to incomplete or inconsistent data, leaving a study group sample of 80. Consortia also provided quarterly and then biannual data on their progress, including barriers to and facilitators of achievements. Similar to PIMS, consortia leads collected progress report data from their members for submission on behalf of the entire consortium. This work was deemed exempt by JBS’s institutional review board, given that data were part of required grant reporting.
Across the 80 included consortia, there were 1,107 member agencies, representing an array of health, behavioral health, and social service agencies, with an average of 14 member agencies per consortium (SD = 11). Medical providers constituted the majority of consortium leads, with 30% led by a hospital (n = 24) and 35% led by other medical providers, such as a Federally Qualified Health Center (FQHC) or a primary care provider (n = 28). Nearly three-quarters (72%, n = 58) of lead agencies were located in a rural-designated area [17]. Although some lead agencies were located outside of a rural-designated area, their service areas had to be in rural-designated areas. Consortia operated in 329 counties across 37 states in the continental U.S. Most consortia operated in more than one county within a single state (65%, n = 52). While they had a wide range of service area populations, few areas had more than 250,000 individuals (10%, n = 8). Demographic data of lead agency project directors (i.e., respondents) were not collected in PIMS or progress reports. Hence, demographic data are unavailable.
Data sources and measures
Performance Improvement and Measurement System (PIMS) data
Biannually, consortia submitted required data on SUD/OUD performance metrics to HRSA. This analysis includes three PIMS metrics: (a) the reported service area population in the first six-month reporting period, (b) the number of providers in each consortium with a DATA 2000 waiver in each six-month reporting period, and (c) the number of individuals who received MAT4 (alone or in combination with psychotherapy) from agencies and organizations represented within the consortium at each six-month reporting period. Given RCORP’s focus on increasing buprenorphine prescribing, while other medications could be reported, the number of individuals who received MAT is predominately the number of individuals who received buprenorphine.
RCORP progress report data
RCORP Progress Reports gather data on grant activities, successes, challenges, and contextual factors that could impede or facilitate RCORP grant performance. Beginning September 1, 2019, FY19 consortia answered questions each quarter; beginning September 1, 2021, reporting shifted from quarterly to biannually. Likert scales, continuous, dichotomous, checkboxes, and text (e.g., detail when an “other” category was selected) response formats were used based upon the question. This analysis includes questions on (a) the extent (consortia reported large or moderate impacts) to which potential barriers impeded their consortium’s ability to provide MAT and (b) whether pharmacy-related barriers were endorsed by consortia as reasons for pharmacies not stocking medications among consortia reporting that at least one medication (from a list of approved medications for OUD) was unavailable in pharmacies in their service area (e.g., injectable naltrexone, buprenorphine). Barrier data contained in this paper are from the September 2022 progress reports.
U.S. Census Bureau’s 2016−2020 American Community Survey (ACS) 5-Year Estimates Data
The ACS is a rolling population survey implemented annually. Its 5-year estimates provide increased precision and reliability, particularly for rural areas [18]. We matched a county-specific estimate of the percentage of the population aged eighteen and older with each RCORP consortium’s service area and multiplied it by their reported population in the first six-months to estimate an age-adjusted population. For consortia operating in more than one county, we used the mean percentage aged eighteen and over across their service area counties.
National Survey of Drug Use and Health (NSDUH)
NSDUH provides nationally representative estimates of substance use disorders and related issues. We utilized the following published results from the 2020 NSDUH as benchmarks and comparators to consortia’s data: (a) the national OUD prevalence rate of 1% [19] and (b) the percentage of individuals 18 years and older with OUD who received MAT (11.5%) [20].
Data analyses
The study team used SPSS 27 for all data processing and analyses. In preparation for analyses, three variables were computed.
Estimated number of individuals with OUD at each six-month reporting period
We multiplied each consortium’s age-adjusted service area population by the 2020 NSDUH past-year OUD prevalence rate of 1% [19], which covered most of the first project year. This number was divided by 2 to account for the six-month reporting period in the PIMS data.
Potential number of MAT slots
We multiplied each consortium’s reported number of providers with a DATA 2000 waiver by 30, which was the first-year MAT limit for new providers at that time, to project the number of potentially available patient slots for MAT in each six-month reporting period. We used this conservative caseload estimate, given that individual provider prescribing limits were unknown.
MAT benchmark
We created a consortium-specific MAT benchmark to examine whether consortia were meeting the national OUD treatment rate. We multiplied the estimated number of individuals with OUD at each six-month reporting period (OUD prevalence rate multiplied by age-adjusted service area population) by 11.5%, the MAT treatment rate of individuals 18 and over with OUD who received MAT in the 2020 NSDUH. This calculation provided the minimum number of individuals in a service area who should have received MAT based on national treatment rates. We then compared the benchmark with the consortium’s number of individuals who received MAT to determine whether each consortium met or exceeded the national MAT rate.
Results
Change in MAT capacity and access
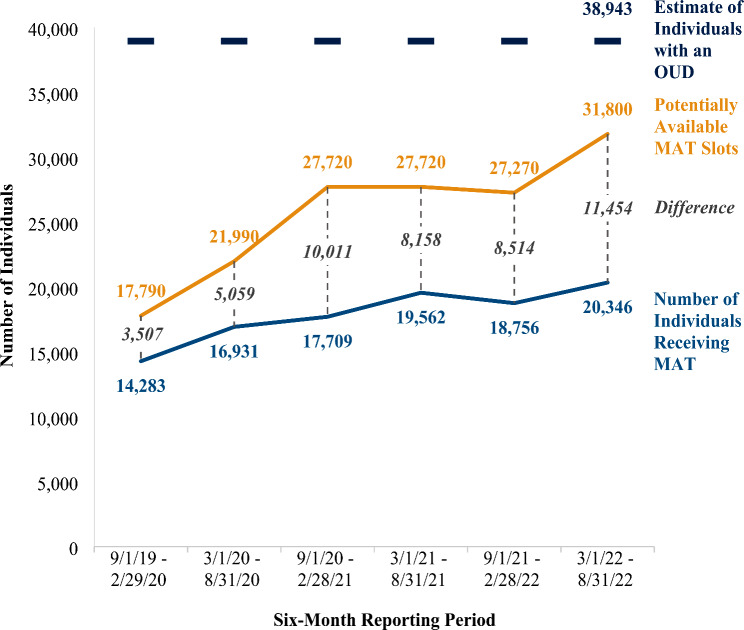
Service capacity increased as a result of the added workforce able to prescribe buprenorphine throughout the three-year award period. As illustrated in Table 1, changes in the number of waivered providers ranged from − 1.62 to 26.06%, depending upon the six-month reporting period. Using a conservative estimate of 30 MAT patients per each waivered provider, Fig. 1 illustrates the changes in the number of potential MAT slots over the award period. Overall, the capacity to deliver MAT increased by 79% from 17,790 potential buprenorphine slots among 593 waivered providers at inception to 31,800 potential buprenorphine slots among 1,060 waivered providers at the conclusion of the award period.
Table 1.
Number of providers with a DATA waiver and percentage change across reporting periods
| Reporting period | Number of waivered providers | Percentage change from prior reporting period |
|---|---|---|
| 9/1/19–2/29/20 | 593 | n/a |
| 3/1/20–8/31/20 | 733 | 23.60% |
| 9/1/20–2/28/21 | 924 | 26.06% |
| 3/1/21–8/31/21 | 924 | 0.00% |
| 9/1/21–2/28/22 | 909 | -1.62% |
| 3/1/22–8/31/22 | 1,060 | 16.61% |
Fig. 1.
MAT potential capacity, access, and estimated service area need. Note. Number of consortia included in analysis is 80
Not surprisingly, MAT access also increased throughout this three-year period. The number of individuals who received MAT (see Fig. 1) increased by 42% from the first (N = 14,283) to the final (N = 20,346) reporting period. Despite these gains, a gap between the number who received MAT and the number of potential MAT slots grew from 3,507 potentially unused slots in the first reporting period to 11,454 potentially unused slots in the final reporting period. In other words, potential MAT capacity exceeded MAT provision.
MAT capacity and service area need
To examine whether unused capacity (i.e., the difference between MAT receipt and potential MAT slots) was a result of consortia meeting the MAT need that existed in their communities, we compared (1) the number of individuals who received MAT with the estimated number of individuals who needed MAT (i.e., the age-adjusted number of individuals with an OUD in consortia’s service areas), and (2) the potential MAT slots with the estimated number of individuals who needed MAT. As shown in Fig. 1, the estimated service area MAT need was larger than the number of individuals receiving MAT, suggesting that there was an ample number of individuals with OUD who could have benefitted from filling a potential unused MAT slot. While the number of potential MAT slots throughout the award period was not enough to entirely meet community MAT need, had waivered providers prescribed to a conservative number of thirty individuals each, at least 82% of the estimated MAT need could have been met.
Consortia prescribing at the national MAT rate
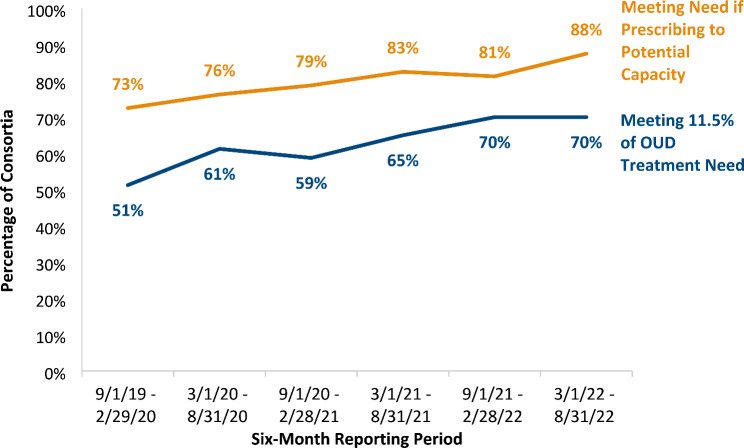
Given the individual, system, and provider barriers to MAT provision, which can be entrenched and significantly impact access to care, we believe it was important to assess whether consortia met or exceeded the national rate of MAT provision for individuals with OUD. In this way, access numbers can be contextualized to national MAT treatment receipt. As shown in Fig. 2, 51% of consortia provided MAT to 11.5% or more of their service area’s estimated need (i.e., to individuals with OUD) at the beginning of the award period. By the end of the award period, 70% of consortia provided MAT to at least 11.5% of their estimated service area need, a 37% increase. As Fig. 2 also shows, had each consortium prescribed MAT to at least thirty patients per waivered provider, an additional 14–23% of consortia would have met the national MAT rate.
Fig. 2.
Percent of consortia meeting 11.5% of estimated service area MAT need. Note. Number of consortia included in analysis is 80. The national rate of MAT provision (11.5%) is among individuals 18 years and older with an OUD
Barriers to MAT provision
As illustrated in Table 2, barriers to MAT provision fell within two categories: provider and patient barriers. At the provider level, almost half (46%) of consortia reported that providers had a difficult time engaging individuals in treatment, with slightly more than one-third of consortia reporting that provider apprehension about the clinical complexity of cases and negative stereotypes about individuals with OUD affected their willingness to treat this population suggesting healthcare stigma toward individuals with OUD [21–25]. A third of consortia reported that provider unwillingness to prescribe medications for OUD at all or to their buprenorphine patient caseload limit impacted MAT access in their service areas, an issue commonly reported in the literature [26, 27]. Finally, 14% of consortia reported that providers did not see MAT as a best practice for treating OUD suggesting intervention stigma toward MOUD [28–30].
Table 2.
Provider and patient barriers limiting MAT access (N = 78)
| Barrier | Number (%) of consortia reporting large or moderate impacts (N = 78a) |
|---|---|
| Provider Barriers to MAT Provision | |
| Perceived Clinical Complexity | 28 (36%) |
| Unwillingness to Provide MAT at All or to Capacity | 26 (33%) |
| Negative Stereotypes About Individuals With SUD/OUDb | 25 (32%) |
| Difficulty in Getting OUD Patients Who Present to Engage in Treatment | 36 (46%) |
| MAT Not Seen as a Best Practice | 14 (18%) |
| Patient Barriers to MAT Provision | |
| Mistrust of the Health Care System | 26 (33%) |
| Refusal of MAT as a Treatment Approach | 11 (14%) |
| Individuals in the Community with OUD Are Not Presenting for Treatmentb | 38 (49%) |
| Lack of Insurance (un- or under-insured) | 32 (41%) |
| Logistical Barriers (e.g., transportation, telehealth-related issues such as no internet connection or cell phone) | 54 (69%) |
a Among the 80 consortia included in our main analysis sample, 2 did not complete progress reports, leaving a sample of 78 for the barriers analysis
b One of the 78 consortia did not respond to the Negative Stereotypes About Individuals with SUD/OUD item, and another consortium did not respond to the Individuals in the Community with OUD Are Not Presenting for Treatment item, leaving a sample size of 77 for these two items
At the patient level, 69% of consortia attributed access issues to logistical barriers (e.g., transportation, limited cell phone limits impacting telehealth), with roughly 40% of consortia attributing it to lack of insurance. Almost one-third reported that prior patient histories of poor treatment resulted in mistrust of the health care system, which may be related to the 49% of consortia reporting individuals were not presenting for treatment, as well as poor engagement mentioned above. Thirteen percent reported that patients refused MAT as a treatment.
Barriers to MAT availability
Consortia also reported that pharmacy- and pharmacist-related barriers impacted MAT availability (see Table 3). At the pharmacy level, roughly 25% of consortia reported that pharmacies’ perceived that a lack of demand for MAT (causing concern that unsold medications would expire with limited options for drug company reimbursement) impacted their willingness to stock these medications. Roughly one consortium in five reported that stocking medication was also negatively impacted by concerns of financial losses, as well as fear about triggering a DEA investigation. At the pharmacist level, 25% of consortia reported that pharmacists’ lack of MAT knowledge or training, coupled with not seeing MAT as a best practice (reported by 16%) and not wanting individuals with OUD in their pharmacies (reported by 16%), impacted their willingness to stock these medications.
Table 3.
Pharmacy and pharmacist barriers limiting MAT availability (N = 61)
| Reasons | Number (%) of consortia endorsing reason (N = 61a) |
|---|---|
| Business-Related Reasons | |
| Concerns About Triggering a DEA Investigation | 11 (18%) |
| Perceived Lack of Demand (causing concern the pharmacy would be left with unsold expired medications) | 16 (26%) |
| Financial Losses Due to Medication Costs | 9 (15%) |
| Pharmacist-Specific Reasons | |
| Lack of MAT Knowledge or Training Among Pharmacists | 15 (25%) |
| Not Wanting Individuals with OUD in the Pharmacy | 10 (16%) |
| Reluctance to Dispense to Patients Not from the Area the Pharmacy Serves | 9 (15%) |
| MAT Not Seen as Best Practice by Dispensing Pharmacists | 8 (13%) |
a Questions on pharmacists and pharmacy barriers were only asked of consortia that reported that at least one medication for OUD had limited or no availability in their area. Among the 80 consortia included in our main analysis sample, 61 reported some limitations in medication availability and were included in this analysis
Discussion
Data contained in this paper illustrate sizable increases in the number of DATA-waivered providers able to prescribe buprenorphine in rural communities throughout the U.S., even in the midst of a global pandemic. By the end of three years of grant funding, consortia had 1,060 providers with the ability to prescribe buprenorphine, representing a 79% increase in potential prescribers from 2019 to 2022. Along with increases in potential provider capacity, the number of individuals who received MAT increased by 42% over the same three years, with over 20,000 individuals receiving MAT by the end of the funding period. Unfortunately, gains made in MAT capacity by rural consortia were not followed by comparable gains in MAT access among individuals with OUD. While both capacity and access did in fact increase, an additional 11,454 individuals could have potentially filled unused buprenorphine slots and received buprenorphine if all waivered providers prescribed to a conservative patient limit of thirty patients. These findings align with previous research showing that most waivered providers are not prescribing buprenorphine to their full capacity [12, 13, 31]. Importantly, the gap between MAT provision and potential capacity does not appear to be the result of a lack of need, as the number of individuals in the service areas who were projected to have OUD was consistently higher than the number who received MAT. It is important to note, however, that it is possible that individuals in need may have received MAT from MAT providers who were not part of the consortia. For example, it is possible that some individuals may have been receiving methadone at an opioid treatment program (OTP) outside of the consortia given that only 32% of consortia reported methadone availability within their consortia [32, 33]. However, we estimate the number of individuals receiving MAT from MAT providers outside of the consortia to be small, as funding decisions were based on rural areas with limited MAT resources. Nonetheless, identifying and subsequently exploring other data sources to further estimate how much unmet need may have been met by providers outside of the consortia could provide additional information on this topic. Unfortunately, many of these datasets bring limitations which would need to be taken into account if used (e.g., N-SSATS includes only a biannual frequency of client level data, point prevalence data, and it focuses on facilities rather than practitioners).
Our analyses also reveal a diverse set of barriers impacting MAT provision, adding additional evidence to qualitative reporting on this topic [9, 14, 23–25, 28–30]. Not surprisingly, providers who do not prescribe at all or only to a few clearly impacted provision, as did stigma toward individuals with OUD and to some extent lack of knowledge about and stigma toward MAT. Prior negative experience with health care providers at the patient level, coupled with limited knowledge of MAT’s benefits, could contribute to consortia reports of treatment initiation and engagement difficulties. It is important to note that pharmacies and pharmacists are also a factor in MAT access. Pharmacy willingness to stock medications to treat individuals with opioid use disorder was negatively impacted by cost concerns, fear of DEA investigations, stigma, and lack of knowledge about OUD best practices. This finding supports a growing body of literature that suggests pharmacies pose another barrier to addressing the opioid epidemic [34–36].
Data contained here and put forth by others [12, 13, 31, 37] suggest that removing regulations will not be enough to reach all who need OUD treatment especially in rural communities. A number of issues will need to be addressed if we are to capitalize on the Consolidated Appropriations Act of 2023 that eliminated the waiver requirement to prescribe buprenorphine. Addressing provider concerns about clinical complexity and identifying or addressing lack of psychosocial or behavioral health support is needed, as is attention to negative attitudes or stigma toward OUD and/or MAT [28–30], including the attitudes of the individuals with OUD themselves [12, 38]. Working with rural pharmacies to address DEA concerns and stigma held by pharmacists will be needed to ensure a reliable source for prescription fulfillment. Employing peer recovery support specialists to work with patients who do not seek or refuse care because of prior negative healthcare experiences could potentially increase treatment initiation and engagement [39].
Limitations
There are four primary limitations to this paper. First, estimates of unmet need may be lower than reported if there were other available MAT providers who were not part of each consortium. Given that RCORP targeted rural communities with limited OUD treatments, these numbers should be low. Second, we did not have data on the number of waivered providers who were actively prescribing, the number of individuals each waiver provider prescribed to, or each waivered provider’s caseload limits. This data unavailability could have resulted in an overestimate (e.g., if waivered providers were not prescribing and thus not considered an MAT provider) or underestimate (e.g., if a waivered provider caseload limit exceeded thirty patients) of potential MAT slots. Therefore, we used conservative methods in all calculations (i.e., the former minimum limit of thirty patients per provider as the multiplier to determine potential MAT slots) and limited our analyses to descriptive sums across all consortia. Limitations associated with waivered provider data availability shed light on the nuances of examining this topic. Additionally, the number estimated with OUD across all consortia and the percentage of consortia prescribing at the national MAT rate were calculated using national estimates that included data across metro and non-metro counties, most of which include a combination of urban and rural populations [40]. Third, secondary datasets including NSDUH can produce underestimates of OUD [41]. Finally, since barrier data were collected from the perspectives of consortia leads and their member agencies, providers might have additional information on prescribing barriers. Taken together, these limitations warrant caution when generalizing these results to all U.S. communities or buprenorphine prescribers and illustrate the challenges encountered when examining this topic area.
Note
This work was undertaken during the Coronavirus disease 2019 (COVID-19) pandemic which certainly put a strain on rural resources. Consortia responded by implementing a variety of OUD service delivery adaptations to lesson COVID-19’s impact on treatment access (e.g., redesigning physical spaces to accommodate social distancing, implementing telehealth-delivered services). During the first year of the pandemic, consortia significantly increased the number of patients per 100,000 receiving MAT, with medians increasing by 105% [42]. The increased use of telehealth may have contributed to consortia’s increased provision of MOUD. This suggests that COVID-19’s impact on MAT access was minimal within the service areas served by these consortia given service adaptations.
Conclusion
MAT treatment capacity is a necessary but not exclusive requirement for increasing access to MAT particularly in rural areas. Addressing the multifaceted barriers to prescribing MAT, particularly buprenorphine, will be critical to ensure the Consolidated Appropriations Act of 2023 does in fact result in a larger workforce that actually prescribes buprenorphine and meets the treatment needs of individuals with OUD. Additionally, collaborating with pharmacists and their associations (e.g., American Pharmacists Association) to improve knowledge of regulatory policies, particularly DEA regulations, reduce stigma toward individuals with OUD, and increase knowledge of best practices in treating OUD appears necessary so that patients can actually fill a buprenorphine prescription when they receive one.
Acknowledgements
The authors thank consortium members for completing their data collection requirements and Richard Koban Payne and Gina Heller for contributions to data collection, data analyses, and project management.
Abbreviations
- ACS
American Community Survey
- COVID-19
Coronavirus disease 2019
- DATA
Drug Addiction Treatment Act of 2000
- DEA
Drug Enforcement Administration
- FQHC
Federally Qualified Health Center
- FY
Fiscal Year
- HRSA
Health Resources and Services Administration
- MAT
Medication-Assisted Treatment
- MAT-PDOA
Medication-Assisted Treatment-Prescription Drug and Opioid Addiction
- MOUD
Medications for Opioid Use Disorder
- NSDUH
National Survey of Drug Use and Health
- OTP
Opioid Treatment Program
- OUD
Opioid Use Disorder
- PIMS
Performance Improvement and Measurement System
- RCORP
Rural Communities Opioid Response Program
- SAMHSA
Substance Abuse and Mental Health Services Administration
- SOR
State Opioid Response
- STR
State Targeted Response
- SUD
Substance Use Disorder
Author contributions
C.M. conceptualized the study, curated data, conducted formal analysis, visualized the tables and figures, designed the methodology, wrote the original draft, and reviewed and edited the final manuscript. R.Z. conducted formal analysis and managed software. E.T. conducted formal analysis, curated the data, managed software, and validated findings. H.S. managed software, curated the data, and reviewed and validated findings. E.B. administered and supervised the study and managed software and resources. K.M. conceptualized and supervised the study, designed the methodology, wrote the original draft, and reviewed and edited the final manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by the Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) as part of an award totaling $12,672,053.00 (grant number U3CRH33332) with 0% financed with non-governmental sources.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This work was deemed exempt by JBS’ institutional review board given that data were part of required grant reporting.
Consent for publication
Data availability.
Competing interests
The authors declare no competing interests.
Footnotes
While the field is moving toward using the term, medications for opioid use disorder (MOUD), MAT was the term used during data collection and hence is used in this paper.
Until December, 2023, practitioners interested in prescribing buprenorphine outside of an Opioid Treatment Program (OTP) had to apply for and receive a Drug Addiction Treatment Act of 2000 (DATA) waiver (also called X-waiver) that required 8 h of specialized training for physicians and 24 h of specialized training for advanced practitioners.
RCORP is a multiyear muti-focused HRSA initiative aimed at reducing the morbidity and mortality of SUD, including OUD, in rural communities. RCORP funds multisector consortia that aim to (1) strengthen capacity to implement and sustain SUD/OUD and behavioral health activities; (2) strengthen and expand SUD/OUD and behavioral health prevention, treatment, and recovery services.
MAT was the term used during data collection and hence is used in this paper.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Spencer MR, Miniño AM, Warner M. Drug overdose deaths in the United States, 2001–2021. Hyattsville, MD: National Center for Health Statistics; 2022. 10.15620/cdc:122556. NCHS Data Brief, no 457. [PubMed]
- 2.Ahmad FB, Cisewski JA, Rossen LM, Sutton P. Provisional drug overdose death counts. National Center for Health Statistics; 2023. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
- 3.Wakeman SE, Larochelle MR, Ameli O, Chaisson CE, McPheeters JT, Crown WH, Azocar F, Sanghavi DM. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3(2):e1920622. 10.1001/jamanetworkopen.2019.20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, Ferri M, Pastor-Barriuso R. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ (Clinical Res Ed). 2017;357:j1550. 10.1136/bmj.j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santo T Jr, Clark B, Hickman M, Grebely J, Campbell G, Sordo L, Chen A, Tran LT, Bharat C, Padmanathan P, Cousins G, Dupouy J, Kelty E, Muga R, Nosyk B, Min J, Pavarin R, Farrell M, Degenhardt L. Association of opioid agonist treatment with all-cause mortality and specific causes of death among people with opioid dependence: a systematic review and meta-analysis. JAMA Psychiatry. 2021;78(9):979–93. 10.1001/jamapsychiatry.2021.0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Academies of Sciences. Engineering, and Medicine. Medications for opioid use disorder save lives. Washington, DC: National Academies; 2019. 10.17226/25310. [PubMed] [Google Scholar]
- 7.Volkow ND, Blanco C. Medications for opioid use disorders: clinical and pharmacological considerations. J Clin Investig. 2020;130(1):10–3. 10.1172/JCI134708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haffajee RL, Lin LA, Bohnert ASB, Goldstick JE. Characteristics of US counties with high opioid overdose mortality and low capacity to deliver medications for opioid use disorder. JAMA Netw Open. 2019;2(6):e196373. 10.1001/jamanetworkopen.2019.6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lister JJ, Weaver A, Ellis JD, Himle JA, Ledgerwood DM. A systematic review of rural-specific barriers to medication treatment for opioid use disorder in the United States. Am J Drug Alcohol Abus. 2020;46(3):273–88. 10.1080/00952990.2019.1694536. [DOI] [PubMed] [Google Scholar]
- 10.Andrilla CHA, Moore TE, Patterson DG, Larson EH. Geographic distribution of providers with a DEA waiver to prescribe buprenorphine for the treatment of opioid use disorder: a 5-year update. J Rural Health. 2019;35(1):108–12. 10.1111/jrh.12307. [DOI] [PubMed] [Google Scholar]
- 11.Andrilla CHA, Patterson DG. Tracking the geographic distribution and growth of clinicians with a DEA waiver to prescribe buprenorphine to treat opioid use disorder. J Rural Health. 2022;38(1):87–92. 10.1111/jrh.12569. [DOI] [PubMed] [Google Scholar]
- 12.Jones CM, Olsen Y, Ali MM, Sherry TB, Mcaninch J, Creedon T, et al. Characteristics and prescribing patterns of clinicians waivered to prescribe buprenorphine for opioid use disorder before and after release of new practice guidelines. JAMA Health Forum. 2023;4(7):e231982. 10.1001/jamahealthforum.2023.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stein BD, Sorbero M, Dick AW, Pacula RL, Burns RM, Gordon AJ. Physician capacity to treat opioid use disorder with buprenorphine-assisted treatment. JAMA. 2016;316(11):1211–2. 10.1001/jama.2016.10542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haffajee RL, Andraka-Christou B, Attermann J, Cupito A, Buche J, Beck AJ. A mixed-method comparison of physician-reported beliefs about and barriers to treatment with medications for opioid use disorder. Subst Abuse Treat Prev Policy. 2020;15(1):69. 10.1186/s13011-020-00312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joudrey PJ, Kolak M, Lin Q, Paykin S, Anguiano JRV, Wang EA. Assessment of community-level vulnerability and access to medications for opioid use disorder. JAMA Netw Open. 2022;5(4):e227028. 10.1001/jamanetworkopen.2022.7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Consolidated A, Act H, R.2617. 2023. https://www.congress.gov/bill/117th-congress/house-bill/2617
- 17.HRSA. Defining rural population. HHS; 2024. https://www.hrsa.gov/rural-health/about-us/what-is-rural
- 18.U.S. Census Bureau. American Community Survey 5-Year data (2009–2021): 2016–2020 estimates; 2022. https://www.census.gov/data/developers/data-sets/acs-5year.html
- 19.Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: Results from the 2020 National Survey on Drug Use and Health (HHS Publication No. PEP21-07-01-003, NSDUH, Series H-. 56). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2021. Retrieved from https://www.samhsa.gov/data/
- 20.Center for Behavioral Health Statistics and Quality (CBHSQ). Results from the 2020 National Survey on Drug Use and Health: Detailed tables. Rockville, MD: Substance Abuse and Mental Health Services Administration (SAMHSA). 2021. Retrieved from https://www.samhsa.gov/data/
- 21.Franz B, Dhanani LY, Miller WC. Rural-urban differences in physician bias toward patients with opioid use disorder. Psychiatr Serv. 2021;72(8):874–9. [DOI] [PubMed] [Google Scholar]
- 22.Garpenhag L, Dahlman D. Perceived healthcare stigma among patients in opioid substitution treatment: a qualitative study. Subst Abuse Treat Prev Policy. 2021;16:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carl A, Pasman E, Broman MJ, Lister JJ, Agius E, Resko SM. Experiences of healthcare and substance use treatment provider-based stigma among patients receiving methadone. Drug Alcohol Depend Rep. 2023;6:100138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLean K, Murphy J, Kruis N. I think we’re getting better but we’re still not there: provider-based stigma and perceived barriers to care for people who use opioids (PWUO). J Subst Use Addict Treat. 2024;159:209270. [DOI] [PubMed] [Google Scholar]
- 25.Magnan E, Weyrich M, Miller M, Melnikow J, Moulin A, Servis M, Henry SG. Stigma against patients with substance use disorders among health care professionals and trainees and stigma-reducing interventions: a systematic review. Acad Med. 2024;99(2):221–2. [DOI] [PubMed] [Google Scholar]
- 26.Andrilla CHA, Coulthard C, Patterson DG. Prescribing practices of rural physicians waivered to prescribe buprenorphine. Am J Prev Med. 2018;54(6):S208–14. [DOI] [PubMed] [Google Scholar]
- 27.Conway KP, Khoury D, Hilscher R, Aldridge AP, Parker SJ, Zarkin GA. Rural and urban differences in undersupply of buprenorphine provider availability in the United States, 2018. Addict Sci Clin Pract. 2022;17(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richard EL, Schalkoff CA, Piscalko HM, et al. You are not clean until you’re not on anything: perceptions of medication-assisted treatment in rural Appalachia. Int J Drug Policy. 2020;85:102704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madden EF, Prevedel S, Light T, Sulzer SH. Intervention stigma toward medications for opioid use disorder: a systematic review. Subst Use Misuse. 2021;56(14):2181–201. [DOI] [PubMed] [Google Scholar]
- 30.Piscalko H, Dhanani LY, Brook D, Hall OT, Miller WC, Go V, Franz B. Knowledge of medications for opioid use disorder and associated stigma among primary care professionals. Ann Med. 2024;56(1):2399316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stein BD, Saloner B, Schuler MS, Gurvey J, Sorbero M, Gordon AJ. Concentration of patient care among buprenorphine-prescribing clinicians in the US. JAMA. 2021;325(21):2206–8. 10.1001/jama.2021.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mun C, Zambrano R, Tallman E, Schuler H, Waibel E, Heller G, Payne RK, Meyers K. Laying the foundation for HRSA’s RCORP initiative: findings from RCORP’s first implementation cohort. Report prepared for the Health Resources and Services Administration. North Bethesda, MD: JBS International, Inc; 2023. [Google Scholar]
- 33.Tallman E, Mun C, Zambrano R, Schuler H, Waibel E, Heller G, Mueller D, Payne RK, Meyers K. Laying the foundation for HRSA’s RCORP initiative: findings from RCORP’s medication-assisted treatment-expansion (MAT-E) cohort. Report prepared for the Health Resources and Services Administration. JBS International, Inc; 2023.
- 34.Cooper HL, Cloud DH, Freeman PR, Fadanelli M, Green T, Van Meter C, et al. Buprenorphine dispensing in an epicenter of the U.S. opioid epidemic: a case study of the rural risk environment in Appalachian Kentucky. Int J Drug Policy. 2020;85:102701. 10.1016/j.drugpo.2020.102701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kazerouni NJ, Irwin AN, Levander XA, Geddes J, Johnston K, Gostanian CJ et al. Pharmacy-related buprenorphine access barriers: an audit of pharmacies in counties with a high opioid overdose burden. Drug Alcohol Depend. 2021;224:108729. [DOI] [PubMed]
- 36.Thakur T, Frey M, Chewning B. Pharmacist roles, training, and perceived barriers in naloxone dispensing: a systematic review. J Am Pharmacists Association: JAPhA. 2020;60(1):178–94. 10.1016/j.japh.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 37.HHS Buprenorphine Practice Guidelines Evaluation Workgroup. Early changes in waivered clinicians and utilization of buprenorphine for opioid use disorder after implementation of the 2021 HHS buprenorphine practice guidelines. U.S. Department of Health and Human Services Assistant Secretary for Planning and Evaluation (ASPE). 2022. Accessed from: https://aspe.hhs.gov/sites/default/files/documents/ed38939a27154d27d87f2543f7de4cfa/buprenorphine-practice-guideline-early-impacts.pdf
- 38.Haffajee RL, Bohnert ASB, Lagisetty PA. (2018). Policy pathways to address provider workforce barriers to buprenorphine treatment. American Journal of Preventive Medicine. 2018;54(6 Suppl 3); 230–S242. 10.1016/j.amepre.2017.12.022 [DOI] [PMC free article] [PubMed]
- 39.Substance Abuse and Mental Health Services Administration. Publication No. PEP23-02-01-001. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2023. Incorporating Peer Support Into Substance Use Disorder Treatment Services. Treatment Improvement Protocol (TIP) Series 64. [PubMed]
- 40.U.S. Department of Agriculture Economic Research Service. Rural Classifications, Overview; 2024. https://www.ers.usda.gov/topics/rural-economy-population/rural-classifications/
- 41.Reuter P, Caulkins JP, Midgette G. Heroin use cannot be measured adequately with a general population survey. Addiction. 2021;116(10):2600–9. [DOI] [PubMed] [Google Scholar]
- 42.Regalbuto E, Schachtner R, Schuler HR, Kuritzky A, Rupp S, Birmingham C, Bresani E, Mun C, Tate E, Meyers K. Dual public health emergencies: How rural RCORP communities are responding to COVID-19 and the opioid epidemic. Report prepared for the Health Resources and Services Administration. North Bethesda, MD: JBS International, Inc; 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Spencer MR, Miniño AM, Warner M. Drug overdose deaths in the United States, 2001–2021. Hyattsville, MD: National Center for Health Statistics; 2022. 10.15620/cdc:122556. NCHS Data Brief, no 457. [PubMed]
Data Availability Statement
No datasets were generated or analysed during the current study.