Abstract
In preclinical studies, GST-HG141, a novel hepatitis B virus (HBV) capsid assembly modulator displayed potent anti-HBV activity in vitro and strong efficacy in HBV animal models. A randomized, double-blind, ascending phase 1b trial assessed the pharmacokinetics, safety, and efficacy of GST-HG141 in chronic hepatitis B (CHB) individuals. Thirty treatment-naïve CHB patients were enrolled in three cohorts (25, 50, and 100 mg twice orally after meals daily) over 28 days, with 10 subjects per cohort (8:2 ratio for GST-HG141 and placebo). Dose-related safety and tolerability, pharmacokinetic profiles, and drug responses were evaluated. GST-HG141 exhibited a generally favorable safety profile across all doses with predominantly mild adverse reactions, including three cases of grade 1 transaminase elevations. Significant reductions in HBV DNA and pregenomic RNA (pgRNA) levels were observed across all doses of (25, 50, and 100 mg of GST-HG141, twice-daily) after 28 days of treatment. Pharmacokinetic analysis showed a consistent linear trend in GST-HG141 concentrations, with mean trough concentrations ranging from 75 to 240 ng/mL. These concentrations adequately covered the protein binding-adjusted EC50 (16.89 ng/mL) by factors of 4.4, 11.1, and 14.6 for doses of 25, 50, and 100 mg, respectively. Our study demonstrated GST-HG141’s well-tolerated profile up to 100 mg over 4 weeks, alongside robust antiviral activity in CHB patients, supporting its progression into further clinical investigation for CHB management.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12985-024-02584-8.
Keywords: Antiviral therapy, Hepatitis B, Pharmacokinetics, Tolerability, Capsid assembly modulator
Introduction
Chronic hepatitis B virus (HBV) infection represents a significant global health threat, leading to substantial liver-related morbidity and mortality [1, 2]. The primary risk factor for disease progression in chronically infected individuals is an elevated serum HBV DNA concentration. Reducing the HBV DNA load is the best strategy for hepatitis B treatment [3, 4]. Although therapies such as nucleoside analogs and pegylated interferon-alpha can control HBV infection, they often fail to completely eradicate the virus, and they can cause adverse reactions such as drug resistance and viral recurrence [5, 6].
To address these challenges, there is a pressing need for novel antivirals with alternative mechanisms to effectively inhibit viral replication. One promising mechanism for novel therapeutics is modulating HBV capsid assembly, a crucial step of viral life cycle facilitated by core viral proteins. These proteins play diverse roles in the HBV life cycle [7, 8], making them attractive targets for intervention. The emerging classes of anti-HBV therapeutics include core protein allosteric regulators (CpAMs) [9, 10]. These can be categorized into capsid assembly modulator-anomaly (CAM-A) and capsid assembly modulator-empty (CAM-E). CAM-As induce the formation of abnormal non-capsid polymers, whereas CAM-Es accelerate the formation of HBV capsids and induce the formation of “empty” capsids devoid of genetic material. Both drug classes inhibit pgRNA encapsidation and viral replication [11–15].
GST-HG141 is a novel capsid assembly modulator grouped into the CAM-E category, but it has some novel features distinct from those of other CAM-Es [10]. GST-HG141 targets HBV core capsid assembly by regulating core protein conformation, effectively inhibiting HBV replication. Preclinical studies demonstrated its potent antiviral activities in vitro and strong efficacy in HBV animal models in vivo. GST-HG141 also displayed an excellent safety profile and activity in preclinical safety and pharmacokinetic studies.
Based on the safety and tolerability data from a phase 1a study using single ascending doses (up to 500 mg) and multiple ascending doses (up to 200 mg) of GST-HG141 (BID for 7 days), GST-HG141 was found to be well tolerated in healthy subjects [16]. Our phase 1b trial evaluated the efficacy, tolerability, and pharmacokinetics of multiple ascending doses of GST-HG141 in patients with chronic HBV infection. This study marks a crucial step in advancing this potential novel therapy for the management of CHB.
Materials and methods
Study design and administration
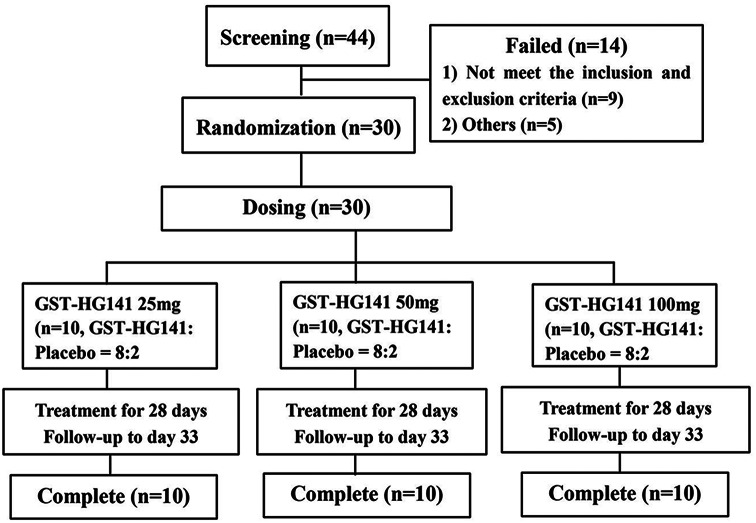
The study was structured as a randomized, double-blind, placebo-controlled, phase 1b clinical trial (https://clinicaltrials.gov, trial Identifier: NCT04868981), and it was conducted at the Phase I Clinical Trial Unit of the First Hospital of Jilin University (Jilin, China). The clinical study protocol was approved by the Ethics Committee of the Jilin University First Affiliated Hospital-Clinical Research Institute (Approval Number 21Y088-001). Prior to participation, all patients provided written informed consent. A cohort of 30 patients with chronic hepatitis (CHB) was recruited to receive either GST-HG141 at doses of 25, 50, or 100 mg BID or placebo over a 28-day period (Fig. 1). Each dose cohort underwent continuous administration for 28 days. From day 1 to day 27, the medication was administered orally twice a day with an interval of approximately 12 h. On day 28, it was given once in the morning. All subjects had regular breakfast and/or dinner 30 min prior to taking the medicine. After taking the medication, the subjects were provided with approximately 240 mL of warm water in the post-meal state. Within each cohort, 10 subjects were randomly assigned at a ratio of 8:2 to receive either GST-HG141 or placebo. GST-HG141 was administered in the clinical research center on days 1–3 and 26–33, with patients continuing treatment at home under the same conditions between hospital visits (Figure S1). Patients were scheduled for follow-up visits on days 8, 15, 22, and 33 and at the time of early termination when necessary. The randomization process was executed using an electronic data capture system, with further details provided in Additional file 1.
Fig. 1.
Design of this study
Patient selection
Regarding patient selection, subjects aged 18–70 with CHB were eligible for participation. Additional inclusion criteria were as follows: (1) chronic HBV infection (defined as hepatitis B surface antigen [HBsAg] positivity for ≥ 6 months or negativity for hepatitis B core antibody immunoglobulin M); (2) serum HBV DNA levels of ≥ 2 × 105 IU/mL for hepatitis B e antigen (HBeAg)-positive individuals or ≥ 2 × 104 IU/mL for HBeAg-negative individuals; (3) serum alanine aminotransferase (ALT) levels within 5× the upper limit of normal (ULN); and (4) no prior treatment or discontinuation of interferon treatment at least 12 months prior to enrollment or nucleos(t)ide analog treatment least 6 months prior to enrollment.
The exclusion criteria were as follows: (a) serum total bilirubin > 2× ULN; (b) co-infection with syphilis, human immunodeficiency virus, and hepatitis C virus; (c) concurrent severe chronic medical conditions or malignancies; (d) serum alpha-fetoprotein levels exceeding 50 µg/L or malignancy detected via imaging; and (e) presence of liver cirrhosis (as indicated by FibroScan score ≥ 12.4 kPa when ALT was normal or < 2× ULN or FibroScan score > 17.5 when ALT was ≥ 2× ULN). Additionally, each group required a minimum of four subjects with elevated ALT (> 1× ULN). Further inclusion and exclusion criteria are delineated in Additional file 1.
Evaluation of tolerability
To assess the tolerability of the drug, routine clinical examinations were conducted alongside sign monitoring and various laboratory tests including hematology, biochemistry, coagulation, and urinalysis. Additionally, electrocardiogram, as well as abdominal and adrenal ultrasound, was performed. The determination of treatment-emergent adverse events (TEAEs) adhered to the guidelines outlined in the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE, 5.0).
Pharmacokinetic assessment
Blood samples were collected intermittently to analyze GST-HG141PK using an anticoagulant (K2-EDTA). After centrifuging the samples at 2000 × g for 10 min, the resultant supernatant from each sample was carefully separated and preserved at − 80 °C until further analysis.
Assessment of effectiveness
The effectiveness of the treatment was evaluated by monitoring changes in the serum levels of HBsAg, hepatitis B core-related antigen (HBcrAg), HBeAg, HBV pgRNA, and HBV DNA at various intervals. Blood samples were collected on day 1 before administering the treatment (baseline) and on days 1, 15, 29, and 33 after treatment initiation. Additionally, samples for detecting viral resistance to GST-HG141 were obtained on days 1 (prior to treatment) and 29 (24 h after the final dose on day 28). HBV DNA levels were assessed using COBAS kits (Roche Diagnostics, Pleasanton, CA, USA). The lowest detectable level with this kit is 10 IU/mL. HBeAg and HBsAg serum levels were quantitatively evaluated using COBAS kits (Roche e601, CA, USA), while HBcrAg was detected using a Lumipulse G1200 assay (Fujirebio Europe, Belgium). All measurements were conducted at Teddy Clinical Research Laboratory in Shanghai, China.
Statistical analysis
Plasma concentrations of GST141 were utilized to assess pharmacokinetic parameters using non-compartmental methodology, including the terminal elimination half-life (t1/2), volume of distribution, clearance, λz, area under the plasma concentration–time curve (AUC0 − t, AUC0−∞), time to reach the maximum plasma concentration, and maximum plasma concentration (Cmax). These parameters were calculated using WinNonlin® Enterprise (version 8.3) software. Additional statistical analyses were conducted using SAS 9.4 software (SAS, Cary, NC, USA). The dose proportionality of GST-HG141 was assessed utilizing the power model and linear fixed-effects model. Descriptive analysis was employed to evaluate antiviral activity indices, tolerability, and safety.
Results
Participant characteristics
Of 44 individuals screened, 30 were selected for randomization, and notably, there were no withdrawals from the study. The flow chart is presented in Fig. 1. Generally, demographic and disease characteristics were well balanced across the groups (Table 1). All 30 recruited individuals were included in the full analysis set, safety analysis set, pharmacodynamic analysis set, and pharmacokinetic parameter analysis dataset. Among the enrolled subjects, 90% (27 of 30) were HBeAg-negative, and the cohort included an equal number of males and females. Baseline HBV DNA levels ranged from 7.54 to 8.25 log10 IU/mL. Before treatment initiation, serum levels of HBsAg and HBV DNA were similar across the different treatment groups, whereas HBeAg levels were slightly lower in the 50-mg cohort than in the 25- and 100-mg cohorts. All enrolled patients were HBeAg-positive and treatment-naïve. Additionally, albumin and platelet levels, as presented in Table 1, were comparable across all groups, and they remained within normal ranges.
Table 1.
Patient demographics and clinical features
| 25 mg BID(N = 8) | 50 mg BID (N = 8) |
100 mg BID (N = 8) |
Placebo (N = 6) |
Total(N = 30) | ||
|---|---|---|---|---|---|---|
| Age, mean (SD), years | 42.6 (9.0) | 34.8 (8.5) | 37.3 (9.4) | 43.0 (5.0) | 39.2 (8.7) | |
| Gender, male/female, n | 3/5 | 4/4 | 6/2 | 2/4 | 15/15 | |
| BMI, mean (SD), kg/m2 | 25.4 (2.9) | 23.6 (2.6) | 25.0 (2.6) | 25.7 (2.4) | 24.9 (2.6) | |
| HBV DNA (Log10IU/mL) | 8.25(0.08) | 7.54(1.40) | 8.05(0.53) | 7.74(1.11) | 7.91 (0.91) | |
| HBsAg (Log10IU/mL) | 4.64 (0.38) | 4.12 (0.95) | 4.40 (0.45) | 4.22 (0.98) | 4.35 (0.71) | |
| HBeAg (Log10U/mL) | 3.2 (0.23) | 1.9 (1.6) | 2.4 (1.4) | 2.1 (1.7) | 2.7 (1.2) | |
| HBeAg negative, n (%) | 0 | 1 (12.5) | 1 (12.5) | 1 (16.7) | 3 (10.0) | |
| FibroScan (kPa) | 6.44 (2.30) | 6.21 (2.15) | 6.44(4.55) | 7.67 (4.81) | 6.78(2.65) | |
| AFP, a ng/mL | 80 (51–102) | 88 (59–125) | 73 (52–95) | 80 (54–104) | 78 (51–125) | |
| History of anti-hepatitis B virus treatment | No | No | No | No | No | |
| ALT (U/L) | 41 (23) | 51 (39) | 61 (44) | 36 (25) | 48 (34) | |
| ALT > 1×ULN, n (%) | 3 (37.5) | 4 (50.0) | 3 (37.5) | 2 (33.3) | 12 (40.0) | |
| Platelet (10^9/L) | 198 (44) | 226 (61) | 178 (48) | 196 (85) | 201 (53) | |
| Albumin (g/L) | 43 (1.5) | 44 (2.9) | 44 (2.5) | 42 (1.6) | 44 (2.3) | |
| Creatinine (umol/L) | 64 (10) | 69 (16) | 77 (14) | 63 (12) | 70 (14) | |
| HBV Genotype, n(%) | B | 1 (12.5) | 2 (25.0) | 2 (25.0) | 0 | 5 (16.7) |
| C | 7 (87.5) | 6 (75.0) | 6 (75.0) | 6 (100) | 25 (83.3) | |
*Data are presented as the mean (SD) unless otherwise noted
Abbreviations: BMI, body mass index; HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B e antigen; ALT, alanine aminotransferase; ULN, upper limit of normal
Assessment of tolerability
Throughout the 28-day treatment period, there were no instances of mortality, serious adverse events, or discontinuations attributed to adverse drug reactions (ADRs). The incidence of ADRs by treatment group is detailed in Table 2. Overall, GST-HG141 demonstrated a favorable safety profile, and it was well tolerated among the subjects. Among the randomized individuals, 21 clinical ADRs (adverse events deemed definitely, very likely, or possibly related to the study drug) were reported, affecting 50% (12 of 24) of the dosed subjects. The incidence rates of adverse reactions in each dosage cohort were as follows: 25 mg, 62.5% (5 of 8); 50 mg, 37.5% (3 of 8); 100 mg, 50.0% (4 of 8); and placebo, 33.3% (2 of 6). Although the incidence of ADRs was slightly higher in the treatment groups than in the placebo group, however no significant difference was found (P = 0.65). Notably, all TEAEs were mild, and their frequency or severity did not increase with increasing GST-HG141 doses (Table 2). No grade ≥ 3 ADRs were reported, and all ADRs either reverted to baseline levels or remained stable. The most commonly reported ADRs (in ≥ 2 subjects) included decreased neutrophil counts (12.5%, 3 of 24), decreased leukocyte counts (8.3%, 2 of 24), hypophosphatemia (8.3%, 2 of 24), increased alanine aminotransferase levels (8.3%, 2 of 24), and increased aspartate aminotransferase levels (8.3%, 2 of 24). Notably, no instances of decrease albumin levels or total bilirubin elevation were observed. The transaminase elevations noted in three patients (randomization Nos. 1001, 1002, and 3002) were mild (grade 1). In conclusion, GST-HG141 tablets at dosages of 25, 50, and 100 mg BD were well tolerated by patients with CHB following continuous administration for 28 days.
Table 2.
Type and incidents of adverse drug reaction(ADR) reported
| ADR, n (%) | 25 mg BID (N = 8) | 50 mg BID (N = 8) |
100 mg BID (N = 8) |
GST-HG141 (N = 24) |
Placebo (N = 6) |
GST-HG141 vs. placebo p-value * |
|---|---|---|---|---|---|---|
| Incidence of adverse reactions | ||||||
| Alanine aminotransferase increase | 1 (12.5) | 0 | 1 (12.5) | 2 (8.3) | 0 | > 0.99 |
| Aspartate aminotransferase increase | 2 (25.0) | 0 | 0 | 2 (8.3) | 0 | > 0.99 |
| Leukocyte count decrease | 1 (12.5) | 0 | 1 (12.5) | 2 (8.3) | 0 | > 0.99 |
| Platelet count decrease | 0 | 0 | 1 (12.5) | 1 (4.2) | 0 | > 0.99 |
| Neutrophil count decrease | 2 (25.0) | 0 | 1 (12.5) | 3 (12.5) | 1 (16.7) | > 0.99 |
| Blood creatinine increase | 0 | 1 (12.5) | 0 | 1 (4.2) | 0 | > 0.99 |
| Hypophosphatemia | 0 | 0 | 2 (25.0) | 2 (8.3) | 0 | > 0.99 |
| Hyperglyceridemia | 1 (12.5) | 0 | 0 | 1 (4.2) | 0 | > 0.99 |
| Peripheral swelling | 0 | 1 (12.5) | 0 | 1 (4.2) | 0 | > 0.99 |
| Supraventricular arrhythmia | 0 | 1 (12.5) | 0 | 1 (4.2) | 0 | > 0.99 |
| Supraventricular extrasystolic contraction | 0 | 0 | 0 | 0 | 1 (16.7) | 0.2 |
Abbreviations: ADR, adverse drug reaction; N, number ADRs; n, number of subjects with adverse reactions in each dose cohort, %, incidence of subjects reporting ADRs
Antiviral effectiveness
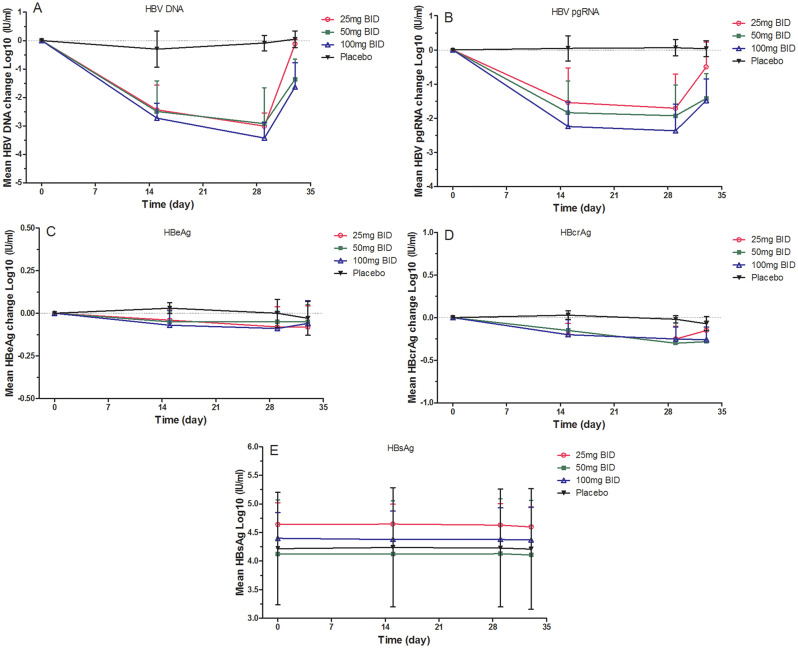
Over the treatment period, HBV DNA levels exhibited a consistent decrease, reaching their lowest point on day 29. Notably, the reduction in HBV DNA levels after 28 days of treatment varied across the dosage groups of GST-HG141, namely − 3.01 (0.47), − 2.92 (1.26), and − 3.43 (055) log10 IU/mL in the 25-, 50-, and 100-mg groups, respectively (P < 0.05, Fig. 2). Although the decrease in HBV DNA levels was not significantly different between the 25- and 50-mg cohorts, a slightly greater reduction were observed in the 100-mg cohort. Rebound effects were observed after day 33, although the HBV DNA levels remained below the baseline and the speed of the rebound decreased with increasing treatment dosage. No significant changes were noted in the placebo group.
Fig. 2.
The mean change of therapeutic efficacy variables at different time points following GST-HG141 treatment in different cohorts (25–100 mg BID) and placebo treatment. A–D present the means changes in mean HBV DNA (A), HBV pgRNA (B), HBeAg (C), HBcrAg levels (D) and HBsAg (E). Abbreviations: HBV, hepatitis B virus; pgRNA, pregenomic RNA; HBeAg, hepatitis B e antigen; HBcrAg, hepatitis B core-related antigen
Following the 28-day treatment period, a dose-dependent decrease in HBV pgRNA levels was observed across the GST-HG141 cohorts, with the most significant reduction compared to baseline observed on day 29. Specifically, the reductions were − 2.37 (0.78) log10 IU/mL for 100-mg cohort, − 1.93 (0.90) log10 IU/mL for 50-mg cohort, and − 1.71 (1.00) log10 IU/mL for 25-mg cohort. Although rebound effects were noted after day 33, pgRNA levels remained lower than baseline.
Decreases in HBcrAg levels from baseline were evident in the GST-HG141 cohorts, with rebound effects observed up to day 33, although its levels remained below baseline. Similar to HBV DNA and pgRNA, no significant changes were observed in the placebo group. Following 28 days of treatment, the mean declines in HBcrAg levels were recorded as − 0.25 log10 U/mL in the 25-mg cohort, − 0.30 log10 U/mL in the 50-mg cohort, and − 0.25 log10 U/mL in the 100-mg cohort on day 29. Notably, the extent of the decline did not vary significantly across the dosage groups. Additionally, the mean declines in HBeAg levels were − 0.08, − 0.05, and − 0.09 log10 U/mL in the 25-, 50-, and 100-mg groups, respectively, on day 29. No notable changes were observed in HBsAg levels after the 28-day treatment period, and neither HBeAg nor HBsAg underwent seroconversion. Serum ALT and AST levels exhibited minor decreases, and the mean changes were not significantly different across the treatment groups.
Pharmacokinetic analysis
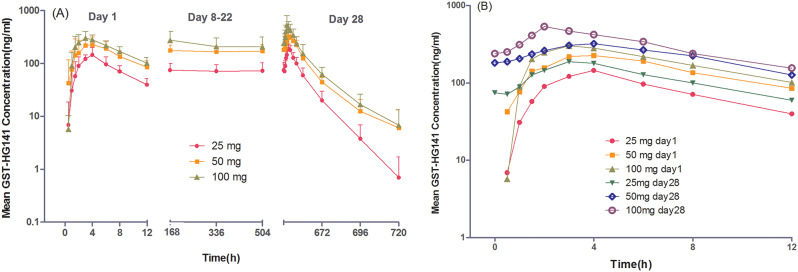
The concentration–time profiles of GST-HG141 in the GST-HG141 cohorts are depicted in Fig. 3, with the corresponding pharmacokinetic parameters outlined in Table 3. Following BID administration of GST-HG141 at doses of 25–100 mg for 28 consecutive days, the plasma concentrations of GST-HG141 were increased post-drug administration, peaking at approximately 3–3.5 h on day 1 and 2–3.5 h on day 28. Notably, t1/2 of GST-HG141 was prolonged with multiple doses. The plasma concentrations of GST-HG141 appeared to stabilize after day 8. On day 28, the average plasma trough concentrations for patients administered 25, 50, or 100 mg of GST-HG141 were 4.4-, 11.1-, and 14.6-fold higher, respectively, than its protein binding-adjusted HBV DNA EC50 (17 ng/mL).
Fig. 3.
The plasma concentration–time profiles for doses of 25–100 mg following the first (day 1) and last day of treatment (day 28). A, Mean log (± SD) GST-HG141 plasma concentration–time profiles. B, Mean log GST-HG141 plasma concentration–time profiles at 0–12 h
Table 3.
Comparison of pharmacokinetic parameters of GST-HG141 between the First and last dose in each treatment cohort
| Parameter | 25 mg BID | 50 mg BID | 100 mg BID | |||
|---|---|---|---|---|---|---|
| D1 | D28 | D1 | D28 | D1 | D28 | |
| Tmax ,(h) | 3.5(1.5,6.0) | 3.0(2.0, 4.0) | 3.0(1.50, 6.0) | 3.5(0.52, 8.0) | 3.5(1.5,4.0) | 2.0(0.52, 2.0) |
| AUC0 − t, (h*ng/mL) | 935(153) | 2209(711) | 1760(517) | 4738(668) | 2198(525) | 6295(1939) |
| AUC0−∞, (h*ng/mL) |
1158 (137) |
2232 (716) |
2010 (NE) |
4976 (879) |
2140 (296) |
6473 (1928) |
| AUC0 − 12, (h*ng/mL) | / | 1402 (328) | / | 2806 (473) | / | 3765 (1259) |
| Cmax, (ng/mL) | 162(50) | 199(37) | 279(95) | 357(57) | 327(97) | 546(254) |
| Ctrough (ng/mL) | / | 75 (31) | / | 182 (35) | / | 240 (88) |
| t½ ,(h) | 4.19(0.40) | 9.07(2.59) | 5.23(NE) | 15.19(11.83) | 4.20(0.37) | 13.46(5.21) |
| CL/F, (mL/h) |
21,814 (3267) |
18,712 (4406) |
20,614 (5454) |
18,275 (3034) |
33,987 (9632) |
28,662 (7580) |
| Vz/F, (mL) |
153,571 (29866) |
236,500 (51835) |
168,286 (45103) |
404,750 (314204) |
281,500 (92333) |
591,375 (347094) |
| DF, (%) | / | 123(28) | / | 100(21) | / | 123(24) |
| RA, Cmax | / | 1.31(0.43) | / | 1.39(0.41) | / | 1.67(0.43) |
| RA, AUC0 − 12 h | / | 1.51(0.36) | / | 1.69(0.44) | / | 1.74(0.46) |
Data are shown as geometric mean (SD), except Tmax which are as median (Min–Max),
Abbreviations: Tmax, time to maximum observed plasma concentration; AUC0–t, area under the concentration-time curve from time of dosing to the last time point with measurable plasma concentration prior to next dose; AUC0–∞, AUC from time of dosing extrapolated to infinity; Cmax, maximum observed plasma concentration; Ctrough, trough concentration; t1/2, terminal elimination half-life of the drug in plasma; CL/F, clearance; Vz/F, volume. Df, degree of fluctuation; RA, accumulation rate
The geometric mean ratios and lower limits of the 90% confidence interval of AUC0 − 12 h and Cmax on day 28 compared with day 1 were all greater than 1 across all dose cohorts, indicating mild accumulation of GST-HG141 in subjects after 28 days of BID dosing. The accumulation rates were consistent among the cohorts. Moreover, GST-HG141 exposure (Cmax and AUC) exhibited a dose- and time-dependent increase, displaying a linear pharmacokinetic trend. Notably, the slopes of AUC and Cmax power ranged from 0.46 to 0.61, all of which were less than 1, suggesting no significant proportional relationship between pharmacokinetic parameters and the GST-HG141 dose within the 25–100-mg range.
Discussion
In this phase 1b study, which employed a randomized, double-blind, placebo-controlled, dose-escalation design, we assessed the pharmacokinetic profiles, tolerability, and efficacy of GST-HG141, a novel capsid assembly modulator, in patients with chronic HBV infection over a 28-day treatment period. Our findings revealed that GST-HG141 exhibited safety and tolerability, with significant reductions observed in the serum levels of HBV DNA and HBV pgRNA following 28 days of treatment.
Safety considerations are paramount in the management of chronic diseases such as hepatitis B. In our study, we observed an overall adverse reactions incidence rate of 50% among randomized dosed subjects, with no significant correlation with drug dosage noted. Importantly, all adverse reactions recorded in this study were of grade 1–2 in severity. In patients receiving GST-HG141, the incidence of elevated ALT and AST levels was equal at 8.3%. This incidence of elevated aminotransferases was lower than that reported for similar capsid assembly modulators in the literature such as ZM-H1505R (canocapavir) and ABI-H0731 [14, 17]. Because of the relatively short treatment duration of 4 weeks in our study, longer-term treatment and observation are warranted. Nevertheless, our findings suggest that GST-HG141 is safe and well tolerated in patients with CHB.
In previous studies [13, 17], patients with elevated baseline ALT levels were found to be more prone to experiencing ALT flares in the course of antiviral treatment. This phenomenon typically coincided with the reduction of both viral antigen and HBV DNA levels, suggesting that ALT flares represent a therapeutic response. We observed a notable example of this phenomenon in one patient (randomization No. 2010) receiving 100 mg of GST-HG141. This patient had a baseline ALT level of 136 U/L, which gradually increased to 196 U/L on day 29 and peaked at 356 U/L on day 33, coinciding with reductions in HBV marker levels (− 2.8 log10 IU/mL for HBV DNA, − 1.8 log10 U/mL for HBV pgRNA, − 0.3 log10 U/mL for HBcrAg, and − 0.1 log10 IU/mL for HBsAg).
Similarly, in another patient (randomization No. 1002, 25 mg), the ALT level increased from 62 U/L at baseline to a peak of 171 U/L on day 33, whereas in a patient (randomization No. 3002) taking 100 mg of GST-HG141, the ALT level increased from 101.2 U/L at baseline to a peak of 189.5 U/L on day 33. These increases in ALT levels were accompanied by significant declines in HBV DNA marker levels (− 3.8 log10 IU/mL [patient 3002] and − 2.6 log10 IU/mL [patient 1002]). These patients did not display any irregularities in serum albumin and bilirubin levels or in the international normalized ratio. The increase in ALT levels in some patients during treatment might signify the elimination of HBV-infected liver cells by the activated immune system [18, 19].
Despite some biochemical distinct features, GST-HG141 belongs to the CAM-E class of drugs, which have displayed promise in the treatment of CHB. However, other CpAMs have been associated with adverse effects such as aminotransferase elevation or ALT flares. For instance, the CAM-A GLS4 was reported to induce ALT flares in some patients after 28 days of treatment, leading to study withdrawal and necessitating silybin and glutathione therapy [13]. Similarly, the CAM-E ABI-H0731 caused grade 3 treatment-associated liver enzyme elevation in some patients after 28 days of continuous treatment [14]. Notably, although a phase 1a study of GST-HG141 reported a case of grade 4 creatine kinase elevation in a healthy individual [16], no such adverse reaction was observed in this 28-day continuous administration study. Thus, despite the relatively short treatment duration in our study (4 weeks), GST-HG141 did not appear to increase the risk of adverse effects in patients.
It is noteworthy that our results demonstrated robust declines in the serum levels of HBV DNA and HBV pgRNA in patients across the 25–100 mg BID treatment cohorts. Specifically, the mean decreases from baseline in HBV DNA levels after 28 days of treatment were − 3.01, − 2.92, and − 3.43 log10 IU/mL at doses of 25, 50, and 100 mg BID, respectively. Although no clear dose–efficacy correlation was observed, substantial reductions in HBV DNA were evident, suggesting that even the 25 mg dose achieved favorable therapeutic effects. When the baseline level of HBV DNA among different treatment groups was adjusted, a more dose-related effect on HBV DNA reduction was observed − 2.98, − 3.10, and − 3.44 log10 IU/mL at doses of 25, 50, and 100 mg BID, respectively).
Compared to the antiviral efficacy of ABI-H0731, another CAM-E with structural similarity to GST-HG141, GST-HG141 appeared favorable regarding HBV DNA level reductions. ABI-H0731 achieved mean HBV DNA reductions of approximately 2.1 log10 IU/mL at 100 mg and only 2.8 log10 IU/mL at a dose as high as 300 mg in a similarly designed study [14]. Moreover, the tolerability of GST-HG141 appeared also superior to that of ABI-H0731.
The most significant reductions in HBV DNA levels were noted from baseline to day 15, with a slower rate of decline thereafter. This trend aligns with the typical biphasic decline observed with nucleos(t)ide analog inhibitors [20]. Earlier measurements, particularly before day 15, could offer more precise insights into the antiviral kinetics of GST-HG141. Furthermore, our study confirmed the effectiveness of GST-HG141 in inhibiting the assembly of HBV nucleocapsid, reducing the levels of cccDNA, inhibiting the production of HBV pgRNA-containing particles, and preventing viral replication observed in preclinical studies [21, 22, 23]. As an HBV core inhibitor, GST-HG141 operates by disturbing core protein function and aggregation, but it does not directly influence HBV gene expression. Consequently, during the relatively brief 4-week dosing period, no notable clinical alterations in HBeAg or HBsAg concentrations (≥ 0.5 log10 IU/mL) were detected. Nevertheless, certain patients displayed noteworthy decreases in HBcrAg levels, suggesting an interruption in the expression of specific proteins translated from pgRNA.
This study presents initial clinical evidence demonstrating significant inhibition of viral production in patients with CHB through GST-HG141 monotherapy. Future extensive clinical investigations will delve deeper into the mechanism and antiviral efficacy of GST-HG141 and explore potential combinations with other antiviral agents. Moreover, the administration of GST-HG141 post-prandially might enhance its bioavailability, as indicated by pharmacokinetic profiles in phase 1a study illustrating rapid absorption and minimal drug accumulation [16].
Nevertheless, this study had several limitations, including a short treatment and follow-up duration, a small sample size, and a lack of analysis regarding immunoinflammatory factors. These constraints will be addressed in forthcoming multicenter phase 2 studies encompassing larger patient cohorts with extended treatment and follow-up periods.
Conclusions
Among individuals with CHB, GST-HG141 exhibited a reassuring safety profile even at doses as high as 100 mg, with no discernible pattern of serious safety concerns. The majority of adverse reactions were mild to moderate (grade 1 or 2), and there was no apparent dose-related trends in either the frequency or severity of adverse events. Importantly, no instances of serious adverse reactions linked to elevated aminotransferase levels were reported. Pharmacokinetic analysis revealed a consistent linear trend and low-to-mild accumulation rates (1.31–1.69) across the 25-100 mg doses of GST-HG141. After a 28-day treatment course, GST-HG141 substantially reduced the serum levels of both HBV DNA and HBV pgRNA among patients with CHB. These results underscore the merit of advancing GST-HG141 into additional clinical studies as a promising therapeutic option for managing CHB.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
M.W. and J.M. conducted a comprehensive review of the topic and contributed to manuscript writing. H.Z., W.W., and J.M. were involved in the analysis of pharmacokinetic data. M.W. and G.Z. drafted sections of the manuscript. J.M., Y.T., and W.Y. assisted with data analysis and interpretation and the preparation of figures and tables. Y.D. and G.Z. contributed to manuscript writing and provided critical revision. All authors have approved the final version of the manuscript and agreed to be accountable for all aspects of the work.
Funding
This research received financial support from the Capital Construction Funds within the provincial budget in 2020 (Project No. 2020C038-1) aimed at enhancing innovation capacity construction.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The clinical study protocol was approved by the Ethics Committee of the Jilin University First Affiliated Hospital-Clinical Research Institute (Approval Number 21Y088-001). Prior to participation, all patients provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yanhua Ding and Junqi Niu contributed equally to this work.
Contributor Information
Yanhua Ding, Email: dingyanh@jlu.edu.cn.
Junqi Niu, Email: junqiniu@jlu.edu.cn.
References
- 1.Seto WK, Lo YR, Pawlotsky JM, Yuen MF. Chronic hepatitis B virus infection. Chronic hepatitis B virus infection. Lancet. 2018;392:2313–24. Review. [DOI] [PubMed] [Google Scholar]
- 2.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–Review55. [DOI] [PubMed] [Google Scholar]
- 3.Lee HW, Lee JS, Ahn SH. Hepatitis B Virus Cure: targets and future therapies. Int J Mol Sci. 2020;22:213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsukuda S, Watashi K. Hepatitis B virus biology and life cycle. Antiviral Res. 2020. PMID: 32866519 Review. [DOI] [PubMed]
- 5.Wong G, Gane E, Lok A. How to achieve functional cure of HBV: stopping NUCs, adding interferon or new drug development. J Hepatol. 2022;76(6):1249–62. 10.1016/j.jhep.2021.11.024. [DOI] [PubMed] [Google Scholar]
- 6.Viganò M, Grossi G, Loglio A, Lampertico P. Treatment of hepatitis B: is there still a role for interferon? Liver Int. 2018;38(Suppl 1):79–83. 10.1111/liv.13635. [DOI] [PubMed] [Google Scholar]
- 7.Viswanathan U, Mani N, Hu Z, Ban H, Du Y, Hu J, Chang J, Guo JT. Targeting the multifunctional HBV core protein as a potential cure for chronic hepatitis B.Antiviral res. 2020;182:104917. 10.1016/j.antiviral.2020.104917. Epub 2020 Aug 17. [DOI] [PMC free article] [PubMed]
- 8.Diab A, Foca A, Zoulim F, Durantel D, Andrisani O. The diverse functions of the hepatitis B core/capsid protein (HBc) in the viral life cycle: implications for the development of HBc-targeting antivirals. Antiviral Res. 2018;149:211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amblard F, Boucle S, Bassit L, Cox B, Sari O, Tao S, Chen Z, Ozturk T, Verma K, Russell O, Rat V, de Rocquigny H, Fiquet O, Boussand M, Di Santo J, Strick-Marchand H, Schinazi RF. Novel Hepatitis B Virus Capsid Assembly Modulator induces potent antiviral responses in Vitro and in Humanized mice. Antimicrob Agents Chemother. 2020;64(2):e01701–19. 10.1128/AAC.01701-19. Print 2020 Jan 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zoulim F, Zlotnick A, Buchholz S, Donaldson E, Fry J, Gaggar A, et al. Nomenclature of HBV core protein-targeting antivirals. Nat Rev Gastroenterol Hepatol. 2022;19(12):748–50. 10.1038/s41575-022-00700-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Z, Hu T, Zhou X, Wildum S, Garcia-Alcalde F, Xu Z, et al. Heteroaryldihydropyrimidine (HAP) and Sulfamoylbenzamide (SBA) inhibit Hepatitis B Virus replication by diferent molecular mechanisms. Sci Rep. 2017;7:42374. 10.1038/srep42374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rat V, Seigneuret F, Burlaud-Gaillard J, Lemoine R, Hourioux C, Zoulim F, et al. BAY 41–4109-mediated aggregation of assembled and misassembled HBV capsids in cells revealed by electron microscopy. Antiviral Res. 2019;169:104557. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Wang F, Zhu X, Chen Y, Chen H, Li X, Wu M, Li C, Liu J, Zhang Y, Ding Y, Niu J. Antiviral activity and pharmacokinetics of the Hepatitis B Virus (HBV) Capsid Assembly Modulator GLS4 in patients with chronic HBV infection. Clin Infect Dis. 2021;73(2):175–82. 10.1093/cid/ciaa961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuen MF, Agarwal K, Gane EJ, Schwabe C, Ahn SH, Kim DJ, Lim YS, Cheng W, Sievert W, Visvanathan K, Ruby E, Liaw S, Yan R, Huang Q, Colonno R, Lopatin U. Safety, pharmacokinetics, and antiviral effects of ABI-H0731, a hepatitis B virus core inhibitor: a randomised, placebo-controlled phase 1 trial. Lancet Gastroenterol Hepatol. 2020;5(2):152–66. 10.1016/S2468-1253(19)30346-2. Epub 2019 Nov 9. [DOI] [PubMed] [Google Scholar]
- 15.Fanning GC, Zoulim F, Hou J, Bertoletti A. Therapeutic strategies for hepatitis B virus infection: towards a cure. Nat Rev Drug Discov. 2019;18(11):827–44. 10.1038/s41573-019-0037-0. [DOI] [PubMed] [Google Scholar]
- 16.Li C, Wu M, Zhang H, Mai J, Yang L, Ding Y, Niu J, Mao J, Wu W, Zhang D, Tang Y, Yan W. Safety, Tolerability, and pharmacokinetics of the Novel Hepatitis B Virus Capsid Assembly Modulator GST-HG141 in healthy Chinese subjects: a first-in-human single- and multiple-dose escalation Trial. Antimicrob Agents Chemother. 2021; Sep 17; 65(10). [DOI] [PMC free article] [PubMed]
- 17.Jia H, Mai J, Wu M, Chen H, Li X, Li C, Liu J, Liu C, Hu Y, Zhu X, Jiang X, Hua B, Xia T, Liu G, Deng A, Liang B, Guo R, Lu H, Wang Z, Chen H, Zhang Z, Zhang H, Niu J. DingY. Safety, tolerability, pharmacokinetics, and antiviral activity of the novel core protein allosteric modulator ZM-H1505R (Canocapavir) in chronic hepatitis B patients: a randomized multiple-dose escalation trial. BMC Med. 2023;21(1):98. 10.1186/s12916-023-02814-w. PMID: 36927420 Free PMC article. Clinical Trial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang Y, Ma Z, Xin G, Yan H, Li W, Xu H, et al. Th1 and Th2 immune response in chronic hepatitis B patients during a long-term treatment with adefovir dipivoxil. Mediators Infamm. 2010;2010:143026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deres K, Schröder CH, Paessens A, Goldmann S, Hacker HJ, Weber O, et al. Inhibition of hepatitis B virus replication by drug-induced depletion of nucleocapsids. Science. 2003;299(5608):893–6. 10.1126/science.1077215. [DOI] [PubMed] [Google Scholar]
- 20.Ribeiro RM, Germanidis G, Powers KA, et al. Hepatitis B virus kinetics under antiviral therapy sheds light on differences in hepatitis B e antigen positive and negative infections. J Infect Dis. 2010;202:1309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viswanathan U, Mani N, Hu Z, Ban H, Du Y, Hu J, Chang J, Guo JT. Targeting the multifunctional HBV core protein as a potential cure for chronic hepatitis B. Antiviral Res. 2020;182:104917. 10.1016/j.antiviral.2020.104917. Epub 2020 Aug 17. PMID: 32818519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zlotnick A, Venkatakrishnan B, Tan Z, Lewellyn E, Turner W, Francis S. Core protein: a pleiotropic keystone in the HBV lifecycle.Antiviral res. 2015; 121: 82–93. 10.1016/j.antiviral.2015.06.020. Epub 2015 Jun 27. PMID: 26129969 Free PMC article. Review. [DOI] [PMC free article] [PubMed]
- 23.Tseng et al, Gastroenterology. Low Hepatitis B Core-Related Antigen Levels Correlate Higher Spontaneous Seroclearance of Hepatitis B Surface Antigen in Chronic Hepatitis B Patients With High Hepatitis B Surface Antigen Levels. 2023 Apr;164(4):669–679.e6 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.