Abstract
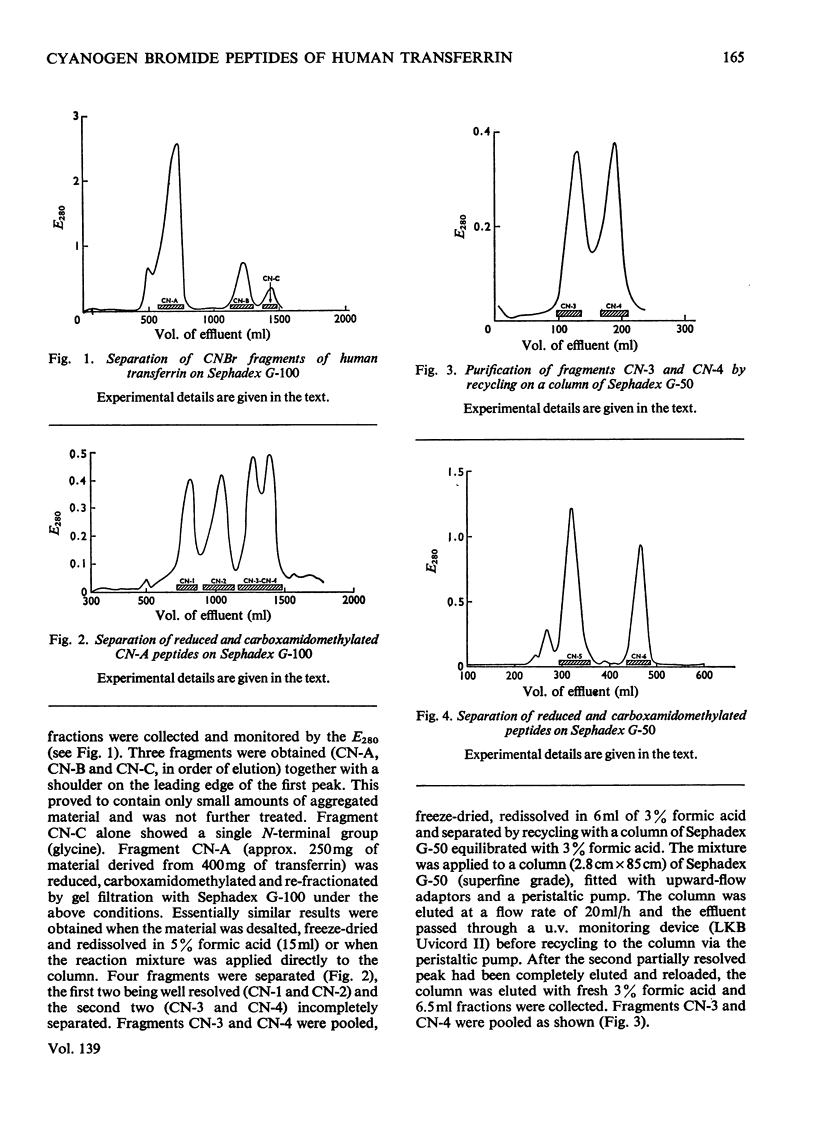
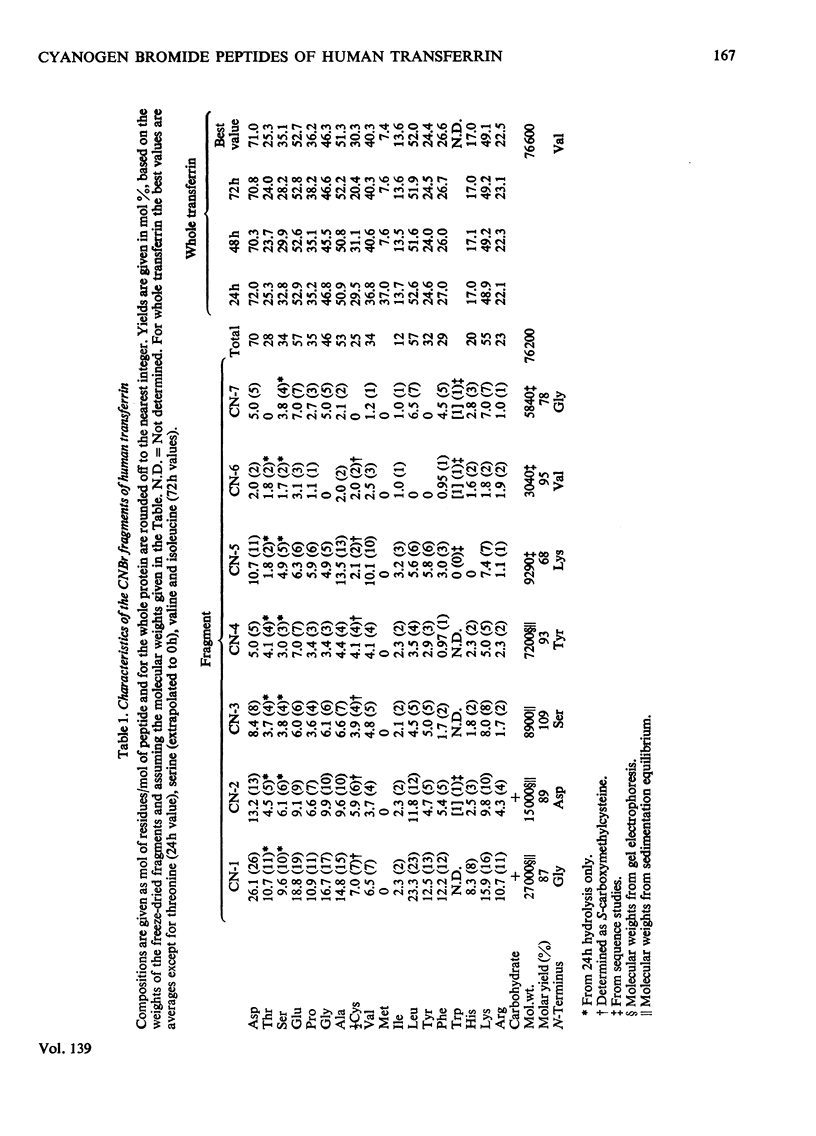
1. Procedures are described for the isolation of seven distinct cyanogen bromide fragments in high yield from human serum transferrin. 2. Cyanogen bromide-cleaved transferrin is separated into three fragments (CN-A, CN-B and CN-C) by gel filtration with Sephadex G-100. 3. Four peptides are obtained from CN-A (the largest fragment) after reduction and carboxamidomethylation, by gel filtration in acidic solvents. Two peptides are similarly obtained from fragment CN-B, whereas fragment CN-C is a single cystine-free peptide. 4. The molecular weights of the seven peptides, as determined by polyacrylamide-gel electrophoresis in the presence of sodium dodecyl sulphate, by sedimentation-equilibrium ultracentrifugation and by sequence studies, range from 3100 to 27000. Together they account for a molecular weight of 76200 for transferrin. 5. The two largest fragments contain the carbohydrate attachment sites of the protein, and the smallest fragment is derived from the N-terminus. 6. The amino acid compositions and N-terminal groups of the fragments are reported and the results compared with those of previous investigations.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bezkorovainy A., Grohlich D. Cyanogen bromid fragments of human serum transferrin. Biochim Biophys Acta. 1973 Jun 15;310(2):365–375. doi: 10.1016/0005-2795(73)90118-9. [DOI] [PubMed] [Google Scholar]
- Bezkorovainy A., Grohlich D., Gerbeck C. M. Some physical-chemical properties of reduced-alkylated and sulphitolysed human serum transferrins and hen's-egg conalbumin. Biochem J. 1968 Dec;110(4):765–770. doi: 10.1042/bj1100765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew K. The complete amino-acid sequence of guinea-pig -lactalbumin. Eur J Biochem. 1972 May 23;27(2):341–353. doi: 10.1111/j.1432-1033.1972.tb01844.x. [DOI] [PubMed] [Google Scholar]
- ERIKSSON S., SJOQUIST J. Quantitative determination of N-terminal amino acids in some serum proteins. Biochim Biophys Acta. 1960 Dec 4;45:290–296. doi: 10.1016/0006-3002(60)91453-0. [DOI] [PubMed] [Google Scholar]
- Findlay J. B., Brew K. The complete amino-acid sequence of human -lactalbumin. Eur J Biochem. 1972 May;27(1):65–86. doi: 10.1111/j.1432-1033.1972.tb01812.x. [DOI] [PubMed] [Google Scholar]
- Greene F. C., Feeney R. E. Physical evidence for transferrins as single polypeptide chains. Biochemistry. 1968 Apr;7(4):1366–1371. doi: 10.1021/bi00844a018. [DOI] [PubMed] [Google Scholar]
- JAMIESON G. A. STUDIES ON GLYCOPROTEINS. II. ISOLATION OF THE CARBOHYDRATE CHAINS OF HUMAN TRANSFERRIN. J Biol Chem. 1965 Jul;240:2914–2920. [PubMed] [Google Scholar]
- Jeppsson J. O. Structural studies of fragments resulting from cyanogen bromide degradation of human transferrin. Biochim Biophys Acta. 1967 Aug 15;140(3):477–486. doi: 10.1016/0005-2795(67)90520-x. [DOI] [PubMed] [Google Scholar]
- LaBar F. E. A procedure for molecular weight measurements: application to Chymotrypsinogen A. Proc Natl Acad Sci U S A. 1965 Jul;54(1):31–36. doi: 10.1073/pnas.54.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K. G., Fish W. W., Cox A. C., Tanford C. Single-chain nature of human serum transferrin. Biochemistry. 1970 Mar 17;9(6):1348–1354. doi: 10.1021/bi00808a008. [DOI] [PubMed] [Google Scholar]
- Palmour R. M., Sutton H. E. Vertebrae transferrins. Molecular weights, chemical compositions, and iron-binding studies. Biochemistry. 1971 Oct 26;10(22):4026–4032. doi: 10.1021/bi00798a003. [DOI] [PubMed] [Google Scholar]
- ULMER D. D., VALLEE B. L. OPTICALLY ACTIVE METALLOPROTEIN CHROMOPHORES. III. HEME AND NONHEME IRON PROTEINS. Biochemistry. 1963 Nov-Dec;2:1335–1340. doi: 10.1021/bi00906a027. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Woodworth R. C., Morallee K. G., Williams R. J. Perturbations of the proton magnetic resonance spectra of conalbumin and siderophilin as a result of binding Ga3+ or Fe3+. Biochemistry. 1970 Feb 17;9(4):839–842. doi: 10.1021/bi00806a017. [DOI] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]


