Abstract
Purpose
The C4 dermatome anesthesia holds significance for arthroscopic shoulder surgery. However, the reliability of achieving C4 dermatome anesthesia with the current low-dose ultrasound-guided interscalene block (ISB) and supraclavicular block (SCB) remains uncertain. This prospective, single-center study examined the effects of ultrasound-guided interscalene block (ISB) and supraclavicular block (SCB) on the C4 sensory dermatome in patients undergoing shoulder, hand, or wrist surgery.
Patients and Methods
Patients undergoing outpatient shoulder, hand, or wrist surgeries received ultrasound-guided ISB (for shoulder surgeries) with 12–15 mL of 0.5% bupivacaine or ultrasound-guided SCB (for hand and wrist surgeries) with 20–24 mL of 0.5% bupivacaine. The primary objective of the study was to investigate the sensory effect on the C4 dermatome after ISB and SCB. The secondary outcomes included the sensory effect on the C5 dermatome after performing ISB and SCB, pain scores upon arrival at the post-anesthesia care unit (PACU), and the incidence of adverse effects.
Results
Sixty-one patients were recruited: 30 for ISB and 31 for SCB. C4 dermatome coverage was achieved in 53% and 29% of patients in the ISB and SCB groups, respectively (p=0.0268). Additionally, C5 dermatome coverage was achieved in 97% and 68% of patients in the ISB and SCB groups, respectively (p=0.0034). A moderate correlation coefficient (r=0.47) was observed between C4 sensory scores and postoperative pain scores.
Conclusion
Ultrasound-guided ISB successfully provided C4 coverage in 53% of cases, suggesting that performing an additional C4 block alongside ISB could be advantageous when aiming for complete surgical anesthesia. SCB may not be the primary choice for shoulder surgeries as it often fails to achieve satisfactory sensory blocks at the C4 and C5 levels.
Keywords: shoulder surgeries, nerve block, ultrasound-guided block, regional anesthesia
Plain language summary
This study examined how the interscalene block and supraclavicular block work for numbing the C4 area in patients undergoing shoulder, hand, or wrist surgery. We found that the interscalene block provided C4 coverage in only 53% of cases, suggesting performing an additional C4 block might help achieve complete surgical anesthesia. The supraclavicular block is not the best option for shoulder surgeries because it often fails to provide adequate anesthesia at the C4 and C5 levels.
Introduction
Shoulder surgeries, whether arthroscopic or open, are among the most painful outpatient orthopedic procedures.1 For these procedures, peripheral nerve blocks offer many advantages, including excellent pain control.2,3 Interscalene brachial plexus block is most commonly used for shoulder surgery anesthesia and analgesia, but supraclavicular block (SCB) has also been utilized successfully.4–6 For incisions about the shoulder, the C4 and C5 nerve roots provide the primary dermatomal innervation. As shown, the dermatomal distributions of these nerves encompass the front and top of the shoulder, as well as a degree of the posterior trapezius ridge and the upper, lateral arm. In addition, the C4 root, which gives rise to the supraclavicular nerves, also innervates a substantial portion of the clavicle, the sternoclavicular joint and the acromio-clavicular joint.7
When Winnie first described the interscalene approach to brachial plexus blockade, he noted that large volumes of local anesthetic solution would likely provide effective analgesia of the C4 dermatomal region.8 However, since the C4 root does not typically contribute to the brachial plexus, it is less likely to be affected by lower-volume ISB and SCB that are embraced in the current ultrasound era, than are the elements of the brachial plexus9 While this effect has been noted, a quantitative assessment of the effect of brachial plexus nerve blocks on the supraclavicular nerves during the ultrasound era has not appeared in the literature.
Given the substantial contribution that C4 makes to innervation of the shoulder region, anesthesia and analgesia for surgery of this joint will likely be more effective if this nerve is affected by the peripheral nerve block. We conducted a prospective, observational study to evaluate the effect of both ISB and SCB on the C4 and C5 cutaneous distributions in patients undergoing ambulatory shoulder surgery (ISB) or hand surgery (SCB). We also investigated the relationship between the anesthesia of the C4 distribution and reported initial pain scores in PACU for patients receiving shoulder surgery. Based on prior, informal observations, we hypothesized that 80% of patients after ISB and 40% of patients after SCB will experience effective anesthesia of the C4 dermatomal distribution as manifest by specific sensory testing.
Materials and Methods
This prospective, observational, single-center study, approved by the Institutional Review Board of University of Pittsburgh (IRB, STUDY22040053) and conducted in accordance with the Declaration of Helsinki, examined the effects of ultrasound-guided ISB and SCB on the C4 dermatome in patients undergoing shoulder, hand, or wrist surgery. All patients undergoing shoulder surgery received ISB, while all of those undergoing hand or wrist surgery received SCB. All patients provided written, informed consent for participation. Nerve blocks were provided by staff anesthesiologists or supervised residents from the University of Pittsburgh Department of Anesthesiology.
Inclusion criteria were age ≥18 year, undergoing surgery on the shoulder with ISB and sedation, or undergoing elbow, wrist, or hand surgery with SCB and sedation. Patients were also required to have normal baseline motor and sensory function in their upper extremities. Exclusion criteria encompassed cognitive impairment that impairs autonomy and ability to provide informed consent, medical conditions for which an ISB or SCB is contraindicated (severe pulmonary disease with or without baseline use of supplemental oxygen, diaphragmatic dysfunction, respiratory muscle weakness, pre-existing nerve damage, and recent use of anticoagulation); and limb or neck abnormalities such as amputation, radiation therapy, or infection at the proposed implant site are exclusion criteria.
Block Procedure
Participating patients received a standard “low-volume” ISB for shoulder surgery or a standard SCB for elbow/wrist/hand surgery. We defined ‘low volume’ as use of 12–14 mL of injectate for ISB, as distinct from the pre-ultrasound period when volumes between 30 and 40 mL were common for ISB. The dose range was chosen rather than a fixed volume of the drug for both the block and local application to account for potential variations in patient anatomy, procedural requirements, and desired analgesic effects. Block success was ascertained by checking the motor and distal sensory function. Patients experiencing block failure were excluded from further data collection.
For all blocks, a Sonosite X-Porte ultrasound (Sonosite, Bothell, Washington) with a high frequency (15–6 MHz) linear probe was utilized. The patient was placed in a semi-recumbent position at an angle of 30–45 degrees, with the head turned 45 degrees to the non-operative side. Routine monitors and oxygen were applied. Sedation with Midazolam (1–2 mg) and fentanyl (25–50 mcg) were administered for managing anxiety in accordance with the perceived clinical need. The needle insertion site was sterilised using a 70% isopropyl alcohol 2% chlorhexidine solution.
Interscalene Block
Before performing ISB, the transducer was positioned above the supraclavicular region to offer a short-axis view of the brachial plexus and subclavian artery. The plexus was traced proximally, and the C5, C6, and C7 roots were identified, as described in previous studies.10 The blocks were performed in plane at a level at which the C5 and C6 nerve roots were visible, and the needle was directed in plane, avoiding contact with any visible nerves in the middle scalene muscle, including the dorsal scapular nerve and the long thoracic nerve. A 22-gauge 8-mm insulated block needle (Sonoplex; Pajunk GmbH, Geisingen, Germany) was placed in-plane with the ultrasonic beam on the lateral side of the transducer after 1–3 mL of 2% lidocaine had been infused. The needle was carefully advanced through the middle scalene muscle towards the lateral border of the brachial plexus sheath under ultrasound guidance. The needle tip was advanced to lie between C5 and C6. Following negative aspiration, 12–14 mL of 0.5% bupivacaine was injected incrementally to facilitate its distribution to the brachial plexus roots.
Supraclavicular Block
The transducer was positioned within the supraclavicular fossa provide a short-axis view of the brachial plexus and subclavian artery directly above the first rib before performing SCB. Using the in-plane technique, a 22-gauge 8-mm block needle (SonoPlex; Pajunk GmbH, Geisingen, Germany) was advanced under direct visualization after infiltration of 1–3 mL of 2% lidocaine. The brachial plexus was visualized as a round- or oval-shaped compact hyperechoic cluster of nerves present lateral and superficial to the pulsating subclavian artery and superior to the first rib. Following negative aspiration, 20–24 mL of 0.5% bupivacaine was injected incrementally to facilitate its distribution to the brachial plexus trunks. The volume of the anesthetic was injected in small increments in three areas: between the middle and inferior trunk, in the “corner pocket” where the inferior trunk lies upon the first rib, and between the frequently distinct superior trunk and the middle trunk.11,12
Block Assessment
Nerve block effect on the C4 and C5 dermatomes was evaluated using a “sensory score” that reflects overall sensation, with 0–2 points awarded for cold sensation (as perceived by an ice bag) and 0–2 points awarded for sharp sensation (utilizing a plastic needle). Sensory evaluation conducted in the ‘cape’ area for the C4 nerve root, and over the deltoid muscle for the C5 nerve root. A score of 0 or 1 indicated a successful sensory block, while a score of 2 or 3 indicated a partial block. A baseline sensory score was obtained to establish that the patient had normal perception prior to the block. The evaluation was then repeated 20 minutes after the block administration. We also obtained the initial pain score at the time of admission to the post-anesthesia care unit (PACU) to assess the correlation between the PACU pain score and the C4 sensory score.
Primary Outcome Measure
The effect of ultrasound-guided ISB and SCB on the C4 dermatome, as assessed by the perception of cold sensation and sharp sensation over the top of the shoulder in the “cape” area 20 minutes after performing the block, measured as a sensory score range from 0 to 4, was the primary outcome. We hypothesized that 80% of patients after ISB and 40% of patients after SCB will experience a sensory score <2 at the C4 dermatome.
Secondary Outcome Measures
The secondary outcomes included the sensory effect at the C5 dermatome 20 minutes after performing both blocks, the pain score upon PACU admission, and the incidence of adverse outcomes of the block, including hypoxemia, subjective dyspnea, or persistent numbness.
Sample Size Calculation
Prior to conducting this study, we informally observed numbness to light touch over the anterior/superior shoulder in approximately 80% of patients after ISB and 40% of patients after SCB, which served as the basis for our sample size calculation. We hypothesized that 80% of patients after ISB and 40% of patients after SCB will experience a sensory score <2 at the C4 dermatome. To demonstrate a significant reduction of the sensory score from 4 (normal sensation pre-block) to 0 or 1, 23 patients were required in each group to achieve an 85% power to detect a difference between the group proportions of 40%. To compensate for any potential loss of participants from failed or insufficient blocks, we intended to enroll 30 patients in each group (60 overall).
Statistical Analyses
Descriptive statistics are presented as frequencies (percentages) for categorical data or as mean (standard deviation, SD) or median (interquartile range, IQR) for normally or nonnormally distributed continuous data, respectively. Examination of normal distribution assumptions for continuous data was determined using histograms and Q-Q plots. The Chi-square or Fisher’s exact test, as appropriate, was used to evaluate the differences in categorical characteristics between the groups. The two-sample t test or Wilcoxon rank sum test was used to assess differences in continuous characteristics between the groups. Chi-square or Fisher’s exact test was also used to examine difference in proportions in experiencing a sensory score <2 at the C4 dermatome between the groups. Since an effect on the C4 dermatome is not likely to be increased as the block site is moved distally, a one-sided test was used for the primary outcome of ISB effect on C4 in comparison to SCB effect on C4. All other tests were two-sided, and the significance level was set to 0.05. One additional patient, beyond the intended number of 30, was enrolled in the SCB group, resulting in unequal numbers in the two groups. To evaluate the robustness of the findings, we performed a sensitivity analysis by excluding the additional patient from the primary and secondary analyses.
Finally, to evaluate for correlation, the Spearman correlation coefficient was computed between the C4 sensory score and the NRS pain score in the PACU for the ISB group. As an effect on the C4 dermatome is not likely increase as the block site is moved distally, a one-sided test was used for the primary outcome of ISB effect on C4 in comparison to the SCB effect on C4. All other tests were two-sided, and the significance level was set at 0.05. All statistical analyses were performed using Statistical Analysis System (SAS) software (Version 9.4, SAS Institute Inc., Cary, NC, USA).
Results
Sixty-one patients were recruited in this study between November 2022 to March 2023, with 30 patients receiving ISB and 31 patients receiving SCB. All patients completed the study. Demographic characteristics of the patients are summarized in Table 1. Other than the expected difference in volumes of local anesthetic injected, there were no significant differences in demographic characteristics between groups. No failed blocks were observed.
Table 1.
Patient Characteristics
| Characteristic | ISB (n=30) | SCB (n=31) | p-value |
|---|---|---|---|
| Age, y, mean (SD) | 50 (15) | 49 (17) | 0.79 |
| Female (%) | 12 (40) | 14 (45) | 0.68 |
| BMI, mean (SD) | 31 (10) | 27 (5) | 0.19 |
| NRS baseline pain score, median (IQR) | 0 (0, 3) | 0 (0, 0) | 0.11 |
| Left laterality (%) | 19 (63) | 15 (48) | 0.24 |
| ASA PS (%) | 0.87 | ||
| Score 1 | 7 (23) | 9 (29) | |
| Score 2 | 13 (43) | 13 (42) | |
| Score 3 | 10 (33) | 9 (29) |
Abbreviations: BMI, Body Mass Index; ISB, interscalene block; SCB, supraclavicular block; SD, standard deviation; IQR, interquartile range (Q1=first quartile, Q3=third quartile); ASA PS, American Society of Anesthesiologists physical status; NRS, Numerical Rating Scale.
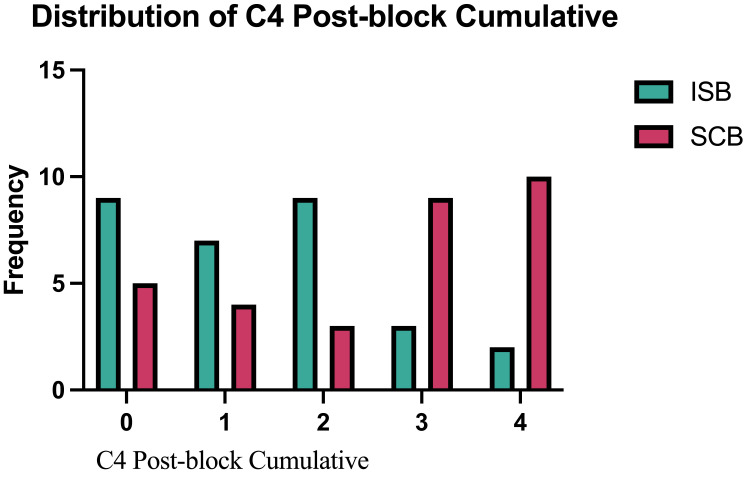
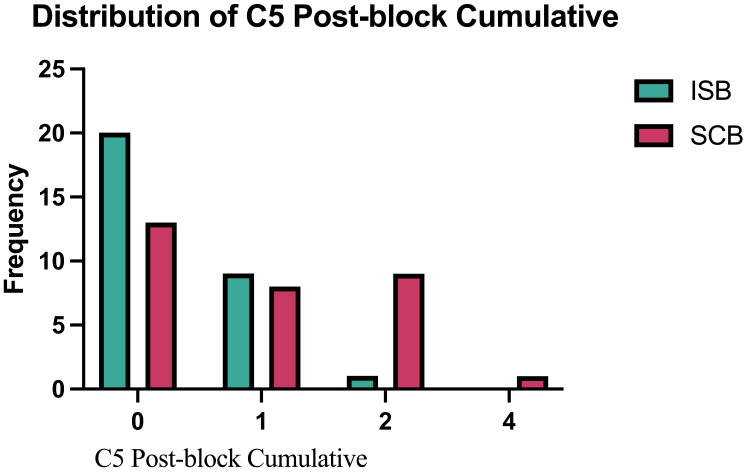
The primary outcome, C4 dermatome anesthesia, was significantly higher in the ISB group than the SCB group, 53% vs 29% (p = 0.0268) (Table 2). While this supports our hypothesis that the anesthetic effect on this region would be higher with ISB than SCB, it fell short of the 80% effect that we predicted. C5 dermatome sensation was successfully blocked in 97% of patients after ISB and 68% after SCB (p = 0.0034). Figure 1 depicts the distribution of sensory scores of the C4 dermatome after performing ISB and SCB. Figure 2 shows a comparison of the sensory scores of the C5 dermatome after performing ISB and SCB. Results from the sensitivity analyses which included the additional patient in the SCB group showed consistent results with the primary and secondary analyses.
Table 2.
Success Rates of the Sensory Blocks
| ISB (n=30) | SCB (n=31) | Difference in Proportions (95% CI) | p-value* | |
|---|---|---|---|---|
| C4 dermatome sensory block success rate | 16 (53%) | 9 (29%) | 24% (0%, 48%) | 0.0268 |
| C5 dermatome sensory block success rate | 29 (97%) | 21 (68%) | 29% (10%, 49%) | 0.0034 |
Notes: *Statistical tests: Chi-square test for C4; Fisher’s exact test for C5.
Abbreviations: ISB, interscalene block; SCB, supraclavicular block.
Figure 1.
Distribution of C4 post block cumulative.
Figure 2.
Distribution of C5 post block cumulative.
The median pain score on arrival in PACU for the ISB group was 0 (interquartile range 0, 1). The Spearman correlation coefficient between C4 sensory score after ISB and the initial PACU pain score was 0.47, indicating a moderate degree of correlation. Finally, there were no episodes of hypoxemia (SaO2 below 95%) or subjective complaints of dyspnea among the patients.
Discussion
This study examined the effects of ultrasound-guided ISB and SCB on the C4 dermatome in patients undergoing shoulder, hand, or wrist surgery. Ultrasound-guided low-volume ISB achieved C4 coverage in 53% of cases, whereas ultrasound-guided SCB achieved C4 coverage in 29% of cases. Additionally, ultrasound-guided low-volume ISB achieved C5 coverage in 97% of cases, whereas ultrasound-guided SCB achieved C5 coverage in 68% of cases. This suggests that ISB provides superior anesthetic coverage at those sites. A moderate relationship was also observed between C4 sensory score and the initial NRS pain score after surgery, suggesting that anesthesia of the C4 dermatome provides a clinically meaningful contribution to postoperative pain relief. The results of this study indicate that ISB is feasible for shoulder surgery; however, an additional C4 block should be performed if a complete surgical block is desired. As far as we are aware, this is the first study to investigate the effects of low-volume ISB and SCB on the C4 dermatome. The results suggest that an additional C4 block should be performed if a complete surgical blockade is desired with either approach.
The exact means by which C4 is affected when local anesthetic is deliberately injected at or between the C5 and C6 nerve roots is not certain. It has been described that the injectate spreads directly into the cervical plexus.13 When larger volumes were utilized, this was mechanistically plausible, as effects on the entire superficial cervical plexus were commonly noted, with numbness involving the side of the head and ear region, as well as the shoulder “cape” region.8,14 Lanz et al evaluated the spread of local anesthetic to various nerves in the head and neck, using an injection volume of 50 mL of 0.5% bupivacaine. The authors found that the supraclavicular nerves were affected in approximately 90% of patients, with lesser effects on the transverse cervical and lesser occipital nerves.15 Volumes of injectate of 20mL have been demonstrated to fill the interscalene groove, extends deep to the cervical fascia, covers the scalene muscles, and reaches the carotid sheath medially while engulfing the middle scalene muscle laterally.16 Presumably, given the use of significantly lower volumes with ultrasound guidance today, this mechanism likely still remains the explanation for C4 effects, though they are probably reduced. Evidence of cervical plexus involvement beyond the supraclavicular nerves is much less common. Before conducting this study, we informally noted numbness to light touch over the anterior/superior shoulder in 80% of patients, which served as the basis of our sample size calculation. Our actual data revealed that a complete effect, encompassing both light touch and temperature to be somewhat lower, at 56%.
ISB can provide effective analgesia for shoulder surgeries; however, hemidiaphragmatic paralysis (HDP), which is caused by local anesthetic spreading to the phrenic nerve, remains a concern14,17,18 especially in patients with pulmonary issues.19–21 The introduction of ultrasound-guided low-volume techniques have enabled additional refinement and enhanced block consistency with decreased local anesthetic volume. Ultrasound-guided low-volume interscalene brachial plexus block reduces HDP incidence4,22 while providing comparable analgesia.23,24 However, it is important to note that general anesthesia was utilized in most of these studies. Despite satisfactory postoperative analgesia was reported in these studies, whether these blocks are sufficient to achieve success as surgical blocks, rather than solely providing postoperative analgesia, remains uncertain.
SCB may be insufficient in this capacity as well. In addition to minimal effect on the C4 dermatome, SCB may not reliably affect the suprascapular nerve,25 which innervates approximately 70% of the shoulder joint as well as the subacromial bursa, coracoclavicular ligament and acromioclavicular joint.26 In our study, SCB achieved C4 and C5 coverage in 29% and 68% of cases, respectively, suggesting that SCB may not be a optimal choice for shoulder surgery as a primary anesthesia method. It is notable that most previous studies primarily focused on postoperative outcomes rather than assessing the quality of sensory and motor blockade achieved through anesthesia.
Administration of regional anesthesia in the preoperative block area, followed by monitored anesthesia care (MAC) or sedation in the operating room remains a popular approach for shoulder arthroscopic procedures such as stabilization, capsular release, acromioplasty, and rotator cuff restoration. Failure to block the C4 region may contribute to insufficient blockade and post-operative shoulder pain. The use of ISB is favored for shoulder surgeries at our facility.
Based on our results, a block that can provide C4 dermatome coverage is recommended to supplement ISB if a surgical block is desired. There are a number of potential techniques for blocking the C4 nerve root or supraclavicular nerves preoperatively to improve the coverage of ISB. An alternative option is to perform a C4 block after the surgery, if necessary, based on the severity of postoperative pain reported by the patient. Local anesthetics (5–10 mL) can be injected deep to the subcutaneous tissues at the sternocleidomastoid muscle (SCM) posterior border to produce a superficial cervical plexus block guided by landmarks. During ISB or SCB, 5 mL of local anesthetic can also be injected deep to the posterior border of the SCM under ultrasound guidance.27 The needle should be placed superficial to the deep cervical fascia to avoid potential effects on the phrenic nerves. C4 area coverage can also be achieved by blocking the supraclavicular nerves.28 Maybin et al9 reported an ultrasound-guided method for blocking the supraclavicular nerve. The supraclavicular nerve, distinguishable as a tiny hypoechoic structure, that arises from the C4 root. At the C4 and C5 nerve root levels, it is superficial to the deep investing layer of the cervical fascia. When scanning caudally, the nerve passes superficially across the scalenus medius muscle before going deeper and ultimately running posterior to the SCM, typically branching into terminal, superficial limbs. During an interscalene block, 2–3 mL of local anesthetic can be deposited around the branches through an established skin puncture.9 Using this C4 block method, light touch and cold sensitivity tests revealed that anesthesia of the epidermis in the supraclavicular nerve area has a high success rate.9
The present study revealed that SCB provides only partial coverage at the C4 and C5 levels, indicating SCB to be insufficient for a surgical block for shoulder surgery. Our findings suggest that SCB may not be as effective at providing complete analgesia for arthroscopic shoulder surgery, as compared to ISB. Since the suprascapular nerve innervates 70% of the shoulder joint, Trabelsi et al29 evaluated the combination of ultrasound-guided suprascapular block (SSB) with SCB and reported that this combination provides efficacy comparable with that of ISB for postoperative.
Limitations
Studies of sensory changes are necessarily subjective to some degree. However, we utilized a quantitative scoring system that has frequently been utilized for the assessment of peripheral nerve block effect. Since C4 has no motor component, we chose to focus solely on the sensory aspects of both nerves, however, we did assess motor block informally to ascertain block success. Also, since no prior data and pre-existing literature were available, sample size and power calculation were based on our pilot observations. The actual sensory block success rate on the C4 dermatome was much lower than we anticipated, which may contribute to the smaller proportional difference detected in the results than was predicted. Finally, lower volume ultrasound-guided ISB and SCB are performed for shoulder surgery to decrease the incidence of both local anesthetic toxicity and HDP. We did not explicitly evaluate the incidence of HPD in either group, but there were no overt complications such as development of hypoxemia or subjective complaints of dyspnea.
Conclusion
Ultrasound-guided low-volume ISB successfully provided C4 dermatome coverage in only about half of cases, indicating the potential advantage of performing an additional C4 block to achieve complete surgical anesthesia. Conversely, ultrasound-guided SCB demonstrated a significantly lower success rate in anesthetizing the C4 and C5 dermatomes, suggesting limitations in its efficacy as the primary choice for shoulder surgery. Moreover, a moderate correlation was observed between the raw C4 sensory score and post-surgery pain score, suggesting that ensuring adequate C4 coverage through deliberate injection of local anesthetic following the ISB procedure may lead to enhanced postoperative pain relief. Since no prior data was available, results from this study provide important information for future research.
Acknowledgments
The authors would like to thank Kristine Ruppert for providing support with statistical analysis.
Funding Statement
This study was performed during YQ’s resident research rotation at UPMC, under the instruction of Dr. Sakai, Tetsuro.
Abbreviations
HDP, hemidiaphragmatic paralysis; ISB, interscalene block; MAC, monitored anesthesia care; PACU, post-anesthesia care unit; SCM, sternocleidomastoid muscle; SCB, supraclavicular block; SSB: suprascapular block.
Data Sharing Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Ethics Approval and Informed Consent
This study is approved by the institutional review board (IRB, STUDY22040053).
Consent for Publication
All patients provided written, informed consent for participation.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; SO took part in drafting, revising, or critically reviewing the article; SO gave final approval of the version to be published; All authors have agreed on the journal to which the article has been submitted; All authors agree to be accountable for all aspects of the work.
Disclosure
The author(s) report no conflicts of interest in this work.
References
- 1.Hewson DW, Oldman M, Bedforth NM. Regional anaesthesia for shoulder surgery. BJA Educ. 2019;19(4):98–104. doi: 10.1016/j.bjae.2018.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warrender WJ, Syed UAM, Hammoud S, et al. Pain management after outpatient shoulder arthroscopy: a systematic review of randomized controlled trials. Am J Sports Med. 2017;45(7):1676–1686. doi: 10.1177/0363546516667906 [DOI] [PubMed] [Google Scholar]
- 3.Xiao M, Cohen SA, Cheung EV, Freehill MT, Abrams GD. Pain management in shoulder arthroplasty: a systematic review and network meta-analysis of randomized controlled trials. J Shoulder Elbow Surg. 2021;30(11):2638–2647. doi: 10.1016/j.jse.2021.06.008 [DOI] [PubMed] [Google Scholar]
- 4.Renes SH, Rettig HC, Gielen MJ, Wilder-Smith OH, van Geffen GJ. Ultrasound-guided low-dose interscalene brachial plexus block reduces the incidence of hemidiaphragmatic paresis. Reg Anesth Pain Med. 2009;34(5):498–502. doi: 10.1097/AAP.0b013e3181b49256 [DOI] [PubMed] [Google Scholar]
- 5.Hussain N, Costache I, Kumar N, et al. Is supraclavicular block as good as interscalene block for acute pain control following shoulder surgery? A systematic review and meta-analysis. Anesth Analg. 2020;130(5):1304–1319. doi: 10.1213/ANE.0000000000004692 [DOI] [PubMed] [Google Scholar]
- 6.Auyong DB, Hanson NA, Joseph RS, Schmidt BE, Slee AE, Yuan SC. Comparison of anterior suprascapular, supraclavicular, and interscalene nerve block approaches for major outpatient arthroscopic shoulder surgery: a randomized, double-blind, noninferiority trial. Anesthesiology. 2018;129(1):47–57. doi: 10.1097/ALN.0000000000002208 [DOI] [PubMed] [Google Scholar]
- 7.Leurcharusmee P, Maikong N, Kantakam P, Navic P, Mahakkanukrauh P, Tran D. Innervation of the clavicle: a cadaveric investigation. Reg Anesth Pain Med. 2021;46(12):1076–1079. doi: 10.1136/rapm-2021-103197 [DOI] [PubMed] [Google Scholar]
- 8.Winnie AP. Interscalene brachial plexus block. Anesth Analg. 1970;49(3):455–466. doi: 10.1213/00000539-197005000-00029 [DOI] [PubMed] [Google Scholar]
- 9.Maybin J, Townsley P, Bedforth N, Allan A. Ultrasound guided supraclavicular nerve blockade: first technical description and the relevance for shoulder surgery under regional anaesthesia. Anaesthesia. 2011;66(11):1053–1055. doi: 10.1111/j.1365-2044.2011.06907.x [DOI] [PubMed] [Google Scholar]
- 10.Franco CD, Williams JM. Ultrasound-guided interscalene block: reevaluation of the ”stoplight” sign and clinical implications. Reg Anesth Pain Med. 2016;41(4):452–459. doi: 10.1097/AAP.0000000000000407 [DOI] [PubMed] [Google Scholar]
- 11.Hanumanthaiah D, Vaidiyanathan S, Garstka M, Szucs S, Iohom G. Ultrasound guided supraclavicular block. Med Ultrason. 2013;15(3):224–229. doi: 10.11152/mu.2013.2066.153.dh1mg2 [DOI] [PubMed] [Google Scholar]
- 12.Siddiqui U, Perlas A, Chin K, et al. Intertruncal approach to the supraclavicular brachial plexus, current controversies and technical update: a daring discourse. Reg Anesth Pain Med. 2020;45(5):377–380. doi: 10.1136/rapm-2019-101260 [DOI] [PubMed] [Google Scholar]
- 13.Urmey WF, Grossi P, Sharrock NE, Stanton J, Gloeggler PJ. Digital pressure during interscalene block is clinically ineffective in preventing anesthetic spread to the cervical plexus. Anesth Analg. 1996;83(2):366–370. doi: 10.1213/00000539-199608000-00028 [DOI] [PubMed] [Google Scholar]
- 14.Neal JM, Gerancher JC, Hebl JR, et al. Upper extremity regional anesthesia: essentials of our current understanding, 2008. Reg Anesth Pain Med. 2009;34(2):134–170. doi: 10.1097/AAP.0b013e31819624eb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanz E, Theiss D, Jankovic D. The extent of blockade following various techniques of brachial plexus block. Anesth Analg. 1983;62(1):55–58. doi: 10.1213/00000539-198301000-00009 [DOI] [PubMed] [Google Scholar]
- 16.Yang WT, Chui PT, Metreweli C. Anatomy of the normal brachial plexus revealed by sonography and the role of sonographic guidance in anesthesia of the brachial plexus. AJR Am J Roentgenol. 1998;171(6):1631–1636. doi: 10.2214/ajr.171.6.9843302 [DOI] [PubMed] [Google Scholar]
- 17.Urmey WF, Talts KH, Sharrock NE. One hundred percent incidence of hemidiaphragmatic paresis associated with interscalene brachial plexus anesthesia as diagnosed by ultrasonography. Anesth Analg. 1991;72(4):498–503. doi: 10.1213/00000539-199104000-00014 [DOI] [PubMed] [Google Scholar]
- 18.Bergmann L, Martini S, Kesselmeier M, et al. Phrenic nerve block caused by interscalene brachial plexus block: breathing effects of different sites of injection. BMC Anesthesiol. 2016;16(1):45. doi: 10.1186/s12871-016-0218-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erickson JM, Louis DS, Naughton NN. Symptomatic phrenic nerve palsy after supraclavicular block in an obese man. Orthopedics. 2009;32(5):368. doi: 10.3928/01477447-20090501-02 [DOI] [PubMed] [Google Scholar]
- 20.Urmey WF, McDonald M. Hemidiaphragmatic paresis during interscalene brachial plexus block: effects on pulmonary function and chest wall mechanics. Anesth Analg. 1992;74(3):352–357. doi: 10.1213/00000539-199203000-00006 [DOI] [PubMed] [Google Scholar]
- 21.Sinha SK, Abrams JH, Barnett JT, et al. Decreasing the local anesthetic volume from 20 to 10 mL for ultrasound-guided interscalene block at the cricoid level does not reduce the incidence of hemidiaphragmatic paresis. Reg Anesth Pain Med. 2011;36(1):17–20. doi: 10.1097/AAP.0b013e3182030648 [DOI] [PubMed] [Google Scholar]
- 22.Riazi S, Carmichael N, Awad I, Holtby RM, McCartney CJ. Effect of local anaesthetic volume (20 vs 5 mL) on the efficacy and respiratory consequences of ultrasound-guided interscalene brachial plexus block. Br J Anaesth. 2008;101(4):549–556. doi: 10.1093/bja/aen229 [DOI] [PubMed] [Google Scholar]
- 23.Lee JH, Cho SH, Kim SH, et al. Ropivacaine for ultrasound-guided interscalene block: 5 mL provides similar analgesia but less phrenic nerve paralysis than 10 mL. Can J Anaesth. 2011;58(11):1001–1006. doi: 10.1007/s12630-011-9568-5 [DOI] [PubMed] [Google Scholar]
- 24.Zhai W, Wang X, Rong Y, Li M, Wang H. Effects of a fixed low-dose ropivacaine with different volume and concentrations on interscalene brachial plexus block: a randomized controlled trial. BMC Anesthesiol. 2016;16(1):80. doi: 10.1186/s12871-016-0248-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sadowski M, Tułaza B, Lysenko L. Renaissance of supraclavicular brachial plexus block. Anaesthesiol Intensive Ther. 2014;46(1):37–41. doi: 10.5603/AIT.2014.0008 [DOI] [PubMed] [Google Scholar]
- 26.Fredrickson MJ, Krishnan S, Chen CY. Postoperative analgesia for shoulder surgery: a critical appraisal and review of current techniques. Anaesthesia. 2010;65(6):608–624. doi: 10.1111/j.1365-2044.2009.06231.x [DOI] [PubMed] [Google Scholar]
- 27.Hipskind JE, Hendrix JM, Ahmed AA. Cervical Plexus Block. Treasure Island, FL: StatPearls Publishing; 2024. [PubMed] [Google Scholar]
- 28.Çelebioğlu EC, Bilgiç MS. Ultrasound-guided supraclavicular nerve block for implantable port catheters: does it show a significant difference in pain control? J Vasc Access. 2022;23(2):206–211. doi: 10.1177/1129729820987358 [DOI] [PubMed] [Google Scholar]
- 29.Trabelsi W, Ben Gabsia A, Lebbi A, Sammoud W, Labbene I, Ferjani M. Suprascapular block associated with supraclavicular block: an alternative to isolated interscalene block for analgesia in shoulder instability surgery? Orthop Traumatol Surg Res. 2017;103(1):77–83. doi: 10.1016/j.otsr.2016.10.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.