Abstract
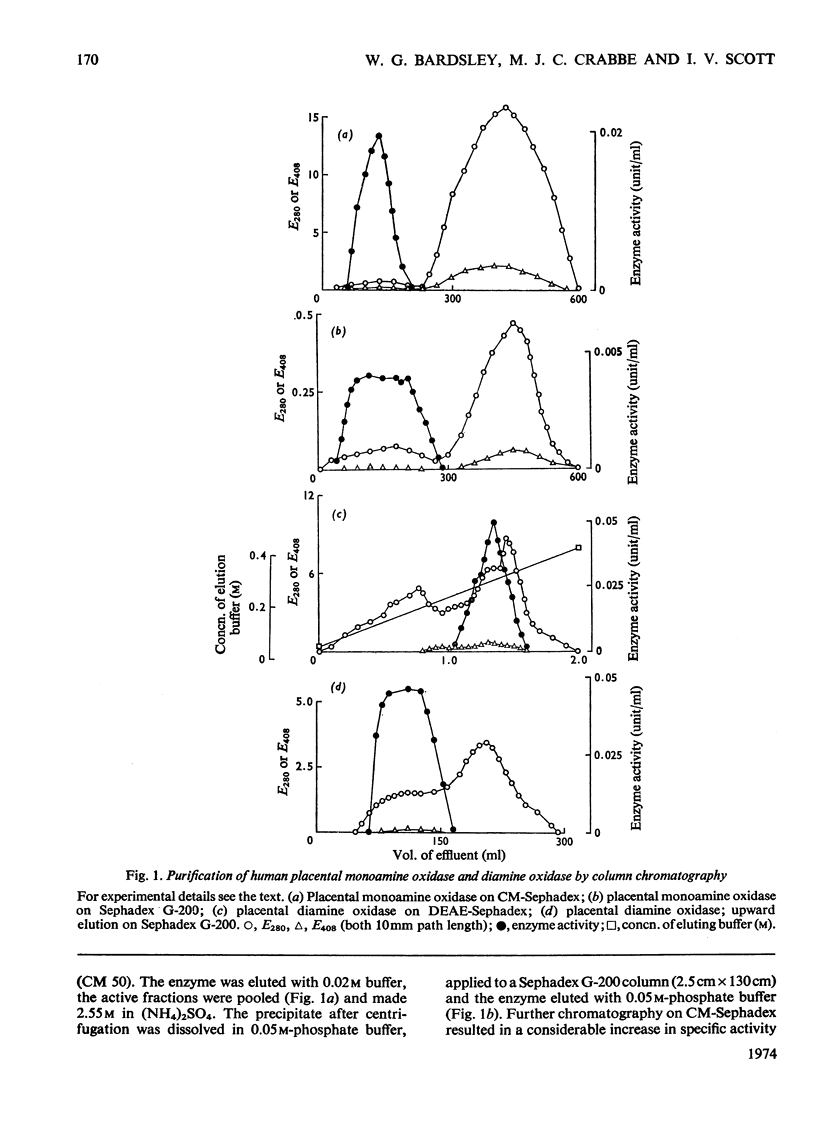
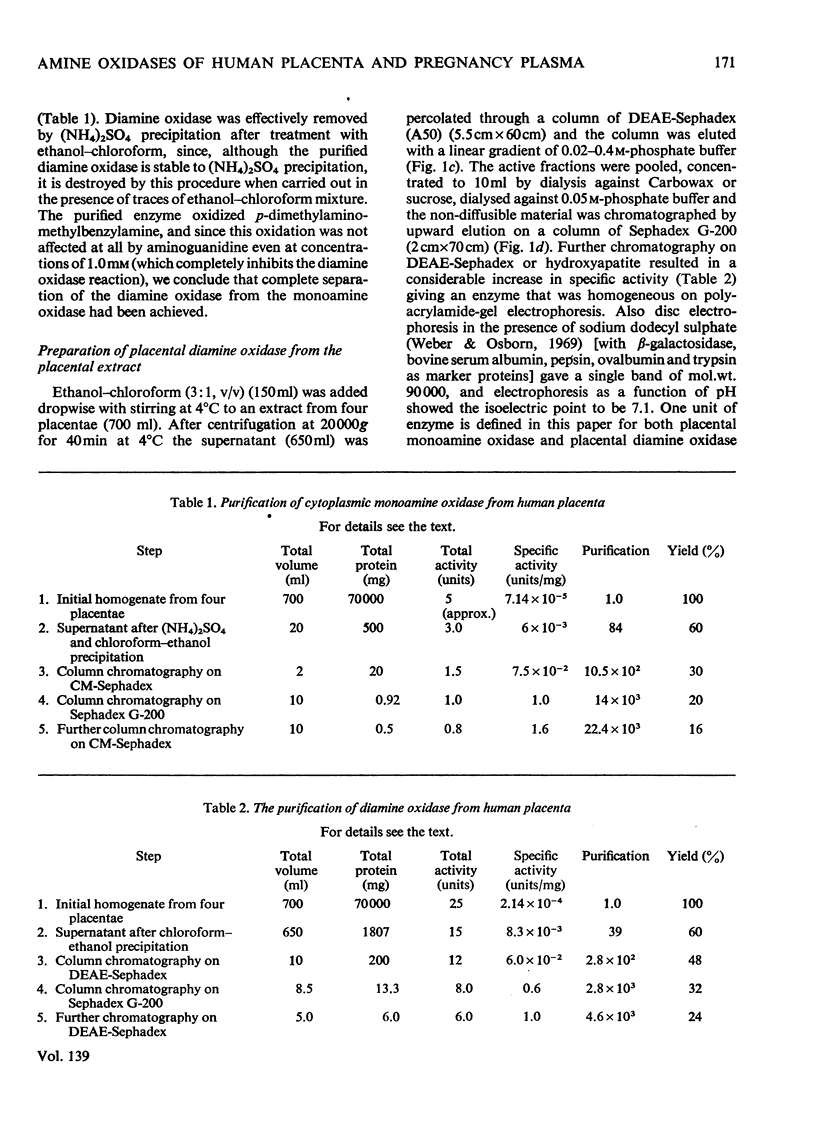
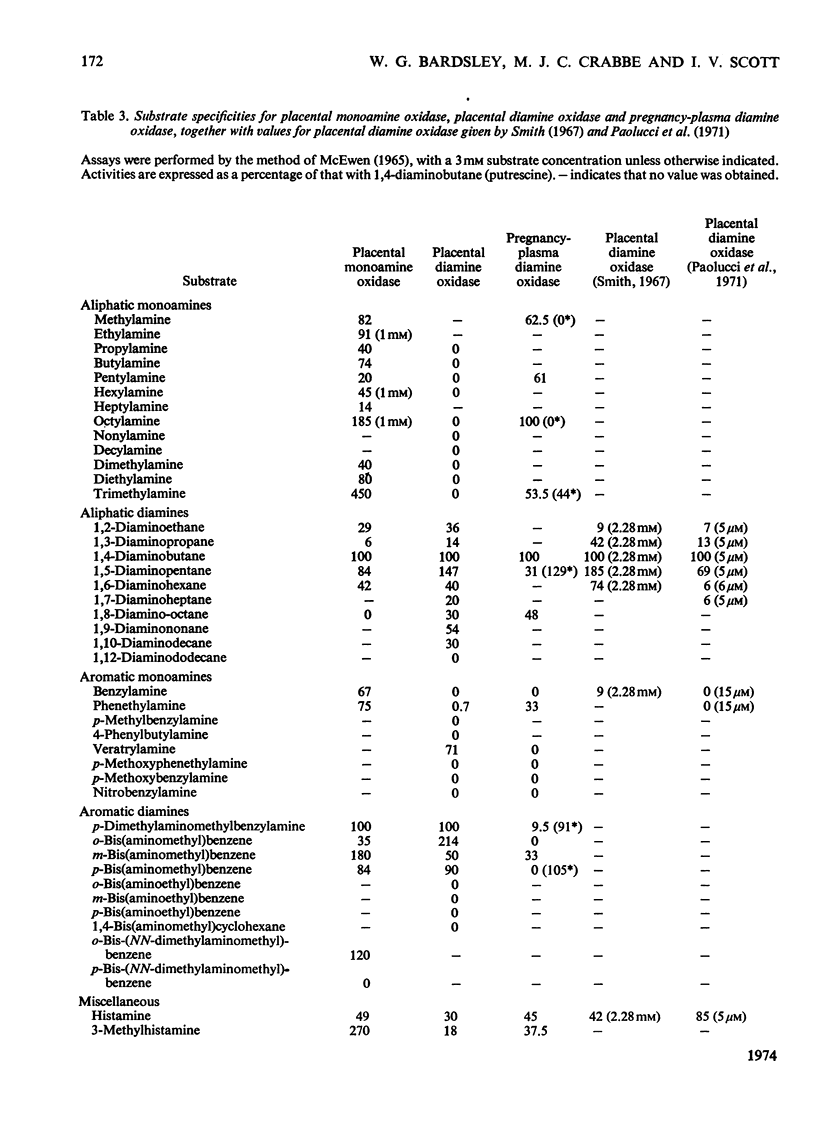
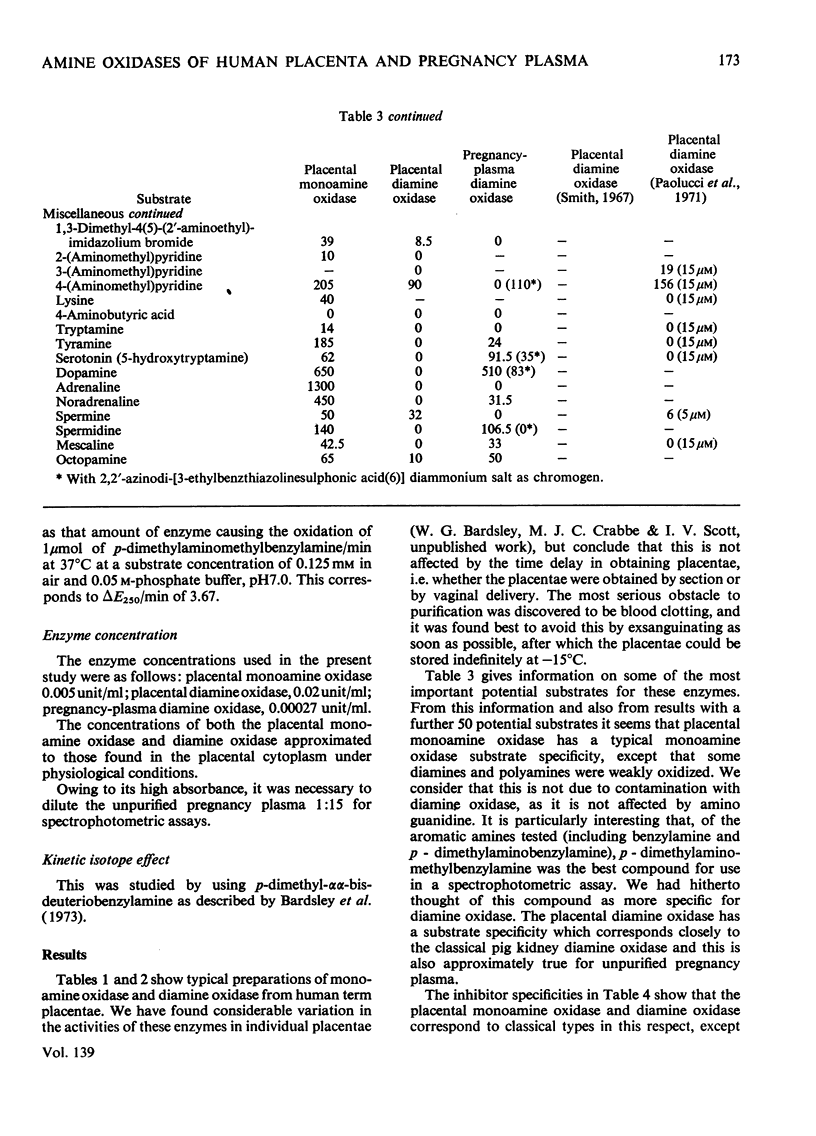
1. The purification of monoamine oxidase and diamine oxidase from normal human term placental tissue is described. 2. The properties of these enzymes are reported and compared with the properties of unpurified human pregnancy plasma. 3. This comparison shows that the amine oxidase of pregnancy plasma has properties corresponding to purified placental diamine oxidase, suggesting a placental origin for the plasma enzyme system. 4. Detailed kinetic study of the purified placental diamine oxidase suggests that it has a Ping Pong sequence, a mechanism of action and rate-limiting step similar to the diamine oxidase of pig kidney. 5. It is suggested that the enzyme system is important in protecting the foeto-placental unit from excesses of biogenic amines.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bardsley W. G., Childs R. E., Crabbe M. J. Inhibition of enzymes by metal ion-chelating reagents. The action of copper-chelating reagents on diamine oxidase. Biochem J. 1974 Jan;137(1):61–66. doi: 10.1042/bj1370061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardsley W. G., Childs R. E. Inhibition of enzymes by metal ion-chelating reagents. Theory and new graphical methods of study. Biochem J. 1974 Jan;137(1):55–60. doi: 10.1042/bj1370055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardsley W. G., Crabbe M. J., Shindler J. S., Ashford J. S. Oxidation of p-dimethylaminomethylbenzylamine by pig kidney diamine oxidase. A new method for spectrophotometric assay. Biochem J. 1972 May;127(5):875–879. doi: 10.1042/bj1270875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardsley W. G., Crabbe M. J., Shindler J. S. Kinetics of the diamine oxidase reaction. Biochem J. 1973 Mar;131(3):459–469. doi: 10.1042/bj1310459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitrich R. A., Erwin V. G. A convenient spectrophotometric assay for monoamine oxidase. Anal Biochem. 1969 Sep;30(3):395–402. doi: 10.1016/0003-2697(69)90133-x. [DOI] [PubMed] [Google Scholar]
- HOLMSTEDT B., LARSSON L., THAM R. Further studies of a spectrophotometric method for the determination of diamine oxidase activity. Biochim Biophys Acta. 1961 Mar 18;48:182–186. doi: 10.1016/0006-3002(61)90530-3. [DOI] [PubMed] [Google Scholar]
- HOLMSTEDT B., THAM R. A spectrophotometric method for determination of diamine oxidase (DAO) activity. Acta Physiol Scand. 1959 Mar 31;45:152–163. doi: 10.1111/j.1748-1716.1959.tb01687.x. [DOI] [PubMed] [Google Scholar]
- McEwen C. M., Jr Human plasma monoamine oxidase. 1. Purification and identification. J Biol Chem. 1965 May;240(5):2003–2010. [PubMed] [Google Scholar]
- Paolucci F., Cronenberger L., Plan R., Pacheco H. Purification et propriétés de la diamine: oxygène oxydo-reductase du placenta humain. Biochimie. 1971;53(6):735–749. [PubMed] [Google Scholar]
- Smith J. K. The purification and properties of placental histaminase. Biochem J. 1967 Apr;103(1):110–119. doi: 10.1042/bj1030110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TABOR C. W., TABOR H., ROSENTHAL S. M. Purification of amine oxidase from beef plasma. J Biol Chem. 1954 Jun;208(2):645–661. [PubMed] [Google Scholar]
- Thompson R. H., Tickner A. Observations on the mono-amine oxidase activity of placenta and uterus. Biochem J. 1949;45(2):125–130. doi: 10.1042/bj0450125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


