Abstract
Background
Cognitive impairment (CI) is frequently observed in patients with chronic pain (CP). CP progression increases the risk of dementia and accelerates Alzheimer’s disease pathogenesis. However, risk diagnostic models and biomarkers for CP-related CI remain insufficient. Previous research has highlighted the relationships between several complete blood count parameters for CP or CI-related diseases, such as Alzheimer’s disease, while the specific values of complete blood count parameters in CP-related CI patients remain unclear. This study aimed to explore the correlation between complete blood count parameters and CP-related CI to establish a risk diagnostic model for the early detection of CP-related CI.
Methods
This cross-sectional study was conducted at West China Hospital, Sichuan University. The Montreal Cognitive Assessment (MoCA) was used to classify patients into either the CP with CI group or the CP without CI group. Univariate analysis and multivariate logistic regression analysis were used to screen the related factors of CP-related CI for constructing a risk diagnostic model, and the model was evaluated using receiver operating characteristic (ROC) curve analysis.
Results
The study ultimately included 163 eligible patients. Based on analysis, age (OR, 1.037 [95% CI, 1.007–1.070]; P=0.018), duration of pain (OR, 2.546 [95% CI, 1.099–6.129]; P=0.032), VAS score (OR, 1.724 [95% CI, 0.819–3.672]; P=0.153), LMR (OR, 0.091 [95% CI, 0.024–0.275]; P<0.001), absolute neutrophil value (OR, 0.306 [95% CI, 0.115–0.767]; P=0.014), and lymphocyte percentage (OR, 6.551 [95% CI, 2.143–25.039]; P=0.002) were identified as critical factors of CP-related CI. The diagnostic model was evaluated by the ROC curve, demonstrating good diagnostic value with an area under the curve (AUC) of 0.803, a sensitivity of 0.603 and a specificity of 0.871.
Conclusion
The risk diagnostic model developed in this study for CP-related CI has significant value and enables clinicians to customize interventions based on each patient’s needs.
Keywords: cognitive impairment, chronic pain, diagnostic model, complete blood count parameters, risk factors
Introduction
Chronic pain (CP) refers to pain that lasts or recurs for more than 3 months.1 Cognitive impairment (CI) is a prevalent complication in CP patients, primarily characterized by diminished memory, attention, and executive function.2 CP affects approximately 53% of the communities in the United States,3 and persistence of pain interference is associated with 21% increased odds of CI for every 2 years.4 CP persistent progression can increase the risk of Alzheimer’s disease (AD) and accelerate AD pathogenesis,5,6 leading to reduced quality of life, medical adherence, and work capacity for patients.5 Of note, another meta-analysis have demonstrated that the prevalence of CP in AD patients was as high as 45.8%.7 A study of CP patients found that two-thirds had impaired working memory and attention.8 Cognitive function did not improve even after short-term local analgesia,8 indicating a close bidirectional relationship rather than two separate conditions.
The specific mechanisms underlying CP-related CI remain incompletely understood. Current evidence suggests that the two conditions share several common pathophysiological changes.5 CP and cognitive task execution involve overlapping brain regions, suggesting that pain may reduce the availability of cognitive resources within specific circuits.5,9 Additionally, abnormalities in the locus coeruleus norepinephrine system,10 microglial activation and increased central neuroinflammation have been observed in both CP and AD.11 However, since the unclear mechanisms underlying CP-related CI, effective risk assessment and intervention strategies are still lacking in clinical practice. While commonly used analgesics provide some relief from pain, many may potentially cause or exacerbate cognitive dysfunction.12 Therefore, investigating the risk factors of CP-related CI and developing a risk diagnostic model for early prevention and treatment are of clinical significance.
Complete blood count (CBC) tests are cost-effective and easy to obtain, making them ideal biomarkers for early disease diagnosis. Some parameters in the CBC test are considered to be either protective or exacerbating factors for CP. The platelet-to-lymphocyte ratio (PLR) in fibromyalgia patients is significantly higher than that in the control group.13 Changes in the neutrophil-to-lymphocyte ratio (NLR) can be used for early identification risk of chronic postoperative pain, prompting clinicians to conduct further evaluations.14 High-quality basic research has also confirmed that the neutrophil activation induced acute inflammatory response protects against the development of CP.15 Furthermore, neutrophil count, and the NLR in CBC tests were associated with postoperative cognitive dysfunction and AD.16,17 However, the relationship between CBC parameters and CP-related CI remains unclear due to a lack of sufficient clinical research evidence. Therefore, this study aimed to explore the correlation between CBC parameters and CP-related CI, identify risk factors of CP-related CI, and establish a risk diagnostic model combining general clinical data for the early detection of CP-related CI.
Methods
Ethical Approval
All procedures involving human participants adhered to the 1964 helsinki Declaration and its subsequent amendments. The study plan was approved by the Ethics Committee of West China Hospital, Sichuan University (ethics approval number: 2021 review (221)) and registered in the Chinese Clinical Trial Registry (registration number: ChiCTR2100047290).
Study Design and Study Setting
This study was a single-center, cross-sectional study conducted on CP patients at the Department of Pain Medicine, West China Hospital, Sichuan University, from October 2020 to February 2023. We conducted an epidemiological survey on eligible CP patients.
Study Population
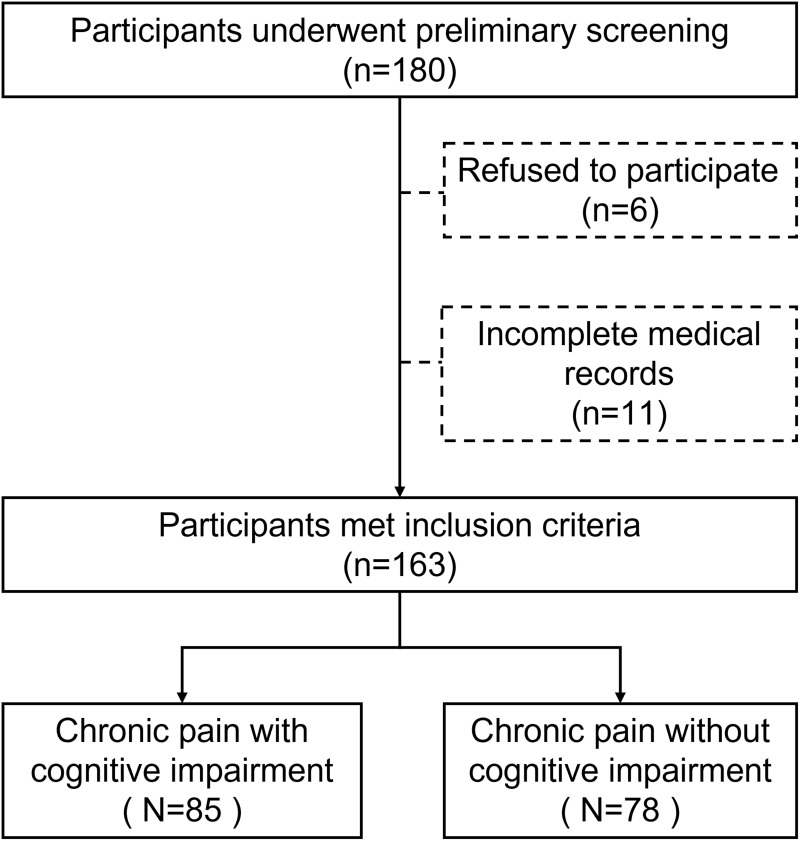
Under the principles of informed consent and voluntary participation, a total of 180 people were surveyed, and 163 patients were ultimately included after excluding patients diagnosed with chronic primary pain, chronic cancer-related pain, or those who did not complete all the questionnaires or who were unqualified (Figure 1).
Figure 1.
Flowchart of the study.
The inclusion criteria were as follows: (1) voluntary participation and signing an informed consent form; (2) age over 18 years; (3) VAS score > 0; (4) pain duration > 3 months, diagnosed as CP by a specialist in pain; and (5) no participation in other clinical trials. The exclusion criteria were as follows: (1) Poor general condition, inability to objectively describe symptoms or actively cooperate, severe infection, respiratory failure, or heart failure. (2) Previous diagnosis of dementia, Alzheimer’s disease, cerebral ischemia, brain injury, central nervous system infection, epilepsy, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis, or spinocerebellar ataxia. (3) Pre-existing diagnosis of severe anxiety, severe depression, schizophrenia or other mental diseases before the onset of CP; (4) Undergoing hemodialysis; (5) Suffering from severe anemia; (6) Suspected or confirmed drug and alcohol addiction; (7) Pregnant or breastfeeding; (8) Refusal to be enrolled.
Measurement and Data Collection
The patients who met the inclusion and exclusion criteria were assessed with the Montreal Cognitive Assessment (MoCA) questionnaire on the admission day, and were divided into the CP with CI group and the CP without CI group according to their MoCA scores. Additionally, common demographic factors such as age, gender, height, weight, body mass index (BMI), education level, degree of pain (VAS score and ID pain score), pain duration, main diagnosis, and medications were recorded. Basic vital signs of the patients were also collected for statistical analysis.
The visual analogue scale (VAS)18 was used to assess the degree of pain. The VAS score is widely employed for measuring pain, quality of life, and anxiety. It consists of a 100 mm straight line with one end at 0 indicating no pain and the other end at 10 indicating unbearable pain. The patient marks their subjective perception of pain on this line within the range of 0 to 10.19 Pain levels are categorized as follows: no pain (0), mild pain (1–3), moderate pain (4–6), and severe pain (7–10). In addition, the ID pain scale was used to preliminarily screen for neuropathic pain.20 This scale consists of 6 items evaluating sensory descriptions (acupuncture, burning, numbness, over-electricity, and hyperalgesia; each item is scored positively with 1 point) and joint pain (describing whether pain is limited to the joint to exclude injury-related pain; reversely scored with −1 point).21 The total score ranges from −1 to 5 points. Clinically, treatment options for neuropathic pain are determined based on the ID Pain score, which is generally ≥3 points.
The MoCA was used to assess cognitive function, with 28 items covering eight areas: executive function, attention, memory, language, visual-spatial ability, abstract thinking, calculation, and orientation. Scores range from 0–30; higher scores indicate better cognitive function. The cut-off values are based on education level: ≤6 years (19 scores), 7–12 years (22 scores), and >12 years (24 scores). Patients completed the MoCA in approximately 15 minutes.
Statistical Analysis
Data entry, database establishment, and data analysis for this study were conducted via Excel software, R language 4.1.3 software, and SPSS 27.0 software. Double entry was performed to ensure accuracy. This study analyzed the epidemiology of patients with CP-related CI by describing the demographic data and CBC test parameter data of the subjects. The prevalence characteristics of CP-related CI were examined through frequency analysis. The NLR, PLR, lymphocyte-monocyte ratio (LMR), and neutrophil-monocyte ratio (NMR) were calculated, and ROC curve were used to determine the best cut-off points for continuous variables such as pain-related factors and CBC parameters. Univariate logistic regression analysis was used to screen related factors for CP-related CI diagnosis, which were then included in multivariate logistic regression analysis to construct a diagnostic model. The diagnostic level of the model was evaluated using ROC curve and a nomogram was created accordingly. All the statistical tests were conducted at a significance level of α=0.05 for two-sided testing.
Results
Baseline Characteristics and Outcomes of Subjects
This study included 163 patients with CP, 85 of whom were in the CI group and 78 of whom were in the non-CI group. There were no significant differences in gender, educational level, or BMI between the two groups. The study included 8 general information variables (Table 1) and 14 CBC parameters variables (Table 2) related to CP and CI. The baseline characteristics of the two groups are presented in Table 1.
Table 1.
Univariate Logistic Analysis of the General Information of Chronic Pain Patients
| CP with CI | CP without CI | OR (95% CI) | P | |
|---|---|---|---|---|
| Gender [n(%)] | ||||
| Male | 36 (54.55) | 30 (45.45) | ||
| Female | 49 (50.52) | 48 (49.48) | 1.20 (0.63–2.20) | 0.61 |
| Age (year) | 55.62±12.57 | 48.26±13.37 | 1.05 (1.02–1.07) | <0.01* |
| Educational level [n(%)] | ||||
| High school and above | 69 (52.67) | 62 (47.33) | ||
| Elementary School | 14 (58.33) | 10 (41.67) | 1.30 (0.52–3.00) | 0.61 |
| Illiterate | 2 (25.00) | 6 (75.00) | 0.30 (0.06–1.50) | 0.15 |
| BMI (kg·m−2) [n(%)] | ||||
| Normal (18.5–24) | 43 (51.81) | 40 (48.19) | ||
| Obesity (≥28) | 9 (56.25) | 7 (43.75) | 2.10 (0.60–7.30) | 0.25 |
| Overweight (24–28) | 33 (55.00) | 27 (45.00) | 1.00 (0.53–2.00) | 0.95 |
| Emaciated (<18.5) | 0 (0.00) | 4 (100) | 0.0 (0.00-Inf) | 0.99 |
| VAS Score [n(%)] | ||||
| <5.5 score | 36 (43.37) | 47 (56.63) | ||
| ≥5.5 score | 49 (61.25) | 31 (38.75) | 2.10 (1.10–3.90) | 0.02* |
| ID Pain Score [n(%)] | ||||
| <3 score | 65 (56.52) | 50 (43.48) | ||
| ≥3 score | 20 (41.67) | 28 (58.33) | 0.55 (0.28–1.10) | 0.09 |
| Categories of Pain [n(%)] | ||||
| CNP | 18 (58.06) | 13 (41.94) | ||
| CPSP | 3 (60.00) | 2 (40.00) | 0.00 (0-Inf) | 0.99 |
| CSMSP | 64 (50.39) | 63 (49.61) | 1.10 (0.16–7.40) | 0.94 |
| Pain Duration [n(%)] | ||||
| <6.5 months | 17 (38.64) | 27 (61.36) | ||
| ≥6.5 months | 68 (57.14) | 51 (42.86) | 2.10 (1.00–4.30) | 0.04* |
Note: *Represents a statistically significant difference with P < 0.05.
Abbreviations: BMI, Body mass index; CP, chronic pain; CNP, chronic neuropathic pain; CPSP, chronic postsurgical or post-traumatic pain; CSMSP, chronic secondary musculoskeletal pain; CI, Cognitive impairment; OR, odds ratio; VAS, Visual Analogue Scale.
Table 2.
Univariate Logistic Analysis of Complete Blood Count Parameters from Chronic Pain Patients
| CP with CI | CP without CI | OR (95% CI) | P | |
|---|---|---|---|---|
| NLR [n(%)] | ||||
| <1.7 | 50 (60.98) | 32 (39.02) | ||
| ≥1.7 | 35 (43.21) | 46 (56.79) | 0.49 (0.26–0.91) | 0.02* |
| PLR [n(%)] | ||||
| <90.26 | 34 (60.71) | 22 (39.29) | ||
| ≥90.26 | 51 (47.66) | 56 (52.34) | 0.59 (0.31–1.10) | 0.11 |
| LMR [n(%)] | ||||
| <3.69 | 40 (65.57) | 21 (34.43) | ||
| ≥3.69 | 45 (44.12) | 57 (55.88) | 0.41 (0.21–0.80) | 0.01* |
| NMR [n(%)] | ||||
| <8.00 | 64 (60.38) | 42 (39.62) | ||
| ≥8.00 | 21 (36.84) | 36 (63.16) | 0.38 (0.20–0.74) | <0.01* |
| White blood cell count (109/L) | ||||
| <6.53 | 72 (59.50) | 49 (40.50) | ||
| ≥6.53 | 13 (30.95) | 29 (69.05) | 0.31 (0.14–0.64) | <0.01* |
| Red blood cell count (109/L) | ||||
| <4.23 | 37 (48.05) | 40 (51.95) | ||
| ≥4.23 | 48 (55.81) | 38 (44.19) | 1.40 (0.74–2.50) | 0.32 |
| Hemoglobin (g/L) | ||||
| <129.5 | 37 (45.68) | 44 (54.32) | ||
| ≥129.5 | 48 (58.54) | 34 (41.46) | 1.70 (0.90–3.10) | 0.10 |
| Platelet count (109/L) | ||||
| <121.5 | 16 (69.57) | 7 (30.43) | ||
| ≥121.5 | 69 (49.29) | 71 (50.71) | 0.43 (0.16–1.10) | 0.08 |
| Percentage of monocytes (%) | ||||
| <7.75 | 35 (42.31) | 46 (56.79) | ||
| ≥7.75 | 50 (60.98) | 32 (39.02) | 2.10 (1.10–3.80) | 0.02* |
| Absolute Monocyte Count (109/L) | ||||
| <0.34 | 16 (41.03) | 23 (58.97) | ||
| ≥0.34 | 69 (55.65) | 55 (44.35) | 1.80 (0.87–3.70) | 0.11 |
| Lymphocyte Percentage (%) | ||||
| <31.3 | 30 (42.86) | 40 (57.12) | ||
| ≥31.3 | 55 (59.14) | 38 (40.86) | 1.90 (1.00–3.60) | 0.04* |
| Absolute lymphocyte count (109/L) | ||||
| <2.43 | 79 (55.24) | 64 (44.76) | ||
| ≥2.43 | 6 (30) | 14 (70) | 0.35 (0.13–0.95) | 0.04* |
| Neutrophil percentage (%) | ||||
| <55.85 | 50 (60.98) | 32 (39.24) | ||
| ≥55.85 | 35 (43.21) | 46 (56.79) | 0.49 (0.26–0.91) | 0.02* |
| Absolute neutrophil count (109/L) | ||||
| <3.85 | 72 (61.02) | 46 (38.98) | ||
| ≥3.85 | 13 (28.89) | 32 (71.11) | 0.26 (0.12–0.55) | <0.01* |
Note: *Represents a statistically significant difference with P < 0.05.
Abbreviations: CP, chronic pain; CI, cognitive impairment; NLR, Ratio of Neutrophil Count to Lymphocyte Count; PLR, Ratio of Platelet Count to Lymphocyte Count; LMR, Ratio of Lymphocyte Count to Monocyte Count; NMR, Ratio of Neutrophil Count to Monocyte Count; OR, Odds ratio; CI, Confidence Interval.
Univariate Analysis to Identify Related Factors
For CP-related CI, univariate analysis identified 3 differentiated factors among the 8 general information variables and 9 differentiated factors among 14 CBC parameter variables. The variables with P<0.05 from Table 1 and Table 2 were assigned as independent variables. Specifically, the results showed significant differences between the two groups in terms of age, pain duration, VAS score, NLR, LMR, NMR, white blood cell count, absolute value of lymphocytes, absolute value of neutrophils, percentage of neutrophils, percentage of lymphocytes, and percentage of monocytes.
Multivariate Logistic Regression Analysis and Diagnostic Model of Chronic Pain with Cognitive Impairment Establishment
Based on the results of univariate analysis, CP-related CI were treated as the dependent variable (those without CI were assigned a value of 0, and those with CI were assigned a value of 1), and the 12 statistically significant variables from Table 1 and Table 2 were set as independent variables (the assignment values are shown in Table 3) and included in a multivariate logistic regression analysis. After a rigorous feature selection process of multivariate logistic analysis, six factors (age, duration of pain, VAS score, LMR, neutrophil count, and lymphocyte percentage) were ultimately included into the optimal diagnostic model (Table 4).
Table 3.
Variable Assignment Methods
| Variable | Assignment |
|---|---|
| Age (years) | Direct Inclusion |
| Pain Duration (months) | <6.5 months=0, ≥6.5 months=1 |
| VAS Score | <5.5 Score=0, ≥5.5 months=1 |
| LMR | <6.5 months=0, ≥6.5 months=1 |
| Neutrophil count (109/L) | <3.85=0, ≥3.85=1 |
| Lymphocyte Percentage (%) | <31.3%=0, ≥31.3% =1 |
Abbreviations: VAS Score, Visual Analog Scale Score; LMR, Ratio of Lymphocyte Count to Monocyte Count.
Table 4.
Multivariate Logistic Analysis from Chronic Pain Patients
| β value | Waldχ2 | OR (95% CI) | P | |
|---|---|---|---|---|
| Age (years) | 0.04 | 5.64 | 1.037 (1.007~1.070) | 0.018* |
| Pain Duration ≥ 6.5 months | 0.94 | 4.60 | 2.546 (1.099~6.129) | 0.032* |
| VAS Score ≥ 5.5 score | 0.55 | 2.04 | 1.724 (0.819~3.672) | 0.153 |
| LMR ≥ 3.69 | −2.40 | 15.21 | 0.091 (0.024~0.275) | <0.001* |
| Neutrophil count ≥ 3.85 (109/L) | −1.18 | 6.09 | 0.306 (0.115~0.767) | 0.014* |
| Lymphocyte Percentage ≥ 2.43 (%) | 1.88 | 9.37 | 6.551 (2.143~25.039) | 0.002* |
Note: *Represents a statistically significant difference with P < 0.05.
Abbreviations: CP, Chronic pain; CI, Cognitive impairment; VAS Score, Visual Analogue Scale Score; LMR, Ratio of lymphocyte count to monocyte count; Wald χ2, Wald chi-square value; OR, Odds ratio; CI, Confidence interval.
Construction of a Nomogram Model for Assessing the Risk of Cognitive Impairment in Patients with Newly Diagnosed Chronic Pain
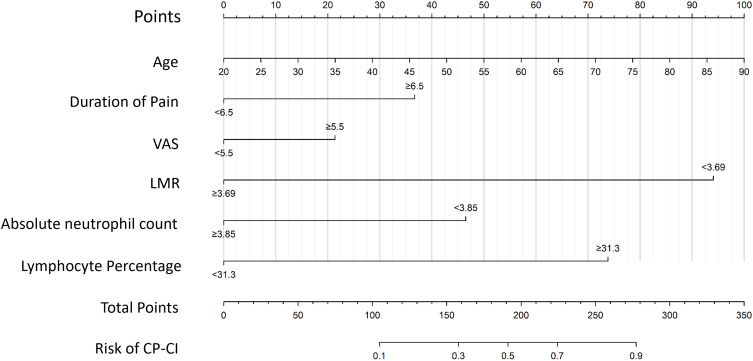
A prediction model was constructed based on the regression coefficients and constant terms derived from the multivariate logistic regression analysis. This logistic regression model was visualized using R software to create a nomogram (Figure 2). The total score was obtained by calculating the individual scores for each factor in the nomogram.
Figure 2.
Nomogram of a diagnostic model of CP-related CI. This figure shows that six factors, including age, duration of pain, VAS score, LMR, absolute neutrophil count, and lymphocyte percentage, were included in the diagnostic model.
Performance Analysis of the Nomogram Model Assessing the Risk of Cognitive Impairment in Patients with Chronic Pain
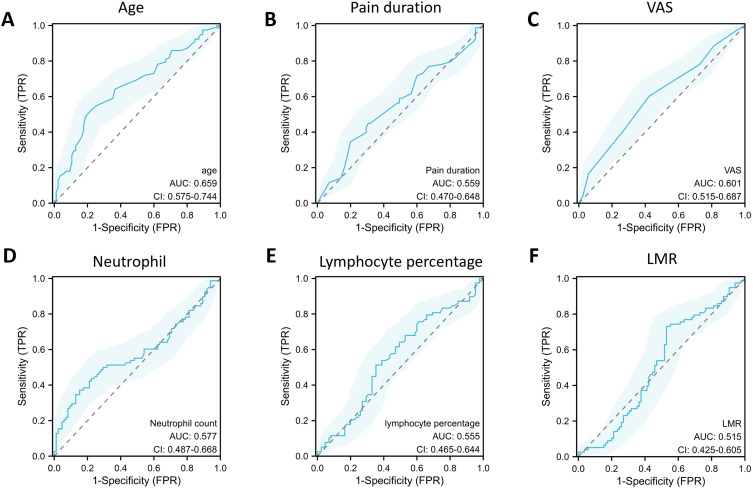
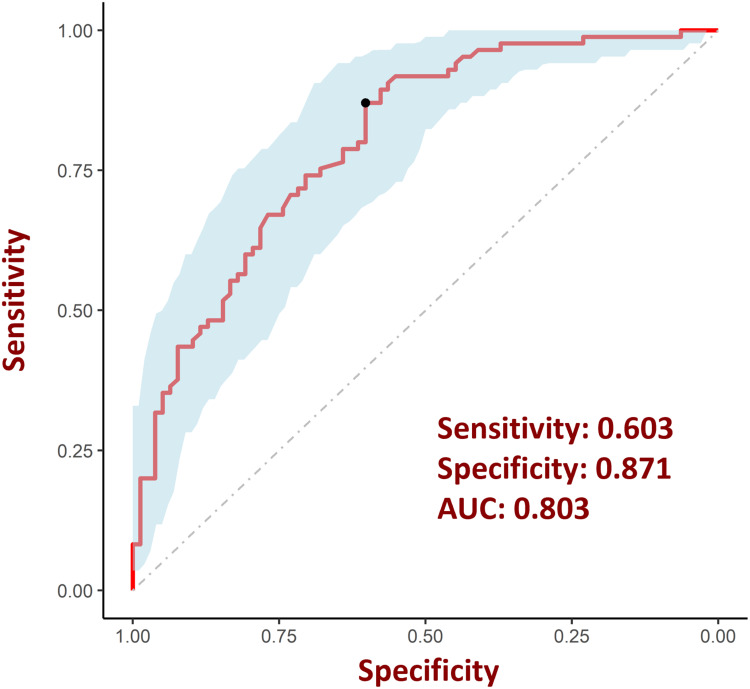
Then, we separately plotted ROC curves for the six individual variables (Figure 3) according to previous research by Janes et al.22 The nomogram model constructed above was used to evaluate their respective diagnostic value of these factors for CI risk in CP patients and was also evaluated by ROC curve analysis. According to the results of ROC curve analysis, the AUC for the nomogram model in diagnosing CI risk in CP patients was 0.803, with a sensitivity of 0.603 and a specificity of 0.871 (Figure 4).
Figure 3.
ROC curve for assessing the performance of each factor in the diagnostic models. (A) ROC curve of age in the diagnostic models. (B) ROC curve of pain duration in the diagnostic models. (C) ROC curve of VAS score in the diagnostic models. (D) ROC curve of neutrophil in the diagnostic models. (E) ROC curve of lymphocyte percentage in the diagnostic models. (F) ROC curve of LMR in the diagnostic models.
Figure 4.
ROC curve for evaluating the performance of risk diagnostic models. The model was assessed using ROC curves and achieved an AUC of 0.803, with sensitivity and specificity values of 60.3% and 87.1%, respectively.
Discussion
Although the analgesic management of CP has improved significantly through the use of novel drugs or other therapeutic interventions, CI remains one of the common complications for these patients, seriously affecting their daily activities and quality of life.23,24 Previous studies have demonstrated the close relationship between CP and CI,25,26 providing reliable support for the construction of the risk diagnostic model presented in our research. As the level of CI increased from no impairment to substantial CI, the prevalence of any pain increased from 62.44% to 83.20%.27 Therefore, our study identified the risk factors among the general information and CBC parameters associated with CP-related CI and constructed a risk diagnostic model based on these factors. Specifically, it was found that age, duration of pain, the VAS score, the LMR, the neutrophil count, and the lymphocyte percentage were included in the risk diagnostic model. This result is crucial for the early detection and prevention of CI for CP patients.
Given the high degree of heterogeneity among different studies, the prevalence of CI in CP patients ranges from 6%–54%.28,29 In this study, we found a prevalence of 52.15%, which is slightly higher than that most reported in previous studies. The reasons for the observed differences may be attributed to the variations in patient characteristics compared with those reported in previous studies, including age, gender composition, medications, pain types, and the cognitive function assessment tools used. Some studies have utilized the Mini-Mental State Examination (MMSE) scale to evaluate CI,29 whereas the MoCA scale was employed in this study. Differences in assessment tools might lead to discrepancies in the reported incidence of CI. Furthermore, the gender distribution in this study was not entirely balanced, with female patients accounting for nearly 60% of the cohort, which may also contribute to the observed differences in CI incidence.
In this study, age was identified as a critical risk factor for CI in CP patients, with an increasing age related to a higher risk. The normal aging process is associated with declines in certain cognitive abilities, such as processing speed and certain memory, language, visuospatial, and executive function abilities.30,31 Research has indicated that aging is associated with an elevation in the pain threshold, a decline in pain perception, and a reduction in pain tolerance.32,33 Meanwhile, aging of the central nervous system might result in a decrease in the brain reserve,34 leading to increased brain frailty.35 The brain reserve refers to the ability of the brain to withstand neuropathological changes due to aging or disease without presenting clinical symptoms.36 While the brain reserve declines in elderly patients, CP, as a long-standing stressor, is more likely to cause brain dysfunction.
In addition, the results showed that pain duration is a risk factor for the development of CI in CP patients. Specifically, the risk of CI in patients with a pain duration exceeding 6.5 months is more than twice that of patients with a pain duration of less than 6.5 months, which aligns with previous research conclusions. Some studies have reported negative correlations between a lower grey matter volume and a longer duration of pain.37,38 Similarly, longer pain duration in knee osteoarthritis patients was associated with extended, bilateral cortical thinning after controlling for age effects using whole vertexwise brain analysis.39 The accumulation of time-induced physiological and pathological changes in the brain may be a key factor in the development of CI.
This study found no gender differences in the occurrence of CI in CP patients. However, the correlation between sex and CP-related CI patients remains controversial. Research has indicated that memory performance is better in female patients with fibromyalgia than in male patients,40 whereas a study conducted by Randy S Roth et al suggested that women with CP are particularly vulnerable to cognitive dysfunction.41 The underlying reason may be attributed to the effects of sex hormones. Sex hormones exhibit highly complex interactions with both pain and cognition.42,43 For women, fluctuations in estrogen levels can enhance the intensity and perception of pain.42,44 In addition, estrogen also demonstrates certain neuroprotective effects.45 The lack of gender differences observed in this study may be closely related to the age range of the included patients, as estrogen levels vary significantly across different ages of female CP patients.
This study innovatively revealed that the LMR, neutrophil count, and lymphocyte percentage, were significant factors associated with the occurrence of CI in individuals with CP. The LMR is often used as an indicator of systemic inflammation.46,47 A low LMR has been implicated in several studies as a potential risk factor for cognitive decline after surgery and in patients with neuroinflammatory conditions.48 In this study, it was found that the group with a LMR greater than or equal to 3.69 had a lower incidence of CI in CP patients than did the group with an LMR less than 3.69, indicating that a higher LMR may have a protective effect against cognition. Postoperative cognitive dysfunction, which shares similarities with CP-related CI, was found to be correlated with a reduced LMR,48 suggesting that abnormal immune states may contribute to neurological dysfunction following chronic conditions.
The neutrophil count, an indicator of systemic inflammation, has been studied as a biomarker or risk factor of several diseases.49,50 A population-based cohort study demonstrated that increased neutrophils were associated with poor cognitive performance and accelerated decline in episodic memory.51 However, our study revealed that the prevalence of CI was lower in the group with a neutrophil count ≥ 3.85 × 109/L compared to the group with a neutrophil count < 3.85 × 109/L, indicating that hypo-neutrophil-count has a negative effect on cognition, which contradicts with previous studies. Furthermore, this study also found that a higher lymphocyte percentage was associated with CI, which also contrasts with the findings of previous cognition-related studies. For example, lower lymphocyte counts and altered lymphocyte subset ratios have been observed in patients with Alzheimer’s disease and mild cognitive impairment.52,53 Few studies showed the direct link the lymphocyte percentage and neutrophil count with the coexistence of CP and CI, while some research reported this correlation in other types of cognitive disorders, such as Alzheimer’s disease.54–57 However, these findings do not represent contradictory results under the same conditions. These differences may be attributed to various factors, such as patient age and immune state. Immune homeostasis is beneficial for maintaining cognitive health.58,59 Thus, hypoactive or hyperactive peripheral immune reactions might be deleterious for cognitive function in CP patients.
Our study identified the factors related to CI in CP patients, mainly focusing on general information and CBC test parameters. We constructed a risk diagnostic model by including six factors: age, duration of pain, VAS score, LMR, neutrophil count, and lymphocyte percentage. ROC curve analysis demonstrated the value of our risk diagnostic model. As a simple clinical tool for personalized evaluation, it can help identify individuals at high risk for CP-related CI, enabling clinicians to customize interventions based on each patient’s needs.
Limitations
This study has certain limitations. First, this study is a cross-sectional survey without a time sequence of disease development, thus compromising the strength of the evidence for causal inference. Second, the study used the MoCA scale to assess cognitive function, without imaging or other clinical examinations, and lacked a gold standard for diagnosing CI. Additionally, during the recruitment period, multiple outbreaks of COVID-19 occurred in Sichuan, China. The implementation of control measures against the epidemic by relevant authorities introduces bias among volunteers, resulting in insufficient representativeness of the sample and limited external validity of the conclusions. In the future, more large-scale surveys and high-quality clinical studies are needed to further explore the influencing factors of CP-related CI and the diagnostic significance of blood biomarkers.
Conclusion
Our study demonstrated that a risk diagnostic model based on information of chronic pain patients is both sensitive and specific for diagnosing CI. The key factors contributing to CI diagnosis include age, duration of pain, VAS score, the LMR, the neutrophil count, and the lymphocyte percentage.
Acknowledgments
The authors would like to thank all of the participants of this study.
Funding Statement
Not applicable.
Data Sharing Statement
The datasets used or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
All procedures involving human participants adhered to the 1964 helsinki Declaration and its subsequent amendments. The study plan was approved by the Ethics Committee of West China Hospital, Sichuan University (ethics approval number: 2021 review (221)) and registered in the Chinese Clinical Trial Registry (registration number: ChiCTR2100047290).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Treede RD, Rief W, Barke A, et al. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the international classification of diseases (ICD-11). Pain. 2019;160:19–27. doi: 10.1097/j.pain.0000000000001384 [DOI] [PubMed] [Google Scholar]
- 2.Mazza S, Frot M, Rey AE. A comprehensive literature review of chronic pain and memory. Prog Neuropsychopharmacol Biol Psychiatry. 2018;87:183–192. doi: 10.1016/j.pnpbp.2017.08.006 [DOI] [PubMed] [Google Scholar]
- 3.Liu M, Hou T, Nkimbeng M, et al. Associations between symptoms of pain, insomnia and depression, and frailty in older adults: a cross-sectional analysis of a cohort study. Int J Nurs Stud. 2021;117:103873. doi: 10.1016/j.ijnurstu.2021.103873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell T, Franz CE, Kremen WS. Persistence of pain and cognitive impairment in older adults. J Am Geriatr Soc. 2022;70:449–458. doi: 10.1111/jgs.17542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao S, Fisher DW, Yu T, Dong H. The link between chronic pain and Alzheimer’s disease. J Neuroinflammation. 2019;16(204). doi: 10.1186/s12974-019-1608-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guerreiro SR, Guimarães MR, Silva JM, et al. Chronic pain causes Tau-mediated hippocampal pathology and memory deficits. Mol Psychiatry. 2022;27:4385–4393. doi: 10.1038/s41380-022-01707-3 [DOI] [PubMed] [Google Scholar]
- 7.van Kooten J, Binnekade TT, van der Wouden JC, et al. A review of pain prevalence in Alzheimer’s, vascular, frontotemporal and Lewy body dementias. Dement Geriatr Cognit Disord. 2016;41:220–232. doi: 10.1159/000444791 [DOI] [PubMed] [Google Scholar]
- 8.Dick BD, Rashiq S. Disruption of attention and working memory traces in individuals with chronic pain. Anesth Analg. 2007;104:1223–1229. [tables of contents]. doi: 10.1213/01.ane.0000263280.49786.f5 [DOI] [PubMed] [Google Scholar]
- 9.Moriarty O, McGuire BE, Finn DP. The effect of pain on cognitive function: a review of clinical and preclinical research. Prog Neurobiol. 2011;93:385–404. doi: 10.1016/j.pneurobio.2011.01.002 [DOI] [PubMed] [Google Scholar]
- 10.Hayashida KI, Obata H. Strategies to treat chronic pain and strengthen impaired descending noradrenergic inhibitory system. Int J Mol Sci. 2019;20:822. doi: 10.3390/ijms20040822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salter MW, Stevens B. Microglia emerge as central players in brain disease. Nat Med. 2017;23:1018–1027. doi: 10.1038/nm.4397 [DOI] [PubMed] [Google Scholar]
- 12.Warner NS, Mielke MM, Verdoorn BP, et al. Pain, opioid analgesics, and cognition: a conceptual framework in older adults. Pain Med. 2023;24:171–181. doi: 10.1093/pm/pnac113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khamisy-Farah R, Fund E, Raibman-Spector S, Adawi M. Inflammatory markers in the diagnosis of fibromyalgia. Isr Med Assoc J. 2021;23:801–804. [PubMed] [Google Scholar]
- 14.Shu B, Xu F, Zheng X, et al. Change in perioperative neutrophil-lymphocyte ratio as a potential predictive biomarker for chronic postsurgical pain and quality of life: an ambispective observational cohort study. Front Immunol. 2023;14:1177285. doi: 10.3389/fimmu.2023.1177285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parisien M, Lima LV, Dagostino C, et al. Acute inflammatory response via neutrophil activation protects against the development of chronic pain. Sci Transl Med. 2022;14:eabj9954. doi: 10.1126/scitranslmed.abj9954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo J, Thomassen JQ, Nordestgaard BG, Tybjærg-Hansen A, Frikke-Schmidt R. Blood leukocyte counts in Alzheimer disease. JAMA Network Open. 2022;5:e2235648. doi: 10.1001/jamanetworkopen.2022.35648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yong R, Meng Y. Preoperative neutrophil-lymphocyte ratio, an independent risk factor for postoperative cognitive dysfunction in elderly patients with gastric cancer. Geriatr Gerontol Int. 2020;20:927–931. doi: 10.1111/ggi.14016 [DOI] [PubMed] [Google Scholar]
- 18.Sung YT, Wu JS. The visual analogue scale for rating, ranking and paired-comparison (VAS-RRP): a new technique for psychological measurement. Behav Res Methods. 2018;50:1694–1715. doi: 10.3758/s13428-018-1041-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shafshak TS, Elnemr R. The visual analogue scale versus numerical rating scale in measuring pain severity and predicting disability in low back pain. J Clin Rheumatol. 2021;27:282–285. doi: 10.1097/RHU.0000000000001320 [DOI] [PubMed] [Google Scholar]
- 20.Portenoy R. Development and testing of a neuropathic pain screening questionnaire: ID pain. Curr Med Res Opin. 2006;22:1555–1565. doi: 10.1185/030079906X115702 [DOI] [PubMed] [Google Scholar]
- 21.Reyes-Gibby C, Morrow PK, Bennett MI, Jensen MP, Shete S. Neuropathic pain in breast cancer survivors: using the ID pain as a screening tool. J Pain Symptom Manage. 2010;39:882–889. doi: 10.1016/j.jpainsymman.2009.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janes H, Pepe MS. Adjusting for covariates in studies of diagnostic, screening, or prognostic markers: an old concept in a new setting. Am J Epidemiol. 2008;168:89–97. doi: 10.1093/aje/kwn099 [DOI] [PubMed] [Google Scholar]
- 23.Scholich SL, Hallner D, Wittenberg RH, Hasenbring MI, Rusu AC. The relationship between pain, disability, quality of life and cognitive-behavioural factors in chronic back pain. Disabil Rehabil. 2012;34:1993–2000. doi: 10.3109/09638288.2012.667187 [DOI] [PubMed] [Google Scholar]
- 24.Tarasidis GS, DeConde AS, Mace JC, et al. Cognitive dysfunction associated with pain and quality of life in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2015;5:1004–1009. doi: 10.1002/alr.21578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J, Wang X, Xu Z. The relationship between chronic pain and cognitive impairment in the elderly: a review of current evidence. J Pain Res. 2023;16:2309–2319. doi: 10.2147/JPR.S416253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phelps CE, Navratilova E, Porreca F. Cognition in the chronic pain experience: preclinical insights. Trends Cognit Sci. 2021;25:365–376. doi: 10.1016/j.tics.2021.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nahin RL, DeKosky ST. Comorbid pain and cognitive impairment in a nationally representative adult population: prevalence and associations with health status, health care utilization, and satisfaction with care. Clin J Pain. 2020;36:725–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCracken LM, Iverson GL. Predicting complaints of impaired cognitive functioning in patients with chronic pain. J Pain Symptom Manage. 2001;21:392–396. doi: 10.1016/S0885-3924(01)00267-6 [DOI] [PubMed] [Google Scholar]
- 29.Povedano M, Gascón J, Gálvez R, Ruiz M, Rejas J. Cognitive function impairment in patients with neuropathic pain under standard conditions of care. J Pain Symptom Manage. 2007;33:78–89. doi: 10.1016/j.jpainsymman.2006.07.012 [DOI] [PubMed] [Google Scholar]
- 30.Harada CN, Natelson Love MC, Triebel KL. Normal cognitive aging. Clin Geriatr Med. 2013;29:737–752. doi: 10.1016/j.cger.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panza F, Lozupone M, Solfrizzi V, et al. Different cognitive frailty models and health- and cognitive-related outcomes in older age: from epidemiology to prevention. J Alzheimers Dis. 2018;62:993–1012. doi: 10.3233/JAD-170963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El Tumi H, Johnson MI, Dantas PBF, Maynard MJ, Tashani OA. Age-related changes in pain sensitivity in healthy humans: a systematic review with meta-analysis. Eur J Pain. 2017;21:955–964. doi: 10.1002/ejp.1011 [DOI] [PubMed] [Google Scholar]
- 33.Lautenbacher S, Peters JH, Heesen M, Scheel J, Kunz M. Age changes in pain perception: a systematic-review and meta-analysis of age effects on pain and tolerance thresholds. Neurosci Biobehav Rev. 2017;75:104–113. doi: 10.1016/j.neubiorev.2017.01.039 [DOI] [PubMed] [Google Scholar]
- 34.Jones RN, Fong TG, Metzger E, et al. Aging, brain disease, and reserve: implications for delirium. Am J Geriatr Psychiatry. 2010;18:117–127. doi: 10.1097/JGP.0b013e3181b972e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buchman AS, Yu L, Wilson RS, Schneider JA, Bennett DA. Association of brain pathology with the progression of frailty in older adults. Neurology. 2013;80:2055–2061. doi: 10.1212/WNL.0b013e318294b462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stern Y, Arenaza-Urquijo EM, Bartrés-Faz D, et al. Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. 2020;16:1305–1311. doi: 10.1016/j.jalz.2018.07.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Apkarian AV, Sosa Y, Sonty S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24:10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidt-Wilcke T, Leinisch E, Gänssbauer S, et al. Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain. 2006;125:89–97. doi: 10.1016/j.pain.2006.05.004 [DOI] [PubMed] [Google Scholar]
- 39.Alshuft HM, Condon LA, Dineen RA, Auer DP. Cerebral cortical thickness in chronic pain due to knee osteoarthritis: the effect of pain duration and pain sensitization. PLoS One. 2016;11:e0161687. doi: 10.1371/journal.pone.0161687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Segura-Jiménez V, Estévez-López F, Soriano-Maldonado A, et al. Gender differences in symptoms, health-related quality of life, sleep quality, mental health, cognitive performance, pain-cognition, and positive health in Spanish fibromyalgia individuals: the al-ándalus project. Pain Res Manag. 2016;2016:5135176. doi: 10.1155/2016/5135176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roth RS, Geisser ME, Theisen-Goodvich M, Dixon PJ. Cognitive complaints are associated with depression, fatigue, female sex, and pain catastrophizing in patients with chronic pain. Arch Phys Med Rehabil. 2005;86:1147–1154. doi: 10.1016/j.apmr.2004.10.041 [DOI] [PubMed] [Google Scholar]
- 42.Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth. 2013;111:52–58. doi: 10.1093/bja/aet127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.ter Horst JP, de Kloet ER, Schächinger H, Oitzl MS. Relevance of stress and female sex hormones for emotion and cognition. Cell Mol Neurobiol. 2012;32:725–735. doi: 10.1007/s10571-011-9774-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aloisi AM, Sorda G. Relationship of female sex hormones with pain perception: focus on estrogens. Pain Manag. 2011;1:229–238. doi: 10.2217/pmt.11.13 [DOI] [PubMed] [Google Scholar]
- 45.Pozzi S, Benedusi V, Maggi A, Vegeto E. Estrogen action in neuroprotection and brain inflammation. Ann N Y Acad Sci. 2006;1089:302–323. doi: 10.1196/annals.1386.035 [DOI] [PubMed] [Google Scholar]
- 46.Zhang YX, Shen ZY, Jia YC, et al. The association of the neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, lymphocyte-to-monocyte ratio and systemic inflammation response index with short-term functional outcome in patients with acute ischemic stroke. J Inflamm Res. 2023;16:3619–3630. doi: 10.2147/JIR.S418106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao L, Cui M, Yang S, Zhou H, Li M. Systemic inflammatory indicators and risk of incident metabolically unhealthy phenotype. J Inflamm Res. 2024;17:6905–6916. doi: 10.2147/JIR.S474201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao Q, Gao R, Liu C, et al. Dynamic change of lymphocyte-to-monocyte is associated with the occurrence of POCD after cardiovascular surgery: a prospective observational study. Front Behav Neurosci. 2021;15:646528. doi: 10.3389/fnbeh.2021.646528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adams-Huet B, Jialal I. The neutrophil count is superior to the neutrophil/lymphocyte ratio as a biomarker of inflammation in nascent metabolic syndrome. Ann Clin Biochem. 2019;56:715–716. doi: 10.1177/0004563219866221 [DOI] [PubMed] [Google Scholar]
- 50.Karon BS, Tolan NV, Wockenfus AM, et al. Evaluation of lactate, white blood cell count, neutrophil count, procalcitonin and immature granulocyte count as biomarkers for sepsis in emergency department patients. Clin Biochem. 2017;50:956–958. doi: 10.1016/j.clinbiochem.2017.05.014 [DOI] [PubMed] [Google Scholar]
- 51.Fa W, Liang X, Liu K, et al. Associations of blood absolute neutrophil count and cytokines with cognitive function in dementia-free participants: a population-based cohort study. J Gerontol a Biol Sci Med Sci. 2024;79. doi: 10.1093/gerona/glad231 [DOI] [PubMed] [Google Scholar]
- 52.Dong X, Nao J, Shi J, Zheng D. Predictive value of routine peripheral blood biomarkers in Alzheimer’s disease. Front Aging Neurosci. 2019;11(332). doi: 10.3389/fnagi.2019.00332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shad KF, Aghazadeh Y, Ahmad S, Kress B. Peripheral markers of Alzheimer’s disease: surveillance of white blood cells. Synapse. 2013;67:541–543. doi: 10.1002/syn.21651 [DOI] [PubMed] [Google Scholar]
- 54.Chen C, Kumbhar R, Wang H, et al. Lymphocyte-activation gene 3 facilitates pathological tau neuron-to-neuron transmission. Adv Sci. 2024;11:e2303775. doi: 10.1002/advs.202303775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dong Y, Lagarde J, Xicota L, et al. Neutrophil hyperactivation correlates with Alzheimer’s disease progression. Ann Neurol. 2018;83:387–405. doi: 10.1002/ana.25159 [DOI] [PubMed] [Google Scholar]
- 56.Eckert A, Förstl H, Zerfass R, Hartmann H, Müller WE. Lymphocytes and neutrophils as peripheral models to study the effect of beta-amyloid on cellular calcium signalling in Alzheimer’s disease. Life Sci. 1996;59:499–510. doi: 10.1016/0024-3205(96)00329-3 [DOI] [PubMed] [Google Scholar]
- 57.Feng W, Zhang Y, Ding S, et al. B lymphocytes ameliorate Alzheimer’s disease-like neuropathology via interleukin-35. Brain Behav Immun. 2023;108:16–31. doi: 10.1016/j.bbi.2022.11.012 [DOI] [PubMed] [Google Scholar]
- 58.de Sousa LP, Rosa-Gonçalves P, Ribeiro-Gomes FL, Daniel-Ribeiro CT. Interplay between the immune and nervous cognitive systems in homeostasis and in malaria. Int J Biol Sci. 2023;19:3383–3394. doi: 10.7150/ijbs.82556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Princiotta Cariddi L, Mauri M, Cosentino M, Versino M, Marino F. Alzheimer’s disease: from immune homeostasis to neuroinflammatory condition. Int J Mol Sci. 2022;23:13008. doi: 10.3390/ijms232113008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used or analyzed during the current study are available from the corresponding author upon reasonable request.