Abstract
Background
Obstructive sleep apnea (OSA) is the most common sleep-related breathing disorder characterized by recurrent upper airway collapse and obstruction, leading to reduced or absent breathing during sleep, especially rapid eye movement (REM) sleep, and continuous positive airway pressure treatment (CPAP) is often used for treatment of OSA. Sawtooth waves (STWs) are a characteristic of REM sleep.
Objective
To examine effects of CPAP treatment on STWs during REM sleep in the OSA patients.
Methods
Polysomnographic recordings were performed on 20 moderate-to-severe OSA patients and 16 normal controls, and comparisons of STWs during REM sleep in the OSA patients with and without CPAP treatment (paired t-test or Wilcoxon signed-rank test wherever appropriate), and between OSA patients and normal controls (Student’s t-test or Wilcoxon rank-sum test) were carried out. In addition, linear correlation analyses were used to estimate the relationship of STWs and REM sleep with duration of non-REM (NREM) sleep stage 3 (N3).
Results
The STWs were classified to be apnea/hypopnea associated and not associated (isolated), and the amplitude of the isolated STWs was significantly higher than that of the apnea/hypopnea associated. With CPAP treatment, the percentage of REM sleep with STWs and the amplitude of STWs were significantly increased to the levels, which were not significantly different from those in the normal controls, while the frequency of STWs was not significantly changed. In addition, the total duration of REM sleep and the duration of REM sleep with STWs were both positively correlated with the duration of N3 sleep in the normal controls and the OSA patients with CPAP treatment. Furthermore, CPAP treatment also caused a significant increase in the duration of rapid eye movements in REM sleep.
Conclusion
These findings suggest that there are some interconnections between NREM and REM sleep, and STWs not only represent the quality of REM sleep but also are correlated with N3 sleep.
Keywords: sawtooth wave, obstructive sleep apnea, continuous positive airway pressure, rapid eye movement sleep, non-rapid eye movement sleep
Introduction
Obstructive sleep apnea (OSA) is the most common sleep-related breathing disorder characterized by recurrent partial or full obstruction in the upper respiratory tract resulting in apnea/hypopnea, disruption of sleep with multiple microarousals, sleep fragmentation and reduction of deep sleep.1–3 OSA has negative influences on multiple systems, such as circulatory system and endocrine system,4 and high blood pressure, diabetes mellitus and stroke commonly occur in OSA patients.2,4,5 In addition, OSA patients often have excessive daytime sleepiness (EDS), fatigue, and cognitive impairments.6,7
Continuous positive airway pressure (CPAP) is the most recommended treatment method for moderate-to-severe OSA patients,8,9 and can improve sleep quality and oxygen saturation during sleep, both of which contribute to relief of OSA symptoms such as snore, excessive daytime sleep, cognitive dysfunction and other complications of OSA.10 With CPAP treatment, the duration of non-rapid eye movement sleep stage 3 (N3) is prolonged, and amplitude and power of delta waves of N3 sleep are also increased.11,12
According to the criteria established by the American Academy of Sleep Medicine (AASM),13 sawtooth waves are defined as 2–6 Hz electroencephalographic (EEG) waves during rapid eye movement (REM) sleep, which are found in all brain regions, except the occipital lobe, and more predominant in the central vertex. From intracranial EEG recordings, sawtooth waves are observed to be associated with a widespread increase in high-frequency activity including ripples (80–250 Hz) in the brain such as the sensorimotor cortex, associative areas, and limbic structures.14 These electrophysiological properties suggest sawtooth waves may be involved in cognitive processes by driving faster EEG activities and orchestrating synchronized reactivations in different networks necessary for cognitive processes such as memory consolidation during REM sleep.14 In addition, a previous study has shown two distinctive clusters of delta waves with different properties during REM sleep in healthy human subjects, namely a frontal-central cluster characterized by faster, relatively large, and notched EEG waves (“sawtooth waves”) with a positive correlation with gamma wave activity and rapid eye movements, and a medial-occipital cluster containing more isolated, smaller and slower (<2 Hz) waves negatively related to gamma wave activity, and not associated with rapid eye movements.15 However, whether sawtooth waves, as a delta wave subtype in human REM sleep, were correlated with N3 sleep was still unclear. Furthermore, previous studies reported that CPAP treatment could increase the duration of REM sleep,13,16 but it was unclear how CPAP treatment affected EEG activities during REM sleep. Therefore, the current study aimed to examine whether there was any correlation of sawtooth waves during REM sleep with N3 sleep and whether there were any effects of CPAP treatment on sawtooth waves during REM sleep in OSA patients.
Materials and Methods
Subjects
The normal controls were recruited from the subjects who underwent routine checkup (eg, physical examination, blood glucose, blood pressure, and electrocardiographic examination) at the Jiangxi Provincial People’s Hospital during the period from May 2020 to June 2023. Those who had good general health (eg without ischemic artery disease, hypertension, and diabetes mellitus, etc.) and good sleep quality with apnea/hypopnea index (AHI) <5 events per hour of sleep confirmed by polysomnographic (PSG) examination, and did not have a history of excessive daytime sleepiness, irregular sleep or any conditions listed in the exclusion criteria (eg insomnia) below were included as the normal controls.17
To test effects of CPAP treatment on sawtooth waves during REM sleep in the OSA patients, the OSA patients were recruited from the inpatients of the Jiangxi Provincial People’s Hospital who underwent a series of routine medical tests and PSG examination due to OSA during the period from June 2020 to June 2023. Inclusion criteria for the patients were as follows: 1) AHI > 15 events/hour of sleep; 2) ages between 30 and 70 years old; 3) a minimum arterial oxygen saturation (SaO2) < 90% during sleep.
The OSA patients were not allowed to drink caffeine, tea and other stimulating beverages 12 h before PSG recordings, and they were excluded from the study if they had any of the following conditions: 1) primary and secondary pulmonary diseases, or chronic obstructive pulmonary disease; 2) hypertension (Stage II or higher) or symptomatic ischemic artery disease [New York Heart Association (NYHA) Functional Class II or worse]; 3) heart diseases such as cardiomyopathy and rheumatic heart disease; 4) uncontrolled diabetes mellitus with complications; 5) neurological diseases such as stroke and neurodegenerative diseases; 6) a history of other sleep disorders (eg insomnia); 7) alcohol and substance abuse; 8) use of psychotropics or other medications known to influence sleep, EEG or respiration within a month prior to the study.
PSG recordings were performed on the patients without and with CPAP treatment in two separated nights and on the normal controls as in previous studies.12,17 Prior to the experiment, the CPAP machine was programmed and tried to make sure that the patients could tolerate without feeling uncomfortable. Then, PSG recordings began and continued in the titration night until the patients woke up next morning around 7–8 a.m.12,17
Before the start of experiments, the sample size was estimated with G*Power (version 3.1.9.7, http://www.gpower.hhu.de/) and it was found that a minimum of 15 patients and 15 normal controls were needed to achieve a desired statistical power (>0.8) based on preliminary experiments. After the experiments were finished, the statistical power of each test with P ≥ 0.05 was calculated and all of the tests had a desired statistical power.
Polysomnographic Recordings
PSG recordings were performed using a computerized recording system (Pro Fusion PSG 4 System, Compumedics Limited, Abbotsford, Victoria, Australia) as described in the previous studies.12,16 Briefly, EEG (F4-M1, C4-M1, O2-M1, F3-M2, C3-M2, O1-M2), electrocardiographic (ECG), electrooculographic (EOG) activities, and electromyographic (EMG) activities from jaw muscles (mylohyoid and masseter), upper (bilateral flexor and extensor carpi radialis) and lower limb muscles (bilateral gastrocnemius and tibialis anterior muscle), and respiration via thermistor, nasal pressure transducer and thoracoabdominal plethysmography were continuously recorded. In addition, the peripheral oxygen saturation via a percutaneous finger pulse oximetry, body position via a position sensor, and snoring sound via an audio recorder were also recorded at the same time. Furthermore, the patients’ or normal control subjects’ movements were simultaneously monitored and recorded with two video cameras focusing on the patients’ or normal control subjects’ head (DS-2CD3386FWDV2-IS, Hangzhou Hikvision Digital Technology Co., Ltd., Hangzhou, Zhejiang, China) and body (HANWHA QND-6022R, Shenzhen Simkeway Technology Co., Ltd., Shenzhen, Guangdong, China), respectively.
A Philips CPAP device equipped with a nasal mask (BIPAP Auto767P, Bi-Flex mode, Murrysville, PA, USA) was used in this study, and an algorithm developed by the company and a pressure generator were utilized to analyze airflow and provide a synchronized breathing curve.12,17 The device was a bilevel positive airway pressure (BiPAP) machine, which could automatically sense the patients’ breathing pattern and switch between inhalation positive airway pressure (IPAP) and exhalation positive airway pressure (EPAP) pressure and adjust the two pressure levels automatically. To decrease the influence of EMG artifacts, the EEG signals were filtered with a high-pass filter set at 0.01 Hz and a low-pass filter set at 35 Hz.12,17
Data Analysis
Data were initially analyzed with the Pro Fusion PSG 4 software (Compumedics Limited, Abbotsford, Victoria, Australia) and then with Spike2 (Version 8.07, CED, Milton, Cambridge, UK) according to the criteria established by the AASM.13 An episode of OSA events was defined as a ≥90% decrease in airflow from baseline with respiratory efforts lasting at least 10 seconds, while an episode of obstructive sleep hypopnea events was defined as a ≥30% decrease in airflow from respiratory baseline accompanied by a ≥3% decrease in SpO2 or an arousal.13 The severity of OSA was assessed by the AHI, minimum SpO2, and the duration of sleep with SpO2 below 90%.12,17
Since the sawtooth waves were predominant in the central region of the brain and there was no significant difference in sawtooth waves between the left and right hemispheres according to previous studies, EEG activities recorded from C4 channel were selected for analysis.15,18,19 According to the criteria established by the AASM,13 sawtooth waves were defined as 3 or more continuous EEG waves with amplitudes greater than 20 μV and a frequency of 2–6 Hz, predominantly in bilateral central vertex during REM sleep. When partial or whole cluster of sawtooth waves overlapped with apnea/hypopnea in time, they were considered to be apnea/hypopnea-associated.
Statistical Analysis
Data were first examined for their normality with Shapiro–Wilk test and if P value was greater than 0.05, data were considered to be normally distributed and were shown as mean ± standard error of the mean (SEM) as the null-hypothesis of this test was that the population was normally distributed; otherwise, data were shown as median (minimum–maximum). Paired t-test or Wilcoxon signed-rank test were used to compare paired data (ie, with and without CPAP treatment) wherever appropriate. Student’s t-test and Wilcoxon rank-sum test were used for comparisons of unpaired data with normal and skewed distributions, respectively. Statistical analysis was performed using the SPSS statistical software package (Version 25, IBM Corp., Armonk, NY, USA). P < 0.05 was considered to be statistically significant.
Results
General Sleep Features
A total of 32 OSA patients were recruited, and among them, 12 patients were excluded since they had one or more of the conditions listed in the exclusion criteria or could not meet the inclusion criteria. A total of 20 patients [16 males and 4 females; ages: 51.35 ± 2.58 years old (mean ± SEM); age range: 30–69 years old] with moderate-to-severe OSA and the Epworth Sleepiness Scale score (ESS) >10 and 16 age-matched normal controls [13 males and 3 females; age: 50.69 ± 2.15 years old (mean ± SEM); age range: 30-69 years old] were included in the study, and their demographic data, sleep parameters and respiratory variables were summarized in Table 1. CPAP treatment did not significantly change the total sleep time, but significantly prolonged the duration of N3 sleep to 76.20 ± 7.55 min from 47.20 ± 5.96 min without CPAP treatment. Similarly, the duration of REM sleep was also significantly increased to 73.23 ± 7.56 min with CPAP treatment from 54.88 ± 4.25 min without CPAP treatment. Furthermore, the durations and percentages of N3 and REM sleep were both significantly increased, while the durations and percentages of N1 and N2 sleep were both significantly decreased. Additionally, the PLMI, SBI, wake after sleep onset (WASO) and arousal indexes during N1-N3 and REM sleep were all significantly decreased with CPAP treatment.
Table 1.
Changes in General Sleep and Respiratory Variables with CPAP Treatment
| Variables | Normal control | OSA patients | P& | P&& | |
|---|---|---|---|---|---|
| NO CPAP | CPAP | ||||
| Age (years) | 50.69 ± 2.15 | 51.35 ± 2.58 | NS | ||
| Sex | 13M, 3F | 16 M, 4 F | |||
| BMI (kg/m²) | 23.47 ± 0.66 | 28.29 ± 0.95 | < 0.001* | ||
| Total sleep time (min) | 397.00±16.68 | 393.90±11.70 | 396.30±11.54 | NS | NS |
| N1 sleep (min) | 37.25 (17.50–66.00) | 47.25 (20.50–100.50) | 31.75 (17.00–91.00) | < 0.01# | NS |
| N2 sleep (min) | 219.10 ± 11.46 | 241.50±10.74 | 212.50±14.00 | < 0.05* | NS |
| N3 sleep (min) | 70.47 ± 4.80 | 47.20±5.96 | 76.20±7.55 | < 0.001* | NS |
| REM sleep (min) | 69.63 ± 4.79 | 54.88±4.25 | 73.23±7.56 | < 0.05* | NS |
| N1 sleep (%) | 9.58±0.70 | 12.25 (6.30–19.60) | 7.95 (4.40–20.10) | < 0.01# | NS |
| N2 sleep (%) | 55.09±1.56 | 61.17±1.84 | 53.70±3.15 | < 0.05* | NS |
| N3 sleep (%) | 17.88±1.10 | 12.32±1.63 | 19.30±1.78 | < 0.001* | NS |
| REM sleep (%) | 17.46±0.92 | 13.82±0.83 | 18.24±1.68 | < 0.05# | NS |
| Sleep efficiency (%) | 89.75±1.24 | 80.55±1.75 | 85.15±1.46 | < 0.05* | < 0.05* |
| WASO (min) | 34.47±3.98 | 84.50±9.24 | 59.80±7.07 | < 0.05* | < 0.01* |
| Arousal index | |||||
| N1 sleep | 15.34±1.68 | 33.92±4.94 | 18.83±3.47 | < 0.01* | NS |
| N2 sleep | 17.17±2.01 | 38.80±5.34 | 16.70±2.61 | < 0.0001* | NS |
| N3 sleep | 7.14 (2.68–19.24) | 15.92 (1.01–84.71) | 5.80 (0.00–24.26) | < 0.05# | NS |
| REM sleep | 8.80 (3.69–19.78) | 16.67 (5.38–65.71) | 6.18 (1.40–25.56) | < 0.001# | NS |
| Total sleep | 13.87(4.04–22.57) | 29.50 (1.80–90.30) | 13.10 (1.90–31.00) | < 0.0001# | NS |
| AHI (events/h) | 2.45 (0.40–4.40) | 42.90 (15.2–93.20) | 2.80 (0.00–18.80) | < 0.0001# | NS |
| Minimal SpO2 (%) | 90.00 (86.00–92.00) | 66.00 (53.00–81.00) | 90.00 (73.00–93.00) | < 0.0001# | NS |
| Mean SpO2 (%) | 96.00 (95.00–98.00) | 95.00 (84.00–96.00) | 96.00 (94.00–98.00) | < 0.0001# | NS |
| Time with SpO2<90% (min) | 0.00 (0.00–14.50) | 22.20 (3.70–331.70) | 0.10 (0.00–27.20) | < 0.0001# | NS |
| Duration of apnea (s) | 16.50±2.39 | 27.45±1.35 | 15.30±2.25 | < 0.0001* | NS |
| Duration of hypopnea (s) | 20.56±1.80 | 23.80±1.10 | 18.70±1.52 | < 0.01* | NS |
| PLMI (events/h) | 2.50 (0.00–11.20) | 9.60 (0.80–66.60) | 1.20 (0.00–61.90) | < 0.01# | NS |
| SBI (events/h) | 0.45 (0.00–1.60) | 0.46 (0.19–2.47) | 0.47 (0.13–1.45) | NS | NS |
Notes: #Data with a skewed distribution were presented as median (minimum-maximum) and Wilcoxon signed-rank test and Wilcoxon rank-sum test were used for comparisons of paired and unpaired samples, respectively. *Normally distributed data were presented as mean ± SEM and paired t-test and Student’s t-test were used for comparisons of paired and unpaired samples, respectively.
Abbreviations: M, male; F: female; AHI, apnea/hypopnea index; CPAP, continuous positive airway pressure; N1, N2, and N3, non-REM sleep stage 1, 2 and 3; REM, rapid eye movement; PLMI, periodic leg movements index; SBI, sleep bruxism index; WASO, wake after sleep onset. P&, NO CPAP VS CPAP. P&&, Normal control VS CPAP. NS, nonsignificant.
The respiratory variables were significantly improved with CPAP treatment. With CPAP treatment, the minimal SpO2 was significantly increased to 90.00% (73.00%–93.00%) from 66.00% (53.00%–81.00%) and mean SpO2 was significantly increased to 96.00% (94.00%–98.00%) from 95.00% (84.00%–96.00%). Meanwhile, with CPAP treatment, the mean duration of apnea was significantly decreased to 15.30 ± 2.25 s from 27.45 ± 1.35 s, and the mean duration of hypopnea was also significantly decreased to 18.70 ± 1.52 s from 23.80 ± 1.10 s. In addition, AHI was significantly decreased to 2.30 (0.00–18.80) from 42.90 (15.2–93.20).
In the OSA patients with CPAP treatment, almost all PSG parameters were significantly changed to the levels which were not significantly different from those in normal controls, except wake after sleep onset (WASO) and sleep efficiency (Table 1). Sleep efficiency and WASO in the OSA patients with CPAP treatment were still significantly lower (P<0.05) and longer (P<0.01) than those in the normal controls, respectively.
Effects of CPAP Treatment on Duration of Sawtooth Waves During REM Sleep
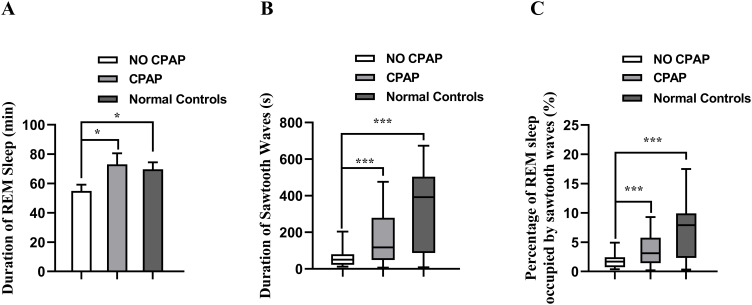
Sawtooth waves usually occurred after the muscle tone reduction or simultaneously, but before eruption of rapid eye movements. With CPAP treatment, the duration of REM sleep was significantly increased to 73.03 ± 7.53 min from 54.88 ± 4.25 min (Figure 1A; P < 0.05), and the duration of EEG segments containing sawtooth waves during REM sleep was significantly increased to 117.40 s (6.84–476.20 s) from 50.55 s (12.57–203.69 s) (Figure 1B; P < 0.001). In addition, with CPAP treatment, the percentage of REM sleep occupied by sawtooth waves was significantly increased to 3.12% (0.21%–9.28%) from 1.68% (0.37%–4.92%) (Figure 1C; P < 0.001). In the normal controls, the duration of REM sleep, the duration of EEG segments containing sawtooth waves in REM sleep, and the percentage of REM sleep occupied by sawtooth waves were significantly higher than those in the OSA patients without CPAP treatment (P < 0.05–0.01), but were not significantly different from those in the OSA patients with CPAP treatment (Figure 1).
Figure 1.
Comparison of the duration of REM sleep (A), duration of sawtooth waves (B) and percentages of REM sleep occupied by sawtooth waves (C) in the OSA patients and normal controls. Data with a normal distribution are expressed as mean +SEM, and examined with paired t-test (No CPAP vs CPAP) or Student’s t-test (Normal controls vs CPAP/No CPAP) (A), and data with a skewed distribution are shown as median (minimum-maximum) and examined with Wilcoxon signed rank test (No CPAP vs CPAP) or Wilcoxon rank-sum test (Normal controls vs CPAP/No CPAP) (B and C), *P < 0.05, ***P < 0.001.
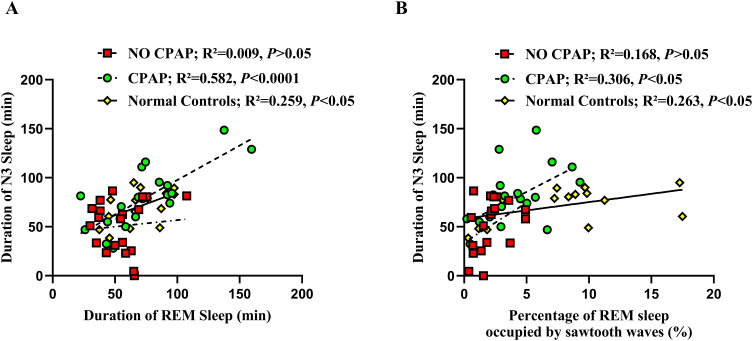
Correlation of Duration of REM Sleep with Duration of N3 Sleep
As shown in Figure 2A, the duration of REM sleep was not significantly correlated with that of N3 sleep in the OSA patients without CPAP treatment (R2 = 0.009; P > 0.05). However, with CPAP treatment, the duration of REM sleep was positively correlated with that of N3 sleep (R2 = 0.582; P < 0.001), which was similar to that in normal controls (R2 = 0.259, P < 0.05). Meanwhile, the percentage of REM sleep occupied by sawtooth waves was positively correlated with the duration of N3 sleep in the OSA patients with CPAP treatment (R2 = 0.306; P < 0.05) and in the normal controls (R2 = 0.263; P < 0.05), but not in the OSA patients without CPAP treatment (R2 = 0.168; P > 0.05) (Figure 2B).
Figure 2.
Correlations of the duration of REM sleep (A) and the percentage of REM sleep occupied by sawtooth waves with the duration of N3 sleep (B) in the OSA patients with and without CPAP treatment, and normal controls.
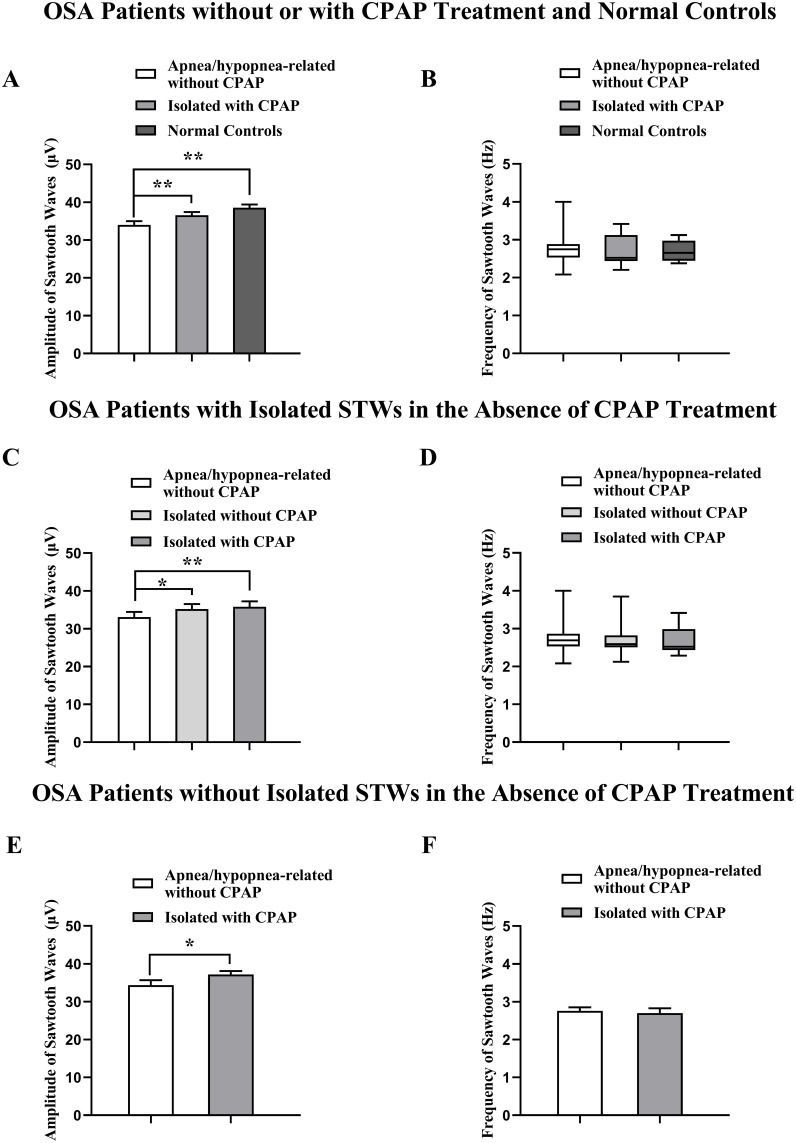
Effects of CPAP Treatment on the Amplitude and Frequency of Sawtooth Waves
Apnea/hypopnea associated sawtooth waves were found in all the 20 patients without CPAP treatment. The amplitudes of the apnea/hypopnea-associated sawtooth waves (34.00 ± 1.00 μV) were significantly smaller than those of isolated sawtooth waves in the OSA patients with CPAP treatment (36.55 ± 0.87 μV, P < 0.01; Figure 3A) and in the normal controls (38.56 ± 0.83 μV; P < 0.01). However, there was no significant difference in the amplitude of isolated sawtooth waves between the OSA patients with CPAP treatment and normal controls (P < 0.05). In contrast, the frequency of isolated sawtooth waves with CPAP treatment was not significantly different from that of apnea/hypopnea-associated sawtooth waves [No CPAP: 2.75 (2.08–4.00) Hz; CPAP: 2.52 (2.20–3.42) Hz; Figure 3B, P > 0.05)]. In addition, the frequency of isolated sawtooth waves in the normal controls [2.47 (2.32–3.07) Hz] was not significantly different from that in the OSA patients with CPAP treatment.
Figure 3.
Effects of CPAP treatment on the amplitude and frequency of sawtooth waves (STWs) during rapid eye movement sleep in the OSA patients with or without STWs (A and B), and in the OSA patients with (C and D) and without (E and F) isolated sawtooth waves in the absence of CPAP treatment. *P < 0.05, **P < 0.01.
Among the 20 OSA patients in the absence of CPAP treatment, isolated sawtooth waves during REM sleep were only found in 12 OSA patients, and no isolated sawtooth waves were found in the remaining 8 OSA patients. Although there were no significant differences in age, BMI, total sleep time, sleep efficiency, N1-N3 and REM sleep time and their proportions in total sleep, and WASO, AHI in each sleep stage and arousal indexes in N2, N3 and REM sleep, as well as AHI and arousal index during the entire sleep period were significantly lower (P < 0.01–0.05) in the OSA patients with isolated sawtooth waves than those in the patients without isolated sawtooth waves during REM sleep (Table 2). In addition, minimal SpO2, mean SpO2, and time with SpO2 <90% were significantly higher (P < 0.05), higher (P < 0.05) and shorter (P < 0.01) in the OSA patients with isolated sawtooth waves than those in the OSA patients without isolated sawtooth waves during sleep, respectively (Table 2).
Table 2.
Comparison of General Sleep and Respiratory Variables in the OSA Patients with and without Isolated Sawtooth Waves During REM Sleep Without CPAP Treatment
| Variables | With Isolated Sawtooth Waves | Without Isolated Sawtooth Waves | P |
|---|---|---|---|
| Age (years) | 53.08±3.12 | 48.75±1.44 | NS |
| Sex | 9M,3F | 7M, 1F | |
| BMI (kg/m²) | 26.90±0.95 | 30.36±1.71 | NS |
| Total sleep time (min) | 389.00±13.62 | 401.20±21.88 | NS |
| N1 sleep (min) | 51.25±4.77 | 48.88±9.72 | NS |
| N2 sleep (min) | 236.40±15.12 | 249.10±15.15 | NS |
| N3 sleep (min) | 47.25±7.15 | 47.13±10.97 | NS |
| REM sleep (min) | 54.13±4.58 | 56.00±8.52 | NS |
| N1 sleep (%) | 13.35±1.29 | 11.71±1.85 | NS |
| N2 sleep (%) | 60.18±2.15 | 62.65±3.34 | NS |
| N3 sleep (%) | 12.64±2.09 | 11.83±2.78 | NS |
| REM sleep (%) | 13.83±0.98 | 13.81±1.70 | NS |
| WASO (min) | 90.71±13.00 | 75.19±12.67 | NS |
| Sleep efficiency (%) | 80.00±2.37 | 81.38±2.73 | NS |
| Sleep cycles with N3 sleep | 3 (0–4) | 3.5 (1–4) | NS |
| Arousal index (events/h) | |||
| N1 sleep | 28.97±4.71 | 32.86±10.82 | NS |
| N2 sleep | 28.82±4.43 | 53.78±9.65 | < 0.05* |
| N3 sleep | 8.76±2.67 | 36.41±12.57 | < 0.05* |
| REM sleep | 18.07±3.63 | 37.38±8.37 | < 0.05* |
| Total sleep | 24.97±3.45 | 47.51±8.96 | < 0.05* |
| AHI (events/h) | |||
| N1 sleep | 42.42±5.57 | 76.42±9.05 | < 0.01* |
| N2 sleep | 32.82±4.77 | 59.25±9.67 | < 0.05* |
| N3 sleep | 13.98±3.52 | 47.10±12.22 | < 0.01* |
| REM sleep | 44.37±3.47 | 67.99±5.90 | < 0.01* |
| Total sleep | 33.45±3.49 | 61.45±8.46 | < 0.01* |
| Minimal SpO2 (%) | 77.00 (58.00–82.00) | 60.00 (53.00–78.00) | < 0.05# |
| Mean SpO2 (%) | 95.00 (90.00–96.00) | 92.00 (84.00–96.00) | < 0.05# |
| Time with SpO2 <90% (min) | 16.05 (3.70–142.10) | 78.40 (20.70–331.70) | < 0.01# |
| Duration of apnea (s) | 27.92±2.11 | 26.75±1.32 | NS |
| Maximal duration of apnea (s) | 59.25±5.33 | 64.00±6.67 | NS |
| Duration of hypopnea (s) | 25.25±1.53 | 21.63±1.22 | NS |
| Maximal duration of hypopnea (s) | 60.58±5.67 | 56.13±6.37 | NS |
| PLMI (events/h) | 9.55 (0.80–66.60) | 11.85 (1.70–52.90) | NS |
| SBI (events/h) | 0.52 (0.37–2.47) | 0.38 (0.19–0.83) | NS |
Notes: #: Data with a skewed distribution were presented as median (minimum-maximum) and Wilcoxon rank-sum tests were used for comparisons. *: Normally distributed data were presented as mean ± SEM and Student’s t-tests were used for comparisons. NS: nonsignificant.
Abbreviations: M: male; F: female; CPAP: continuous positive airway pressure; REM: rapid eye movement; N1, N2, and N3: non-REM sleep stage 1, 2 and 3; PLMI: periodic leg movements index; AHI: apnea/hypopnea index; WASO: wake after sleep onset; SBI: sleep Bruxism index.
In the OSA patients with isolated sawtooth waves without CPAP treatment, the amplitudes of isolated sawtooth waves (35.18 ± 1.35 μV, P < 0.05) without CPAP treatment were significantly higher than those of the apnea/hypopnea associated sawtooth waves (33.09 ± 1.36 μV, P < 0.05) in the same OSA patients (Figure 3C). However, the amplitudes of isolated sawtooth waves in the OSA patients with CPAP treatment (35.81 ±1.41 μV) were not significantly different from those without CPAP treatment (P > 0.05). The amplitudes of isolated sawtooth waves in the OSA patients with CPAP treatment were significantly higher than those of apnea/hypopnea associated sawtooth waves in the OSA patients without CPAP treatment (Figure 3C, P < 0.01), but there were no significant differences in the frequencies between the isolated sawtooth waves [2.59 (2.12–3.85) Hz] and apnea/hypopnea associated sawtooth waves [2.69 (2.08–4.00) Hz] in the OSA patients without CPAP treatment, and between isolated sawtooth waves in the OSA patients with [2.52 (2.29–3.42) Hz] (Figure 3D, P > 0.05) and without CPAP treatment. Moreover, in the 8 OSA patients without isolated sawtooth waves during REM sleep, the amplitudes of isolated sawtooth waves in the OSA patients with CPAP treatment (37.21 ± 0.87 μV) were significantly higher than those of apnea/hypopnea associated sawtooth waves without CPAP treatment in the same patients (34.36 ± 1.33 μV) (Figure 3E, P < 0.05), while the frequencies were not significantly changed (Figure 3F, P > 0.05).
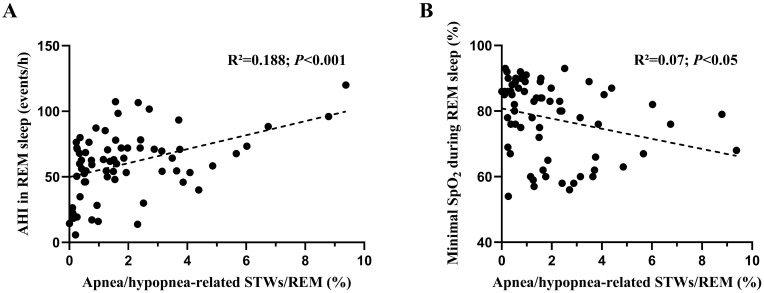
The percentage of REM sleep occupied by apnea/hypopnea-related sawtooth waves was positively and negatively correlated with AHI and minimal SpO2 in REM sleep, respectively (Figure 4A and B).
Figure 4.
Correlations of the percentage of REM sleep occupied by apnea/hypopnea-related sawtooth waves (STWs) with AHI in REM sleep (A) and with minimal SpO2 during REM sleep (B).
Effects of CPAP Treatment on Eye Movements in REM Sleep
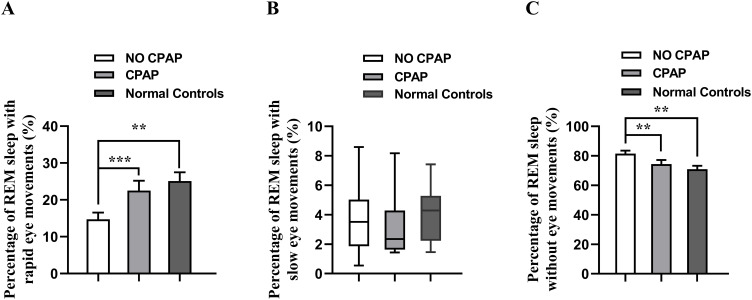
The percentage of REM sleep containing rapid eye movements in the OSA patients without CPAP treatment (14.72%±1.81%) was significantly smaller than those in the OSA patients with CPAP treatment (22.52%±2.69%, Figure 5A, P < 0.001) and in normal controls (25.11% ± 2.37%, Figure 5A, P < 0.01), while and the percentage of REM sleep without eye movements in the OSA patients without CPAP treatment (81.53%±2.04%) was significantly larger than those in the OSA patients with CPAP treatment (74.39%±2.80%, Figure 5C, P < 0.01) and in the normal controls (70.97% ± 2.24%, Figure 5C, P < 0.01). However, there were no significant differences in the percentage of REM sleep without eye movements or containing rapid eye movements between the normal controls and OSA patients with CPAP treatment.
Figure 5.
Comparisons of the percentage of REM sleep with rapid eye movements (A), slow eye movements (B), and without eye movements (C) in the OSA patients with and without CPAP treatment, and normal controls. **P < 0.01, ***P < 0.001.
The percentage of REM sleep containing slow eye movements was not significantly different in the OSA patients with and without CPAP treatment, and between the OSA patients and normal controls (Figure 5B, P > 0.05).
Discussion
Methods for treatment of OSA include sleep position adjustment, lifestyle modifications, orthodontic treatments, CPAP, pharmacological therapies, upper airway surgery, and hypoglossal nerve stimulation,20 and orthodontic treatments for mild to moderate OSA such as personalized oral appliances, mandibular positioning devices, rapid maxillary expansion, which are treatment options only when skeletal discrepancies exist, have demonstrated a significant reduction in AHI and alleviation of OSA symptoms.21,22
CPAP is the most commonly used treatment for OSA.8,9 In the current study, the authors systemically examined sawtooth waves during REM sleep and influence of CPAP treatment in the OSA patients, and found that there were two types of sawtooth waves, namely isolated and apnea/hypopnea-associated sawtooth waves during REM sleep and CPAP caused a significant increase in the duration of REM sleep, the percentage of REM sleep with sawtooth waves and the amplitudes of sawtooth waves without changes in the frequences. In addition, the duration of total sawtooth waves was positively correlated with the duration of N3 sleep in the OSA patients with CPAP treatment and the normal controls. These findings suggest that increases in isolated sawtooth waves during REM sleep might represent an improvement of sleep quality in OSA patients with CPAP treatment.
As OSA occurs most commonly in middle-aged and older adults and CPAP treatment was often used in moderate-to-severe OSA patients, only moderate-to-severe OSA patients between 30 and 70 years old were recruited for the current study. To eliminate potential confounding variables that might influence REM sleep and sawtooth waves, age-matched OSA patients and normal control subjects (P > 0.05) without taking psychotropics or other medications known to influence sleep, EEG or respiration within a month prior to the study were included. However, BMI of the OSA patients was significantly greater than that in the normal controls (P < 0.001) (Table 1) as OSA patients are often overweight.
Consistent with previous studies,12,23,24 it was found in the current study that the sleep quality in the OSA patients was significantly improved with CPAP treatment, which manifested as increased N3 and REM sleep as well as sleep efficiency, and decreased N1 and N2 sleep, arousal index and WASO (Table 1). These improvements might result from decreased AHI and increased SpO2. Significantly lower sleep efficiency and longer WASO in the OSA patients with CPAP treatment than the normal controls (Table 1) indicate that the patients might need some time to adapt to CPAP treatment.
Sawtooth waves were commonly found in EEG recordings during REM sleep.25 In the current study, sawtooth waves were found to occur after sequence of muscle tone reduction, and prior to the onset of the rapid eye movements as shown in the previous studies18,25 and might predict the eruption of rapid eye movements. Furthermore, it was found that there were two types of sawtooth waves, namely apnea/hypopnea associated and not associated (isolated), in REM sleep in the OSA patients. Although no significant differences in the frequencies, the amplitudes of isolated sawtooth waves were significantly higher than those associated with apnea/hypopnea (Figure 3). Therefore, OSA events were associated with decreased amplitudes of sawtooth waves, which was similar to decreased amplitudes of delta waves in N3 sleep as previously reported in OSA patients.12 Consistent with these findings, the OSA patients with isolated sawtooth waves in REM sleep had less severe OSA with significantly lower AHI and arousal index and shorter time with SpO2<90% than those without isolated sawtooth waves, and AHI in REM sleep and the minimal SpO2 were correlated with the percentage of REM sleep with apnea/hypopnea-related sawtooth waves (Table 2, Figure 4). Moreover, arousal indexes in N2, N3, REM sleep and total sleep period in the OSA patients without isolated sawtooth waves were significantly higher than those with isolated sawtooth waves, which might be related to higher AHI in the OSA patients without isolated sawtooth waves. Therefore, apnea/hypopnea-associated sawtooth waves might be used to indicate the severity of OSA. However, there were no significant differences in the total sleep time, sleep efficiency or duration of N3 and REM sleep, and the proportions of N3 and REM sleep to total sleep and WASO between the OSA patients with and without isolated sawtooth waves (Table 2). These findings might indicate isolated sawtooth waves might be a more sensitive indicator than N3 sleep duration, total sleep time, and sleep efficiency to reflect sleep quality in the OSA patients without CPAP treatment. In addition, with CPAP treatment, the duration of REM sleep and the percentage of REM sleep occupied by sawtooth waves were significantly increased (Figure 1) and correlated with the duration of N3 sleep (Figure 2). These findings suggest that isolated sawtooth waves in REM sleep might also reflect sleep quality in the OSA with CPAP treatment.
The significance of sawtooth waves in REM sleep is not fully clear. A previous study showed sawtooth waves as frontal-central clusters of delta waves characterized by faster, relatively large, and notched EEG waves with a positive correlation with gamma wave activity and rapid eye movements, which suggests sawtooth waves may be involved in cognitive processes such as memory consolidation during REM sleep.14 In addition, it was also shown that sleep deprived healthy volunteers with haloperidol intake demonstrated an increase in the duration and density of sawtooth waves without significant alterations in rapid eye movements, which suggest that the dopaminergic receptors exert a modulating role in sawtooth waves since REM sleep deprivation and administration of neuroleptics sensitize dopaminergic receptors.26 Furthermore, it was also demonstrated that significant differences in the mean latencies of the features associated with onset of rapid eye movements, muscle tone reduction and sawtooth waves between postpolio patients with and without involvement of brainstem, thus changes in these features might be a biomarker of functional or structural changes in the brainstem.27 Consistent with the previous study, CPAP treatment in the current study also caused a significant increase in the duration of rapid eye movements in REM sleep, which might be associated with a reversal of brainstem dysfunction in OSA patients by CPAP treatment.
Sawtooth waves together with gamma waves, bursts of eye movements linked to ponto-geniculo-occipital waves, irregular respiratory and cardiac activities, myoclonic twitches of limb muscles and contractions of the middle ear muscles occur during phasic REM sleep. In contrast, no sawtooth waves occur during tonic REM sleep, which comprises obviously longer and more quiescent periods.28 In previous studies,29,30 the arousal thresholds were found to be lower and gamma activity was found to be higher in phasic REM sleep in comparison with tonic REM sleep. These previous findings indicate that phasic REM sleep presents cognitively active state, which might explain the phenomenon of dreams.14
OSA events during REM sleep have been shown to stimulate the limbic system, which might evoke the occurrence of emotive dreams.31,32 Furthermore, AHI in REM sleep was found to be an independent factor for occurrence of nightmares in OSA patients, and nightmares disappeared in most of OSA patients with optimal CPAP treatment.32,33 In the current study, apnea/hypopnea associated sawtooth waves, a marker of dream related phasic REM sleep, found in the OSA patients disappeared with CPAP treatment. However, it is unclear the relationship between apnea/hypopnea associated sawtooth waves and nightmares or dreams and further studies are needed.
A previous study showed that patients with temporal lobe epilepsy had significantly lower density, shorter duration and lower frequency of sawtooth waves than the controls, which suggests a cortical influence on REM sleep either directly or through limbic–hypothalamic-brainstem connections..34 This is consistent with more sawtooth waves in acute hemispheric stroke patients with good short-term clinical outcome.35 In addition, sawtooth waves were found in all cortical regions except the occipital lobe, with a significant increase in power of the 2–4 Hz scalp EEG band in the fronto-parieto-temporal cortex and were related with widely distributed, but locally regulated REM sleep slow oscillations. Furthermore, sawtooth waves were found to be associated with a strong and widespread increase in high frequencies, and these slow and fast activities exhibited a high spatiotemporal heterogeneity. Sawtooth waves might orchestrate synchronized multifocal reactivations, which granted tagging of complex representations necessary for REM sleep-dependent memory consolidation by driving fast activities.14 These findings suggest that sawtooth waves may be related to cognitive processes during REM sleep. In the current study, it was found that the percentage of REM sleep with sawtooth waves and the amplitudes of sawtooth waves were significantly increased, while the frequency of sawtooth waves did not significantly change with CPAP treatment. These findings are consistent with cognitive dysfunction reported in OSA patients and suggest that OSA causes significant changes in multiple cortical regions and CPAP treatment might reverse some of these changes.36,37
Rapid eye movements and slow eye movements during REM sleep were shown to be associated with dream emotions,38 and there was a positive correlation between activities of facial muscles and rapid eye movements.39 In the current study, the percentages of REM sleep containing rapid eye movements and no eye movements were significantly increased and decreased with CPAP treatment, respectively (Figure 5A and C), while the percentage of REM sleep containing slow eye movements was not significantly changed (Figure 5B). These findings might indicate obstructive sleep apnea/hypopnea has no significant effect on slow eye movements but decreases the chances for occurrence of rapid eye movements and CPAP treatment abolishes these changes. It seems that a higher percentage of REM sleep containing rapid eye movements might reflect an improvement of sleep quality and an increase in dream emotions. In addition, increased REM and N3 sleep, and positive correlations of the duration of REM sleep with sawtooth waves and the total duration of REM sleep with the duration of N3 sleep were also found in the OSA patients with CPAP treatment (Figure 2), which is consistent with previous findings of increased duration of N3 sleep and percentage of REM and N3 sleep after N3 sleep deprivation or with CPAP treatment in OSA patients.16,40,41 Since sawtooth waves in REM sleep and delta waves in NREM sleep share some common characteristics, sawtooth waves might be considered as delta waves in REM sleep with similar function to delta waves in N3 sleep.15,42–44 Thus, the findings in the current study support the view that there are some interconnections between NREM and REM sleep,45,46 and might indicate that sawtooth waves not only represent REM sleep quality but also are correlated with N3 sleep. However, these correlations were not found in the same patients without CPAP treatment. This indicates that interconnections between N3 and REM sleep might be interrupted with apnea/hypopnea events.
In short, in the current study, it was found that isolated and apnea/hypopnea-associated sawtooth waves occurred during REM sleep in the OSA patients and CPAP treatment caused significant increases in the amplitude of sawtooth waves, durations of REM and N3 sleep, and duration of REM sleep with sawtooth waves in the OSA patients. Moreover, with CPAP treatment, the total duration of REM sleep as well as the duration of REM sleep with sawtooth waves were positively correlated with the duration of N3 sleep. These findings support the view that there are some interconnections between NREM and REM sleep and indicate that sawtooth waves might represent the quality of REM sleep and predict the quality of NREM sleep.
Limitation
The significance of sawtooth waves during REM sleep was examined in the OSA patients without and with CPAP treatment in the current study, but there were some limitations to be considered. First of all, although all of the statistical tests in the current study had a desired power, the number of subjects was relatively small. Second, since no mild OSA patients were included, the findings in the current study might not be generalizable to all OSA patients, especially those with mild symptoms. Third, CPAP treatment was carried out only for one night and long-term effects of CPAP treatment on sawtooth waves were still unknown. Fourth, apnea/hypopnea events still existed in the OSA patients with CPAP treatment although the AHI during REM sleep with CPAP treatment was low (2.23±0.41 events/h). The number of apnea/hypopnea associated sawtooth waves in the OSA patients with CPAP treatment was negligible.
This was a preliminary study to explore the significance of sawtooth waves in the OSA patients, and more normal control subjects and patients with different severity of OSA are needed to be included in the future studies. In addition, effects of long-term CPAP treatment in OSA patients on sawtooth waves need to be tested in the future. Moreover, effects of CPAP treatment on nightmares or dreams in OSA patients in relation to phasic REM sleep such as sawtooth waves, gamma waves, eye movements linked to ponto-geniculo-occipital waves, irregular respiratory and cardiac activities, myoclonic twitches of limb muscles and contractions of the middle ear muscles need to be examined. Furthermore, the current study in OSA patients is observational, and further studies of the function and significance of sawtooth waves at cellular and molecular level are needed.
Funding Statement
This research was supported by the Jiangxi Provincial People’s Hospital Grant 2019-009, Jiangxi Provincial Overseas High-level Talent Project (No. 20242BCE50018) and Jiangxi Province Key Laboratory of Neurology Grant (No. 2024SSY06081).
Data Sharing Statement
The data used to support the findings of this study are included in the article.
Ethics Declarations
All experimental protocols and procedures were approved in accordance with the Declaration of Helsinki by the Research Ethics Committee of the Jiangxi Provincial People’s Hospital (No. 2019-014). All subjects could withdraw from the study at any time and the informed consent was acquired from them all.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest.
References
- 1.Pham LV, Jun J, Polotsky VY. Obstructive sleep apnea. Handb Clin Neurol. 2022;189:105–136. doi: 10.1016/B978-0-323-91532-8.00017-3 [DOI] [PubMed] [Google Scholar]
- 2.Yeghiazarians Y, Jneid H, Tietjens JR, et al. Obstructive sleep apnea and cardiovascular disease: a scientific statement from the American Heart Association [published correction appears in Circulation. 2022;145(12):e775. doi: 10.1161/CIR.0000000000001043]. Circulation. 2021;144(3):e56–e67. doi: 10.1161/CIR.0000000000000988 [DOI] [PubMed] [Google Scholar]
- 3.Korkalainen H, Leppanen T, Duce B, et al. Detailed assessment of sleep architecture with deep learning and shorter epoch-to-epoch duration reveals sleep fragmentation of patients with obstructive sleep apnea. IEEE J Biomed Health Inform. 2021;25(7):2567–2574. doi: 10.1109/JBHI.2020.3043507 [DOI] [PubMed] [Google Scholar]
- 4.Cai A, Wang L, Zhou Y. Hypertension and obstructive sleep apnea. Hypertens Res. 2016;39(6):391–395. doi: 10.1038/hr.2016.11 [DOI] [PubMed] [Google Scholar]
- 5.Gleeson M, McNicholas WT. Bidirectional relationships of comorbidity with obstructive sleep apnoea. Eur Respir Rev. 2022;31(164):210256. doi: 10.1183/16000617.0256-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson ML, Howard ME, Barnes M. Cognition and daytime functioning in sleep-related breathing disorders. Prog Brain Res. 2011;190:53–68. doi: 10.1016/B978-0-444-53817-8.00003-7 [DOI] [PubMed] [Google Scholar]
- 7.Vanek J, Prasko J, Genzor S, et al. Obstructive sleep apnea, depression and cognitive impairment. Sleep Med. 2020;72:50–58. doi: 10.1016/j.sleep.2020.03.017 [DOI] [PubMed] [Google Scholar]
- 8.Lorenzi-Filho G, Almeida FR, Strollo PJ. Treating OSA: current and emerging therapies beyond CPAP. Respirology. 2017;22(8):1500–1507. doi: 10.1111/resp.13144 [DOI] [PubMed] [Google Scholar]
- 9.Gambino F, Zammuto MM, Virzì A, Conti G, Bonsignore MR. Treatment options in obstructive sleep apnea. Intern Emerg Med. 2022;17(4):971–978. doi: 10.1007/s11739-022-02983-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powell TA, Mysliwiec V, Brock MS, Morris MJ. OSA and cardiorespiratory fitness: a review. J Clin Sleep Med. 2022;18(1):279–288. doi: 10.5664/jcsm.9628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCarter SJ, Hagen PT, St Louis EK, et al. Physiological markers of sleep quality: a scoping review. Sleep Med Rev. 2022;64:101657. doi: 10.1016/j.smrv.2022.101657 [DOI] [PubMed] [Google Scholar]
- 12.Chen S, Li Q, Zou X, et al. Effects of CPAP treatment on electroencephalographic activity in patients with obstructive sleep apnea syndrome during deep sleep with consideration of cyclic alternating pattern. Nat Sci Sleep. 2022;14:2075–2089. doi: 10.2147/NSS.S382305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berry R, Quan S, Abreu A. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.6. Darien, Illinois: American Academy of Sleep Medicine; 2020. [Google Scholar]
- 14.Frauscher B, von Ellenrieder N, Dolezalova I, Bouhadoun S, Gotman J, Peter-Derex L. Rapid eye movement sleep sawtooth waves are associated with widespread cortical activations. J Neurosci. 2020;40(46):8900–8912. doi: 10.1523/JNEUROSCI.1586-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernardi G, Betta M, Ricciardi E, Pietrini P, Tononi G, Siclari F. Regional delta waves in human rapid eye movement sleep. J Neurosci. 2019;39(14):2686–2697. doi: 10.1523/JNEUROSCI.2298-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verma A, Radtke RA, VanLandingham KE, King JH, Husain AM. Slow wave sleep rebound and REM rebound following the first night of treatment with CPAP for sleep apnea: correlation with subjective improvement in sleep quality. Sleep Med. 2001;2(3):215–223. doi: 10.1016/s1389-9457(00)00069-1 [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Li Q, Zou X, et al. Effects of CPAP treatment on electroencephalographic activity in patients with obstructive sleep apnea syndrome during deep sleep: Preliminary findings of a cross-sectional study. Chron Respir Dis. 2023;20:14799731231215094. doi: 10.1177/14799731231215094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peter-Derex L, von Ellenrieder N, van Rosmalen F et al Regional variability in intracerebral properties of NREM to REM sleep transitions in humans. Proc Natl Acad Sci U S A. 2023;120(26):e2300387120. doi: 10.1073/pnas.2300387120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grenèche J, Krieger J, Erhardt C, et al. EEG spectral power and sleepiness during 24 h of sustained wakefulness in patients with obstructive sleep apnea syndrome. Clin Neurophysiol. 2008;119(2):418–428. doi: 10.1016/j.clinph.2007.11.002 [DOI] [PubMed] [Google Scholar]
- 20.Verbraecken J, Dieltjens M, Op de Beeck S, et al. Non-CPAP therapy for obstructive sleep apnoea. Breathe (Sheff). 2022;18(3):220164. doi: 10.1183/20734735.0164-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorikapudi PK, Chhabria V, Kaur K, et al. Evaluation of Orthodontic Treatment Modalities for Obstructive Sleep Apnoea: A Systematic Review. Cureus. 2024;16(7):e65161. doi: 10.7759/cureus.65161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lima Illescas MV, Aucapiña Aguilar DC, Vallejo Ledesma LP. A review on the influence of rapid maxillary expansion and mandibular advancement for treating obstructive sleep apnea in children. J Clin Pediatr Dent. 2023;47(1):9–16. doi: 10.22514/jocpd.2022.035 [DOI] [PubMed] [Google Scholar]
- 23.Batool-Anwar S, Goodwin JL, Kushida CA, et al. Impact of continuous positive airway pressure (CPAP) on quality of life in patients with obstructive sleep apnea (OSA). J Sleep Res. 2016;25(6):731–738. doi: 10.1111/jsr.12430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang G, Goebel JR, Li C, Hallman HG, Gilford TM, Li W. Therapeutic effects of CPAP on cognitive impairments associated with OSA. J Neurol. 2020;267(10):2823–2828. doi: 10.1007/s00415-019-09381-2 [DOI] [PubMed] [Google Scholar]
- 25.Pearl PL, LaFleur BJ, Reigle SC, et al. Sawtooth wave density analysis during REM sleep in normal volunteers. Sleep Med. 2002;3(3):255–258. doi: 10.1016/s1389-9457(01)00142-3 [DOI] [PubMed] [Google Scholar]
- 26.Lr P Jr, Peres CA, Russo RH, Remesar-Lopez AJ, Tufik S. Sawtooth waves during REM sleep after administration of haloperidol combined with total sleep deprivation in healthy young subjects. Braz J Med Biol Res. 2002;35(5):599–604. doi: 10.1590/s0100-879x2002000500013 [DOI] [PubMed] [Google Scholar]
- 27.Siegel H, McCutchen C, Dalakas MC, et al. Physiologic events initiating REM sleep in patients with the postpolio syndrome. Neurology. 1999;52(3):516–522. doi: 10.1212/wnl.52.3.516 [DOI] [PubMed] [Google Scholar]
- 28.Simor P, van der Wijk G, Nobili L, Peigneux P. The microstructure of REM sleep: Why phasic and tonic?. Sleep Med Rev. 2020;52:101305. doi: 10.1016/j.smrv.2020.101305 [DOI] [PubMed] [Google Scholar]
- 29.Ermis U, Krakow K, Voss U. Arousal thresholds during human tonic and phasic REM sleep. J Sleep Res. 2010;19(3):400–406. doi: 10.1111/j.1365-2869.2010.00831.x [DOI] [PubMed] [Google Scholar]
- 30.Simor P, Gombos F, Szakadát S, Sándor P, Bódizs R. EEG spectral power in phasic and tonic REM sleep: different patterns in young adults and children. J Sleep Res. 2016;25(3):269–277. doi: 10.1111/jsr.12376 [DOI] [PubMed] [Google Scholar]
- 31.Carrasco E, Santamaria J, Iranzo A, et al. Changes in dreaming induced by CPAP in severe obstructive sleep apnea syndrome patients. J Sleep Res. 2006;15(4):430–436. doi: 10.1111/j.1365-2869.2006.00553.x [DOI] [PubMed] [Google Scholar]
- 32.BaHammam AS, Pirzada AR, Pandi-Perumal SR. Neurocognitive, mood changes, and sleepiness in patients with REM-predominant obstructive sleep apnea. Sleep Breath. 2023;27(1):57–66. doi: 10.1007/s11325-022-02602-5 [DOI] [PubMed] [Google Scholar]
- 33.BaHammam AS, Al-Shimemeri SA, Salama RI, Sharif MM. Clinical and polysomnographic characteristics and response to continuous positive airway pressure therapy in obstructive sleep apnea patients with nightmares. Sleep Med. 2013;14(2):149–154. doi: 10.1016/j.sleep.2012.07.007 [DOI] [PubMed] [Google Scholar]
- 34.Vega-Bermudez F, Szczepanski S, Malow B, Sato S. Sawtooth wave density analysis during REM sleep in temporal lobe epilepsy patients. Sleep Med. 2005;6(4):367–370. doi: 10.1016/j.sleep.2005.02.005 [DOI] [PubMed] [Google Scholar]
- 35.Bassetti CL, Aldrich MS. Sleep electroencephalogram changes in acute hemispheric stroke. Sleep Med. 2001;2(3):185–194. doi: 10.1016/s1389-9457(00)00071-x [DOI] [PubMed] [Google Scholar]
- 36.Kerner NA, Roose SP. Obstructive sleep apnea is linked to depression and cognitive impairment: evidence and potential mechanisms. Am J Geriatr Psychiatry. 2016;24(6):496–508. doi: 10.1016/j.jagp.2016.01.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olaithe M, Bucks RS, Hillman DR, Eastwood PR. Cognitive deficits in obstructive sleep apnea: insights from a meta-review and comparison with deficits observed in COPD, insomnia, and sleep deprivation. Sleep Med Rev. 2018;38:39–49. doi: 10.1016/j.smrv.2017.03.005 [DOI] [PubMed] [Google Scholar]
- 38.Maranci JB, Nigam M, Masset L, et al. Eye movement patterns correlate with overt emotional behaviours in rapid eye movement sleep. Sci Rep. 2022;12(1):1770. doi: 10.1038/s41598-022-05905-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rivera-García AP, Ramírez-Salado I, Corsi-Cabrera M, Calvo JM. Facial muscle activation during sleep and its relation to the rapid eye movements of REM sleep. J Sleep Res. 2011;20(1 Pt 1):82–91. doi: 10.1111/j.1365-2869.2010.00853.x [DOI] [PubMed] [Google Scholar]
- 40.Bougard C, Gomez-Merino D, Rabat A, et al. Daytime microsleeps during 7 days of sleep restriction followed by 13 days of sleep recovery in healthy young adults. Conscious Cogn. 2018;61:1–12. doi: 10.1016/j.concog.2018.03.008 [DOI] [PubMed] [Google Scholar]
- 41.Cheng JX, Ren J, Qiu J, et al. Rapid eye movement sleep and slow wave sleep rebounded and related factors during positive airway pressure therapy. Sci Rep. 2021;11(1):7599. doi: 10.1038/s41598-021-87149-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Langille JJ. Human REM sleep delta waves and the blurring distinction between NREM and REM Sleep. J Neurosci. 2019;39(27):5244–5246. doi: 10.1523/JNEUROSCI.0480-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Funk CM, Honjoh S, Rodriguez AV, Cirelli C, Tononi G. Local slow waves in superficial layers of primary cortical areas during REM sleep. Curr Biol. 2016;26(3):396–403. doi: 10.1016/j.cub.2015.11.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siclari F, Tononi G. Local aspects of sleep and wakefulness. Curr Opin Neurobiol. 2017;44:222–227. doi: 10.1016/j.conb.2017.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vyazovskiy VV, Delogu, and A. NREM and REM Sleep: Complementary Roles in Recovery after Wakefulness. Neuroscientist. 2014;20(3):203–219. doi: 10.1177/1073858413518152 [DOI] [PubMed] [Google Scholar]
- 46.Falup-Pecurariu C, Diaconu Ș, Țînț D, Falup-Pecurariu O. Neurobiology of sleep. Exp Ther Med. 2021;21(3):272. doi: 10.3892/etm.2021.9703 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included in the article.