Abstract
MicroRNAs (miRNAs) are pervasive regulators of gene expression, necessitating the development of tools to inhibit individual miRNAs for functional studies or therapeutic targeting. Specialized base-pairing configurations between a miRNA and an RNA target site can trigger the degradation of the targeting miRNA through target-directed miRNA decay (TDMD). Previous work has identified several natural sites that induce TDMD of specific miRNAs. We explored retargeting known TDMD sites for the inhibition of heterologous miRNAs, including several encoded by Kaposi's Sarcoma-associated herpesvirus (KSHV). We focused particularly on miR-K11, a viral mimic of the oncogenic miRNA miR-155. miRNA pairing architectures based on the TDMD site in the long non-coding RNA Cyrano outperformed other retargeted sites. Cyrano-like inhibitors were specific for viral miR-K11 over cellular miR-155 and vice versa. Lentiviral delivery of a Cyrano-like miR-K11 inhibitor into KSHV-transformed primary effusion lymphoma (PEL) cells impaired their viability, showing that miR-K11 promotes KSHV-dependent PEL cell survival. Surprisingly, inactivation of ZSWIM8, a key mediator of TDMD, did not substantially affect miRNA inhibition by retargeted Cyrano-based inhibitors in 293T or PEL cells. Together, our results demonstrate the feasibility of retargeting natural TDMD sites to highly expressed viral or cellular miRNAs and further define features of effective encoded miRNA inhibitors.
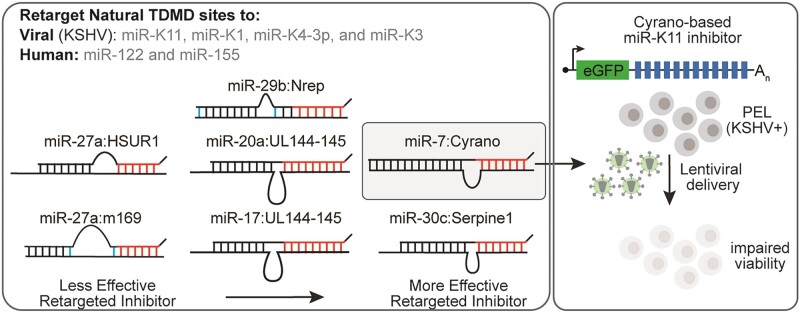
Graphical Abstract
Graphical Abstract.
Introduction
MicroRNAs (miRNAs) are a class of ∼22 nucleotides long non-coding RNAs that post-transcriptionally repress messenger RNA (mRNA) expression (reviewed in (1)). Most mRNAs are subject to miRNA-mediated regulation by one or more of several hundred human or viral miRNAs, resulting in increased robustness or substantial functional impact on biological pathways and diseases. Late in their biogenesis, miRNAs are loaded into Argonaute (Ago) proteins, forming the core of the miRNA-induced silencing complex (miRISC). miRNAs canonically use their seed sequence, comprised of nucleotides (nts) 2–7 or 8 from their 5′ end, to guide the miRISC to sites in 3′ untranslated regions (3′ UTRs). The additional pairing of 4–6 nts in the miRNA 3′ portion (nts 12–19) can further supplement seed pairing interactions (2), leading to stronger target mRNA repression. Canonical seed base pairing typically results in modestly reduced target mRNA expression or translational repression. In contrast, complete base-pairing of the entire miRNA to a target promotes Ago2-mediated endonucleolytic target cleavage across nts 10 and 11 of the miRNA, in an RNA-interference-like mechanism that is rare for natural sites but can be exploited in miRNA activity sensors.
miRNAs demonstrate considerable stability in the cytoplasm, remaining intact for several hours to days (3). This stability is attributed mainly to the protective role of Ago proteins, which bury the miRNA 5′ and 3′ ends within their MID (middle) and PAZ (PIWI/Argonaute/Zwille) domains, respectively (4,5). The stability of miRNAs poses challenges to miRNA inhibition in settings not amenable to genetic editing, for example, for miRNAs that are expressed from multicopy γ-herpesviral genomes in latently infected cells. miRNA inhibition is also desirable for therapeutic applications. Despite their commonly long half-lives, specific miRNAs can show rapid turnover under certain circumstances, suggesting the existence of sequence-specific miRNA turnover mechanisms, now called target-directed microRNA degradation (TDMD). The first example of TDMD was reported during infection by Herpesvirus Saimiri (HVS), which encodes a U-rich RNA (HSUR1) that triggers a reduction in the levels of miR-27 in HVS-transformed T cells (6). Concurrently, it was shown that synthetic sites with extensive base pair complementarity to the targeting miRNA induce 3′ tailing and trimming of the miRNA, suggestive of an accessible 3′ end (7).
Sites that have been validated to inhibit vertebrate miRNAs engage in both seed pairing and extensive 3′ pairing, separated by several unpaired central nts in the miRNA and the target sites (Figure 1A). Structural analyses of human Ago2 in complex with miRNAs and TDMD sites have shown that this configuration causes Ago2 to undergo a conformational change, releasing the miRNA 3′ end from the PAZ domain, thereby exposing it to cellular terminal transferases and exonucleases (8). Moreover, two independent studies applied genome-wide CRISPR screens to define the genes required for TDMD (9,10). These studies implicated the Cullin 3 (CUL3)-RING E3 ubiquitin ligase (CRL3) in complex with the substrate adaptor ZSWIM8 (CRL3ZSWIM8) in the polyubiquitination and proteasomal degradation of Ago as critical for miRNA turnover through TDMD. In addition to HSUR1, sites in murine cytomegalovirus (MCMV) m169 and human cytomegalovirus (HCMV) UL144-145 negatively regulate miRNA expression (11–13). The vertebrate transcripts Cyrano (a long noncoding RNA encoded by OIP5-AS1), Nrep, Serpine1 and BIM negatively regulate miRNAs through TDMD (14–17). Additional candidates for insect, nematode and mammalian TDMD pairs have been proposed, suggesting that TDMD may be relatively widespread (17–24). Finally, several synthetic miRNA inhibitors, including miRNA ‘sponges’ and tough decoys (TuDs), have been demonstrated to repress miRNA function through similar pairings (25–28).
Figure 1.
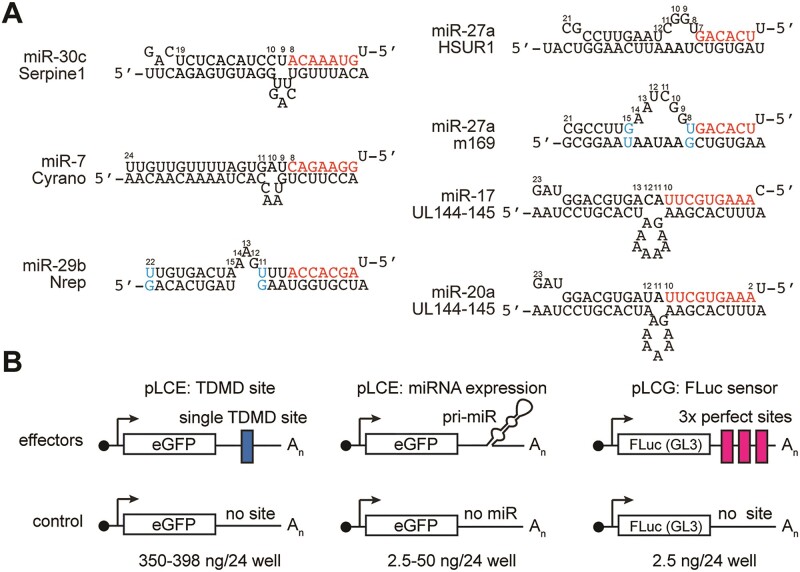
Assessment of retargeted TDMD sites in miRNA luciferase sensor assays. (A) Validated natural TDMD sites chosen for retargeting. The site in UL144-145 can bind to either miR-17 or miR-20, which differ from each other at nts 1 and 12. See Supplementary Figure S1 for all retargeted sites. (B) Vectors used to assess the efficiency of retargeted TDMD sites in miRNA luciferase sensor assays. pLCE was used to express single TDMD sites (left) or the targeting miRNAs (middle). pLCG was used as a miRNA sensor through three completely complementary sites, resulting in Ago2-mediated cleavage. The corresponding empty control vectors used for normalization are shown in the bottom row. pLCR, used to express Renilla Luciferase in each well, is not shown.
Which validated natural TDMD pairing architectures can be efficiently retargeted to other miRNAs remains unknown. To address this, we retargeted the best-documented TDMD sites to four highly expressed viral miRNAs encoded by Kaposi's sarcoma-associated herpesvirus (KSHV) and two human miRNAs, miR-155 and miR-122. miR-155 and miR-122 are highly expressed and represent therapeutic targets due to their roles in lymphomagenesis and hepatitis C virus infection, respectively (29–31). KSHV is a human γ-herpesvirus that causes primary effusion lymphoma (PEL) and Kaposi's sarcoma (KS). In these cancers, KSHV expresses at least 18 mature miRNAs from twelve pre-miRNA stem loops within two miRNA clusters in its latency locus (32–37). The effective experimental inhibition of the KSHV miRNAs is challenging since individual KSHV miRNAs are expressed at very high levels, and the multicopy KSHV genome in patient-derived PEL cell lines precludes Cas9-mediated editing due to a cytotoxic DNA damage response (38–40). The lasting and complete inhibition of highly expressed KSHV miRNAs in rapidly dividing PEL cell lines using transfected inhibitors is difficult in our experience and there is no systematic KSHV miRNA inhibition study in PEL cells. The roles of the KSHV miRNAs in PEL and KS have, therefore, largely remained unknown, and using lentivirally encoded inhibitors of mature KSHV miRNAs for their functional investigation is an attractive approach.
Among the KSHV miRNAs, miR-K11 is the most likely candidate for a viral oncogene in PEL since miR-K11 functionally mimics the B cell oncogenic miRNA miR-155 (41,42). Human miR-155 is overexpressed in several types of lymphomas, including diffuse large B cell lymphomas, and is essential for the survival of the corresponding cancer cell lines (29,30). The avian α-herpesvirus Marek's disease virus (MDV) similarly encodes a viral mimic of miR-155, which is required for MDV-mediated T-cell lymphomagenesis in chickens (43). In contrast to KSHV and MDV, which encode viral mimics of miR-155, the second human B cell oncogenic γ-herpesvirus, EBV, induces cellular miR-155 expression upon infection of naïve B cells. The resulting EBV-immortalized lymphoblastoid cell lines depend on continued miR-155 expression to survive in culture (30). The mimicry of miR-155 by miR-K11 is well established. miR-K11 shares its seed sequence (nts 2–8) with miR-155, resulting in its association with miR-155 target sites and repression of miR-155 target mRNAs (29,30,32,41,42,44,45). In addition, either miR-155 or miR-K11 induce B cell proliferation in mouse models (46–48). Despite these findings, a role for miR-K11 in B cell transformation has yet to be demonstrated in the context of KSHV infection. In contrast to EBV, KSHV does not readily immortalize B cells in vitro, making de novo experiments infeasible. Viral mutagenesis studies of the KSHV miRNA clusters are furthermore complicated by the complexity of the latency locus with several overlapping features, including an origin of lytic replication, a lytic promoter, an antisense lncRNA and transcripts for several protein-coding genes. Complete inhibition of miR-K11 in PEL cells is challenging, and miR-K11 antisense inhibition has not demonstrated an essentiality of this miRNA in PEL-derived cells (49).
We have systematically retargeted validated natural TDMD sites to several KSHV miRNAs, including miR-K11, miR-K3, miR-K1 and miR-K4-3p, or human miR-155 and miR-122. Our results show that miRNAs respond differently to various pairing architectures. Notably, a Cyrano-based pairing architecture was an effective miRNA inhibitor across all tested miRNAs. We leveraged this pairing to characterize requirements for effective miRNA inhibition and show that ZSWIM8, a key component of the TDMD machinery, is largely dispensable for miRNA inhibition by retargeted TDMD sites. We finally demonstrate for the first time that miR-K11 promotes the viability of PEL cells.
Material and methods
Cell culture
293T/17 cells (‘293T’, ATCC CRL-11268) were grown in Dulbecco's Modified Eagle's Medium (DMEM, Corning, 10–017-CV) supplemented with 10% Serum Plus™ II Medium Supplement (Sigma-Aldrich, 14009C) and 10 μg/ml gentamicin. BJAB and BCBL-1 cells were grown in RPMI 1640 medium containing L-glutamine (Corning, 10–040-CV) supplemented with 10% Fetal Bovine Serum (Corning, 35–010-CV), 0.05mM β-mercaptoethanol and 10 μg/ml gentamicin.
Plasmids and cloning procedures
Oligonucleotide sequences used in cloning procedures are listed in Supplementary Table S1. pLCE, pLCG and pLCR, expressing eGFP, firefly luciferase, or Renilla luciferase under the control of the CMV promoter were reported previously (32,51). miRNAs were expressed from the 3′UTR of eGFP in the lentiviral vector pLCE. pLCE-miR-30-K11 and pLCE-miR-K4-3p are miR-30-based miRNA expression vectors that were previously validated for authentic 5′-ends (32). pLCE-miR-155 expresses miR-155 from its human context, as reported previously. pLCE-miR-122 expresses miR-122 from its human context and was cloned by inserting gBlock 5356 between the XbaI and EcoRI sites of pLCE using Gibson Assembly. To clone lentiviral sensors, we inserted annealed oligonucleotides containing three completely complementary sites to miR-K11, miR-K4-3p, miR-K3, miR-K1, miR-155 or miR-122 into the 3′UTR of pLCG. Sensors containing mutant miRNA bind sites were similarly cloned into pLCG.
All miRNA inhibitor sites were cloned into the 3′ UTR of EGFP in pLCE, as previously reported using XbaI, XhoI, or EcoRI restriction sites. Inhibitors with 1 or 2 TDMD sites were cloned using a single set of annealed oligonucleotides, while inhibitors with 4, 8 or 12 sites were cloned by iteratively inserting annealed ultramers containing four sites between the XbaI and EcoRI sites of pLCE.
Western blots
Cells were washed with ice-cold PBS and lysed in RIPA buffer containing 1x protease inhibitor for 20–30 min on ice. The cell lysates were subsequently sonicated for five cycles (30 s on/30 s off) and sonicated lysates were centrifuged at 14 000 × g for 20 min at 4°C to remove debris. Protein concentrations in supernatants were measured using a bicinchoninic acid (BCA) protein quantification assay kit (ThermoFisher, 23227). Equal amounts of protein (20 μg) were separated by SDS-PAGE and transferred to a 0.45 μm nitrocellulose membrane. The membrane was blocked in TBS with 5% non-fat dry milk for 1 hour at room temperature. The membrane was then incubated overnight at 4°C in 5% milk TBST with a 1:300 dilution of ZSWIM8 antibody (ThermoFisher, PA5-59492). Western for GAPDH (anti-GAPDH, Santa Cruz Biotechnology, Cat. sc-47724) was used as a loading control to ensure equal protein loading and transfer efficiency at a dilution of 1:5000. After rinsing with TBST, the membrane was incubated with IRDye 800 CW-conjugated secondary antibodies (LI-COR Biosciences) for 1 h at room temperature. Membranes were rinsed with TBST. Bands were visualized using the LI-COR Odyssey imaging platform.
Dual luciferase assay
Around 80 000 293T cells were seeded per well in 24-well plates. The cells were subsequently co-transfected with (a) 5 ng of either pLCG or pLCG containing three perfect 3′UTR miRNA binding sites (miRNA sensor), (b) 2.5 ng of the internal transfection control pLCR luciferase construct, (c) 2.5–50 ng of miRNA expression vector or empty pLCE control and (d) 350–397.5 miRNA inhibitor vector or empty pLCE control, totaling 407.5ng DNA/well. We selected the amount of miRNA expression vector for each figure based on the potency of the inhibitors in pilot experiments, with low amounts of miRNA vector for single or weak sites and higher amounts for more potent sites. Transfections were carried out using Lipofectamine 2000 (Life Technologies) and Opti-MEM (Gibco, 31985070), as instructed. Two days after transfection, the medium was aspirated, and the cells were lysed with 100 μl of 1× Passive Lysis Buffer (Promega). Around 5 μl of the cell lysate was transferred to 96-well half-well plates and dual luciferase activity was measured using the Promega Dual Luciferase Kit (Promega) on a Victor Nivo plate reader. Firefly luciferase activity was normalized to Renilla luciferase activity within the same well. Results were subsequently normalized to empty miRNA, TDMD and sensor vectors, i.e. pLCE only (no miRNA, no TDMD) and no sensor (pLCG).
Lentivirus production and titration
For lentivirus production, we co-transfected 293T cells with a molar ratio of 0.45:0.35:0.2 of transfer vector, psPAX2 and pMD2.G plasmids, respectively, totaling 45 μg of DNA per 15 cm dish, using polyethylenimine. Media were changed 6 h after transfection and, 42–72 h later, the supernatants were collected and filtered through 0.45 μm pore size filters. Lentiviral particles were concentrated by ultracentrifugation (1 h, 80 000 g, 4°C) and the resulting lentiviral pellet was incubated with cell culture medium for at least 1 h at 4°C before resuspension by pipetting and storage at −80°C.
Lentiviral vectors were functionally titered by serial dilution of concentrated stocks. BJAB and BCBL-1 cells were seeded at 2.5 × 105 cells/ml in 12-well plates and transduced in the presence of 5 μg/mL polybrene. Around 24 h after transduction, cells were spun and resuspended in fresh medium. The percentage of GFP-positive cells was measured 2 days after transduction using flow cytometry on a BD FACSCanto II (BD Biosciences), and the titer was calculated from the percentage of GFP-positive cells in wells with <20% positive cells. Lentivirus generated from the empty vector (pLCE) or control vector (pLCE miR-122 12×) was titered in both BJAB and BCBL-1 cells. In contrast, lentiviruses containing miR-K11 inhibitors were titered only in BJAB cells, which do not express miR-K11. Relative BCBL-1 titers of miR-K11 inhibitors were estimated based on the ratio of transducing units per ml (TU/ml) relative to pLCE in BJAB.
CRISPR-Cas9 ZSWIM8 knockout
To generate the ZSWIM8-targeting vector for inducible Cas9 gene knockout in BCBL-1 cells, the sgAAVS1 sequence in the pLX-sgAAVS1 plasmid (50) (Addgene #50662) was replaced using overlap PCR. For ZSWIM8 sg1, PCR fragment 1 was amplified with primer pair 2430 and 5764, while fragment 2 was amplified with primer pair 5763 and 2431. For ZSWIM8 sg2, PCR fragment 1 was generated using primer pair 2430 and 5894, and fragment 2 was generated using primer pair 5895 and 2431. The two respective fragments were joined by overlap PCR using pairs 2340 and 2431. The resultant amplicons were digested with XhoI and NheI, and subsequently ligated into similarly digested pLX-sgRNA. BCBL-1/CwCas9 knockout cells were generated by transducing either pLX-AAVS, pLX-ZSWIM8 sg1 or pLX-ZSWIM8 sg2 lentivirus at a MOI of ∼1.5 in a 10-cm dish of cells at 2 × 105 cells/mL. Cells were subsequently selected with blasticidin (10 μg/mL) for 1 week and Cas9 induced transiently with DOX as previously described (58).
293T ZSWIM8 or AAVS1 knockout cell pools were generated by transducing cells with lentiCRISPR_v2 (51) (Addgene #52961) vectors encoding ZSWIM8 sg1 or sg2. The lentiCRISPR_v2 plasmid was digested with BsmBI and T4-ligated with annealed double-stranded oligonucleotide pairs 5767 and 5768 for ZSWIM8 sg1, and pairs 5899 and 5800 for ZSWIM8 sg2. The day before transduction, 293T cells were plated at 40 000 cells/cm2. The following morning, the cells were transduced at an MOI of ∼2. After transduction, the cells were selected with puromycin (1.2 μg/mL) for 1 week.
Cumulative growth curves
For cumulative growth curve analyses, BJAB and BCBL-1 and ZSWIM8 KO BCBL-1 cells were transduced at a multiplicity of infection (MOI) of 5 TU/cell. The medium was changed 1 day after transduction. Two days after transduction, live cells transduced with pLCE were counted manually with trypan blue, while other cell counts were estimated relative to pLCE-transduced cells using Celltiter Glo 2.0 (Promega), as instructed, and measured on a Victor Nivo plate reader. At each time point, live cells were split to 2 × 105 cells per ml and cumulative cells counts relative to pLCE-transduced cells were calculated based on dilution factors at each passage.
Taqman qRT-PCR
Total RNA was prepared using TRIzol (Life Technologies) and the Direct-zol™ RNA Miniprep (Zymo Research) two days after lentiviral transduction. qRT-PCR was performed on 5 ng of total RNA using TaqMan™ small RNA assays and RNU48 control (Life Technologies), as instructed. Real-time PCR was performed on a Roche LightCycler® 480 system and data analyzed using the 2(-ΔΔCT) method.
Results
A retargeted Cyrano TDMD-site-based pairing architecture outperforms other pairings
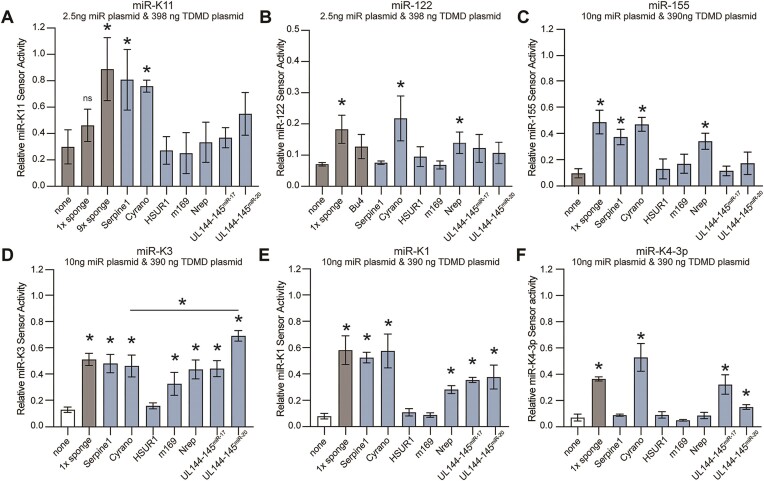
To assess whether validated TDMD pairing architectures can be retargeted to other miRNAs, we retargeted the TDMD sites of vertebrate Cyrano, Serpine1 and Nrep, HVS HSUR1, MCMV m169 and HCMV UL144-145 to KSHV miR-K11, miR-K3, miR-K1, miR-K4-3p and human miRNAs miR-122 and miR-155. We chose these miRNAs for their high expression in their natural context and potential as therapeutic targets. Retargeted sites retained their original length of 5′ pairing, centrally unpaired nucleotides, GU wobble positions where possible, and 3′ pairing (Supplementary Figure S1). We inserted a single copy of each site into the 3′ UTR of enhanced GFP (EGFP) in the context of a lentiviral vector we have previously used for sponge-based miRNA inhibition (pLCE) (30,52,53). As controls for established miRNA inhibitors, we included single synthetic ‘sponge’ sites with previously reported pairing (Supplementary Figure S1) (30,52,53). The designation of this site as a ‘sponge’ site reflects the original designation of this pairing architecture rather than its mechanism of action, which is unknown (25,26). Since these miRNAs are not expressed in 293T cells, we initially measured the effectiveness of each site following co-transfection into 293T with a miRNA expression vector, a firefly luciferase miRNA activity sensor, and a control vector expressing Renilla luciferase (Figure 1B). Results were sequentially normalized to the Renilla control, and empty vector controls for miRNA expression, miRNA inhibition and firefly sensors. To resolve subtle changes in miRNA-mediated sensor de-repression, we initially transfected low amounts of the miRNA expression plasmid (2.5–10 ng/24 well). Under these conditions, targeting miR-K11 using a single sponge site did not result in significant rescue of sensor, while the presence of nine tandem sponge sites induced a notable rescue effect (Figure 2A). For other miRNAs tested, a single sponge site conferred significant reduction in miRNA activity (Figure 2B–F). For miR-122, we additionally included the bu4 pairing, which contains a symmetrical 4 nt bulge, has previously been shown to trigger TDMD of miR-122 in hepatocytes, and was analyzed in structural studies (8,54) (Supplementary Figure S11). This pairing did not significantly reduce miR-122 activity here (Figure 2B), but rescue reached significance under more stringent conditions (see Figure 3C). Among the retargeted designs, we observed significant rescue with designs based on Cyrano for all miRNAs (6/6), and Serpine1 for most miRNAs (5/6). Designs based on Nrep (4/6), miR-17 UL-144/145 (3/6), and miR-20 UL-144/145 (3/6) showed varied effectiveness, while m169 (1/6) and HSUR (0/6) consistently lacked activity across examined miRNAs. Our normalization approach is robust since we obtained comparable results for miR-K11 and miR-122 following normalization to miRNA sensors with scrambled seed match sequences or completely scrambled miRNA binding sites (Supplementary Figure S2) and have previously shown that our sensors do not respond to seed mutant miR-K11 (32). We have previously validated our sensor set-up in the context of doxycycline-inducible miRNA expression (52). Together, these results suggest that the effectiveness of retargeted miRNA inhibitory sites is miRNA-specific and that a Cyrano-like architecture may be broadly effective for miRNA inhibition.
Figure 2.
Retargeting TDMD sites to viral and human miRNAs. (A) Results for miR-K11 using a low amount of miRNA expression plasmid (2.5 ng per 24-well) to distinguish effective and non-effective sites. Firefly luciferase sensor results were normalized to an internal Renilla luciferase transfection control (pLCR), a no TDMD-site empty vector control (pLCE), a no miRNA empty vector control (pLCE), and a firefly luciferase vector without target sites (pLCG). (B) As in (A) but showing results for miR-122. (C-F) As in (A) but the amount of transfected miRNA expression plasmid was increased to 10 ng due to relatively lower miRNA activity for (C) miR-155, (D) miR-K3, (E) miR-K1 and (F) miR-K4-3p. Error bars represent mean ± SD. Unless indicated by a horizontal bar, significance refers to comparisons against the no TDMD-site EV control (none) and was calculated using unpaired two-tailed Student's t tests. n = 3 independent experiments. * indicates P < 0.05. ns not significant.
Figure 3.
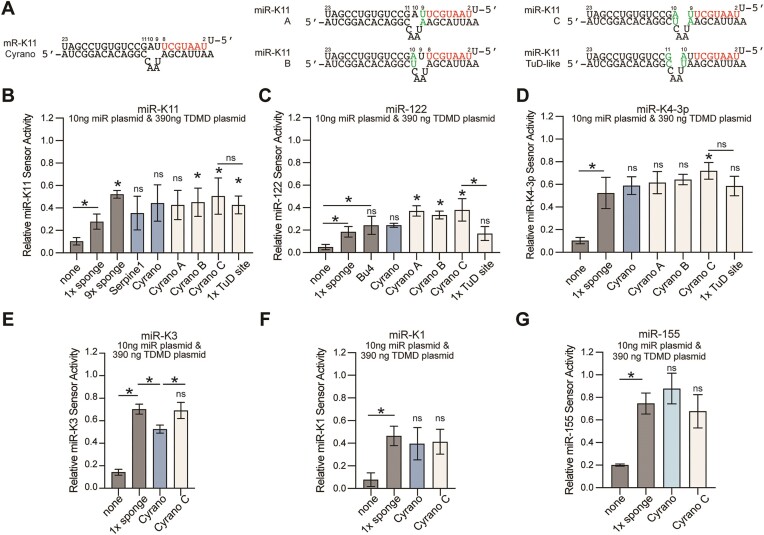
Changes in the central bulge identify an optimized Cyrano-like TDMD site. (A) Schematic of the changes introduced into the bulge configuration of the miR-K11-retargeted Cyrano TDMD site. See Supplementary Figure S3 and Supplementary Table S1 for other miRNA target sites. (B–D) results of luciferase assays as in Figure 2, for (B) miR-K11, (C) miR-122 and (D) miR-K4-3p, using a higher amount of miRNA than for Figure 1 (i.e. 10ng per 24 well), to achieve more robust sensor knock-down and higher resolution between effective TDMD sites. (E–G) Additional results for miR-K3, miR-K1, and miR-155, comparing only the sponge site, the original Cyrano site, and the Cyrano-C site. Error bars show mean ± SD. Significance refers to comparisons against single sponge inhibitors, unless specified by horizontal bars for other comparisons, and was calculated using unpaired two-tailed Student's t tests. n = 3–6. * indicates P < 0.05. ns: not significant.
The Cyrano-based TDMD site tolerates subtle changes in the location and configuration of the central bulge
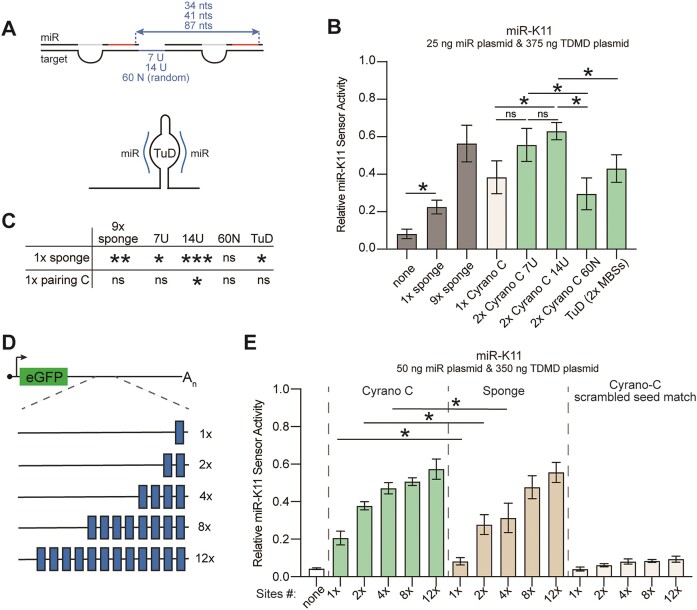
The Cyrano TDMD site includes seed base pairing of miRNA nts 2–8 and a perfectly paired miRNA 3′end, separated by a central bulge consisting of two unpaired miRNA nts (nts 9–10) across 4 unpaired nts in Cyrano (Figures 1A and 3A). To test the importance of the location and configuration of the central bulge, we reduced the number of the unpaired miRNA nucleotides or moved the bulge by one nucleotide to the 5′ end or 3′ end (Figure 3A for miR-K11, and Supplementary Figure S3 and Supplementary Table S1 for other sites). While constructing these sites, we noticed that a similar pairing architecture had previously been employed in TuDs (27,28). TuDs are synthetic miRNA inhibitors that form a stem-loop structure with two miRNA binding sites across from each other. In previous side-by-side comparisons, a TuD was more effective at miR-122 inhibition than a seven-copy sponge inhibitor (55), but it is not clear if this resulted from the TuD secondary structure or the type of site that was used. The reported TuD pairing involves pairing of all miRNA nucleotides, with a central bulge comprised of 4 unpaired nts in the target site, located between miRNA nts 10–11 to prevent Ago2-mediated endonucleolytic cleavage (27). To directly compare the pairing previously used in TuDs to Cyrano-like sites, we generated constructs containing a single TuD-like miRNA binding site but devoid of the TuD secondary structure (Figure 3A). To compare these configurations, we increased the amount of transfected miRNA compared to Figure 1, allowing for more robust sensor repression and increased resolution between efficient miRNA inhibitory sites. Under these settings, only configuration C, with all miRNA nts paired and a central bulge comprised of 4 unpaired target nts, located between miRNA nts 9 and 10, was consistently more effective than a single sponge site for miR-K11, miR-122 and miR-K4-3p (Figure 3B-D). This pairing was significantly more efficient than the previously reported TuD-like pairing for miR-122, but not for miR-K11 or miR-K4-3p. For miR-K11, the efficiency of a single Cyrano-like site with pairing C was comparable to that of 9 sponge sites, an arrangement we have previously used for miRNA inhibition (30,52,53). We extended our analysis of pairing C to miR-K3, miR-K1 and miR-155. For these miRNAs, the sponge and Cyrano-C pairing were equally efficient at miRNA inhibition (Figure 3E-G). Since the Cyrano-C pairing performed well for all miRNAs tested, while other pairings gave variable results or performed poorly, we focused on further exploring the potential of Cyrano-C pairing for targeting heterologous miRNAs.
Spacing and number of sites required for miRNA inhibition
Our experiments so far have employed single TDMD-based sites. We reasoned that TDMD-based miRNA inhibitors would be more efficient with several sites, as seen for miR-K11 ‘sponge’ sites in Figures 2 and 3. While miRNA seed sites mediate cooperative target repression when spaced closely together (2,56,57), TDMD sites do not show similar cooperativity (18,58). To determine the impact of using more than one site, we tested two Cyrano-like C TDMD sites set apart by 7, 14 or 60 nucleotides, respectively, resulting in 34, 41 or 87 nt distances between the 5′ends of the adjacent miRNA seed sequences (Figure 4A-C). For 7 and 14-nt spacing, the two sites were separated by strings of Us, while the 60-nt spacer was designed not to form a secondary structure (see ‘Materials and methods’ section). Results showed that two TDMD sites with a 14 nt spacer were significantly more efficient than a single TDMD site, while a potential improvement from two TDMD sites spaced 7 nts apart did not reach significance over three independent replicates (Figure 4B–C). Sites separated by either 7 or 14 nts outperformed a 60 nt spacer, which did not perform better than a single site (Figure 4C). We did not test additional spacer sequences to determine if this result is due to the specific 60 bp sequence used here or would also apply to alternative sequences. Finally, two linear Cyrano-like C-type TDMD sites spaced 14 nts apart also outperformed a TuD stem-loop containing two Cyrano-C sites, embedded in the 3′UTR of eGFP at the same location as the linear sites (Figure 4B).
Figure 4.
Improved miRNA inhibition through optimized spacing and increased number of Cyrano-like sites. (A) Schematic explaining the spacings between two Cyrano-like type C sites used in panel B. Two Cyrano-like type C sites were separated by 7, 14 and 60 sites or incorporated into a TuD secondary structure. (B) miR-K11 activity sensor assays to determine the benefit of using two miRNA inhibitory sites, as outlined in panel A. Significance refers to comparisons specified by horizontal bars and was calculated using unpaired two-tailed Student's t tests. n = 3–6. Data are represented as mean ± SD. *P < 0.05. ns: not significant. (C) Additional significance calculations from data in panel B. (D) Schematic illustrating miRNA inhibitors used for panel E. The number of Cyrano-like type C sites was increased to 2, 4, 8 and 12, with 14 nts spacers. (E) miR-K11 activity sensor assays to determine the benefit of using additional miRNA-inhibitory sites. Cyrano-C pairing improved miRNA repression compared to equally spaced sponge sites at 1, 2 and 4 sites. All Cyrano-C and sponge configurations resulted in significant sensor derepression. Seed match scrambled miR-K11 Cyrano-C sites did not confer robust sensor derepression. Significance refers to comparisons specified by horizontal bars and was calculated using unpaired two-tailed Student's t tests. n = 3. Data are represented as mean ± SD. *P < 0.05. ns not significant.
Building on these results, we further increased the number of Cyrano-C or sponge sites to 4, 8 or 12 using the 14-nt spacer under high miRNA expression conditions (50 ng miR-K11 plasmid/24 well) (Figure 4D). We additionally tested a Cyrano-C site with a scrambled seed match as a control. Results show improved repression of miR-K11 with an increasing number of either type of site, reaching a plateau between 4 and 12 sites (Figure 4E). The Cyrano-C based inhibitor outperformed a sponge-based inhibitor in the 1×, 2× and 4× site configurations; however, we observed no difference in effectiveness with the 8× and 12× site configurations, suggesting a potential saturation and maximal miRNA turnover levels. The seed match-scrambled Cyrano-C-based inhibitor did not substantially restore sensor activity, indicating that seed base pairing is required for inhibition. These results and the findings above show that up to four Cyrano-like C type sites more efficiently inhibit miR-K11 than the same number of sponge sites. However, there may be an upper limit to miRNA inhibitor efficiency in this system.
TDMD-site-based miRNA Inhibitors distinguish miRNAs with identical seed sequences
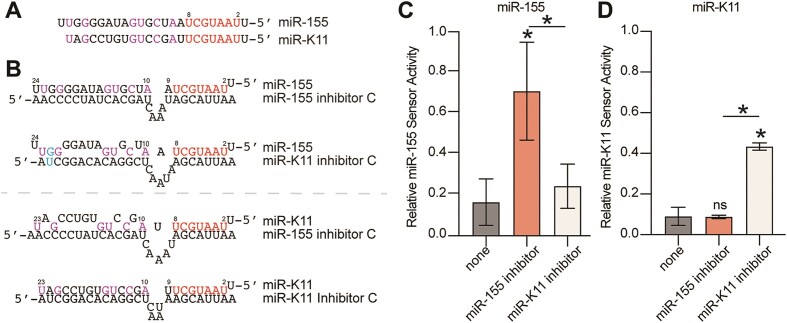
The selectivity of miRNA inhibitors for the targeted miRNA over similar miRNAs is important for both therapeutic and experimental applications. We therefore tested the specificity of the Cyrano-like miRNA inhibitor for viral miR-K11 over its cellular counterpart miR-155 and vice versa. miR-K11 and miR-155 share nts 2–8 but have largely distinct 3′-end (Figure 5A), resulting in functional mimicry in terms of seed-based miRNA repression (41,42). A newly designed Cyrano-like type C site-based miR-155 inhibitor effectively inhibited miR-155 activity, without inhibiting miR-K11 activity (Figure 5B-C). Conversely, the miR-K11 inhibitor did not affect miR-155 activity (Figure 5D). These findings indicate that targeting of heterologous miRNAs exhibits high selectivity toward miRNAs with identical seed sequences but distinct 3′ ends and can therefore be used to target viral miRNAs without perturbing endogenous miRNAs with identical seeds. Of note, KSHV encodes 4 such viral mimics of cellular miRNAs and this approach would also be beneficial to distinguish members of cellular miRNA families with shared seed sequences but distinct 3′ ends.
Figure 5.
Cyrano-like miRNA inhibitors distinguish miRNAs with shared seed sequences. (A) Comparison of human miR-155 and its KSHV mimic miR-K11. Identical seed sequences (nts 2–8) are in red, other shared nts are in purple. (B) Predicted base pairing of miR-155 (top) or miR-K11 (bottom) to the Cyrano-like miRNA inhibitors (type C) used in panels C–D. (C) miR-155 luciferase activity sensor assay shows specificity of the miR-155 inhibitor for miR-155. (D) as for panel C, but for miR-K11. In panels C–D, significance refers to comparisons against the control vector without miRNA inhibitory sites, unless specified by horizontal bars, and was calculated using unpaired two-tailed Student's t tests. n = 3. Data are represented as mean ± SD. *P < 0.05. ns: not significant.
ZSWIM8 is dispensable for miRNA inhibition by sponge sites or retargeted TDMD sites
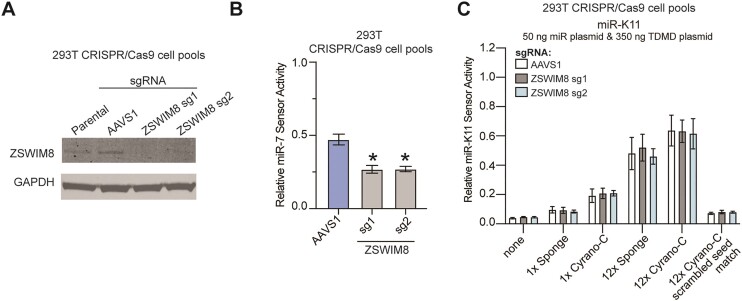
During TDMD, ZSWIM8 functions as the CRL substrate adaptor that recognizes Ago proteins in their TDMD configuration. Reasoning that both sponge and retargeted TDMD sites are likely to function by TDMD, we tested the importance of ZSWIM8 for miRNA inhibition by these sites. For this, we generated ZSWIM8-deficient 293T cells using CRISPR/Cas9 and previously validated sgRNAs (9,17). As a control, we targeted the safe-harbor locus AAVS1. We confirmed efficient loss of ZSWIM8 using Western blotting (Figure 6A). Using a miR-7 miRNA sensor, we showed that miR-7, a validated ZSWIM8/TDMD target in 293T, is overexpressed upon ZSWIM8 deletion (Figure 6B), confirming the functional impact of ZSWIM8 inactivation and replicating published results in our cell lines (9,10). However, ZSWIM8 inactivation did not affect the efficiency of miR-K11 inhibition by single and 12× Cyrano-C or sponge-based inhibitors (Figure 6C). These results suggest that ZSWIM8 is dispensable for miRNA inhibition by our encoded miRNA inhibitors, suggesting that these inhibitors function competitively or, perhaps less likely, by inducing TDMD through redundant cellular machinery.
Figure 6.
ZSWIM8 is dispensable for miRNA inhibition by retargeted TDMD sites. (A) Western blot analysis of 293T ZSWIM8 KO cell pools following Cas9-mediated gene editing. (B) Endogenous miR-7 activity was increased in ZSWIM8 KO cell pools compared to AAVS1, as measured by miR-7 specific activity sensors. (C) No change in miR-K11 activity was observed in ZSWIM8 KO 293T cells compared to sgAAVS1 control pools following transfection with miR-K11 expression plasmids, miR-K11 inhibitory plasmids (sponge or Cyrano-C), miRNA sensors, and internal control. Significance values show unpaired two-tailed Student's t test. n = 3–4. Data are represented as mean ± SD. *P < 0.05.
Endogenously expressed KSHV miR-K11 promotes the survival of PEL cells
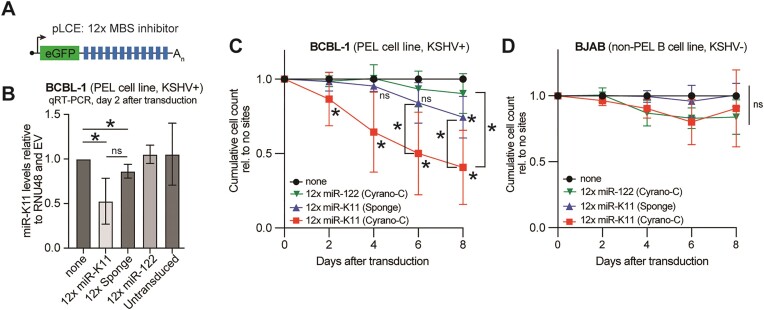
Although often assumed based on the B cell oncogenic roles of miR-155, it has not been demonstrated that miR-K11 contributes to the KSHV-induced survival and proliferation of PEL-derived cell lines. A previous report did not observe decreased viability upon inhibiting only miR-K11 by nanoparticle-based delivery of antisense locked-nucleic acids into PEL cell lines, while the combined inhibition of miR-K11 with several other KSHV miRNAs had a measurable effect (49). To test the effectiveness of the Cyrano-like type C-based inhibitor in inhibiting highly expressed endogenous miR-K11 in PEL cells, we transduced the PEL cell BCBL-1 with an empty lentiviral vector, or vectors containing 12x miR-K11-directed Cyrano-C or sponge sites or a 12x miR-122-targeting Cyrano-C inhibitor as a negative control, since miR-122 is not expressed in PEL cells (32) (Figure 7A). miRNA Taqman qRT-PCR showed a significant decrease in miR-K11 expression upon transduction with vectors containing 12x Cyrano-C and sponge pairings compared to EV or the miR-122-directed negative control inhibitor two days following transduction (Figure 7B). Both Cyrano-C and sponge-type inhibitors attenuated the viability of BCBL-1 cells in a growth curve experiment, but the 12x Cyrano-C type significantly outperformed a 12x sponge-type inhibitor (Figure 7C). Neither miR-K11 inhibitors or the miR-122 targeting control affected the survival of the KSHV-negative B-cell lymphoma cell line BJAB, which does not express miR-K11 (Figure 7D). Cumulatively, these results suggest that miR-K11 inhibition using Cyrano-like sites reduces PEL cell viability more effectively than sponge sites. Our results also demonstrate for the first time that viral miR-K11 promotes the viability of a PEL-derived cell line.
Figure 7.
miR-K11 promotes the growth of the PEL cell line BCBL-1. (A) Schematic of the lentiviral expression cassette for miRNA inhibitors in this figure. The inhibitors used were the 12 × Cyrano-C sites for miR-K11 (12 × miR-K11), the 12× sponge sites for miR-K11 (12 × sponge), the 12 × Cyrano C sites for miR-122 (12 × miR-122), with matched spacing. (B) TaqMan qRT-PCR analysis of miR-K11 expression relative to RNU48 in the KSHV-transformed PEL cell line BCBL-1, two days after lentiviral transduction at a MOI of 5. (C) Cumulative growth curves of BCBL-1 cells transduced with lentiviruses encoding no miRNA inhibitory sites (none), 12× miR-K11 Cyrano-C, 12× miR-K11 sponge or 12× miR-122-directed Cyrano-C inhibitors at an MOI of 5. (D) Cumulative growth curves as in (B), but in BJAB cells, a non-KSHV infected control. Significance values were calculated using an unpaired two-tailed Student's t-test. n = 3–10. Data are represented as mean ± SD. *P < 0.05. ns: not significant.
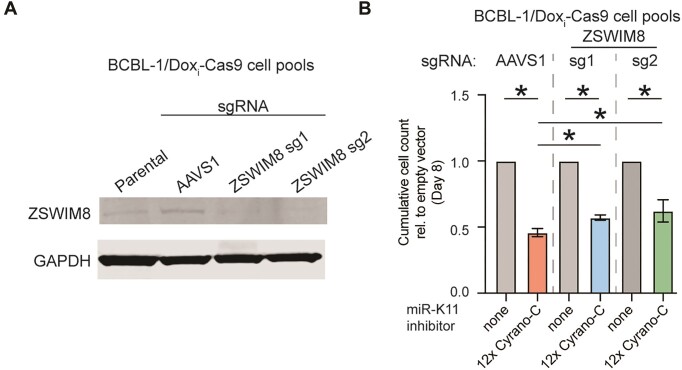
Moderate effect of ZSWIM8 knockout in BCBL-1
To test whether ZSWIM8 mediates miRNA inhibition in PEL cells, we inactivated ZSWIM8 using two guide RNAs in a previously reported BCBL-1 cell line allowing doxycycline-inducible Cas9 expression (59). Loss of ZSWIM8 expression after transient induction of Cas9 was confirmed by Western blot analysis (Figure 8A). miR-K11 inhibitors decreased the viability of both the sgAAVS1 control and ZSWIM8-inactivated BCBL-1 cell pools (Figure 8B). Interestingly, ZSWIM8 knockout cells had slightly higher viability compared to AAVS1 controls, an effect that reached significance on day 8 after transduction. These findings, combined with earlier results, suggest that ZSWIM8 makes a minimal but measurable contribution to inhibiting endogenous miR-K11 by a retargeted Cyrano-TDMD-site-based miRNA inhibitor.
Figure 8.
ZSWIM8 knockout in BCBL-1 does not rescue miR-K11 inhibitor lethality. (A) Western blot analysis of ZSWIM8 expression in sgRNA-transduced BCBL-1/Dox-inducible-Cas9 cell pools following transient induction of Cas9 expression. (B) End point of cumulative growth curves on day 8 after miRNA inhibitor transduction of the cell pools from panel A. Transductions with either an empty vector (none) or a 12× miR-K11 Cyrano-C inhibitor were performed at MOI of 5. n = 3. Significance values show unpaired two-tailed Student's t test. Data are represented as mean ± SD. *P < 0.05.
Discussion
In contexts not amenable to genetic editing, inhibitors of mature miRNAs are required to assess miRNA functions and can be developed for therapeutic intervention. Despite recent studies highlighting the machinery and natural sites involved in miRNA turnover via TDMD, it remains unclear which TDMD sites can be retargeted to heterologous miRNAs. Our results show that the efficiency of retargeting natural TDMD sites depends on the targeted miRNA. Retargeting a Cyrano site to four KSHV miRNAs, miR-K11, miR-K3, miR-K1 and miR-K4-3p, and two human miRNAs consistently showed miRNA repression. Moreover, an optimized Cyrano TDMD-site architecture outperformed previously reported synthetic sponge sites and other binding sites for three miRNAs, i.e. KSHV miR-K11 and miR-K4-3p and human miR-122. The higher efficiency of Cyrano-site retargeting was particularly evident when only one site was used for miRNA inhibition.
Future studies should investigate why a Cyrano-like pairing architecture, characterized by an asymmetrical central bulge with more unpaired target nucleotides than miRNA nucleotides, is particularly effective for miRNA inhibition. Although the Cyrano site is a well-established inducer of miR-7-TDMD, our results in 293T surprisingly suggest that retargeting this site in a synthetic context does not significantly engage the classical ZSWIM8-mediated TDMD pathway. Thus, the binding architecture may instead enhance miRNA association; in support of this, a similar central bulge structure has been shown to facilitate higher Ago association with let-7a or miR-155 in vitro (60). Additionally, there may be unexplored protein redundancies that enable TDMD in the absence of ZSWIM8. A Cyrano-like pairing structure may also be less favorable for alternative outcomes of miRNA binding, such as miRNA-mediated target repression or cleavage. It is possible that the high levels of miRNA inhibitor used in our assays mask a contribution by ZSWIM8. A more detailed investigation of ZSWIM8 dependency of retargeted TDMD sites in future studies should ideally include testing various stoichiometries of miRNA and miRNA inhibitors and additional readouts for miRNA expression and tailing by Northern blotting, qRT-PCR, or next generation sequencing.
Our results in BCBL-1 cells showed a measurable but minor role for ZSWIM8 in inhibiting endogenous miR-K11 by multiple miR-K11-targeted Cyrano-C type sites. In this context, we observed measurable downregulation of miR-K11. Future studies should more closely monitor the fate of the targeted miRNA, including ZSWIM8-dependent and -independent tailing, trimming and turnover. Future studies could additionally focus on developing and characterizing miRNA inhibitors designed specifically to engage TDMD, providing deeper insights into the distinct roles of enzymatic miRNA degradation versus competitive binding.
Interestingly, we did not observe considerable rescue of miRNA sensor activity when retargeting miRNAs with pairing architectures based on HVS HSUR1, MCMV m169 or HCMV UL-144–145. An absence of the natural sequence contexts in our vectors could explain these results (11). For example, it has been shown that 50 nts adjacent to the UL144-145 TDMD site are required for its role. Sequence context has also been shown to promote miRNA turnover by the TDMD element in BCL2L11, encoding Bim (17). Alternatively, these sites may depend on lower miRNA:target stoichiometries or RNAs expressed from our constructs formed unfavorable secondary structures, which have been shown to limit TDMD activity in other settings (17,61).
Notably, Cyrano-like inhibitors of miR-K11 and miR-155, two miRNAs that share nucleotides 2–8, strictly depended on 3′-base pairing, while certain miRNAs in Caenorhabditis elegans are degraded based solely on the seed sequence (24). While two TDMD sites outperformed a single site, our data confirm previous findings that TDMD sites in proximity do not exhibit cooperative function (18,58), in contrast to cooperativity between canonical miRNA binding sites that lead to mRNA repression. However, increasing the number of miRNA-inhibiting sites did not proportionally reduce miRNA activity, plateauing at ∼75–80% luciferase sensor rescue with 4–12 sites. This plateau may indicate a saturation effect, possibly due to limited miRNA binding capacity or the competitive nature of the inhibitor rather than miRNA turnover.
We have shown that the redesigned Cyrano-like miRNA inhibitor effectively targets the KSHV-encoded mimic of the human oncomiR miR-155, miR-K11, in KSHV-transformed PEL cells. Although functional overlap between miR-K11 and miR-155 has been suggested, a direct role for miR-K11 in PEL cells had not been previously demonstrated, likely due to the challenges in achieving lasting loss of function for highly expressed viral miRNAs in rapidly proliferating cell lines. Our results indicate that miR-K11 contributes to the viability of the PEL cell line BCBL-1, thereby supporting the role of miR-K11 in KSHV-mediated lymphomagenesis. These results identify a new target for therapeutic intervention in PEL and identify an additional lymphoma model for studies of which targets underlie miR-K11/miR-155 dependency.
Collectively, our findings underscore the potential of leveraging natural miRNA pairings to enhance miRNA suppression efficiency, with Cyrano emerging as particularly effective for targeting heterologous miRNAs. Additionally, our results demonstrate that KSHV miR-K11 promotes the survival of KSHV-transformed B cell lines.
Supplementary Material
Acknowledgements
Author contributions: J.A.O. and E.G. conceptualized this study. J.A.O. performed most cloning and all experiments, Z.L. and J.K.X. each contributed several vectors and Z.L. performed Western blot experiments. J.A.O. drafted the manuscript and figures, with editing by E.G. and input from all authors. We thank Dr. Mark Manzano for prior studies of a potential miR-K11 essentiality that inspired a portion of this work.
Contributor Information
Jesus A Ortega, Department of Microbiology-Immunology, Northwestern University, Feinberg School of Medicine, Tarry 6-735, Chicago, IL 60611, USA.
Ziyan Liang, Department of Microbiology-Immunology, Northwestern University, Feinberg School of Medicine, Tarry 6-735, Chicago, IL 60611, USA.
Junpeng Kenny Xu, Department of Microbiology-Immunology, Northwestern University, Feinberg School of Medicine, Tarry 6-735, Chicago, IL 60611, USA.
Eva Gottwein, Department of Microbiology-Immunology, Northwestern University, Feinberg School of Medicine, Tarry 6-735, Chicago, IL 60611, USA.
Data availability
Any raw data and constructs will be shared on reasonable request to the corresponding author.
Supplementary data
Supplementary Data are available at NAR Online.
Funding
National Institute of General Medical Sciences (NIGMS)-funded Cellular and Molecular Basis of Disease Training Program [T32-GM008061 supported J.A.O]; National Institute of Allergy and Infectious Diseases (NIAID)-funded Ruth L. Kirschstein National Research Service Award F31 Fellowship [F31 AI183996 to J.A.O.]; National Cancer Institute [R01 CA247619 and R01 CA285193 to E.G.].
Conflict of interest statement. None declared.
References
- 1. Bartel D.P. Metazoan MicroRNAs. Cell. 2018; 173:20–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grimson A., Farh K.K., Johnston W.K., Garrett-Engele P., Lim L.P., Bartel D.P.. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol. Cell. 2007; 27:91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kingston E.R., Bartel D.P.. Global analyses of the dynamics of mammalian microRNA metabolism. Genome Res. 2019; 29:1777–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schirle N.T., Sheu-Gruttadauria J., MacRae I.J.. Structural basis for microRNA targeting. Science. 2014; 346:608–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang Y., Sheng G., Juranek S., Tuschl T., Patel D.J.. Structure of the guide-strand-containing argonaute silencing complex. Nature. 2008; 456:209–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cazalla D., Yario T., Steitz J.A.. Down-regulation of a host microRNA by a Herpesvirus saimiri noncoding RNA. Science. 2010; 328:1563–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ameres S.L., Horwich M.D., Hung J.H., Xu J., Ghildiyal M., Weng Z., Zamore P.D.. Target RNA-directed trimming and tailing of small silencing RNAs. Science. 2010; 328:1534–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sheu-Gruttadauria J., Pawlica P., Klum S.M., Wang S., Yario T.A., Schirle Oakdale N.T., Steitz J.A., MacRae I.J.. Structural basis for target-directed microRNA degradation. Mol. Cell. 2019; 75:1243–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Han J., LaVigne C.A., Jones B.T., Zhang H., Gillett F., Mendell J.T.. A ubiquitin ligase mediates target-directed microRNA decay independently of tailing and trimming. Science. 2020; 370:eabc9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shi C.Y., Kingston E.R., Kleaveland B., Lin D.H., Stubna M.W., Bartel D.P.. The ZSWIM8 ubiquitin ligase mediates target-directed microRNA degradation. Science. 2020; 370:eabc9359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee S., Song J., Kim S., Kim J., Hong Y., Kim Y., Kim D., Baek D., Ahn K.. Selective degradation of host MicroRNAs by an intergenic HCMV noncoding RNA accelerates virus production. Cell Host Microbe. 2013; 13:678–690. [DOI] [PubMed] [Google Scholar]
- 12. Libri V., Helwak A., Miesen P., Santhakumar D., Borger J.G., Kudla G., Grey F., Tollervey D., Buck A.H.. Murine cytomegalovirus encodes a miR-27 inhibitor disguised as a target. Proc. Natl. Acad. Sci. USA. 2012; 109:279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marcinowski L., Tanguy M., Krmpotic A., Radle B., Lisnic V.J., Tuddenham L., Chane-Woon-Ming B., Ruzsics Z., Erhard F., Benkartek C.et al.. Degradation of cellular mir-27 by a novel, highly abundant viral transcript is important for efficient virus replication in vivo. PLoS Pathog. 2012; 8:e1002510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ghini F., Rubolino C., Climent M., Simeone I., Marzi M.J., Nicassio F.. Endogenous transcripts control miRNA levels and activity in mammalian cells by target-directed miRNA degradation. Nat. Commun. 2018; 9:3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bitetti A., Mallory A.C., Golini E., Carrieri C., Carreno Gutierrez H., Perlas E., Perez-Rico Y.A., Tocchini-Valentini G.P., Enright A.J., Norton W.H.J.et al.. MicroRNA degradation by a conserved target RNA regulates animal behavior. Nat. Struct. Mol. Biol. 2018; 25:244–251. [DOI] [PubMed] [Google Scholar]
- 16. Kleaveland B., Shi C.Y., Stefano J., Bartel D.P.. A Network of Noncoding Regulatory RNAs Acts in the Mammalian Brain. Cell. 2018; 174:350–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li L., Sheng P., Li T., Fields C.J., Hiers N.M., Wang Y., Li J., Guardia C.M., Licht J.D., Xie M.. Widespread microRNA degradation elements in target mRNAs can assist the encoded proteins. Genes Dev. 2021; 35:1595–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de la Mata M., Gaidatzis D., Vitanescu M., Stadler M.B., Wentzel C., Scheiffele P., Filipowicz W., Grosshans H.. Potent degradation of neuronal miRNAs induced by highly complementary targets. EMBO Rep. 2015; 16:500–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jones B.T., Han J., Zhang H., Hammer R.E., Evers B.M., Rakheja D., Acharya A., Mendell J.T.. Target-directed microRNA degradation regulates developmental microRNA expression and embryonic growth in mammals. Genes Dev. 2023; 37:661–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simeone I., Rubolino C., Noviello T.M.R., Farinello D., Cerulo L., Marzi M.J., Nicassio F.. Prediction and pan-cancer analysis of mammalian transcripts involved in target directed miRNA degradation. Nucleic Acids Res. 2022; 50:2019–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shi C.Y., Elcavage L.E., Chivukula R.R., Stefano J., Kleaveland B., Bartel D.P.. ZSWIM8 destabilizes many murine microRNAs and is required for proper embryonic growth and development. Genome Res. 2023; 33:1482–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kingston E.R., Blodgett L.W., Bartel D.P.. Endogenous transcripts direct microRNA degradation in Drosophila, and this targeted degradation is required for proper embryonic development. Mol. Cell. 2022; 82:3872–3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nahar S., Morales Moya L.J., Brunner J., Hendriks G.J., Towbin B., Hauser Y.P., Brancati G., Gaidatzis D., Grosshans H.. Dynamics of miRNA accumulation during C. elegans larval development. Nucleic Acids Res. 2024; 52:5336–5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Donnelly B.F., Yang B., Grimme A.L., Vieux K.F., Liu C.Y., Zhou L., McJunkin K.. The developmentally timed decay of an essential microRNA family is seed-sequence dependent. Cell Rep. 2022; 40:111154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ebert M.S., Neilson J.R., Sharp P.A.. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat. Methods. 2007; 4:721–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ebert M.S., Sharp P.A.. MicroRNA sponges: progress and possibilities. RNA. 2010; 16:2043–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haraguchi T., Ozaki Y., Iba H.. Vectors expressing efficient RNA decoys achieve the long-term suppression of specific microRNA activity in mammalian cells. Nucleic Acids Res. 2009; 37:e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haraguchi T., Nakano H., Tagawa T., Ohki T., Ueno Y., Yoshida T., Iba H.. A potent 2'-O-methylated RNA-based microRNA inhibitor with unique secondary structures. Nucleic Acids Res. 2012; 40:e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eis P.S., Tam W., Sun L., Chadburn A., Li Z., Gomez M.F., Lund E., Dahlberg J.E.. Accumulation of miR-155 and BIC RNA in human B cell lymphomas. Proc. Natl. Acad. Sci. USA. 2005; 102:3627–3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Linnstaedt S.D., Gottwein E., Skalsky R.L., Luftig M.A., Cullen B.R.. Virally induced cellular microRNA miR-155 plays a key role in B-cell immortalization by Epstein-Barr virus. J. Virol. 2010; 84:11670–11678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jopling C.L., Yi M., Lancaster A.M., Lemon S.M., Sarnow P.. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005; 309:1577–1581. [DOI] [PubMed] [Google Scholar]
- 32. Gottwein E., Corcoran D.L., Mukherjee N., Skalsky R.L., Hafner M., Nusbaum J.D., Shamulailatpam P., Love C.L., Dave S.S., Tuschl T.et al.. Viral microRNA targetome of KSHV-infected primary effusion lymphoma cell lines. Cell Host Microbe. 2011; 10:515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Samols M.A., Hu J., Skalsky R.L., Renne R.. Cloning and identification of a microRNA cluster within the latency-associated region of Kaposi's sarcoma-associated herpesvirus. J. Virol. 2005; 79:9301–9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grundhoff A., Sullivan C.S., Ganem D.. A combined computational and microarray-based approach identifies novel microRNAs encoded by human gamma-herpesviruses. RNA. 2006; 12:733–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pfeffer S., Sewer A., Lagos-Quintana M., Sheridan R., Sander C., Grasser F.A., van Dyk L.F., Ho C.K., Shuman S., Chien M.et al.. Identification of microRNAs of the herpesvirus family. Nat. Methods. 2005; 2:269–276. [DOI] [PubMed] [Google Scholar]
- 36. Umbach J.L., Cullen B.R.. In-depth analysis of Kaposi's sarcoma-associated herpesvirus microRNA expression provides insights into the mammalian microRNA-processing machinery. J. Virol. 2010; 84:695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cai X., Lu S., Zhang Z., Gonzalez C.M., Damania B., Cullen B.R.. Kaposi's sarcoma-associated herpesvirus expresses an array of viral microRNAs in latently infected cells. Proc. Natl. Acad. Sci. USA. 2005; 102:5570–5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Manzano M., Patil A., Waldrop A., Dave S.S., Behdad A., Gottwein E.. Gene essentiality landscape and druggable oncogenic dependencies in herpesviral primary effusion lymphoma. Nat. Commun. 2018; 9:3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Munoz D.M., Cassiani P.J., Li L., Billy E., Korn J.M., Jones M.D., Golji J., Ruddy D.A., Yu K., McAllister G.et al.. CRISPR screens provide a comprehensive assessment of cancer vulnerabilities but generate false-positive hits for highly amplified genomic regions. Cancer Discov. 2016; 6:900–913. [DOI] [PubMed] [Google Scholar]
- 40. Aguirre A.J., Meyers R.M., Weir B.A., Vazquez F., Zhang C.Z., Ben-David U., Cook A., Ha G., Harrington W.F., Doshi M.B.et al.. Genomic copy number dictates a gene-independent cell response to CRISPR/Cas9 Targeting. Cancer Discov. 2016; 6:914–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gottwein E., Mukherjee N., Sachse C., Frenzel C., Majoros W.H., Chi J.T., Braich R., Manoharan M., Soutschek J., Ohler U.et al.. A viral microRNA functions as an orthologue of cellular miR-155. Nature. 2007; 450:1096–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Skalsky R.L., Samols M.A., Plaisance K.B., Boss I.W., Riva A., Lopez M.C., Baker H.V., Renne R.. Kaposi's sarcoma-associated herpesvirus encodes an ortholog of miR-155. J. Virol. 2007; 81:12836–12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhao Y., Xu H., Yao Y., Smith L.P., Kgosana L., Green J., Petherbridge L., Baigent S.J., Nair V.. Critical role of the virus-encoded microRNA-155 ortholog in the induction of Marek's disease lymphomas. PLoS Pathog. 2011; 7:e1001305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gay L.A., Sethuraman S., Thomas M., Turner P.C., Renne R.. Modified cross-linking, ligation, and sequencing of hybrids (qCLASH) identifies Kaposi's sarcoma-associated herpesvirus microRNA targets in endothelial cells. J. Virol. 2018; 92:e02138-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Haecker I., Gay L.A., Yang Y., Hu J., Morse A.M., McIntyre L.M., Renne R.. Ago HITS-CLIP expands understanding of Kaposi's sarcoma-associated herpesvirus miRNA function in primary effusion lymphomas. PLoS Pathog. 2012; 8:e1002884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Boss I.W., Nadeau P.E., Abbott J.R., Yang Y., Mergia A., Renne R.. A Kaposi's sarcoma-associated herpesvirus-encoded ortholog of microRNA miR-155 induces human splenic B-cell expansion in NOD/LtSz-scid IL2Rgammanull mice. J. Virol. 2011; 85:9877–9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dahlke C., Maul K., Christalla T., Walz N., Schult P., Stocking C., Grundhoff A.. A microRNA encoded by Kaposi sarcoma-associated herpesvirus promotes B-cell expansion in vivo. PLoS One. 2012; 7:e49435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sin S.H., Kim Y.B., Dittmer D.P.. Latency locus complements MicroRNA 155 deficiency in vivo. J. Virol. 2013; 87:11908–11911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ju E., Li T., Liu Z., da Silva S.R., Wei S., Zhang X., Wang X., Gao S.J.. Specific inhibition of viral microRNAs by carbon dots-mediated delivery of locked nucleic acids for therapy of virus-induced cancer. ACS Nano. 2020; 14:476–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang T., Wei J.J., Sabatini D.M., Lander E.S.. Genetic screens in human cells using the CRISPR-Cas9 system. Science. 2014; 343:80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sanjana N.E., Shalem O., Zhang F.. Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods. 2014; 11:783–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gottwein E., Cullen B.R.. A human herpesvirus microRNA inhibits p21 expression and attenuates p21-mediated cell cycle arrest. J. Virol. 2010; 84:5229–5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Morrison K., Manzano M., Chung K., Schipma M.J., Bartom E.T., Gottwein E.. The oncogenic Kaposi's sarcoma-associated herpesvirus encodes a mimic of the tumor-suppressive miR-15/16 miRNA family. Cell Rep. 2019; 29:2961–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Denzler R., McGeary S.E., Title A.C., Agarwal V., Bartel D.P., Stoffel M.. Impact of microRNA levels, target-site complementarity, and cooperativity on competing endogenous RNA-regulated gene expression. Mol. Cell. 2016; 64:565–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xie J., Ameres S.L., Friedline R., Hung J.H., Zhang Y., Xie Q., Zhong L., Su Q., He R., Li M.et al.. Long-term, efficient inhibition of microRNA function in mice using rAAV vectors. Nat. Methods. 2012; 9:403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Briskin D., Wang P.Y., Bartel D.P.. The biochemical basis for the cooperative action of microRNAs. Proc. Natl. Acad. Sci. USA. 2020; 117:17764–17774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Saetrom P., Heale B.S., Snove O. Jr., Aagaard L., Alluin J., Rossi J.J. Distance constraints between microRNA target sites dictate efficacy and cooperativity. Nucleic Acids Res. 2007; 35:2333–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Haas G., Cetin S., Messmer M., Chane-Woon-Ming B., Terenzi O., Chicher J., Kuhn L., Hammann P., Pfeffer S.. Identification of factors involved in target RNA-directed microRNA degradation. Nucleic Acids Res. 2016; 44:2873–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Manzano M., Gunther T., Ju H., Nicholas J., Bartom E.T., Grundhoff A., Gottwein E.. Kaposi's sarcoma-associated herpesvirus drives a super-enhancer-mediated survival gene expression program in primary effusion lymphoma. mBio. 2020; 11:e01457-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. McGeary S.E., Bisaria N., Pham T.M., Wang P.Y., Bartel D.P.. MicroRNA 3'-compensatory pairing occurs through two binding modes, with affinity shaped by nucleotide identity and position. eLife. 2022; 11:e69803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fuchs Wightman F., Lukin J., Giusti S.A., Soutschek M., Bragado L., Pozzi B., Pierelli M.L., Gonzalez P., Fededa J.P., Schratt G.et al.. Influence of RNA circularity on target RNA-directed MicroRNA degradation. Nucleic Acids Res. 2024; 52:3358–3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Any raw data and constructs will be shared on reasonable request to the corresponding author.