Osteoporosis, characterized by decreased bone mineral density (BMD) and strength, significantly increases the risk of fractures, disability, and mortality, particularly among older adults [1]. As the global population ages, the burden of osteoporosis on healthcare systems continues to grow worldwide. Diabetes, another prevalent chronic disease among older adults, significantly contributes to increased morbidity and mortality [2]. Diabetes further exacerbates bone fragility by disrupting bone metabolism and increasing fracture risk through advanced glycation end-products (AGEs) accumulation. Denosumab, a humanized monoclonal antibody targeting the receptor activator of nuclear factor κ B ligand (RANKL), is a potent antiresorptive agent known for its efficacy in reducing bone resorption and increasing BMD [3]. Despite its success in the management of osteoporosis, the specific efficacy, safety profile, and potential impact of denosumab on glucose metabolism in patients with diabetes remains underexplored. While some clinical trials are in process, studies in this field are still lacking.
To better understand the effectiveness and safety of denosumab in osteoporosis management for diabetes patients, we systematically collected high-quality information on the progress of clinical trial related to osteoporosis. We searched the Informa Database using the terms ‘Osteoporosis’ and ‘Denosumab’ and identified 196 independent studies listed up to June 2024. The data we collected included trial ID, drug names, targets, mechanisms and other related clinical information. We analyzed trends across clinical trial phases, drug targets, and other parameters to assess the current landscape of trials involving denosumab in the treatment of osteoporosis.
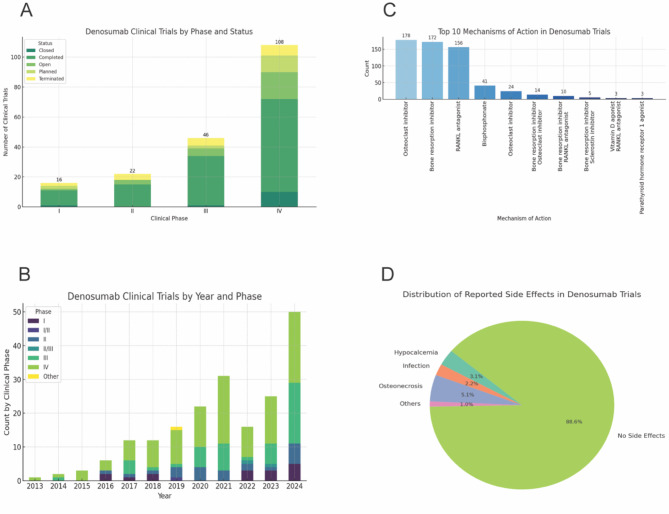
Of the 196 trials, 51% were conducted in North America, followed by Europe (29%) and Asia (14%), reflecting the geographical prevalence and the corresponding research investments. Most trials (55%) were in Phase IV, emphasizing long-term safety, while the others spanned Phase I to III. (Fig. 1A).
Fig. 1.
A: Distribution of Denosumab Clinical Trials by Phase B:Temporal Trends in Clinical Trials Involving Denosumab from 2013 to 2024 C: Safety and Efficacy Outcomes of Denosumab Use in Osteoporosis Management D:Potential Mechanisms of Denosumab in Improving Bone and Metabolic Health
A total of 123 trials have been completed, representing a substantial body of research demonstrating the efficacy and safety of denosumab on osteoporosis. Twenty-seven trials are open, indicating ongoing investigations into further applications or different patient populations. Additionally, 19 of the 196 trials were terminated, possibly due to safety concerns, lack of efficacy, or logistical challenges, such as difficulties in recruitment. Moreover, there are 15 new trials planned, suggesting a continued interest in exploring novel indications or combination therapies. The rise in denosumab trials since 2013, except for a temporary dip during the pandemic (2020–2022), highlights the growing interest in its potential as an osteoporosis treatment(Fig. 1B).
Regardless of diabetes status, denosumab was found to be associated with significant improvements in patients’ BMD and a reduction in fracture risk. In approximately 5% (9 out of 196) of the trials, blood glucose levels were assessed, indicating interest in its potential impact on glucose metabolism. Specifically, 5 trials investigated blood glucose levels in osteoporosis patients with diabetes, primarily conducted in North America, Asia, and Europe—regions with significant research interest in osteoporosis and diabetes. Sample sizes varied across these trials allowing researchers to analyze denosumab’s dual benefits across different patient profiles. While 5 trials exclusively focused on patients with diabetes, the other 4 included individuals with prediabetes or impaired glucose regulation, aiming to explore denosumab’s broader metabolic benefits. The motivation for assessing glucose levels stems from prior research suggesting that denosumab could positively influence glucose metabolism [4], presenting a promising therapeutic strategy for managing both osteoporosis and metabolic health in high-risk populations. These trials highlight the growing interest in personalized treatment for patients with overlapping bone and metabolic health challenges. Previous research has reported that combination therapies, including the co-administration of vitamin D supplements or bisphosphonates, enhance therapeutic outcomes [5], especially in high-risk patients with diabetes. Such combination therapies may offer synergistic effects, improving BMD and reducing the incidence of hypocalcemia, infections, and osteonecrosis. Our analysis identified ten potential mechanisms that may explain these synergistic effects (Fig. InternalRef RefID=“Tab1”>1C). These findings underscore the importance of personalized treatment plans and regular monitoring to effectively manage these potential risks. However, some side effects may arise with long-term use of denosumab. Hypocalcemia, infection, and osteonecrosis of the jaw have been reported in some trials(Fig. 1)is.
Denosumab shows significant promise in the management of osteoporosis in addition to possible positive effects on diabetes management in those patients with osteoporosis and diabetes. To maximize its potential dual therapeutic benefits, future research should prioritize targeted clinical trials, personalized treatment approaches, and robust safety monitoring protocols. Specifically, randomized controlled trials are needed for patients with both diabetes and osteoporosis, designed with dual endpoints to evaluate both bone health and metabolic outcomes. Additionally, developing personalized treatment strategies based on biomarkers, such as HbA1c and bone turnover markers, could enhance the efficacy and safety of denosumab therapy. Furthermore, implementing comprehensive safety monitoring protocols, especially for high-risk populations, is crucial for early the detection and management of potential adverse effects. These procedures will optimize the therapeutic benefits of denosumab while ensuring patient safety.
Acknowledgements
Not applicable.
Author contributions
Conceptualization, William Tang and Luohua Jiang; Manuscript draft, Chenyu Huang, Jiahui Dai, Philip K. Lim, and Katherine Colcord. All the authors have read and approved the final manuscript.
Funding
Not applicable.
Data availability
All data for this study are publicly available.
Declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
The authors have seen and approved the final manuscript.
Competing interests
The authors have declared that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Clynes MA, Harvey NC, Curtis EM, et al. The epidemiology of osteoporosis. Br Med Bull. 2020;133(1):105–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. National Diabetes statistics Report. U.S. Department of Health and Human Services; 2024.
- 3.Lacey DL, Boyle WJ, Simonet WS, et al. Bench to bedside: elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat Rev Drug Discov. 2012;11:401–19. [DOI] [PubMed] [Google Scholar]
- 4.Pacheco-Soto BT, Elguezabal-Rodelo RG, Porchia LM, et al. Denosumab improves glucose parameters in patients with impaired glucose tolerance: a systematic review and meta-analysis. J Drug Assess. 2021;10(1):97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Zhao P, Jiang B, et al. Modulation of the vitamin D/vitamin D receptor system in osteoporosis pathogenesis: insights and therapeutic approaches. J Orthop Surg Res. 2023;18(1):860. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data for this study are publicly available.