Abstract
Background
Polyarteritis nodosa is a relatively uncommon type of systemic necrotizing vasculitis that primarily affects medium-sized arteries. While gastrointestinal involvement is known in polyarteritis nodosa, heavy gastrointestinal bleeding due to gastric ulceration is relatively uncommon. We present the case of an 81-year-old male of Chinese ethnicity who experienced severe gastrointestinal bleeding as a result of polyarteritis nodosa and an innovative treatment approach for a better patient outcomes.
Case presentation
Upon admission to the medical intensive care unit, the patient underwent a comprehensive diagnostic assessment, including examinations for cardiovascular and dermatological abnormalities, laboratory tests, autoantibody and tumor marker assessments, and imaging studies (such as endoscopies, whole-body computed tomography, and positron emission tomography–computed tomography scans), and a skin biopsy. The patient had tachycardia, hypotension, and extensive skin abnormalities on the lower extremities along with anemia, low platelets, and abnormal renal function. Upper gastrointestinal endoscopy revealed gastric and duodenal ulcers. Additional examinations, including electronic colonoscopy, capsule endoscopy, and whole-body computed tomography, were negative. A positron emission tomography–computed tomography scan showed increased uptake in the arterial walls and skin, which supported the diagnosis of polyarteritis nodosa, later confirmed by a biopsy of the skin on the lower extremities. Methylprednisolone, octreotide, and omeprazole were administered, leading to improvement in gastrointestinal symptoms, ulcer healing, and skin recovery. The patient continued with prednisone for 1 month.
Conclusion
This case serves to inform gastroenterologists about the need to consider polyarteritis nodosa in severe upper gastrointestinal bleeding and underscores the importance of prompt, medication-based treatment for successful patient outcome.
Keywords: Polyarteritis nodosa, Gastrointestinal bleeding, Endoscopy, Skin biopsy, Glucocorticoid
Introduction
Polyarteritis nodosa (PAN) is a type of systemic necrotizing vasculitis that primarily affects medium-sized blood vessels [1–3]. PAN typically does not affect veins and is characterized by being antineutrophil cytoplasmic antibodies (ANCA)-negative. In a small percentage of cases, particularly among intravenous drug users, PAN is linked to hepatitis B or C infections. An autoimmune origin has been proposed for the disease, though some cases remain idiopathic, with no clear underlying cause. Clinical manifestations consist of fever, perspiration, loss of weight, and discomfort in the muscles and joints. The vasculitis primarily affects the vessels in the skin, kidneys, nerves, and gastrointestinal tract [4]. Diagnosing PAN can be challenging and often overlooked, initially due to its diverse symptoms and the absence of specific biochemical or hematological markers.
Gastrointestinal (GI) manifestation occurs with a frequency ranging from 10% to 50% in patients with PAN, including melaena, hematochezia, perforation, cholecystitis, appendicitis, acute abdominal pain, bleeding, hemorrhage, and infarction [5, 6]. In the most recent longitudinal study by the French Vasculitis Study Group (FVSG), gastrointestinal involvement was observed in 27% of cases, with abdominal pain in 20%, bleeding in 11%, and perforation in 7%. Despite this, GI symptoms often lack specificity. Particularly severe manifestations, such as bleeding or perforation, are frequently overlooked in differential diagnoses. The Five Factors Score identifies severe GI involvement as a poor prognostic factor [7]. The primary approach to managing medical treatment is immunosuppressant therapy [5, 6, 8]. PAN with GI involvement is a serious and potentially life-threatening condition that necessitates prompt and proactive intervention to reduce the associated morbidity and mortality [9].
Here, we present a case of PAN with extensive GI bleeding caused by gastric ulceration, which is an uncommon manifestation of the disease. Besides, the patient’s complex clinical profile, including cardiovascular abnormalities, skin lesions, and renal dysfunction, posed a significant diagnostic challenge that required comprehensive imaging and histopathology to confirm the diagnosis. Moreover, the case is distinct because of the prompt treatment approach that involved a combination of medications, leading to successful management of both the GI and dermatological symptoms without surgery and invasive treatments.
Case presentation
An 81-year-old man of Chinese ethnicity was admitted to the hospital with a 3-year history of skin ulcers and hypoesthesia primarily in both lower limbs, along with a 2-day history of melena. He did not experience nausea, vomiting, abdominal pain, diarrhea, or fever. The patient had no history of nonsteroidal anti-inflammatory drug use, liver disease, or any chronic conditions other than diabetes and hypertension.
On physical examination, the patient was found to have tachycardia and hypotension, the skin of both lower extremities was hyperpigmented, thickened, and desquamated, with multiple ulcerated scabs and ulcerations on both lower extremities (Fig. 1A). The patient was admitted to the medical intensive care unit, and upon observing melena, an emergency endoscopy was performed. Upper GI endoscopy revealed a gastric body ulcer and multiple duodenal ulcers, with the gastric ulcer classified as superficial and the duodenal ulcers also described as superficial (Fig. 1B). The patient then underwent a comprehensive series of diagnostic tests including laboratory tests, urine microscopy, imaging studies, and skin biopsy, with the results summarized in Table 1.
Fig. 1.
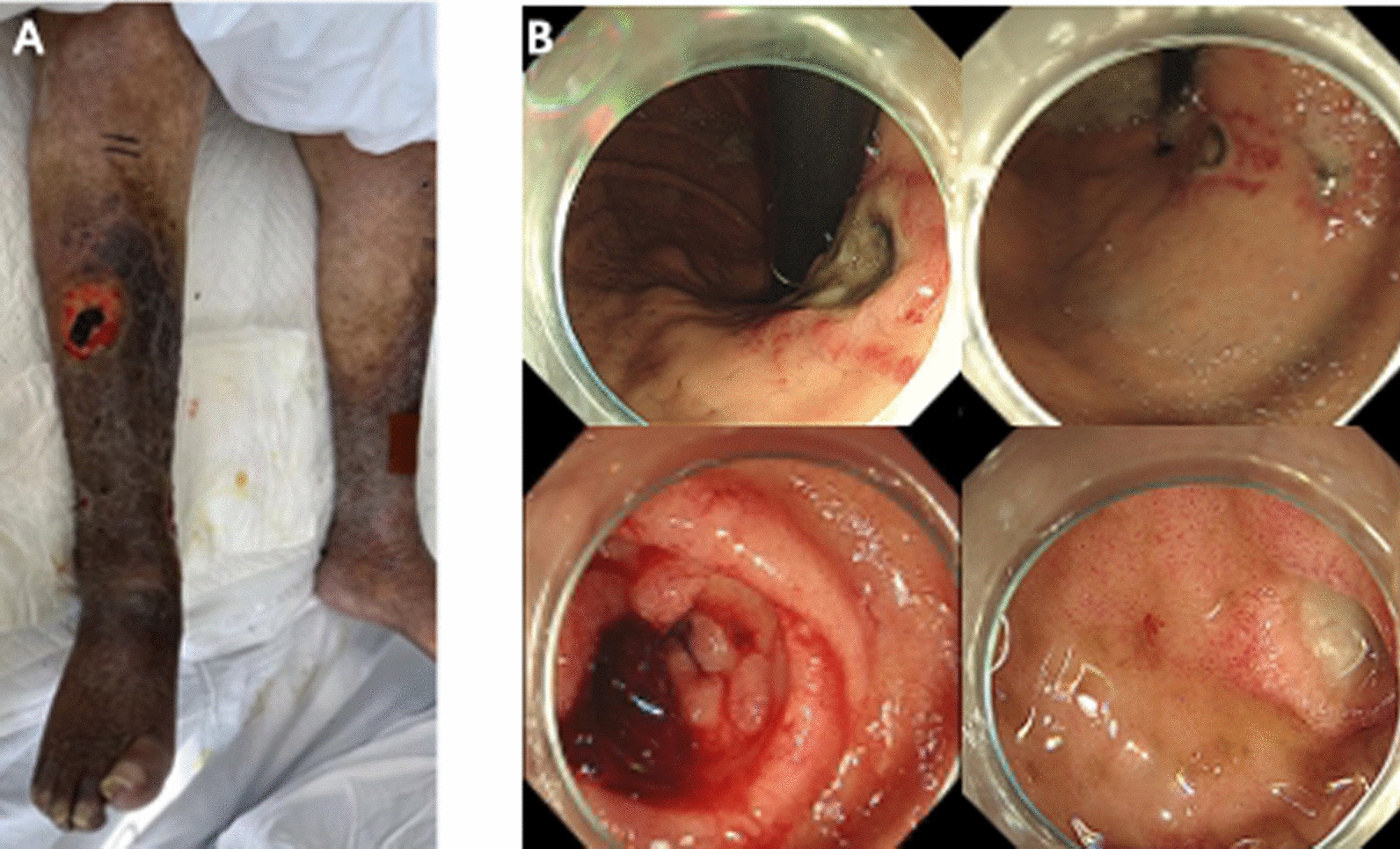
A Diffuse thickening, desquamation and chromatosis of both lower limbs with ulcerations. B Upper gastrointestinal endoscopic picture showing gastric body ulcer (stage A1) and duodenal multiple ulcers
Table 1.
Summary of diagnostic findings and investigations
| Category | Findings |
|---|---|
| Vital signs | |
| Heart rate | 110 beats/minute (tachycardia) |
| Blood pressure | 86/56 mmHg (hypotension) |
| Laboratory tests | |
| Hemoglobin | 66 g/L (anemia) |
| Platelets (PLT) | 44 × 109/L (low) |
| Blood urea nitrogen | 21.46 mmol/L (elevated) |
| Creatinine | 209.8 µmol/L (elevated) |
| Urine red blood cells | 44 per high-power field (indicating bleeding) |
| Urine leukocytes | 43 per high-power field (elevated, suggesting possible infection or inflammation) |
| 24 h urine protein | 2.93 g (elevated, indicating proteinuria) |
| Urine microscopy | |
| Red blood cells | Varied in size, with annular, spiny, and serrated shapes |
| Autoantibody tests | |
| Anticardiolipin antibody | > 90 U/mL (elevated) |
| β2-glycoprotein I | 45 U/mL (elevated) |
| Tumor markers | |
| Cancer antigen 125 (CA125) | 81.7 U/mL (elevated, associated with ovarian cancer or other conditions) |
| Cancer antigen 19-9 (CA19-9) | 64.11 U/mL (elevated, associated with pancreatic cancer or other malignancies) |
| Cytokeratin 19 fragment (CYFRA 21-1) | 4.00 ng/mL (normal) |
| Progastrin-releasing peptide (Pro-GRP) | 155.56 pg/mL (elevated, may indicate lung or neuroendocrine tumors) |
| Squamous cell carcinoma antigen (SCC) | 2.76 ng/mL (normal) |
| Other tests | |
| Cell cytokines | Normal |
| Vasculitis antibodies | Normal |
| Coagulation function | Normal |
| Hepatitis B surface antigen | Normal |
| Abdominal ultrasound | Normal |
| Imaging and pathology | |
| Electronic colonoscopy | Negative |
| Capsule endoscopy | Negative |
| Whole-body computed tomography | Negative |
| Positron emission tomography/computed tomography scan | Diffuse increased radioactive uptake in arterial walls of both lower extremities |
| Skin biopsy | Localized epidermal erosions and ulcers |
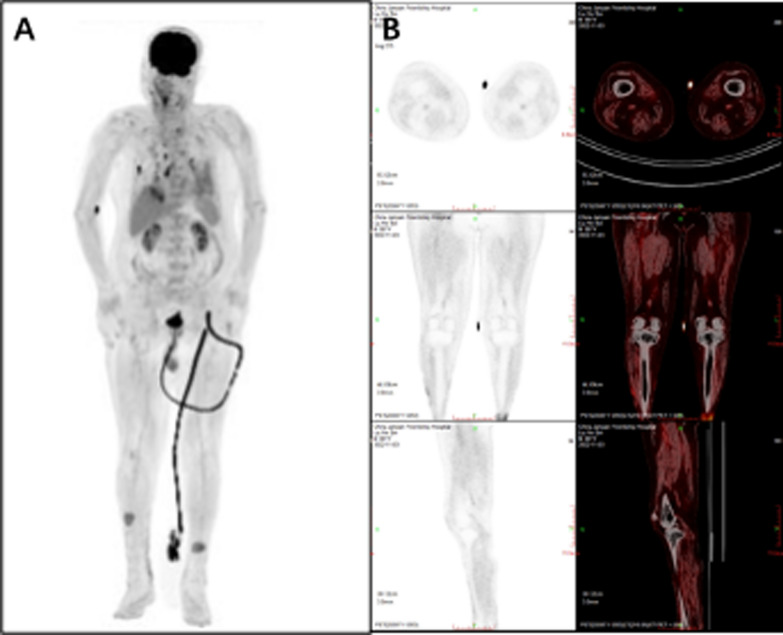
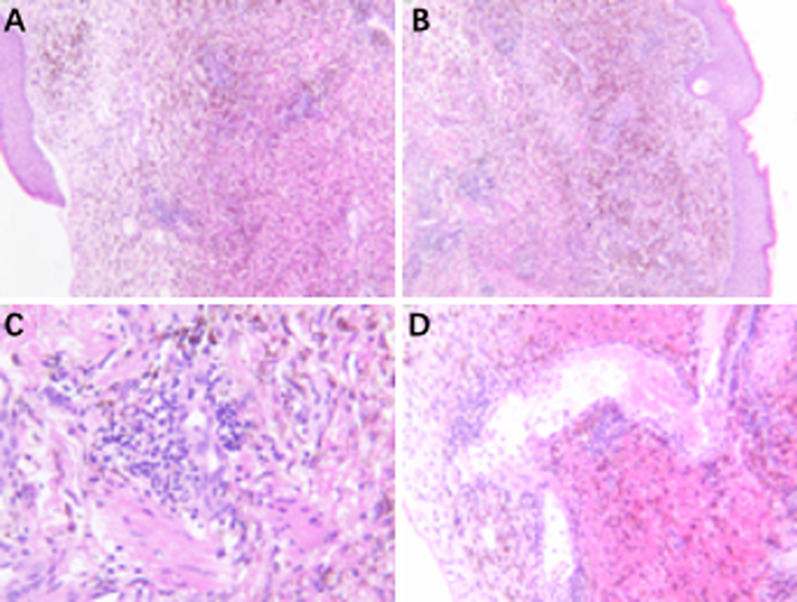
Given the patient’s involvement of multiple organs, including GI bleeding, purpura cutis, and skin ulcers in the perineum, perianal area, and scrotum, we sought a multidisciplinary consultation to explore diagnoses such as Crohn’s disease, vasculitis, and tumors. Subsequent relevant examinations, including electronic colonoscopy, capsule endoscopy, and whole-body computed tomography (CT), returned negative results. Due to the patient’s renal insufficiency and severe lower extremity skin ulcers, we did not perform renal angiography and electromyography. Furthermore, positron emission tomography (PET)–CT scan showed diffusely increased radioactive uptake in arterial walls of both lower extremities, also multiple thickening, and increased metabolism in skin of both lower extremities (Fig. 2). Skin biopsy of the lower extremities showed localized epidermal erosions and ulcers and focal dermal bleeding with extensive hemosiderin deposition and lymphocytic infiltration, accumulation, and proliferation of granulation tissue (Fig. 3).
Fig. 2.
The maximum intensity projection (A) and coronal image (B) from the positron emission tomography–computed tomography scan show increased uptake of fluorodeoxyglucose in the femoral and popliteal arteries, as well as in a localized ulcer on the inner side of both calves and in the muscle compartments throughout the body
Fig. 3.
A skin biopsy (hematoxylin and eosin staining), A, B ×100, C, D ×400) reveals necrosis and ulceration resulting from ischemia caused by vascular involvement, along with a perivascular lymphocytic infiltration surrounding the medium-sized vessel wall, consistent with a diagnosis of polyarteritis nodosa
After integrating information from the patient’s clinical manifestations, laboratory results, skin pathology, and PET–CT examination, we diagnosed PAN and initiated treatment with methylprednisolone: 40 mg intravenous (IV) daily for 3 days, followed by 32 mg for 4 days, and then 24 mg for 3 days. The treatment was combined with intravenous octreotide and omeprazole. We did not include immunosuppressive agents such as cyclophosphamide due to the presence of high metabolic nodules in the patient’s lungs as indicated by the PET–CT, and the patient declined further biopsy.
Post treatment, the patient’s melena resolved, and an electronic gastroscopy revealed that the skin ulcers had gradually scabbed (Fig. 4A), and the gastric ulcers had healed (Fig. 4B). The patient continued with oral prednisone 30 mg daily for 1 month.
Fig. 4.
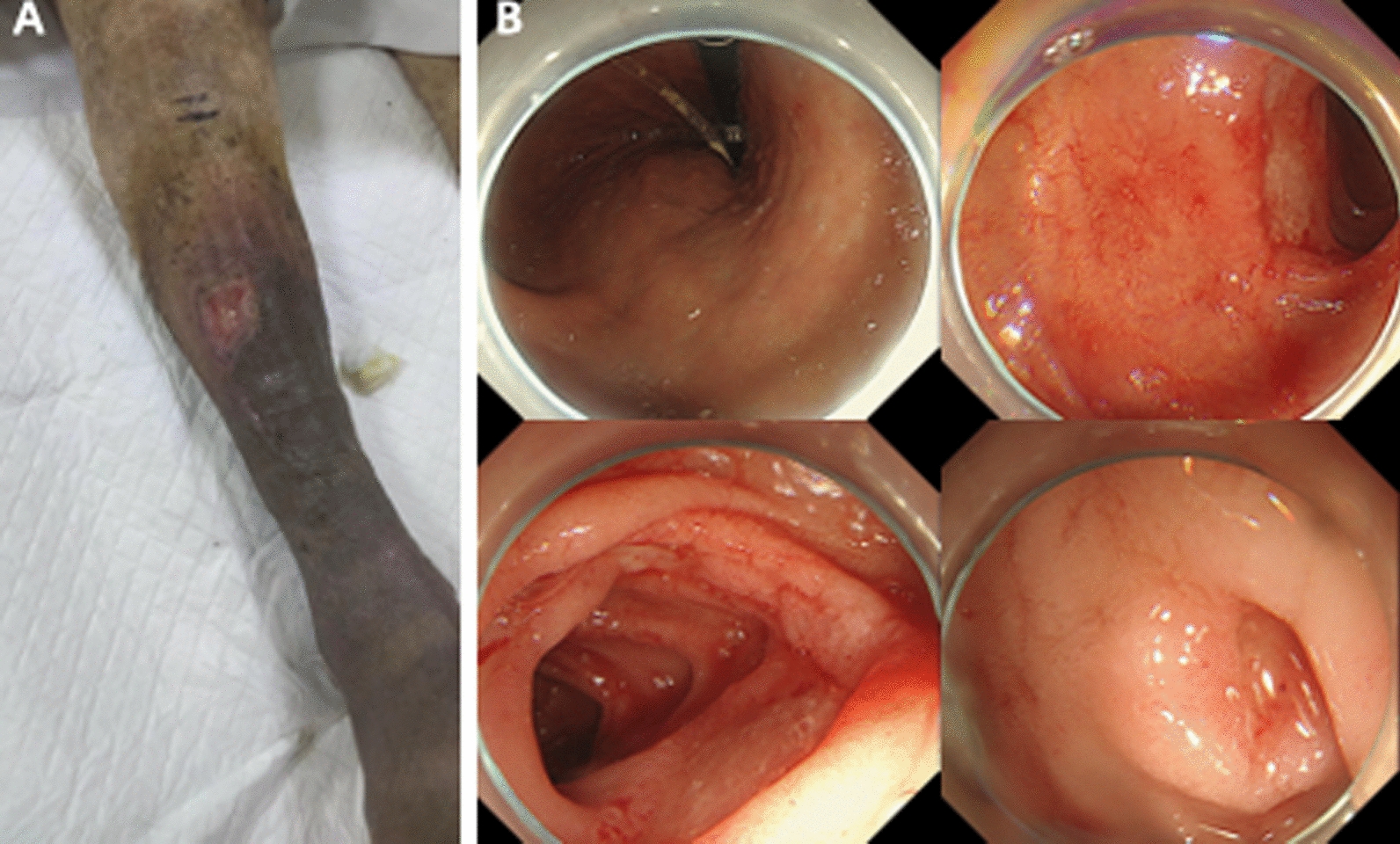
Skin ulcerations gradually scabbed (A) and the gastric ulcer significantly relieved (B) after 2 weeks of glucocorticoid treatment, but the small bowel ulcer remained
Discussion
PAN is a rare idiopathic systemic vasculitis characterized by medium-sized and small arteries necrotizing inflammatory lesions [10, 11]. The pathological feature of PAN is transmural inflammation of the vascular wall, typically manifested as fusiform or saccular microaneurysms, which can lead to luminal stenosis or aneurysm formation. The formation of these structures ultimately leads to local ischemia [12, 13]. There are two types of PAN: typical PAN involving medium-sized blood vessels and cutaneous PAN characterized only by subcutaneous nodules and skin ulcers [14]. The association of PAN with hepatitis B virus (HBV) and hepatitis C virus (HCV) infection is well recognized [15].
The clinical manifestations of PAN can range from single-organ involvement to multiple systems [14]. PAN is often first detected in patients who present with a fever of unknown origin. After this initial presentation of fever, patients with PAN usually develop symptoms related to involvement of various organs within a few weeks. The progression of the disease from an initial fever to organ involvement can be relatively rapid [11, 16]. The organs most affected by PAN included mono neuritis multiplex (75%), kidney (51%), skin (50%), GI tract (38%), and cardiac and vascular disease (22%) [14, 17]. In the skin, PAN can manifest as erythematous nodules, ulcers, bullous or vesicular eruptions, purpura, and reticularis [18, 19]. The incidence of HBV-associated PAN has declined substantially since the vaccination of high-risk populations against hepatitis B [11, 20].
GI involvement of PAN, ranging from 14% to 65% [4, 5, 21, 22] include abdominal pain, nausea, bloody or bloodless diarrhea, vomiting, melena, and pancreatic presentation [6, 23, 24]. GI hemorrhage in PAN is rare, but it can occur in any location, from the esophagus to the rectum, and the prognosis is often poor. It may be manifested by hematemesis, melena, biliary tract bleeding, and intra-abdominal bleeding [25]. GI hemorrhage may be caused by mucosa ischemia ulceration, intestinal infarction, and mucosal ulceration is seen in 5–6% of patients with PAN, mainly in jejunum [17, 22, 26]. There are case reports demonstrating that ruptured hepatic artery and mesenteric aneurysm, duodenal fistula, and haemobilia can cause severe intestinal bleeding in patients with PAN [27–30]. Narusako et al. [31] reported the first case of PAN with multiple refractory bleeding gastric ulcers who underwent gastrectomy. Moreover, Perez et al. [31] described a case of PAN with massive upper GI bleeding due to gastric ulcer. The patient underwent a gastrectomy, and the diagnosis was confirmed through histopathological examination. GI bleeding in PAN is associated with serious complications and adverse outcomes [6, 22, 32, 33].
We diagnosed PAN in this patient according to the 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculatides [11] after integrating information from the patient’s clinical manifestations, laboratory results, and skin biopsy results. A PET–CT examination also confirmed that increased radioactive uptake in arterial walls of both lower extremities, which suggested a local inflammation of the aneurysm [34]. However, for a better diagnosis, angiography examination was needed, which was not performed in this patient due to renal dysfunction. One report describes the successful treatment of PAN with upper GI bleeding caused by gastric ulceration using glucocorticoid, resulting in recovery [25]. Based on our case, we recommend considering PAN in the differential diagnosis of upper GI bleeding, especially when endoscopic findings reveal large ulcers and the patient exhibits multisystem involvement, such as skin lesions, nerve damage, and other systemic manifestations. Prompt treatment with medications, including glucocorticoids (methylprednisolone and prednisone), octreotide, and omeprazole, is crucial to manage PAN effectively and avoid the need for surgery. A schematic diagram illustrating the patient’s clinical presentations in this case of PAN, along with the corresponding management strategies implemented throughout the course of treatment (Fig. 5).
Fig. 5.
A schematic diagram depicting the patient’s clinical manifestations of polyarteritis nodosa, and management approaches employed during treatment
Conclusion
While the existing literature reports only a few cases, this case significantly enhances our limited understanding by emphasizing the critical role of timely diagnosis and prompt administration of a medication regimen in PAN with severe upper GI bleeding. This approach can effectively manage the underlying inflammation in PAN and potentially improve outcomes in cases of severe upper GI bleeding. The case will also serve to inform and guide gastroenterologists in their approach to diagnosing and managing instances of massive upper GI bleeding associated with PAN.
Acknowledgements
Not applicable.
Author contributions
FL is responsible for all the experiments and data. X-YX wrote the article. YN collected the data. P-PL wrote part of the article. Y-MZ collected the data. M-GZ collected the data. G-CW added the references. X-DW recorded the data.
Funding
Not applicable.
Availability data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
No IRB is required for case reports and written informed consent was obtained.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stone JH. Polyarteritis nodosa. JAMA. 2002;288(13):1632–9. 10.1001/jama.288.13.1632. [DOI] [PubMed] [Google Scholar]
- 2.Rohmer J, Nguyen Y, Trefond L, et al. Clinical features and long-term outcomes of patients with systemic polyarteritis nodosa diagnosed since 2005: data from 196 patients. J Autoimmun. 2023;139: 103093. 10.1016/j.jaut.2023.103093. [DOI] [PubMed] [Google Scholar]
- 3.Travers RL, Allison DJ, Brettle RP, et al. Polyarteritis nodosa: a clinical and angiographic analysis of 17 cases. Semin Arthritis Rheum. 1979;8(3):184–99. 10.1016/s0049-0172(79)80007-4. [DOI] [PubMed] [Google Scholar]
- 4.Ahn E, Luk A, Chetty R, et al. Vasculitides of the gastrointestinal tract. Semin Diagn Pathol. 2009;26(2):77–88. 10.1053/j.semdp.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Ebert EC, Hagspiel KD, Nagar M, et al. Gastrointestinal involvement in polyarteritis nodosa. Clin Gastroenterol Hepatol. 2008;6(9):960–6. 10.1016/j.cgh.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Levine SM, Hellmann DB, Stone JH. Gastrointestinal involvement in polyarteritis nodosa (1986–2000): presentation and outcomes in 24 patients. Am J Med. 2002;112(5):386–91. 10.1016/s0002-9343(01)01131-7. [DOI] [PubMed] [Google Scholar]
- 7.Guillevin L, Pagnoux C, Seror R, et al. The five-factor score revisited: assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Medicine. 2011;90(1):19–27. 10.1097/MD.0b013e318205a4c6. [DOI] [PubMed] [Google Scholar]
- 8.Pagnoux C, Mahr A, Cohen P, et al. Presentation and outcome of gastrointestinal involvement in systemic necrotizing vasculitides: analysis of 62 patients with polyarteritis nodosa, microscopic polyangiitis, Wegener granulomatosis, Churg-Strauss syndrome, or rheumatoid arthritis-associated vasculitis. Medicine. 2005;84(2):115–28. 10.1097/01.md.0000158825.87055.0b. [DOI] [PubMed] [Google Scholar]
- 9.Jardel S, Puechal X, Le Quellec A, et al. Mortality in systemic necrotizing vasculitides: a retrospective analysis of the French Vasculitis Study Group registry. Autoimmun Rev. 2018;17(7):653–9. 10.1016/j.autrev.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Ozen S. The changing face of polyarteritis nodosa and necrotizing vasculitis. Nat Rev Rheumatol. 2017;13(6):381–6. 10.1038/nrrheum.2017.68. [DOI] [PubMed] [Google Scholar]
- 11.Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised international Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013;65(1):1–11. 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 12.Robateau Colon A, Rapaka B, Wang XJ. A bad gut feeling: a common disease, an unlikely complication, and a rare cause. Gastroenterology. 2021;160(5):1475–6. 10.1053/j.gastro.2020.11.028. [DOI] [PubMed] [Google Scholar]
- 13.Chircop I, Boespflug A, Cini A, et al. Paraneoplastic polyarteritis nodosa in a patient with cutaneous T-cell lymphoma. Lancet Haematol. 2021;8(3): e240. 10.1016/S2352-3026(20)30393-8. [DOI] [PubMed] [Google Scholar]
- 14.Lai FYX, Groschel MIP, van den Hende L, et al. Prolonged pyrexia and subtle skin lesions: polyarteritis nodosa. Lancet. 2016;387(10022):1025–6. 10.1016/S0140-6736(16)00501-8. [DOI] [PubMed] [Google Scholar]
- 15.Anderson B, Sweetser S. Chronic abdominal pain in a 47-year-old woman. Gastroenterology. 2018;155(3):627–8. 10.1053/j.gastro.2018.02.043. [DOI] [PubMed] [Google Scholar]
- 16.Colmegna I, Maldonado-Cocco JA. Polyarteritis nodosa revisited. Curr Rheumatol Rep. 2005;7(4):288–96. 10.1007/s11926-005-0039-2. [DOI] [PubMed] [Google Scholar]
- 17.Pagnoux C, Seror R, Henegar C, et al. Clinical features and outcomes in 348 patients with polyarteritis nodosa: a systematic retrospective study of patients diagnosed between 1963 and 2005 and entered into the French Vasculitis Study Group Database. Arthritis Rheum. 2010;62(2):616–26. 10.1002/art.27240. [DOI] [PubMed] [Google Scholar]
- 18.Gibson LE, Su WP. Cutaneous vasculitis. Rheum Dis Clin N Am. 1995;21(4):1097–113. [PubMed] [Google Scholar]
- 19.Karlsberg PL, Lee WM, Casey DL, et al. Cutaneous vasculitis and rheumatoid factor positivity as presenting signs of hepatitis C virus-induced mixed cryoglobulinemia. Arch Dermatol. 1995;131(10):1119–23. [PubMed] [Google Scholar]
- 20.Pang CL, Richardson P, Makkuni D. A difficult case of fever of unknown origin. BMJ Case Rep. 2012. 10.1136/bcr.11.2011.5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee EL, Smith HJ, Miller GL 3rd, et al. Ischemic pseudomembranous colitis with perforation due to polyarteritis nodosa. Am J Gastroenterol. 1984;79(1):35–8. [PubMed] [Google Scholar]
- 22.Soowamber M, Weizman AV, Pagnoux C. Gastrointestinal aspects of vasculitides. Nat Rev Gastroenterol Hepatol. 2017;14(3):185–94. 10.1038/nrgastro.2016.179. [DOI] [PubMed] [Google Scholar]
- 23.Flaherty J, Bradley EL 3rd. Acute pancreatitis as a complication of polyarteritis nodosa. Int J Pancreatol. 1999;25(1):53–7. 10.1385/IJGC:25:1:53. [DOI] [PubMed] [Google Scholar]
- 24.Suresh E, Beadles W, Welsby P, et al. Acute pancreatitis with pseudocyst formation in a patient with polyarteritis nodosa. J Rheumatol. 2005;32(2):386–8. [PubMed] [Google Scholar]
- 25.Saikia N, Talukdar R, Mazumder S, et al. Polyarteritis nodosa presenting as massive upper gastrointestinal hemorrhage. Gastrointest Endosc. 2006;63(6):868–70. 10.1016/j.gie.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 26.Bailey M, Chapin W, Licht H, et al. The effects of vasculitis on the gastrointestinal tract and liver. Gastroenterol Clin N Am. 1998;27(4):747–82, v–vi. 10.1016/s0889-8553(05)70032-7. [DOI] [PubMed]
- 27.Cabal E, Holtz S. Polyarteritis as a cause of intestinal hemorrhage. Gastroenterology. 1971;61(1):99–105. [PubMed] [Google Scholar]
- 28.Han SY, Jander HP, Laws HL. Polyarteritis nodosa causing severe intestinal bleeding. Gastrointest Radiol. 1976;1(3):285–7. 10.1007/BF02256382. [DOI] [PubMed] [Google Scholar]
- 29.Nguan C, Leone E. A case of spontaneous perirenal hemorrhage secondary to polyarteritis nodosa. Can J Urol. 2002;9(6):1704–6. [PubMed] [Google Scholar]
- 30.Shuster TA, Almeida J, Coats R, et al. Gastrointestinal bleeding as the initial manifestation of a polyarteritis nodosa-associated hepatic artery aneurysm-duodenal fistula—a case report. Vasc Endovasc Surg. 2004;38(6):563–8. 10.1177/153857440403800611. [DOI] [PubMed] [Google Scholar]
- 31.Narusako T, Ueyama H, Tsunetomi N, et al. Multiple hemorrhagic gastric ulcers due to polyarteritis nodosa. Intern Med. 1997;36(9):657–60. 10.2169/internalmedicine.36.657. [DOI] [PubMed] [Google Scholar]
- 32.Latus J, Koetter I, Fritz P, et al. Gastrointestinal involvement in granulomatosis with polyangiitis and microscopic polyangiitis: histological features and outcome. Int J Rheum Dis. 2014;17(4):412–9. 10.1111/1756-185X.12203. [DOI] [PubMed] [Google Scholar]
- 33.Hiraike Y, Kodaira M, Sano M, et al. Polyarteritis nodosa diagnosed by surgically resected jejunal necrosis following acute abdomen. World J Gastroenterol. 2013;19(18):2830–4. 10.3748/wjg.v19.i18.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watanabe TT, Shiojiri T. PET-CT and polyarteritis nodosa-associated artery aneurysms. QJM. 2019;112(3):219–20. 10.1093/qjmed/hcy308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.