Abstract
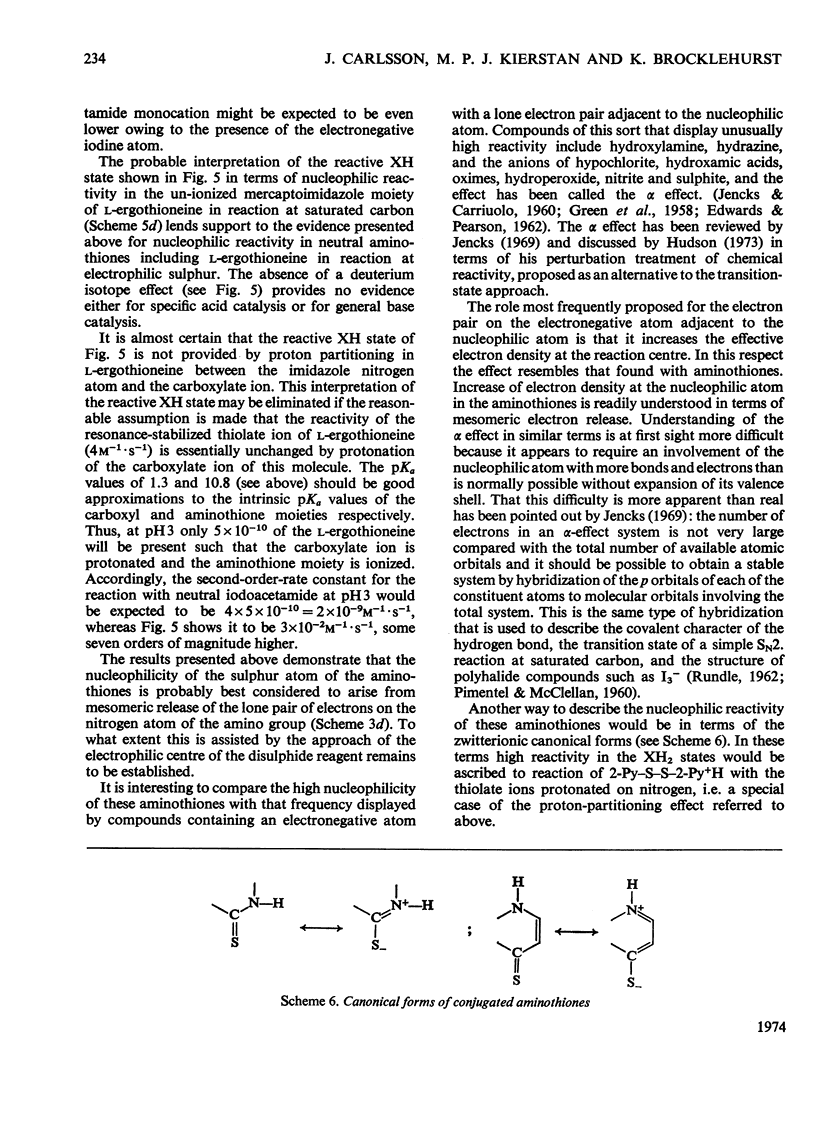
1. The reactions of 2,2′- and 4,4′-dipyridyl disulphide (2-Py–S–S–2-Py and 4-Py–S–S–4-Py) with l-ergothioneine (2-mercapto-l-histidine betaine), 2-mercaptoimidazole, 1-methyl-2-mercaptoimidazole, thiourea, thioacetamide, 2-thiopyridone (Py–2-SH) and 4-thiopyridone (Py–4-SH) were investigated spectrophotometrically in the pH range approx. 1–9. 2. These reactions involve two sequential reversible thiol–disulphide interchanges. 3. The reaction of l-ergothioneine with 2-Py–S–S–2-Py and/or with the l-ergothioneine–Py–2-SH mixed disulphide, both of which provide Py–2-SH, is characterized by at least three reactive protonic states. This provides definitive evidence that neutral l-ergothioneine is a reactive nucleophile, particularly towards the highly electrophilic protonated disulphides. 4. A similar situation appears to obtain in the reactions of l-ergothioneine and Py–2-SH with 4-Py–S–S–4-Py and in the reactions of the other 2-mercaptoimidazoles, thiourea and Py–4-SH with 2-Py–S–S–2-Py. The nucleophilic reactivity of Py–4-SH suggests that general base catalysis provided by the disulphide in a cyclic or quasi-cyclic transition state is not necessary to generate nucleophilic reactivity in the other amino-thiones whose geometry could permit such catalysis. 5. The existence of a positive deuterium isotope effect in the l-ergothioneine–2-Py–S–S–2-Py system at pH6–7 provides no evidence for general base catalysis but is in accord with a mechanism involving specific acid catalysis and post-transition-state proton transfer. 6. The pH-dependences of the overall equilibrium positions of the various thiol–disulphide interchanges are described. 7. Reaction of thioacetamide with a stoicheiometric quantity of 2-Py–S–S–2-Py at pH1 provides 2 molecules of Py–2-SH per molecule of thioacetamide and elemental sulphur; these findings can be accounted for by thiol–disulphide interchange to provide a thioacetamide–Py–2-SH mixed disulphide followed by fragmentation to provide CH3CN, S and Py–2-SH. 8. Provision of high reactivity in the neutral forms of the members of this series of sulphur nucleophiles by electron donation by the amino group is compared with the well known α effect that provides enhanced nucleophilicity in compounds containing an electronegative atom adjacent to the nucleophilic atom. 9. The decrease in the u.v. absorption of l-ergothioneine at 257nm consequent on transformation of its aminothione moiety into an S-alkyl-2-mercaptoimidazole moiety provides a convenient method of following the alkylation of l-ergothioneine by iodoacetamide. 10. The pH dependence of the extinction coefficient of l-ergothioneine at 257nm is described by ε257={8×103/(1+Ka/[H+]} +6×103m−1·cm−1 in which pKa=10.8. 11. In the pH range 3–11 the reaction is characterized by two reactive protonic states (X and XH). 12. The X state, reaction of the ionized 2-mercaptoimidazole moiety of the l-ergothioneine dianion with neutral iodoacetamide, is characterized by the second-order rate constant 4.0m−1·s−1 (25.0°C, I=0.05). The XH state, characterized by the second-order rate constant 0.03m−1·s−1, is interpreted as reaction of the thione form of the neutral 2-mercaptoimidazole moiety of the l-ergothioneine monoanion with neutral iodoacetamide. 13. The XH state of the alkylation reaction does not exhibit a deuterium isotope effect.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brocklehurst K., Crook E. M., Kierstan M. The mutability of stem bromelain: evidence for perturbation by structural transitions of the parameters that characterize the reaction of the essential thiol group of bromelain with 2,2'-dipyridyl disulphide. Biochem J. 1972 Jul;128(4):979–982. doi: 10.1042/bj1280979. [DOI] [PMC free article] [PubMed] [Google Scholar]
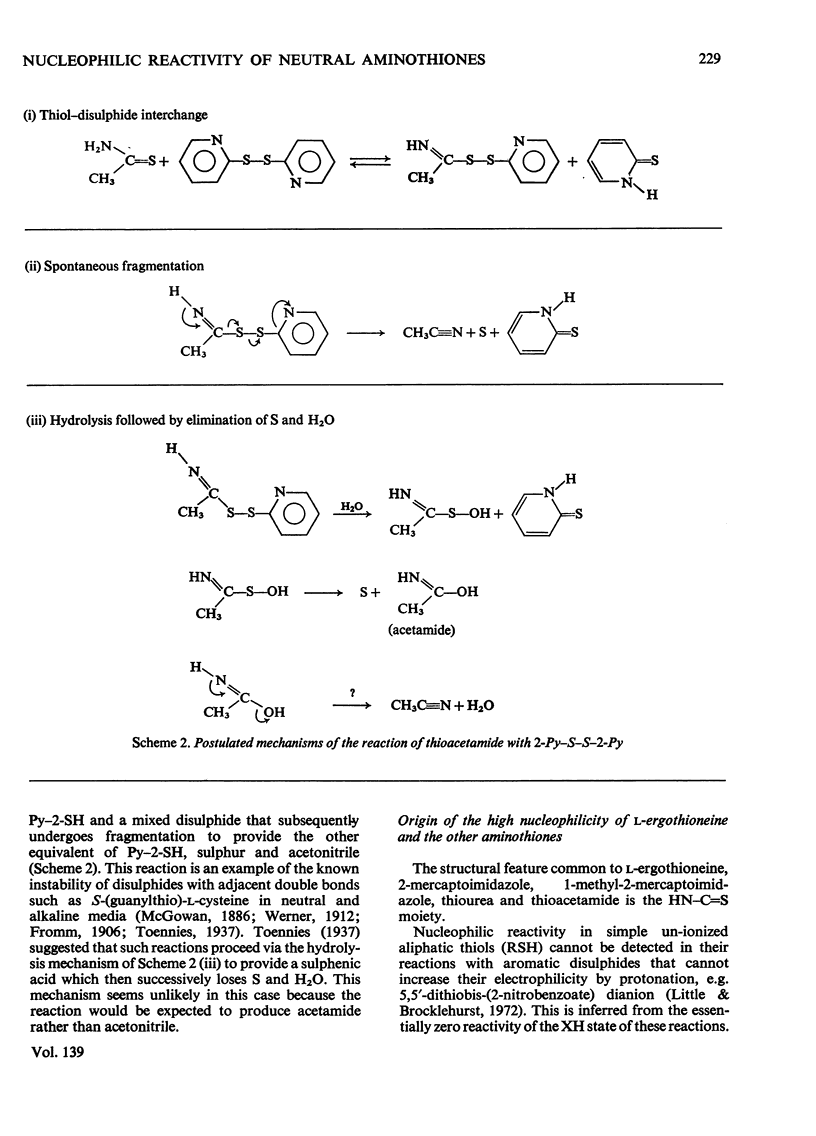
- Brocklehurst K., Little G. A novel reactivity of papain and a convenient active site titration in the presence of other thiols. FEBS Lett. 1970 Jul 29;9(2):113–116. doi: 10.1016/0014-5793(70)80327-1. [DOI] [PubMed] [Google Scholar]
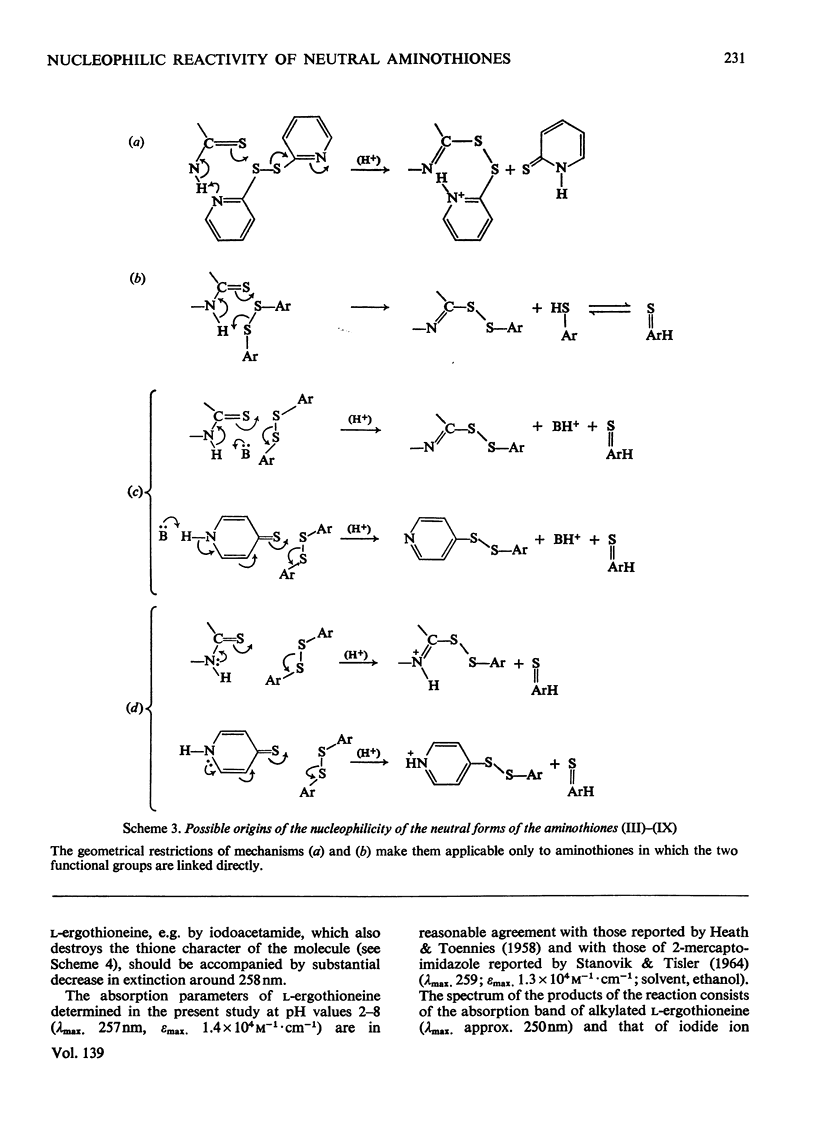
- Brocklehurst K., Little G. Reactions of papain and of low-molecular-weight thiols with some aromatic disulphides. 2,2'-Dipyridyl disulphide as a convenient active-site titrant for papain even in the presence of other thiols. Biochem J. 1973 May;133(1):67–80. doi: 10.1042/bj1330067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Little G. Reactivities of the various protonic states in the reactions of papain and of L-cysteine with 2,2'- and with 4,4'- dipyridyl disulphide: evidence for nucleophilic reactivity in the un-ionized thiol group of the cysteine-25 residue of papain occasioned by its interaction with the histidine-159-asparagine-175 hydrogen-bonded system. Biochem J. 1972 Jun;128(2):471–474. doi: 10.1042/bj1280471. [DOI] [PMC free article] [PubMed] [Google Scholar]
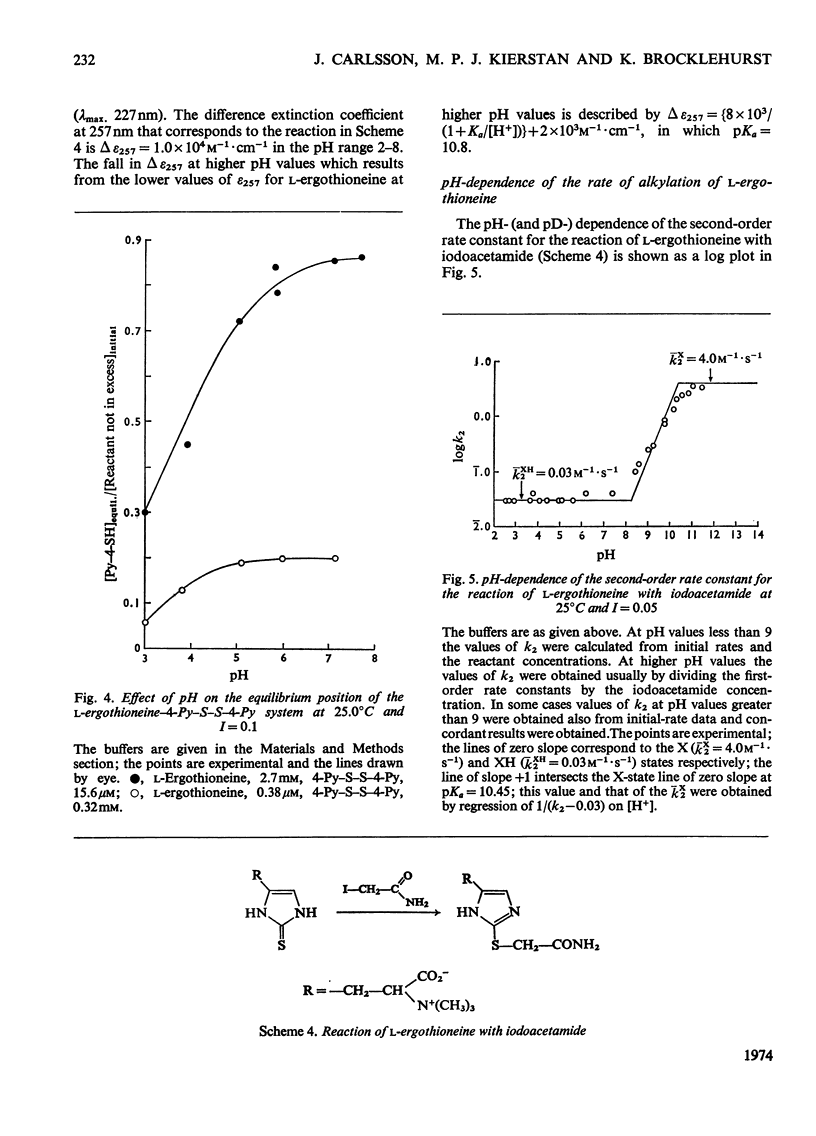
- Carlsson J., Kierstan M. P., Brocklehurst K. A convenient spectrophotometric assay for the determination of L-ergothioneine in blood. Biochem J. 1974 Apr;139(1):237–242. doi: 10.1042/bj1390237. [DOI] [PMC free article] [PubMed] [Google Scholar]
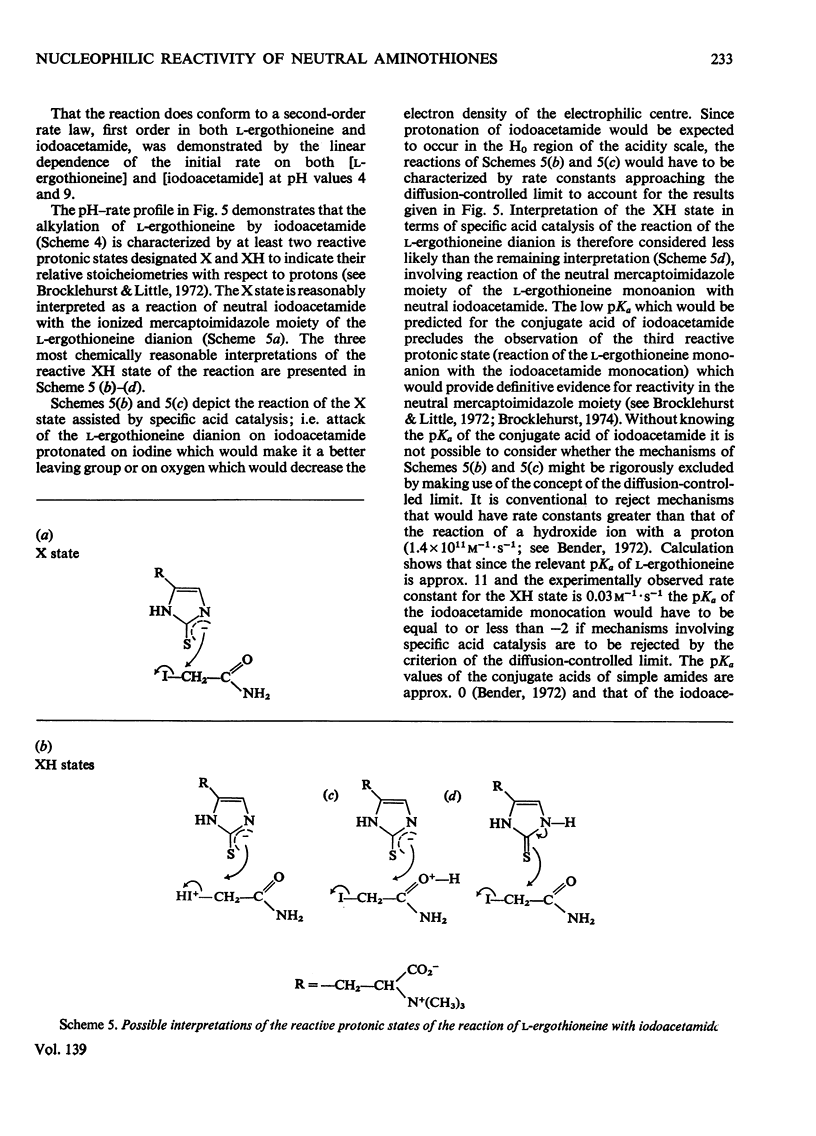
- Grassetti D. R., Murray J. F., Jr Determination of sulfhydryl groups with 2,2'- or 4,4'-dithiodipyridine. Arch Biochem Biophys. 1967 Mar;119(1):41–49. doi: 10.1016/0003-9861(67)90426-2. [DOI] [PubMed] [Google Scholar]
- HEATH H., TOENNIES G. The preparation and properties of ergothioneine disulphide. Biochem J. 1958 Feb;68(2):204–210. doi: 10.1042/bj0680204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little G., Brocklehurst K. Kinetics of the reversible reaction of papain with 5,5'-dithiobis-(2-nitrobenzoate) dianion: evidence for nucleophilic reactivity in the un-ionized thiol group of cysteine-25 and for general acid catalysis by histidine-159 of the reaction of the 5-mercapto-2-nitrobenzoate dianion with the papain-5-mercapto-2-nitrobenzoate mixed disulphide. Biochem J. 1972 Jun;128(2):475–477. doi: 10.1042/bj1280475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgár L. On the mode of activation of the catalytically essential sulfhydryl group of papain. Eur J Biochem. 1973 Feb 15;33(1):104–109. doi: 10.1111/j.1432-1033.1973.tb02660.x. [DOI] [PubMed] [Google Scholar]
- Richardson G. M. Note on the electrometric titration of dl-2-thiolhistidine. Biochem J. 1933;27(4):1036–1039. doi: 10.1042/bj0271036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANOVNIK B., TISLER M. DISSOCIATION CONSTANTS AND STRUCTURE OF ERGOTHIONEINE. Anal Biochem. 1964 Sep;9:68–74. doi: 10.1016/0003-2697(64)90084-3. [DOI] [PubMed] [Google Scholar]


