Abstract
Background
The objectives were to determine the prevalence of de novo and persistent pelvic pain after benign hysterectomy and to assess risk factors.
Methods
A Swedish prospective multicenter study of 440 women undergoing benign hysterectomy was conducted between October 2011 and March 2017. Measures of pain, the spatial extent of bodily pain, and pain sensitivity were assessed using a self-reporting questionnaire, Margolis’s patient pain drawing, and quantitative sensory testing of pain thresholds for pressure, heat, and cold, respectively. Quality of life was evaluated by EQ-5D-3L and SF-36. Psychological distress was assessed by the Hospital Anxiety and Depression Scaleand the Stress-Coping Inventory. Logistic regression models were used to assess risk factors, and the outcome was presented as an adjusted odds ratio (aOR) and 95% confidence interval (CI).
Results
Preoperatively, 18.0% of the women reported no bodily pain, 41.5% had pelvic pain, either as the only location (7.0%) or along with pain in other locations (34.5%), and 40.5% had non-pelvic pain only. Postoperatively, 6.2% developed de novo pelvic pain and 16.4% had persistent pelvic pain. De novo pelvic pain developed exclusively in women who preoperatively had non-pelvic pain only. Risk factors for de novo pelvic pain were a long hospital stay (aOR 1.50 (95%CI) 1.02–2.21)), high preoperative pain intensity (aOR 1.25 (95%CI 1.01–1.62)) and a high number of pain areas (aOR 1.15 (95%CI 1.05–1.27)), along with anxiety (aOR 10.61 (95%CI 1.84–61.03)) and low EQ-5D-3L health index (aOR 0.02 (95%CI 0.00–0.31)). Risk factors for persistent pelvic pain were lower age (aOR 0.89 (95%CI 0.81–0.97)), higher number of pain areas (aOR 1.08 (95%CI 1.02–1.14)), and a higher frequency of preoperative pain (aOR 12.75 (95%CI 2.24–72.66)).
Conclusion
Although hysterectomy appeared to be reasonably effective in curing pelvic pain, a non-negligible proportion of women developed de novo pelvic pain or had persistent pelvic pain. De novo pelvic pain seemed to affect only those who preoperatively had widespread bodily pain. Women at risk for de novo and persistent pelvic pain after hysterectomy could be identified preoperatively.
Trial registrations
The study was retrospectively registered in ClinicalTrial.gov (NCT01526668) on 01/27//2012.
Keywords: Hysterectomy, Pain thresholds, Pelvic pain, Risk factors, Spread of bodily pain
Introduction
The overall purpose of benign hysterectomy is generally to improve the health-related quality of life. The long-term outcome is therefore of particular importance when evaluating the effect of surgery. Postoperative pain can be a problem by delaying the patient’s recovery and causing long-term discomfort. The prevalence of chronic pelvic pain after benign hysterectomy varies in the range of 5–32% [1]. The underlying mechanisms of chronic pain after surgery are not fully understood, although several risk factors have been identified. In connection with benign hysterectomy, risk factors are reported to include open surgery, psychological factors such as depression, anxiety, pain catastrophizing, and pain problems elsewhere, and severe acute postoperative pain intensity [2–11]. Furthermore, preoperative pain seems to play a significant role in the development of chronic pain after surgery [1, 10]. The studies published so far conclude that preexisting pelvic pain or pain outside the surgical area are important risk factors for developing chronic pain after hysterectomy [3, 4, 8, 11]. It is not known how the spatial distribution of preexisting pain influences the development of chronic pain, or what role it may play in identifying patients at risk. Thus, it remains necessary to establish a possible association between the spatial spread of pain, its clinical manifestation, and treatment outcome in connection with hysterectomy.
The identification of women at risk of developing chronic pain is of great clinical importance as it allows them to be prepared for what can be expected from surgery. The experimental method of quantitative sensory testing (QST) can quantify the sensitivity to different painful stimulus modalities such as heat, cold and pressure [12–14]. Associations between pain thresholds and development of chronic postoperative pain have been identified and the cold pain threshold was recently found to be associated with maximum pain intensity postoperatively along with consumption of non-opioid analgesics after hysterectomy [15–18].
The definition of persistent postsurgical pain has varied over time [19]. The latest definition from the International Association for Study of Pain includes both pain that was not present before surgery and pain that was present before surgery but increased in intensity [20]. In gynecological clinical practice, however, it is important to distinguish between new pain after surgery, i.e., de novo pain, and pain that remains from before the surgery because both the etiology and prevalence of these two conditions are different. For clarity, throughout this study, de novo chronic postsurgical pelvic pain will be referred to as de novo pelvic pain (DNPP) and remaining chronic pelvic pain from before the surgery as persistent pelvic pain (PPP).
The purpose of the study was to evaluate changes in quantitatively assessed spatial bodily pain within a year following benign hysterectomy. The primary objective was to determine the prevalence of DNPP. Secondary objectives were to determine the prevalence of PPP, and to evaluate risk factors for DNPP and PPP.
Material and methods
A prospective longitudinal observational multicenter study was conducted between October 2011 and March 2017 investigating the occurrence of DNPP and PPP in women after hysterectomy on benign indication.
The departments of Obstetrics and Gynecology at the public hospitals Linköping University Hospital, Vrinnevi Hospital in Norrköping, Ryhov County Hospital in Jönköping, Värnamo Hospital in Värnamo, and Höglands Hospital in Eksjö in the southeastern health region of Sweden participated in the study.
Women who participated in the randomized multicenter study, the Post-Hysterectomy-Recovery (POSTHYSTREC) trial, which aimed to determine the effect of different models of nurse-led telephone follow-up contact on postoperative recovery after benign hysterectomy were eligible for the study [21].
The women received verbal and written information about the pain study in connection with the POSTHYSTREC trial information approximately one week prior to surgery. Written informed consent was obtained from all participants before inclusion. The participants were allowed to waive the QST measurements. The inclusion and exclusion criteria for the POSTHYSTREC study have previously been described in detail [21]. Briefly, the inclusion criteria were women between 18 and 60 years of age, scheduled for open abdominal or vaginal hysterectomy on benign indication, and able to speak Swedish fluently. One ovary had to be left behind after the operation. Exclusion criteria were concomitant urogynecological surgery, physical disability, severe mental disorder, current drug or alcohol abuse, or expecting more extensive concomitant surgery than hysterectomy, salpingectomy, ovarian resection or appendectomy.
All participating clinics routinely used the perioperative enhanced recovery after surgery (ERAS) program. The mode of anesthesia followed ERAS principles and preferably included intrathecal morphine analgesia alone or in combination with general anesthesia.
Collection of clinical data
Demographic and clinical data were collected upon entry into the study. Postoperative complications were classified according to Clavien-Dindo [22].
Pain assessment
Pain was assessed preoperatively, postoperatively for two days, and one year after the hysterectomy using a self-reported questionnaire consisting of simple questions about bodily pain. The detailed questions concerned the average intensity of preoperative pain indicated on a numeric rating scale from 0 (no pain) to 10 (worst imaginable pain), the frequency of occurrence of bodily pain (none, rarely, sometimes, often, almost always/always), the maximum postoperative pain intensity experienced on the day of surgery (day 0) and on the next day (day 1), reported on a numeric scale rating from 0 (no pain) to 6 (very severe pain) indicating the severity of postoperative pain, and the spatial spread of bodily pain drawn on a Margolis’ pain map (Fig. 1). The pain map outlines the spread of pain in areas of the front and back of the body in a total of 45 areas [23]. These were divided into nine regional areas: head (areas 1,2,23,24), neck and shoulders (areas 3–5,25–27), chest (areas 12,13), thoracic back (areas 34,35), abdomen (areas 14,15), pelvis (16), lower back (areas 36–39), upper extremities (areas 6–11,28–33), and lower extremities (areas 17–22,40–45). In order to assess pelvic pain specifically, the spread of pain areas was categorized into four groups: ‘No pain areas’, ‘Pelvis only’, ‘Pelvis and other areas’, and ‘Non-pelvic areas only’.
Fig. 1.
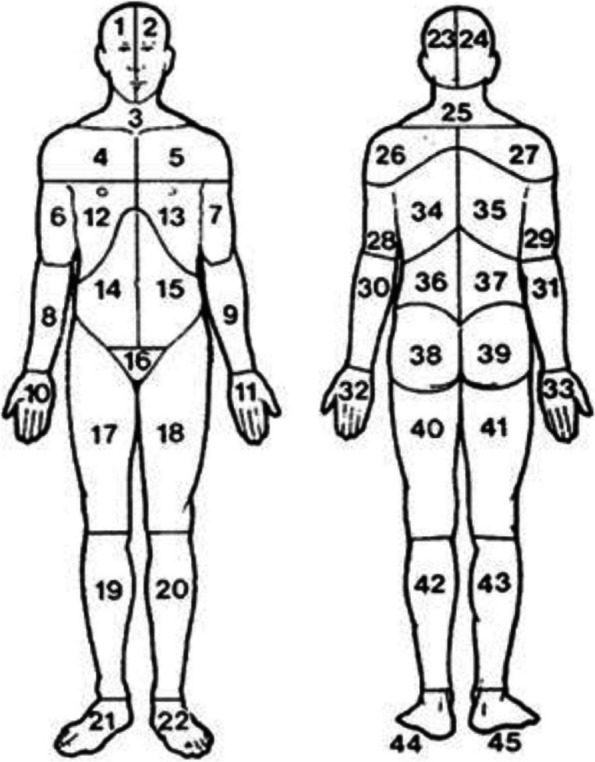
The Margolis pain drawing template [23]. Reprinted with permission from the publisher Wolters Kluwer Health, Inc
Hospital Anxiety and Depression Scale (HADS)
The widely used and validated self-rating HADS questionnaire was completed once preoperatively [24, 25]. The form consists of seven questions related to anxiety (the HADS-A subscale) and seven concerning depression (the HADS-D subscale). Each question is scored between 0 and 3. The sum score of each of the subscales is placed into one of three categories: normal (sum score ≤ 8), borderline (sum score > 8 but < 11), and abnormal, indicating severe symptoms (sum score ≥ 11).
Stress-Coping Inventory (SCI)
To evaluate the stress-coping capability, the SCI form, a validated self-report instrument, was used [26–28]. The form was filled in once preoperatively. It consists of a description of 41 stressful situations. Responses to each item are given using a six-point Likert-type scale, namely: 1-almost never; 2-rarely; 3-occasionally; 4-rather often; 5-very often; 6-almost always. The sum of all scores constitutes a measure of the stress-coping capacity. The cut-off level for low stress coping capacity was set at a score ≤ 169 [27, 28].
Health-related quality of life (HRQoL)
HRQoL was evaluated by two widely used validated generic quality of life instruments, the EQ-5D-3L [29, 30] and the 36-item Short-Form Health Survey (SF-36) [31, 32]. The EQ-5D-3L health index and the physical component summary (PCS) as well as the mental component summary (MCS) scores from the SF-36 were used to assess HRQoL. A higher index or scores indicated better HRQoL. The forms were filled in preoperatively.
Quantitative Sensory Testing (QST)
Thermal and pressure pain thresholds were measured using the methods previously described by Lukas et al. [18]. utilizing the Medoc TSA II Neuro Sensory Analyzer (Medoc Ltd. 1 Ha’dekel St. Ramat Yishai 30,095 Israel) for the thermal pain thresholds and a handheld electronic digital algometer (Somedic SenseLab AB, Sösdala, Sweden) for measuring the pressure pain threshold (PPT). Thermal thresholds for the first perceived sensation of pain for cold (CPT) and heat (HPT) were assessed by computerized thermal testing by increasing or decreasing the temperature at a preset rate of change of 1.5°C/s from the baseline temperature of 32°C to 50°C or to 0°C, respectively. By pressing a handheld button connected to the thermo-testing equipment on the first perception of pain the participants registered the pain threshold.
The probe (1 cm2 in area) of the algometer was pressed against the skin in a standardized manner with a constant increase in pressure at a rate of approximately 40 kPa/s. The participants were instructed to say “stop” at the first sensation of pain and the concurrent pressure value was registered as the PPT.
Ethics
The study was approved by the Regional Ethical Board in Linköping (Dnr. 2011/106–31; date of approval May 23; 2011), complies with the Declaration of Helsinki, and is registered with ClinicalTrial.gov (NCT01526668).
Statistics
Data analyses were performed using the statistical software TIBCO Statistica, version 13.5, (TIBCO Software Inc, Palo Alto CA). Continuous and categorical data are presented as mean (standard deviation) and number (percent), respectively. Continuous variables were analyzed by means of Kruskal–Wallis analysis of variance with subsequent multiple comparisons of mean ranks post-hoc tests or Mann–Whitney U-test, as appropriate. Categorical data were compared using Pearson’s 2 test or Fisher’s exact tests, as appropriate. Two-tailed tests were applied, and the level of significance was set at p < 0.05.
Binominal logistic regression was used to assess risk factors. In the multivariable models, adjustments were made simultaneously for age, body mass index (BMI), preoperative use of analgesics, mode of hysterectomy, Clavien-Dindo categorization of postoperative complications, and HADS-A and -D scores. The outcome of univariate logistic regression is presented as odds ratio (OR) and 95% confidence interval (95%CI), and correspondingly, adjusted OR (aOR) and 95%CI for the multivariable models.
Results
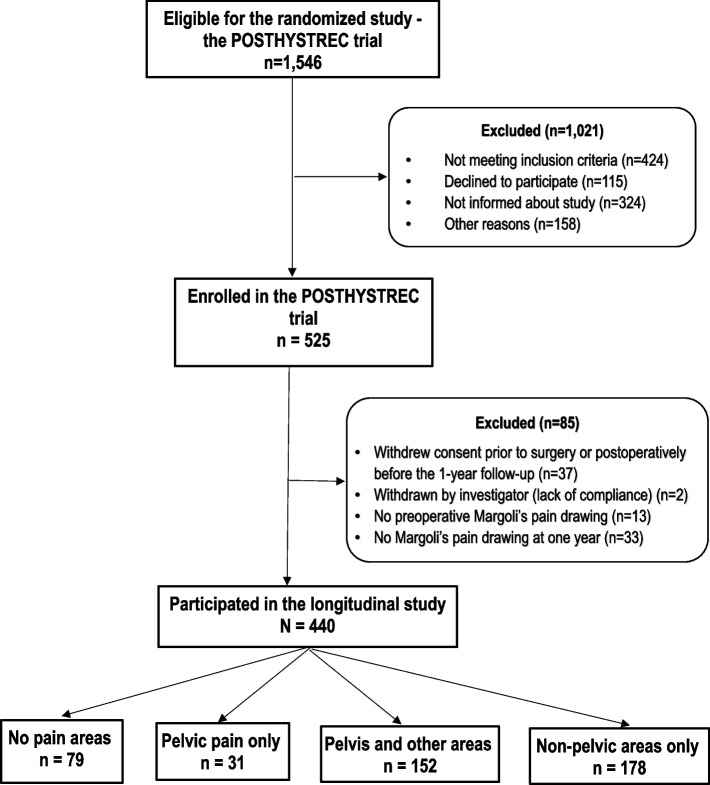
The flow chart (Fig. 2) provides an overview of the selection of the 440 women who made up the study population.
Fig. 2.
Flow chart of the participants in the longitudinal study of pain spread
Spatial bodily pain distribution and relation to demographic and clinical characteristics
Of the 440 women, 79 (18.0%) reported ‘No pain areas’ preoperatively, 31 (7.0%) pain in the ‘Pelvis only’, 152 (34.5%) pain in ‘Pelvis and other areas’, and 178 (40.5%) pain in ‘Non-pelvic areas only’ Thus, in total, 183 (41.6%) reported pain involving the pelvis preoperatively.
The demographic and clinical data, subdivided into the four groups according to the spatial spread of pain, are shown in Table 1.. The groups differed significantly in age and preoperative use of analgesics, which were mainly used by women with pain in other areas. According to the post-hoc tests the difference in age between the groups was mainly seen between ‘No pain areas’ vs. ‘Pelvis and other areas’ (p = 0.01) and ‘Pelvis and other areas’ vs. ‘Non-pelvic areas only’ (p = 0.01). The number of pain areas reported in the body mapping was significantly higher in women with pain in ‘Pelvis and other areas’ compared to women with pain in ‘Non-pelvic areas only’ (8.2 (7.5) vs. 6.9 (6.2), p < 0.01). However, by excluding the pelvic area contribution in ‘Pelvis and other areas’, the number of other areas did not differ between these two groups (p = 0.23). The distribution of the frequency of occurrence of pain also differed between the four groups, with significantly higher frequencies of pain (often or almost always/ always) in the two groups with pain in other areas compared to the pelvis only group. The average intensity of preoperative pain also differed similarly between the groups, mainly attributed to the differences between the ‘Pelvis only’ group vs. the ‘Pelvis and other areas’ group (p = 0.03) and between ‘Pelvis and other areas’ vs. ‘Non-pelvic areas only’ (p = 0.01), respectively. The highest preoperative average pain intensity was found in women with ‘Pelvis and other areas’ (5.0 (2.0)) and the lowest in women with pain in the ‘Pelvis only’ (3.9 (2.1)).
Table 1.
Preoperative demographic and clinical data of 440 women undergoing hysterectomy, categorized by patient-reported pain spread
| Patient-reported pain areas preoperatively | |||||||
|
All (N=440) |
No pain areas (n=79) |
Pelvis only (n=31) |
Pelvis and other areas (n=152) |
Non-pelvic areas only (n=178) |
p-value† | ||
| Preoperative variables | |||||||
| Age (years) | 46.5 (5.5) | 47.6 (5.4) | 46.5 (4.5) | 45.3 (5.4) | 47.2 (5.6) | <0.01 # | |
| Body mass index (kg/m2) | 26.9 (4.6) | 26.3 (4.7) | 26.8 (4.3) | 26.9 (4.9) | 27.0 (4.4) | 0.71 # | |
| Parous | 381 (6.6) | 71 (89.9) | 30 (96.8) | 128 (84.2) | 152 (85.4) | 0.23 | |
| Missing data | 3 (0.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (1.7) | ||
| Smoking | 45 (10.2) | 3 (3.8) | 3 (9.7) | 20 (13.2) | 19 (10.7) | 0.17 | |
| Missing data | 10 (2.3) | 3 (3.8) | 0 (0.0) | 4 (2.6) | 3 (1.7) | ||
| ASA physical status classification | Class I | 285 (64.8) | 53 (67.1) | 19 (61.3) | 104 (68.4) | 109 (61.2) | 0.63 |
| Class II | 143 (32.5) | 25 (31.6) | 12 (38.7) | 43 (28.3) | 63 (35.4) | ||
| Class III | 12 (2.7) | 1 (1.3) | 0 (0.0) | 5 (3.3) | 6 (3.4) | ||
| Comorbidity | Cardiovascular | 66 (15.0) | 17 (21.5) | 3 (9.7) | 19 (12.5) | 27 (15.2 | 0.25 |
| Pulmonary | 41 (9.3) | 6 (7.6) | 0 (0.0) | 14 (9.2) | 21 (11.8) | 0.19 | |
| Medication | Analgesics | 77 (17.5) | 4 (5.1) | 0 (0.0) | 40 (26.3) | 33 (18.5) | <0.0001 |
| Anti-depressives or sedatives | 62 (14.1) | 5 (6.3) | 3 (9.7) | 21 (13.8) | 33 (18.5) | 0.06 | |
| Hypnotics | 19 (14.3) | 4 (5.1) | 0 (0.0) | 6 (3.9) | 9 (5.0) | 0.62 | |
| Physical workload | Sedentary | 119 (27.0) | 22 (27.8) | 11 (35.5) | 39 (27.7) | 47 (26.4) | 0.16 |
| Medium | 122 (27.7) | 32 (40.5) | 5 (16.1) | 41 (27.0) | 44 (24.7) | ||
| Heavy | 178 (40.5) | 25 (31.7) | 14 (45.2) | 62 (40.8) | 77 (43.3) | ||
| Missing data | 21 (4.8) | 0 (0.0) | 1 (3.2) | 10 (6.6) | 10 (5.6) | ||
| Gainfully employed | 412 (93.6) | 77 (97.5) | 31 (100.0) | 140 (92.1) | 164 (92.1) | 0.15 | |
| Previous laparotomy | 150 (34.1) | 29 (36.7) | 9 (29.0) | 56 (36.8) | 56 (31.5) | 0.71 | |
| Missing data | 4 (0.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (2.2) | ||
| Indication for hysterectomy | Bleeding disorder | 109 (24.8) | 15 (19.0) | 7 (22.6) | 43 (28.3) | 44 (24.7) | 0.08 |
| Myoma | 203 (46.1) | 40 (50.6) | 17 (54.8) | 62 (40.8) | 84 (47.2) | ||
| Myoma and bleeding | 53 (12.0) | 10 (12.7) | 4 (12.9) | 16 (10.5) | 23 (12.9) | ||
| Cervical dysplasia | 46 (10.5) | 12 (15.2) | 1 (3.2) | 13 (8.6) | 20 (11.2) | ||
| Pain | 27 (6.1) | 1 (1.3) | 2 (6.4) | 18 (11.8) | 6 (3.4) | ||
| Others | 2 (0.5) | 1 (1.3) | 0 (0.0) | 0 (0.0) | 1 (0.6) | ||
| Number of pain areas on body-mapping | 5.7 (6.7) | NA | 1.0 (--) | 8.2 (7.5) | 6.9 (6.2) | <0.01* | |
| How often do you have pain | No pain | 68 (15.5) | 68 (86.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | <0.0001 |
| Rarely | 33 (7.5) | 4 (5.1) | 1 (3.2) | 15 (9.9) | 13 (7.3) | ||
| Sometimes | 136 (30.9) | 1 (1.3) | 20 (64.5) | 47 (30.9) | 68 (38.2) | ||
| Often | 111 (25.2) | 0 (0.0) | 6 (19.4) | 51 (33.5) | 54 (30.3) | ||
| Almost always/ always | 74 (16.8) | 0 (0.0) | 0 (0.0) | 36 (23.7) | 38 (21.4) | ||
| Missing data | 18 (4.1) | 6 (7.6) | 4 (12.9) | 3 (2.0) | 5 (2.8) | ||
| Average intensity of preoperative pain (VAS scale 1-10) |
(N = 428) 3.8 (2.6) |
(n = 79) NA |
(n = 27) 3.9 (2.1) |
(n = 152) 5.0 (2.0) |
(n = 170) 4.4 (2.0) |
<0.01 # | |
| HADS-A score | 4.9 (4.0) | 3.9 (3.7) | 3.2 (3.2) | 5.4 (4.1) | 5.5 (4.1) | <0.01 # | |
| HADS-A (in categories) | Normal | 323 (73.4) | 66 (83.6) | 26 (83.9) | 100 (65.8) | 131 (73.6) | 0.04 |
| Borderline abnormal | 68 (15.5) | 8 (10.1) | 4 (12.9) | 33 (21.7) | 23 (12.9) | ||
| Abnormal | 49 (11.1) | 5 (6.3) | 1 (3.2) | 19 (12.5) | 24 (13.5) | ||
| HADS-D score | 2.6 (3.0) | 1.7 (2.3) | 1.4 (1.9) | 3.0 (3.0) | 2.9 (3.4) | <0.001 # | |
| HADS-D (in categories) | Normal | 400 (90.9) | 76 (96.2) | 31 (100.0) | 138 (90.8) | 155 (87.1) | 0.13 |
| Borderline abnormal | 28 (6.4) | 2 (2.5) | 0 (0.0) | 11 (7.2) | 15 (8.4) | ||
| Abnormal | 12 (2.7) | 1 (1.3) | 0 (0.0) | 3 (2.0) | 8 (4.5) | ||
| SCI score | 185.4 (25.5) | 186.4 (26.3) | 193.4 (21.4) | 184.2 (23.1) | 184.5 (27.5) | 0.33 # | |
| SCI (in categories) | Low stress coping | 109 (24.8) | 15 (19.0) | 5 (16.1) | 34 (22.4) | 55 (30.9) | 0.08 |
| High stress coping | 331 (75.2) | 64 (81.0) | 26 (83.9) | 118 (77.6) | 123 (69.1) | ||
| SF-36 PCS score | 47.9 (9.3) | 53.5 (5.5) | 50.5 (6.7) | 44.7 (10.0) | 47.7 (9.1) | <0.0001 # | |
| SF-36 MCS score | 47.6 (10.3) | 50.4 (7.7) | 50.7 (8.7) | 36.6 (10.5) | 46.7 (11.0) | <0.01 # | |
| EQ-5D-3L health index | 0.80 (0.20) | 0.90 (0.18) | 0.84 (0.18) | 0.74 (0.21) | 0.80 (0.19) | <0.0001 # | |
| Heat pain threshold (ºC) |
(N = 370) 47.5 (2.6) |
(n=69) 47.3 (2.6) |
(n=25) 48.0 (2.2) |
(n=118) 47.2 (2.9) |
(n=158) 47.7 (2.5) |
0.44 # | |
| Cold pain threshold (ºC) |
(N = 370) 3.7 (6.4) |
(n=69) 2.6 (5.1) |
(n=25) 3.4 (5.6) |
(n=118) 5.3 (7.5) |
(n=158) 3.1 (5.9) |
0.02 # | |
| Pressure pain threshold (kPa) |
(N = 367) 503 (199) |
(n=69) 515 (220) |
(n=25) 530 (225) |
(n=116) 476 (193) |
(n=158) 513 (189) |
0.40 # | |
| Intra- and postoperative variables | |||||||
| Mode of hysterectomy | Total abdominal hysterectomy | 310 (70.4) | 58 (73.4) | 22 (71.0) | 109 (71.7) | 121 (68.0) | 0.86 |
| Subtotal abdominal hysterectomy | 35 (8.0) | 7 (8.9) | 1 (3.2) | 11 (7.2) | 16 (9.0) | ||
| Vaginal hysterectomy | 95 (21.6) | 14 (17.7) | 8 (25.8) | 32 (21.1) | 41 (23.0) | ||
| Mode of anesthesia | General anesthesia (GA) | 162 (36.8) | 30 (38.0) | 9 (29.0) | 59 (38.2) | 64 (36.0) | 0.14 |
| Spinal anesthesia + intrathecal morphine | 164 (37.3) | 30 (38.0) | 17 (54.9) | 60 (39.5) | 57 (32.0) | ||
| Intrathecal morphine + GA | 114 (25.9) | 19 (24.0) | 5 (16.1) | 33 (21.7) | 57 (32.0) | ||
| Incision in abdominal wall | No abdominal incision | 95 (21.6) | 14 (17.7) | 8 (25.8) | 32 (21.0) | 41 (23.0) | 0.63 |
| Low transverse | 313 (71.1) | 58 (73.4) | 22 (71.0) | 110 (72.4) | 123 (69.1) | ||
| Midline | 25 (5.7) | 7 (8.9) | 0 (0.0) | 8 (5.3) | 10 (5.6) | ||
| Missing data | 7 (1.6) | 0 (0.0) | 1 (3.2) | 2 (1.3) | 4 (2.3) | ||
| Operation time (minutes) | 91 (48) | 93 (45) | 100 (114) | 89 (36) | 91 (39) | 0.83 # | |
| Estimated bleeding intraoperatively (mL) | 177 (218) | 184 (225) | 138 (131) | 178 (180) | 180 (255) | 0.44 # | |
| Uterus weight (gram) | 366 (346) | 412 (408) | 344 (255) | 339 (295) | 373 (370) | 0.88 # | |
| Blood transfusion (no. of women) | 15 (3.4) | 2 (2.5) | 1 (3.2) | 3 (2.0) | 9 (5.1) | 0.46 | |
| Duration of hospital stay (days) | 1.8 (1.1) | 1.6 (0.8) | 1.6 (1.8) | 1.8 (1.2) | 1.8 (1.0) | 0.02 # | |
| Maximum pain intensity day 0 § (scale 0-6) |
(n=432) 3.3 (1.7) |
(n=77) 3.1 (1.8) |
(n=31) 2.9 (1.8) |
(n=148) 3.6 (1.6) |
(n=176) 3.3 (1.7) |
0.07# | |
| Maximum pain intensity day 1 § (scale 0-6) |
(n=432) 3.3 (1.4) |
(n=78) 2.8 (1.4) |
(n=31) 3.4 (1.4) |
(n=148) 3.6 (1.4) |
(n=176) 3.2 (1.4) |
<0.001# | |
| Classification of surgical complications | C-D grade 0 | 316 (71.8) | 61 (77.2) | 23 (74.2) | 102 (67.1) | 130 (73.0) | 0.82 |
| C-D grade I | 46 (10.5) | 5 (6.3) | 4 (12.9) | 19 (12.5) | 18 (10.1) | ||
| C-D grade II | 64 (14.5) | 10 (12.7) | 4 (12.9) | 26 (17.1) | 24 (13.5) | ||
| C-D grade III | 14 (3.2) | 3 (3.8) | 0 (0.0) | 5 (3.3) | 6 (3.4) | ||
Figures denote mean and (standard deviation), or number and (percent)
ASA American Society of Anesthesiologists, C-D Contracted Clavien-Dindo classification of postoperative complications within six weeks, EQ-5D-3L European Quality of Life 5 Dimensions 3 Level version, HADS-A Hospital Anxiety and Depression Scale – Anxiety, HADS-D Hospital Anxiety and Depression Scale – Depression, SCI Stress Coping Inventory
† Continuous data are analyzed by means of non-parametric tests and nominal data by means of Pearson’s chi-squared tests. Missing data are excluded in the statistical analyses
# Kruskal-Wallis analysis of variance
* Mann-Whitney U-test for comparison between ‘Pelvis and other areas’and ‘Non-pelvic areas only’
§ Day 0 and day 1 indicate day of surgery and the day after surgery, respectively
The psychometric measures HADS-A and D, but not SCI, along with the HRQoL measures EQ-5D-3L, SF-36’s PCS and MCS all revealed significant differences between the groups. The post-hoc tests showed that the differences in HADS-A and HADS-D scores were mainly attributed to differences between ‘No pain areas’ vs. ‘Pelvis and other areas’ (p = 0.04 and p = < 0.01, respectively) and between ‘Pelvis only’ vs. ‘Pelvis and other areas’ (p = 0.03 and p = 0.02, respectively) with lower scores for the former group in both scenarios. For EQ-5D-3L and SF-36’s PCS the pattern of the post-hoc tests was almost identical, with contributions from differences between ‘No pain areas’ vs. ‘Pelvis and other areas’ (p < 0.0001 and p < 0.0001, respectively), between ‘No pain areas’ vs. ‘Non-pelvic areas only’ (p < 0.0001 and p < 0.0001, respectively), and between ‘Pelvis and other areas’ vs. ‘Non-pelvic areas only’ (p = 0.03 and p = 0.03, respectively). In addition, the SF-36’s PCS also differed between ‘Pelvis only’ and ‘Pelvis and other areas’ (p = 0.01). Concerning the SF-36’s MCS, the main contribution to the significant difference between the groups was, according to the post-hoc test, the difference between ‘No pain areas’ vs. ‘Pelvis and other areas’ (p = 0.04). All comparisons concerning EQ-5D-3L and the SF-36’ PCS and MCS showed higher scores for the former group in the scenarios except for the comparison between ‘Pelvis and other areas’ and ‘Non-pelvic areas only’ where the highest scores were found in the latter group.
Of the three experimental pain threshold measures, only CPT differed significantly between the groups. However, this could not be attributed to differences between any of the subgroups in the post-hoc tests.
The duration of hospital stays differed significantly, albeit modestly, between the groups, mainly because of a difference between the groups ‘Pelvis only’ and ‘Non-pelvic areas only’ (p = 0.04). In addition, the reported maximum pain intensity on day 1, but not on day 0 also differed significantly between the groups, mainly attributed to the difference between ‘No pain areas’ and ‘Pelvis and other areas’ (p < 0.001) and between ‘Pelvis and other areas’’ and ‘Non-pelvic areas only’ (p = 0.02).
Relation between spatial bodily pain frequency preoperatively and one year postoperatively
The pain frequency preoperatively and one year postoperatively in relation to the four categories of spatial spread of pain is presented in Table 2. Preoperatively, of those with pain in the ‘Pelvis only’ 26 of 27 (96.3%) had pain sometimes or more often, not significantly different from the corresponding rate for the women with ‘Pelvis and other areas’ and ‘Non-pelvic areas only’, 294 of 322 (91.2%), (p = 0.71, Fisher’s exact test). The corresponding figures after one year were three of four (75.0%) and 200 of 227 (88.1%), (p = 0.41, Fisher’s exact test).
Table 2.
Pain frequency in relation to grouping of spatial spread of pain preoperatively (A), and one year postoperatively (B)
| A | Grouping of spatial spread of pain preoperatively | |||
| Frequency of pain |
No pain areas (n = 79) |
Pelvis only (n = 31) |
Pelvis and other areas (n = 152) |
Non-pelvic areas only (n = 178) |
| No pain | 68 (86.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Rarely | 4 (5.1) | 1 (3.2) | 15 (9.9) | 13 (7.3) |
| Sometimes | 1 (1.2) | 20 (64.5) | 47 (30.9) | 68 (38.2) |
| Often | 0 (0.0) | 6 (19.4) | 51 (33.5) | 54 (30.3) |
| Almost always/always | 0 (0.0) | 0 (0.0) | 36 (23.7) | 38 (21.3) |
| Missing data | 6 (7.6) | 4 (12.9) | 3 (2.0) | 5 (2.8) |
| B | Grouping of spatial spread of pain one year postoperatively | |||
| Frequency of pain |
No pain areas (n = 208) |
Pelvis only (n = 5) |
Pelvis and other areas (n = 41) |
Non-pelvic areas only (n = 186) |
| No pain | 201 (96.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Rarely | 6 (2.9) | 1 (20.0) | 7 (17.1) | 20 (10.8) |
| Sometimes | 0 (0.0) | 2 (40.0) | 7 (17.1) | 66 (35.5) |
| Often | 0 (0.0) | 0 (0.0) | 18 (43.9) | 54 (29.0) |
| Almost always/always | 1 (0.5) | 1 (20.0) | 9 (21.9) | 46 (24.7) |
| Missing data | 0 (0.0) | 1 (20.0) | 0 (0.0) | 0 (0.0) |
Figures denote number and (percent)
Incidence of DNPP and PPP
The change in spatial pain from preoperatively to one year after the hysterectomy is reported in Table 3. DNPP was reported in 6.2% (16/257) of the women and exclusively in the group ‘Non-pelvic areas only’ Of these, 93.8% (15/16) still had the pain in the other pain areas and one (6.3%) had resolution of the pain in the other pain areas but developed pain in the pelvis as the only location. Pelvic pain disappeared in 83.6% (153/183) and consequently persisted in 16.4% (30/183). No difference was seen in the resolution of pelvic pain between those with pain in the ‘Pelvis only’ (74.2% (23/31)) and those with pain in the ‘Pelvis and other areas’ (82.2% (125/152); p = 0.30, Pearson’s 2 test). None of the women who reported ‘No pain areas’ preoperatively developed pelvic pain while 27.8% (22/79) reported pain in ‘Non-pelvic areas only’, spatially widely spread but predominantly with pain in the abdomen, lower extremities, low back, and head (data not shown), and 72.2% (57/79) still did not report pain areas one year after the surgery. Women with pain in the two groups ‘Pelvis and other areas’ and ‘Non-pelvic areas only’’ reported resolution of all pain areas one year after the hysterectomy in 38.8% (128/330), equally distributed between the two groups. However, pain in the other areas, independent of pelvic involvement, was maintained one year after hysterectomy in 60.0% (198/330).
Table 3.
Association between grouping of spatial spread of pain preoperatively and one year postoperatively
| One year after the hysterectomy | |||||
|---|---|---|---|---|---|
| No pain areas | Pelvis only | Pelvis and other areas | Non-pelvic areas only | ||
| Preoperatively | No pain areas (n = 79) | 57 (72.2%) | 0 (0%) | 0 (0%) | 22 (27.8%) |
| Pelvis only (n = 31) | 23 (74.2%) | 1 (3.2%) | 2 (6.5%) | 5 (16.1%) | |
| Pelvis and other areas (n = 152) | 64 (42.1%) | 3 (2.0%) | 24 (15.8%) | 61 (40.1%) | |
| Non-pelvic areas only (n = 178) | 64 (36.0%) | 1 (0.5%) | 15 (8.4%) | 98 (55.1%) | |
Figures denote number and (percent)
Risk factors for DNPP
The duration of hospital stay, number of pain areas, intensity of average pain preoperatively, and the regional pain areas of the neck and shoulder, lower back, and upper and lower extremities were independent risk factors for DNPP along with the EQ-5D-3L health index, HADS-A score and HADS-A categories (Table 4).
Table 4.
Demographic and clinical data of 257 women without pelvic pain undergoing hysterectomy in relation to de novo pelvic pain one year post-surgery
| De novo pelvic pain | Logistic regression * | |||||
| Yes (n=16) | No (n=241) | Univariate OR (95% CI) | Multivariable † aOR (95% CI) | p-value | ||
| Preoperative variables | ||||||
| Age (years) | 45.8 (7.8) | 47.4 (5.3) | 0.95 (0.87-1.04) | 0.97 (0.88-1.06) | 0.47 | |
| Body mass index (kg/m2) | 27.5 (5.0) | 26.8 (4.5) | 1.03 (0.93-1.15) | 1.03 (0.92-1.16) | 0.59 | |
| Parous | 12 (75.0) | 211 (87.6) | 0.38 (0.12-1.28) | 0.30 (0.08-1.14) | 0.08 | |
| Missing data | 0 (0.0) | 3 (1.2) | ||||
| Smoking | 2 (12.5) | 20 (8.3) | 1.54 (0.33-7.24) | 1.50 (0.30-7.53) | 0.63 | |
| Missing data | 0 (0.0) | 6 (2.5) | ||||
| ASA physical status classification I | I | 7 (43.8) | 155 (64.3) | 1.00 (reference) | 1.00 (reference) | |
| II-III | 9 (56.2) | 86 (35.7) | 2.32 (0.83-6.44) | 2.90 (0.92-9.09) | 0.07 | |
| Comorbidity | Cardiovascular | 3 (18.9) | 41 (17.0) | 1.13 (0.31-4.13) | 1.31 (0.31-5.55) | 0.72 |
| Pulmonary | 4 (25.0) | 23 (9.5) | 3.16 (0.94-10.60) | 2.89 (0.79-10.54) | 0.12 | |
| Medication | Analgesics | 2 (12.5) | 35 (14.5) | 0.84 (0.28-3.86) | 0.68 (0.13-3.39) | 0.63 |
| Anti-depressives or sedatives | 5 (31.3) | 33 (13.7) | 2.87 (0.94-8.77) | 1.68 (0.46-6.16) | 0.43 | |
| Hypnotics | 1 (6.3) | 12 (5.0) | 1.27 (0.15-10.75) | 0.44 (0.04-4.72) | 0.49 | |
| Physical workload | Sedentary | 4 (25.0) | 65 (27.0) | 1.00 (reference) | 1.00 (reference) | |
| Medium | 4 (25.0) | 72 (29.9) | 0.90 (0.22-3.76) | 0.92 (0.21-4.10) | 0.91 | |
| Heavy | 8 (50.0) | 94 (39.0) | 1.38 (0.40-4.78) | 1.30 (0.35-4.78) | 0.70 | |
| Missing data | 0 (0.0) | 10 (4.1) | ||||
| Gainfully employed | 15 (93.8) | 226 (93.8) | 1.00 (0.12-8.05) | 3.20 (0.28-37.16) | 0.35 | |
| Previous laparotomy | 2 (12.5) | 83 (34.4)) | 0.27 (0.06-1.19) | 0.24 (0.05-1.13) | 0.07 | |
| Missing data | 0 (0.0) | 4 (1.7) | ||||
| Indication for hysterectomy | Bleeding disorder | 7 (43.8) | 52 (21.6) | 1.00 (reference) | 1.00 (reference) | |
| Myoma | 5 (31.2) | 119 (49.4) | 0.31 (0.09-1.03) | 0.25 (0.65-0.93) | 0.04 | |
| Myoma and bleeding | 1 (6.2) | 32 (13.3) | 0.23 (0.03-1.98) | 0.20 (0.02-1.91) | 0.16 | |
| Cervical dysplasia | 2 (12.5) | 30 (12.4) | 0.50 (0.10-2.54) | 0.60 (0.11-3.28) | 0.55 | |
| Pain | 1 (6.3) | 6 (2.5) | 1.24 (0.13-11.86) | 0.93 (0.08-10.52) | 0.96 | |
| Other | 0 (0.0) | 2 (0.8) | NA | NA | ||
| Number of pain areas on body-mapping | 9.8 (5.6) | 4.4 (6.0) | 1.09 (1.03-1.16) | 1.15 (1.05-1.27) | <0.01 | |
| How often did you have pain preoperatively | No pain | 0 (0.0) | 68 (28.2) | p=0.06‡ | NA | |
| Rarely | 2 (12.5) | 15 (6.2) | 2.17 (0.36-12.95) | 2.54 (0.38-16.95) | 0.34 | |
| Sometimes | 4 (25.0) | 65 (27.0) | 1.00 (reference) | 1.00 (reference) | ||
| Often | 3 (18.7) | 51 (21.2) | 0.96 (0.20-4.46) | 0.91 (0.17-4.83) | 0.91 | |
| Almost always/ always | 5 (31.3) | 33 (13.7) | 2.46 (0.62-9.79) | 2.59 (0.53-12.63) | 0.24 | |
| Missing data | 2 (12.5) | 9 (3.7) | ||||
| Average intensity of preoperative pain (VAS scale 1-10) | (n =14) 4.6 (2.8) | (n = 6) 2.9 (2.6) | 1.27 (1.04-1.55) | 1.28 (1.01-1.62) | 0.04 | |
| Regional pain areas | Head | 6 (37.5) | 60 (24.9) | 1.81 (0.64-5.19) | 1.59 (0.50-5.01) | 0.43 |
| Neck and shoulder | 11 (68.8) | 72 (29.9) | 5.16 (1.73-15.40) | 4.66 (1.46-14.87) | <0.01 | |
| Chest | 1 (6.3) | 8 (3.3) | 1.94 (0.23-16.56) | 1.65 (0.17-16.26) | 0.67 | |
| Abdomen | 6 (37.5) | 67 (27.8) | 1.56 (0.54-4.46) | 1.39 (0.46-4.21) | 0.57 | |
| Thoracic back | 3 (18.8) | 19 (7.9) | 2.70 (0.71-10.30) | 2.88 (0.66-12.58) | 0.16 | |
| Lower back | 11 (68.8) | 79 (32.8) | 4.51 (1.52-13.43) | 4.24 (1.37-13.17) | 0.01 | |
| Upper extremities | 6 (37.5) | 39 (16.2) | 3.11 (1.07-9.05) | 4.24 (1.18-15.30) | 0.03 | |
| Lower extremities | 9 (56.3) | 54 (22.4) | 4.45 (1.58-12.51) | 5.23 (1.63-16.79) | <0.01 | |
| HADS-A score | 7.3 (4.9) | 4.7 (4.0) | 1.15 (1.03-1.29) | 1.10 (0.93-1.29) | 0.27 | |
| HADS-A (in categories) | Normal | 7 (43.8) | 190 (78.8) | 1.00 (reference) | 1.00 (reference) | |
| Borderline abnormal | 4 (25.0) | 27 (11.2) | 4.02 (1.10-14.65 | 4.94 (1.20-20.28) | 0.03 | |
| Abnormal | 5 (21.2) | 24 (10.0) | 5.65 (1.66-19.22) | 10.61 (1.84-61.03) | <0.01 | |
| HADS-D score | 4.3 (3.1) | 2.4 (3.1) | 1.16 (1.02-1.31) | 1.06 (0.88-1.29) | 0.53 | |
| HADS-D (in categories) | Normal | 14 (87.5) | 217 (90.1) | 1.00 (reference) | 1.00 (reference) | |
| Borderline abnormal | 1 (6.2) | 16 (6.6) | 0.97 (0.12-7.84) | 0.25 (0.02-3.11) | 0.28 | |
| Abnormal | 1 (6.2) | 8 (3.3) | 1.94 (0.23-16.60) | 0.54 (0.04-7.15) | 0.64 | |
| SCI score | 177.8 (32.5) | 185.6 (26.7) | 0.99 (0.97-1.01) | 1.01 (0.98-1.03) | 0.56 | |
| SCI (in categories) | Low stress coping | 7 (43.8) | 63 (26.1) | 2.20 (0.79-6.15) | 0.95 (0.25-3.63) | 0.94 |
| High stress coping | 9 (56.2) | 178 (73.9) | 1.00 (reference) | 1.00 (reference) | ||
| SF-36 PCS | 44.8 (13.7) | 49.8 (8.1) | 0.94 (0.90-0.99) | 0.95 (0.89-1.00) | 0.06 | |
| SF-36 MCS | 42.7 (10.9) | 48.2 (10.1) | 0.96 (0.92-0.99) | 0.99 (0.93-1.06) | 0.79 | |
| EQ-5D-3L health index | 0.63 (0.26) | 0.84 (0.18) | 0.03 (0.00-0.19) | 0.02 (0.00-0.32) | <0.01 | |
| Heat pain threshold (ºC) | (n = 14) 47.7 (2.1) | (n = 213) 47.6 (2.5) | 1.01 (0.81-1.26) | 1.06 (0.83-1.37) | 0.62 | |
| Cold pain threshold (ºC) | (n = 14) 3.4 (6.3) | (n = 213) 2.9 (5.6) | 1.01 (0.93-1.11) | 1.01 (0.92-1.12) | 0.78 | |
| Pressure pain threshold (kPa) | (n = 14) 470 (188) | (n = 212) 517 (199) | 1.00 (1.00-1.00) | 1.00 (1.00-1.00) | 0.41 | |
| Intra- and postoperative variables | ||||||
| Mode of hysterectomy | Total abdominal | 10 (62.5) | 169 (70.1) | 1.00 (reference) | 1.00 (reference) | |
| Subtotal abdominal | 2 (12.5) | 21 (8.7) | 1.61 (0.33-7.85) | 1.51 (0.28-8.24) | 0.63 | |
| Vaginal | 4 (25.0) | 51 (21.2) | 1.33 (0.40-4.41) | 1.06 (0.30-3.77) | 0.93 | |
| Mode of anesthesia | GA | 8 (50.0) | 86 (35.7) | 1.00 (reference) | 1.00 (reference) | |
| SA + IM | 4 (25.0) | 83 (34.4) | 0.52 (0.15-1.79) | 0.60 (0.16-2.28) | 0.46 | |
| GA + IM | 4 (25.0) | 72 (29.9) | 0.60 (0.17-2.06) | 0.67 (0.18-2.47) | 0.54 | |
| Incision in abdominal wall** | Low transverse | 12 (75.0) | 169 (71.3) | 1.00 (reference) | 1.00 (reference) | |
| Midline | 0 (0.0) | 17 (7.2) | p=0.60‡ | NA | ||
| Operation time (minutes) | 89.6 (33.4) | 91.6 (41.9) | 1.00 (0.99-1.01) | 1.00 (0.98-1.01) | 0.89 | |
| Estimated bleeding intraoperatively (mL) | 157 (162) | 182 (250) | 1.00 (1.00-1.00) | 1.00 (1.00-1.00) | 0.67 | |
| Uterus weight (gram) | 387 (456) | 384 (377) | 1.00 (1.00-1.00) | 1.00 (1.00-1.00) | 0.88 | |
| Blood transfusion (no. of women) | 1 (6.2) | 10 (4.1) | 1.54 (0.18-12.84) | 2.12 (0.16-28.30) | 0.57 | |
| Duration of hospital stay (days) | 2.3 (0.9) | 1.7 (0.9) | 1.40 (0.98-1.99) | 1.50 (1.02-2.21) | 0.04 | |
| Maximum pain intensity day 0 § (scale 0-6) | (n=16) 3.4 (1.4) | (n=237) 3.2 (1.7) | 1.08 (0.80-1.46) | 1.03 (0.74-1.42) | 0.79 | |
| Maximum pain intensity day 1 § (scale 0-6) | (n=16) 3.6 (1.2) | (n=238) 3.00 (1.4) | 1.33 (0.92-1.94) | 1.36 (0.89-2.06 | 0.15 | |
| Classification of surgical complications | C-D grade 0 | 12 (74.0) | 179 (74.3) | 1.00 (reference) | 1.00 (reference) | |
| C-D grade I | 1 (6.2) | 22 (9.1) | 0.67 (0.08-5.41) | 0.63 (0.07-5.59) | 0.68 | |
| C-D grades II-III | 3 (18.8) | 40 (16.6) | 1.11 (0.30-4.10) | 1.03 (0.27-3.98) | 0.97 | |
aOR adjusted odds ratio, C-D Clavien-Dindo classification of postoperative complications, CI confidence interval, EQ-5D-3L Euroqol form-five dimensions-three levels, GA general anesthesia, HADS-A Hospital Anxiety and Depression Scale- Anxiety, HADS-D Hospital Anxiety and Depression Scale- Depression, IM intrathecal morphine, MCS, mental component summary, NA not applicable, PCS physical component summary, SA spinal anesthesia, SCI Stress Coping Inventory, SF-36 Short Form 36 items
*Missing data not included in the analyses
† Adjusted for age, Body mass index, smoking, preoperative use of analgesics, mode of hysterectomy, postoperative complications (Clavien-Dindo), HADS-A, and HADS-D
**Includes abdominal hysterectomies only
‡ Because of a cell with no observations, the logistic regression could not be calculated. Instead, the p-value of Fisher’s exact test or Pearson chi-square test is given, as appropriate
§ Day 0 and day 1 indicate day of surgery and the day after surgery, respectively
Risk factors for PPP
Age was an independent risk factor for PPP. For each year that age increased, the risk decreased by 11%. In addition, the number of pain areas, the regional pain area of the lower extremities, and the frequency of occurrence of pain preoperatively were independent risk factors (Table 5).
Table 5.
Demographic and clinical data of 183 women with pelvic pain undergoing hysterectomy in relation to persistent pelvic pain one year post-surgery
| Persistent pelvic pain | Logistic regression * | |||||
|
Yes (n=30) |
No (n=153) |
Univariate OR (95% CI) |
Multivariable † aOR (95% CI) |
p-value | ||
| Preoperative variables | ||||||
| Age (years) | 42.9 (5.5) | 46.0 (5.1) | 0.88 (0.82-0.96) | 0.89 (0.81-0.97) | <0.01 | |
| Body mass index (kg/m2) | 28.2 (5.2) | 26.6 (4.7) | 1.06 (0.99-1.15) | 1.08 (0.99-1.17) | 0.09 | |
| Parous | 26 (86.7) | 132 (86.3) | 1.03 (0.33-3.26) | 2.22 (0.46-10.59) | 0.32 | |
| Smoking | 4 (13.3) | 19 (12.4) | 1.16 (0.36-3.70) | 1.69 (0.48-6.02) | 0.41 | |
| Missing data | 2 (6.7) | 2 (1.3) | ||||
| ASA physical status classification | I | 23 (76.7) | 100 (65.4) | 1.00 (reference) | 1.00 (reference) | |
| II-III | 7 (23.3) | 53 (34.6) | 0.57 (0.23-1.43) | 0.43 (0.14-1.26) | 0.12 | |
| Comorbidity | Cardiovascular | 2 (6.7) | 20 (13.2) | 0.48 (0.10-2.15) | 0.48 (0.10-2.36) | 0.36 |
| Pulmonary | 4 (13.3) | 10 (6.5) | 2.20 (0.64-7.55) | 2.85 (0.76-10.75) | 0.12 | |
| Medication | Analgesics | 9 (30.0) | 31 (20.3) | 1.69 (0.70-4.04) | 1.17 (0.41-3-35) | 0.76 |
| Anti-depressives or sedatives | 4 (13.3) | 20 (13.1) | 1.02 (0.32-3.24) | 0.76 (0.20-2.89) | 0.68 | |
| Hypnotics | 1 (3.3) | 5 (3.3) | 1.02 (0.11-9.06) | NA | ||
| Physical workload | Sedentary | 10 (33.3) | 40 (26.1) | 1.00 (reference) | 1.00 (reference) | |
| Medium | 5 (16.7) | 41 (26.8) | 0.49 (0.15-1.55) | 0.53 (0.15-1.82) | 0.31 | |
| Heavy | 11 (36.7) | 65 (42.5) | 0.68 (0.26-1.74) | 0.67 (0.23-1.92) | 0.46 | |
| Missing data | 4 (13.3) | 7 (4.6) | ||||
| Gainfully employed | 26 (86.7) | 145 (94.8) | 2.79 (0.78-9.94) | 0.32 (0.07-1.52) | 0.15 | |
| Previous laparotomy | 11 (36.7) | 54 (35.3) | 0.94 (0.42-2.12) | 0.96 (0.40-2.33) | 0.93 | |
| Indication for hysterectomy | Bleeding disorder | 6 (20.0) | 44 (28.8) | 1.00 (reference) | 1.00 (reference) | |
| Myoma | 13 (43.3) | 66 (43.1) | 1.44 (0.51-4.09) | 1.29 (0.39-4.28) | 0.68 | |
| Myoma and bleeding | 1 (3.3) | 19 (12.4) | 0.39 (0.04-3.43) | 0.35 (0.04-3.34) | 0.36 | |
| Cervical dysplasia | 3 (10.0) | 11 (7.2) | 2.00 (0.43-9.29) | 2.49 (0.48-12.84) | 0.28 | |
| Pain | 7 (23.3) | 13 (8.5) | 3.95 (1.13-13.83) | 3.28 (0.81-13.25) | 0.10 | |
| Number of pain areas on body-mapping | 11.2 (12.0) | 4.7 (2.1) | 1.35 (1.08-1.67) | 1.08 (1.02-1.14) | <0.01 | |
| Regional pain areas | Head | 10 (33.3) | 37 (24.2) | 1.57 (0.67-3.65) | 1.65 (0.65-4.16) | 0.29 |
| Neck and shoulder | 12 (40.0) | 46 (30.1) | 1..55 (0.69-3.48) | 1.32 (0.53-3.30) | 0.55 | |
| Chest | 0 (0.0) | 5 (3.3) | p=0.59 ‡ | NA | ||
| Abdomen | 18 (60.0) | 71 (46.4) | 1.73 (0.78-3.84) | 1.39 (0.60-3.33) | 0.46 | |
| Thoracic back | 2 (6.7) | 17 (11.1) | 0.57 (0.12-2.61) | 0.48 (0.10-2.31) | 0.36 | |
| Lower back | 16 (53.3) | 69 (45.1) | 1.39 (0.63-3.05) | 1.30 (0.52-3.25) | 0.58 | |
| Upper extremities | 7 (23.3) | 25 (16.3) | 1.56 (0.60-4.02) | 1.15 (0.35-3.76) | 0.81 | |
| Lower extremities | 15 (50.0) | 41 (26.8) | 2.73 (1.23-6.08) | 3.14 (1.25-7.86) | 0.01 | |
| How often did you have pain preoperatively | Rarely | 0 (0.0) | 16 (10.5) | NA | NA | |
| Sometimes | 2 (6.7) | 65 (42.5) | 1.00 (reference) | 1.00 (reference) | ||
| Often | 16 (53.3) | 41 (26.8) | 12.69 (2.77-58.05) | 12.27 (2.44-61.80) | <0.01 | |
| Almost always/always | 11 (36.7) | 25 (16.3) | 14.30 (2.96-69.13) | 12.75 (2.24-72.66) | <0.01 | |
| Missing data | 1 (3.3) | 6 (3.9) | ||||
| Average intensity of preoperative pain (VAS scale 1-10) | 5.8 (1.7) | 4.7 (2.1) | 1.07 (1.02-1.12) | 1.24 (0.98-1.58) | 0.08 | |
| Missing data | 1 (3.3) | 3 (2.0) | ||||
| HADS-A score | 6.2 (4.3) | 4.8 (4.0) | 1.09 (0.99-1.19) | 1.08 (0.95-1.22) | 0.25 | |
| HADS-A (in categories) | Normal | 15 (53.3) | 110 (71.9) | 1.00 (reference) | 1.00 (reference) | |
| Borderline abnormal | 10 (33.3) | 27 (17.6) | 2.55 (1.04-6.23) | 1.94 (0.72-5.24) | 0.19 | |
| Abnormal | 4 (13.3) | 16 (10.5) | 1.72 (0.51-5.79) | 2.14 (0.54-8.41) | 0.28 | |
| HADS-D score | 3.4 (2.9) | 2.6 (2.9) | 1.09 (0.96-1.23) | 1.00 (0.84-1.20) | 0.96 | |
| HADS-D (in categories) | Normal | 28 (93.3) | 141 (92.1) | 1.00 (reference) | 1.00 (reference) | |
| Borderline abnormal | 2(6.7) | 9 (5.9) | 1.12 (0.23-5,46) | 0.76 (0.13-4.44) | 0.76 | |
| Abnormal | 0 (0.0) | 3 (2.0) | NA | NA | ||
| SCI score | 179.3 (23.7) | 187.1 (22.7) | 0.99 (0.97-1.00) | 0.98 (0.96-1.01) | 0.15 | |
| SCI (in categories) | Low stress coping | 10 (33.3) | 29 (19.0) | 2.14 (0.90-5.05) | 2.51 (0.87-7.26) | 0.09 |
| High stress coping | 20 (66.7) | 124 (81.0) | 1.00 (reference) | 1.00 (reference) | ||
| SF-36 PCS | 43.3 (10.4) | 46.1 (9.6) | 0.97 (0.94-1.01) | 0.98 (0.93-1.03) | 0.39 | |
| SF-36 MCS | 44.6 (11.9) | 47.8 (9.9) | 0.97 (0.94-1.01) | 0.99 (0.94-1.04) | 0.71 | |
| EQ-5D-3L health index | 0.69 (0.23) | 0.77 (0.20) | 0.20 (0.04-1.08) | 0.38 (0.04-3.30) | 0.38 | |
| Heat pain threshold (ºC) |
(n = 21) 46.6 (3.4) |
(n = 122) 47.5 (2.7) |
0.91 (0.78-1.05) | 0.93 (0.79-1.10) | 0.42 | |
| Cold pain threshold (ºC) |
(n = 21) 6.1 (8.5) |
(n = 122) 4.7 (7.06) |
1.02 (0.96-1.09) | 1.02 (0.95-1.09) | 0.66 | |
| Pressure pain threshold (kPa) |
(n = 21) 465 (210) |
(n = 120) 489 (198) |
1.00 (1.00-1.00) | 1.00 (1.00-1.00) | 0.46 | |
| Intra- and postoperative variables: | ||||||
| Mode of hysterectomy | Total abdominal | 21 (70.0) | 110 (71.9) | 1.00 (reference) | 1.00 (reference) | |
| Subtotal abdominal | 4 (13.3) | 8 (5.2) | 2.62 (0.72-3.49) | 3.96 (0.89-17.56) | 0.07 | |
| Vaginal | 5 (16.7) | 35 (22.9) | 0.74 (0.26-2.13) | 0.59 (0.19-1.87) | 0.44 | |
| Mode of anesthesia | GA | 11 (36.7) | 57 (37.3) | 1.00 (reference) | 1.00 (reference) | |
| SA + IM | 11 (36.7) | 66 (43.1) | 0.96 (0.35-2.14) | 1.83 (0.62-5.35) | 0.27 | |
| GA + IM | 8 (26.6) | 30 (19.6) | 1.38 (0.50-3.80) | 2.13 (0.69-6.64) | 0.19 | |
| Incision in abdominal wall** | Low transverse | 23 (92.0) | 109 (92.4) | 1.00 (reference) | 1.00 (reference) | |
| Midline | 1 (4,0) | 7 (5.9) | 0.68 (0.08-5.77) | 0.49 (0.04-5.54) | 0.56 | |
| Missing data | 1 (4.0) | 2 (1,7) | ||||
| Operation time (minutes) | 89.1 (32.5) | 91.0 (60.4) | 1.00 (0.99-1.01) | 1.00 (0.99-1.01) | 0.46 | |
| Estimated bleeding intraoperatively (mL) | 186 (188) | 168 (171) | 1.00 (1.00-1.00) | 1.00 (1.00-1.00) | 0.57 | |
| Uterus weight (gram) | 256 (255) | 356 (292) | 1.00 (1.00-1.00) | 1.00 (1.00-1.00) | 0.06 | |
| Blood transfusion (no. of women) | 0 (0.0) | 4 (2.6) | p=1.00 ‡ | NA | ||
| Duration of hospital stay (days) | 2.1 (2.0) | 1.7 (1.2) | 1.19 (0.95-1.50) | 1.15 (0.89-1.49) | 0..29 | |
| Maximum pain intensity day 0 § (scale 0-6) |
(n=30) 4.1 (1.5) |
(n=149) 3.3 (1.7) |
1.41 (1.07-1.85) | 1.26 (0.95-1.68) | 0.11 | |
| Maximum pain intensity day 1 § (scale 0-6) |
(n=30) 3.7 (1.6) |
(n=149) 3.6 (1.4) |
1.06 (0.80-1.41) | 0.97 (0.70-1.33) | 0.72 | |
| Classification of surgical complications | C-D grade 0 | 18 (60.0) | 107 (69.9) | 1.00 (reference) | 1.00 (reference) | |
| C-D grade I | 6 (20.0) | 17 (11.1) | 2.10 (0.73-6.03) | 1.52 (0.44-5.21) | 0.50 | |
| C-D grades II-III | 6 (20.0) | 29 (19.0) | 1.23 (0.45-3.38) | 0.62 (0.19-2.10) | 0.44 | |
aOR adjusted odds ratio, C-D Clavien- Dindo classification of postoperative complications, CI confidence interval, EQ-5D-3L EuroQol form-five dimensions-three levels, GA general anesthesia, HADS-A Hospital Anxiety and Depression Scale - Anxiety HADS-D Hospital Anxiety and Depression Scale - Depression, IM intrathecal morphine, MCS mental component summary, PCS physical component summary, SA spinal anesthesia, SCI Stress Coping Inventory, SF-36 Short Form 36 items
*Missing data not included in the analyses
† Adjusted for age, Body -mass index, smoking, preoperative use of analgesics, mode of hysterectomy, postoperative complications (Clavien-Dindo), HADS-A, and HADS-D
** Includes abdominal hysterectomies only
‡ Because of a cell with 0 observations, the logistic regression could not be calculated. Instead, the p-value of Fisher’s exact test is given
§ Day 0 and day 1 indicate day of surgery and the day after surgery, respectively
Discussion
The study revealed that 6.2% of the women developed DNPP and 16.4% had PPP one year after the hysterectomy. DNPP developed exclusively in women with pain in other areas preoperatively. Over 80% of the women who reported pelvic pain preoperatively achieved complete resolution at the one-year follow-up, ranging from 82% in women who besides pain in the pelvis had pain in other areas, to 90% with preoperative pelvic pain only. Preoperative risk factors for DNPP were identified, including preoperative pain intensity, the number of pain areas, the pain areas neck and shoulders, lower back, and upper and lower extremities along with low quality of life and anxiety. The risk factors for PPP were almost the same but also included a higher frequency of preoperative pain and younger age.
The prevalence of DNPP one year after hysterectomy was in line with the recently published study from the Swedish National Quality Registry for Gynecological Surgery (GynOp) where Grundström et al. found DNPP in 7.8% [33]. The data collection in the GynOp was prospective, as in the present study. A prospective American multicenter study reported a DNPP rate of 3.6% [34]. Brandsborg et al. reported a Danish nationwide postal questionnaire study where DNPP was found in 14.9% [9]. The data in that study were collected retrospectively more than a year after the surgery and there was a significant risk of recall bias. A Dutch study with a similar design to the present one reported a 9.0% prevalence of chronic postsurgical pain one year after hysterectomy [8]. However, the pain in that study was not distinctly related to the pelvis but was described as mainly originating from the lower abdomen. Another study reported persistent postsurgical pelvic pain four months after hysterectomy in 26.1% of the women [6]. These studies highlight the difficulty of comparing results and emphasize the importance of using a uniform design and time indication after surgery, a uniform definition of pain, and delineation of the area of pain.
The prevalence of PPP reported by 16.4% of the respondents in this study corresponded to the rate reported in previous studies [9, 33, 35, 36].
The reported rate of resolution of chronic pelvic pain after hysterectomy varies between 76 and 88% and our result falls within these limits [3, 33–38]. Thus, consistent with other studies, this study indicated that hysterectomy is successful in the treatment of chronic pelvic pain, irrespective of whether that is pelvic pain alone or combined with pain elsewhere in the body. However, the resolution of pelvic pain did not appear to affect the resolution of pain outside the pelvis.
To our knowledge this is the first study to evaluate associations between preoperative pain body mapping and DNPP and PPP one year after surgery. Only a few studies in benign gynecology have reported associations between pain elsewhere and persistent postsurgical pain but without specifying the location or the number of painful areas or distinguishing between DNPP and PPP [6, 7, 9]. A recent systematic review and meta-analysis of the few published studies on the spatial spread of pain and persistent postsurgical pain after hysterectomy reported that patients with preoperative pain elsewhere had a three-fold higher risk of developing persistent postsurgical pain [4].
The present study showed that the risk of DNPP and PPP was significantly associated with an increasing number of pain areas and location of the pain elsewhere. This may indicate that individuals who developed DNPP or PPP already carried a state of aberrant neuro-modulation that may have been triggered to accelerate potential mechanisms involved in the development or maintenance of these pain conditions. Such an association has been shown in fertile-aged women with chronic pain conditions caused by endometriosis [39–41]. Central sensitization probably contributes to chronic pain development in both DNPP and PPP patients, although through different mechanisms. A preoperative pain condition such as widespread pain suggests an established central sensitization that, due to supraspinal mechanisms, facilitates DNPP development [42]. Conversely, central sensitization in PPP patients is caused by excitatory synaptic modulation in the dorsal horn of the spinal cord due to noxious stimuli through peripheral nerves from persistent pelvic nociceptive pain. As a result, the excitatory state of the dorsal horn continues even after the noxious stimulus, such as hysterectomy, is eliminated [43]. Viscerosomatic convergence may also be a major contributor to PPP, further amplifying pain transmission in the spinal cord and perception in higher brain centers [44]. None of the women without preoperative pain developed DNPP, suggesting a rather low risk in those women due to the absence of pain conditions and a state of central sensitization. However, this interpretation should be made with great caution.
Severe acute postoperative pain has been repeatedly identified as a risk factor for the development of chronic pain after hysterectomy [2, 3, 5, 6, 11, 43]. The present study found an association between preoperative pelvic pain and maximum pain intensity on postoperative day 1 but we could not confirm an association between acute postoperative pain and PPP or DNPP.
There is a growing body of literature indicating the association between psychological characteristics and postsurgical pain [45]. While Han et al. and Pinto et al. showed that preoperative anxiety was a risk factor for persistent postsurgical pain they did not discriminate between de novo pain and persistent preoperative pain although the effect of anxiety on these conditions may be different [5, 7]. The latter was supported by our findings that anxiety was a risk factor only for DNPP but not for PPP. Han et al. and Benolo et al. found likewise that depression was a risk factor for persistent post-hysterectomy pain [2, 5]. However, as with anxiety, they did not differentiate persistent postsurgical pain into de novo and persistent preoperative pain. This may explain why we did not find associations between depression and DNPP or PPP.
Information on the relationship between the mode of hysterectomy and persistent postsurgical pain is equivocal. While Pinto et al. reported an association between abdominal hysterectomy and the development of chronic postsurgical pain [6], others did not find such an association [3, 9, 37]. We found no association between surgical mode and DNPP or PPP one year postoperatively.
Chronic pain generally has a negative impact on HRQoL, and patients with multiple pain locations are usually the most severely affected [46]. This seemed consistent with our results. The perceived HRQoL was lowest when pelvic pain co-occurred with pain in other areas. This might imply that pelvic pain contributed to a greater extent to lower HRQoL when it occurred simultaneously with pain in other areas of the body. The measures of HRQoL appeared to predict DNPP, but not PPP. A low EQ-5D-3L health index preoperatively was a risk factor for DNPP one year after the hysterectomy but not for PPP, suggesting a multifactorial etiology of postsurgical DNPP. The group of women with PPP consisted mainly of women who preoperatively had widespread bodily pain including pelvic pain. These women even had the lowest EQ-5D health index preoperatively.
Consistent with other studies, younger age, anxiety, pain elsewhere, and preoperative pain frequency were risk factors for PPP [2, 5–9, 11]. The relationship between preoperative QST and persistent postsurgical pain has been repeatedly investigated, with conflicting results [15–18, 47]. A recent systematic review concluded that no consistency was found for a single QST parameter having a predictive role for the development of chronic postoperative pain [14]. The present study seemed to support that conclusion.
The relationship between preoperative QST and postoperative persistent pain has been extensively researched, with conflicting results [16, 17]. Although some studies have shown that thermal and pressure pain thresholds were predictors of high postoperative pain intensity and persistent pain [18, 47], a systematic review came to the opposite conclusion [15]. Our study failed to demonstrate associations between pain thresholds and PPP.
Strength and limitations
The study has several strengths including the prospective, longitudinal multicenter design, the large number of participants, and the use of an ERAS protocol according to the best standard of care, along with the use of validated forms and methods. In addition, the indications for benign hysterectomy were quite similar to those presented in the GynOp indicating that the study population was representative of the Swedish population [48]. Thus, the result may be generalized at least to communities or countries with similar populations and healthcare facilities.
The study has limitations. It may suffer from selection bias. Anxiety, depression, or fear of experimental pain may have been reasons for refraining from participating in the study. Reluctance and a potential apprehension about participation in the section of the study concerning measurement of pain thresholds was evident with more than 15% refraining from participation in the pain threshold measurements. Moreover, the questions concerning the self-reported pain measures were not strictly validated. However, the questions that were asked were simple in their construction and unambiguous, which should mean a low risk of misinterpretation. Another limitation may be the use of pain frequency as a measure of chronic pain instead of the more commonly used definition of a pain duration of > 3 months. In addition, the information on pain intensity or frequency of pain was not related to the individual areas of pain but represented an overall measure of the condition.
Conclusion
The risk of DNPP after hysterectomy was not negligible, affecting one in 16, but seemed to affect exclusively those who had pain conditions in other parts of the body preoperatively. More than 80% of women with pelvic pain were cured. Women at risk for DNPP and PPP after hysterectomy could be identified preoperatively. Information about the risk factors should be included in the preoperative counseling before benign hysterectomy.
Acknowledgements
The authors thank all women who unselfishly participated in the study. We are grateful for the committed work conducted by all in the POSTHYSTREC study group, particularly the research nurses. We extend special thanks to the late Professor Björn Gerdle at Linköping University for his committed participation in the study and for all his advice and constructive discussions regarding the analyses and preparation of the manuscript. We are deeply honored to have worked with him and to have shared his extensive knowledge in pain research.
The POSTHYSTREC Study Group:
The POSTHYSTREC Study Group consisted of members from five hospitals in the Southeast region of Sweden.
Linköping University Hospital: Preben Kjølhede, MD, PhD, Gunilla Sydsjö, PhD, Ninnie Borendal Wodlin, MD, PhD, Lena Nilsson, MD, PhD, Gulnara Kassymova, MD, Peter Lukas, MD, Petra Langström, RN, Pernilla Nilsson, RN, Linda Shosholli, RN, Sofia Bergström, RN, and Åsa Rydmark Kersley, RN, MSc.
Vrinnevi Hospital, Norrköping: Leif Hidmark, MD, Anders Bolling, MD, Kristina Ekman, RNW, and Karin Granberg-Karlsson, RNW
Ryhov Hospital, Jönköping: Laila Falknäs, MD, Maria Häggström, MD, Ewa Hermansson RNW
Eksjö Highland Hospital: Tomaz Stypa, MD, PhD, Linda Myllimäki, MD, Iréne Johannesson, RNW, and Martina Ekeroth Wikander, RNW
Värnamo Hospital: Christina Gunnervik, MD, Fatima Johansson, MD Magnus Trofast, MD, Mari-Ann Andersson, RNW, and Carita Jacobsson, RN.
Abbreviations
- aOR
Adjusted Odds Ratio
- BMI
Body Mass Index
- CPT
Cold Pain Threshold
- DNPP
De Novo Persistent Pain
- GynOp
The Swedish National Quality Registry for Gynecological Surgery
- HADS
Hospital Anxiety and Depression Scale
- HPT
Heat Pain Threshold
- HRQoL
Health-Related Quality of Life
- MCS
Mental Component Summary
- OR
Odds Ratio
- PCS
Physical Component Summary
- PPP
Persistent Pelvic Pain
- PPT
Pressure Pain Threshold
- SCI
Stress Coping Inventory
- SF-36
Short From 36 Health Survey
- QST
Quantitative Sensory Testing
Authors’ contributions
P.L.: Investigation, Formal analysis, Writing - Original draft preparation. L.N.: Conceptualization, Methodology, Investigation, Writing - Review and Editing, Supervision. N.B.W.: Conceptualization, Methodology, Writing - Review and Editing. L.A.N.: Conceptualization, Methodology, Writing - Review and Editing. Supervision. P.K.: Conceptualization, Methodology, Formal analysis, Investigation, Data Curation, Writing - Review and Editing, Visualization, Supervision, Project administration, Funding acquisition.
Funding
Open access funding provided by Linköping University. The study was supported by grants from the Medical Research Council of Southeast Sweden (grant numbers FORSS-155141; FORSS-222211; FORSS-308441, and FORSS-387761, grant holder Preben Kjølhede)), from Region Östergötland Council (ALF grants RÖ-200641, RÖ-276871, RÖ-356651, RÖ-448391, RÖ-540551, RÖ-607891, RÖ-699021, RÖ-794531, RÖ-931528, RÖ-936208, RÖ-968764, and RÖ-987412, grant holder Preben Kjølhede), and Futurum – the Academy of Health and Care, Region Jönköping Council (grant numbers FUTURUM-487481, and FUTURUM 579171, grant holder Laila Falknäs). The grant applications were assessed for scientific quality by external peer reviewers. The grant providers were otherwise not involved in the research or the writing of the manuscript.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to study participant privacy but are available from the corresponding author (Peter.Lukas@liu.se) on reasonable request and in accordance with Swedish legislation.
Declarations
Ethics approval and consent to participate
The study has been approved by the Regional Ethical Board in Linköping (Dnr. 2011/106–31; date of approval May 23; 2011), complies with the Declaration of Helsinki, and registered in ClinicalTrial.gov (NCT01526668). All participants gave informed consent to participate in the study prior to inclusion.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brandsborg B, Nikolajsen L, Kehlet H, Jensen TS. Chronic pain after hysterectomy. Acta Anaesthesiol Scand. 2008;52(3):327–31. 10.1111/j.1399-6576.2007.01552.x. [DOI] [PubMed] [Google Scholar]
- 2.Benlolo S, Hanlon JG, Shirreff L, Lefebvre G, Husslein H. Predictors of persistent postsurgical pain after hysterectomy-A prospective cohort study. J Minim Invasive Gynecol. 2021;28(12):2036–2046.e1. 10.1016/j.jmig.2021.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Pokkinen SM, Nieminen K, Yli-Hankala A, Kalliomäki ML. Persistent posthysterectomy pain: A prospective, observational study. Eur J Anaesthesiol. 2015;32(10):718–24. 10.1097/EJA.0000000000000318. [DOI] [PubMed] [Google Scholar]
- 4.Sharma LR, Schaldemose EL, Alaverdyan H, Nikolajsen L, Chen D, Bhanvadia S, et al. Perioperative factors associated with persistent postsurgical pain after hysterectomy, cesarean section, prostatectomy, and donor nephrectomy: a systematic review and meta-analysis. Pain. 2022;163(3):425–35. 10.1097/j.pain.0000000000002361. [DOI] [PubMed] [Google Scholar]
- 5.Han C, Ge Z, Jiang W, Zhao H, Ma T. Incidence and risk factors of chronic pain following hysterectomy among Southern Jiangsu Chinese Women. BMC Anesthesiol. 2017;17(1):103. 10.1186/s12871-017-0394-3. [DOI] [PMC free article] [PubMed]
- 6.Pinto PR, McIntyre T, Nogueira-Silva C, Almeida A, Araújo- Soares V, Almeida A, et al. Risk factors for persistent postsurgical pain in women undergoing hysterectomy due to benign causes: a prospective predictive study. J Pain. 2012;13(11):1045–57. 10.1016/j.jpain.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Pinto PR, McIntyre T, Araújo-Soares V, et al. Psychological factors predict an unfavorable pain trajectory after hysterectomy: a prospective cohort study on chronic postsurgical pain. Pain. 2018;159(5):956–67. 10.1097/j.pain.0000000000001170. [DOI] [PubMed] [Google Scholar]
- 8.Theunissen M, Peters ML, Schepers J, Maas JW, Tournois F, van Suijlekom HA, et al. Recovery 3 and 12 months after hysterectomy: epidemiology and predictors of chronic pain, physical functioning, and global surgical recovery. [published correction appears in Medicine (Baltimore). 2017;96(20):e6957; 10.1097/MD.0000000000006957]. Medicine (Baltimore). 2016;95(26):e3980; 10.1097/MD.0000000000003980. [DOI] [PMC free article] [PubMed]
- 9.Brandsborg B, Nikolajsen L, Hansen CT, Jensen TS. Risk factors for chronic pain after hysterectomy: a nationwide questionnaire and database study. Anesthesiology. 2007;106(5):1003–12. 10.1097/01.anes.0000265161.39932.e8. [DOI] [PubMed] [Google Scholar]
- 10.Aasvang EK, Gmaehle E, Hansen JB, Gmaehle B, Forman JL, Schwarz J, et al. Predictive risk factors for persistent postherniotomy pain. Anesthesiology. 2010;112(4):957–69. 10.1097/ALN.0b013e3181d31ff8. [DOI] [PubMed] [Google Scholar]
- 11.Brandsborg B, Dueholm M, Nikolajsen L, Kehlet H, Jensen TS. A prospective study of risk factors for pain persisting 4 months after hysterectomy. Clin J Pain. 2009;25(4):263–8. 10.1097/AJP.0b013e31819655ca. [DOI] [PubMed] [Google Scholar]
- 12.Fruhstorfer H, Lindblom U, Schmidt WC. Method for quantitative estimation of thermal thresholds in patients. J Neurol Neurosurg Psychiatry. 1976;39:1071–5. 10.1136/jnnp.39.11.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rolke R, Baron R, Maier C, Tölle TR, Treede -DR, Beyer A et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Standardized protocol and reference values. [published correction appears in Pain. 2006;125(1–2):197]. Pain. 2006;123(3):231–243; 10.1016/j.pain.2006.01.041. [DOI] [PubMed]
- 14.Petersen KK, Vaegter HB, Stubhaug A, Wolff A, Scammell BE, Arendt-Nielsen L, et al. The predictive value of quantitative sensory testing: a systematic review on chronic postoperative pain and the analgesic effect of pharmacological therapies in patients with chronic pain. Pain. 2021;162(1):31–44. 10.1097/j.pain.0000000000002019. [DOI] [PubMed] [Google Scholar]
- 15.Sangesland A, Støren C, Vaegter HB. Are preoperative experimental pain assessments correlated with clinical pain outcomes after surgery? A systematic review Scand J Pain. 2017;15:44–52. 10.1016/j.sjpain.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 16.van Helmond N, Aarts HM, Timmerman H, Olesen SS, Drewes AM, Wilder-Smith OH, et al. Is preoperative quantitative sensory testing related to persistent postsurgical pain? A systematic literature review Anesth Analg. 2020;131(4):1146–55. 10.1213/ANE.0000000000004871. [DOI] [PubMed] [Google Scholar]
- 17.Braun M, Bello C, Riva T, Hönemann C, Doll D, Urman RD et al. Quantitative sensory testing to predict postoperative pain. Curr Pain Headache Rep. Curr Pain Headache Rep. 2021;25(1):3. 10.1007/s11916-020-00920-5. [DOI] [PMC free article] [PubMed]
- 18.Lukas P, Gerdle B, Nilsson L, Wodlin NB, Fredrikson M, Arendt-Nielsen L et al. Association between experimental pain thresholds and trajectories of postoperative recovery measures after benign hysterectomy. [published correction appears in J Pain Res. 2023;16:677–679. 10.2147/JPR.S410626]. J Pain Res. 2022;15:3657–3674. Published 2022 Nov 23; 10.2147/JPR.S383795. [DOI] [PMC free article] [PubMed]
- 19.Werner MU, Kongsgaard UEI. Defining persistent post-surgical pain: is an update required? Br J Anaesth. 2014;113(1):1–4. 10.1093/bja/aeu012. [DOI] [PubMed] [Google Scholar]
- 20.Schug SA, Lavand’homme P, Barke A, Korwisi B, Rief W, Treede RD. The IASP classification of chronic pain for ICD-11: chronic postsurgical or posttraumatic pain. Pain. 2019;160(1):45–52. 10.1097/j.pain.0000000000001413. [DOI] [PubMed] [Google Scholar]
- 21.Kassymova G, Sydsjö G, Borendal Wodlin N, Nilsson L, Kjølhede P. The effect of follow-up contact on recovery after benign hysterectomy: a randomized, single-blinded, four-arm, controlled multicenter trial. J Womens Health (Larchmt). 2021;30(6):872–81. 10.1089/jwh.2020.875219. [DOI] [PubMed] [Google Scholar]
- 22.Dindo D, Demartines N, Clavien PA. Classification of surgical complications. A new proposal with evaluation in a cohort of 6336 patient and results of a survey. Ann Surg. 2004;240(2):205–213; 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed]
- 23.Margolis RB, Tait RC, Krause SJ. A rating system for use with patient pain drawings. Pain. 1986;24(1):57–65. 10.1016/0304-3959(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 24.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–70. 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 25.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52(2):69–77; 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed]
- 26.Ryding EL, Wijma B, Wijma K, Rydhström H. Fear of childbirth during pregnancy may increase the risk of emergency cesarean section. Acta Obstet Gynecol Scand. 1998;77(5):542–7. [PubMed] [Google Scholar]
- 27.Söderqvist J, Wijma B, Wijma K. The longitudinal course of post-traumatic stress after childbirth J Psychosom Obstet Gynaecol. 2006;27(2):113–9. 10.1080/01674820600712172. [DOI] [PubMed] [Google Scholar]
- 28.Kjølhede P, Borendal Wodlin N, Nilsson L, Wijma K. Impact of stress coping capacity on recovery from abdominal hysterectomy in a fast-track programme: A prospective longitudinal study. [published correction appears in BJOG. 2012;119(10):1291]. BJOG. 2012;119(8):998–1007; 10.1111/j.1471-0528.2012.03342.x. [DOI] [PubMed]
- 29.EuroQol Group. EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 30.Bjurström K, Sun S, Gerdtham UG, Henriksson M, Johannesson M, Levin LÅ, et al. Swedish experience-based value sets for EQ-5D health states. Qual Life Res. 2014;23(2):431–42. 10.1007/s11136-013-0496-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31(3):247–263; 10.1097/00005650-199303000-00006. [DOI] [PubMed]
- 32.Sullivan M, Karlsson J, Ware JE Jr. The Swedish SF-36 Health Survey--I. Evaluation of data quality, scaling assumptions, reliability and construct validity across general populations in Sweden. Soc Sci Med. 1995;41(10):1349–1358; 10.1016/0277-9536(95)00125-q. [DOI] [PubMed]
- 33.Grundström H, Fredrikson M, Alehagen S, Berterö C, Kjølhede P. Incidence of self-reported pelvic pain and risk factors for pain 1 year after benign hysterectomy: A register study from the Swedish National Quality Registry for Gynecological Surgery. Acta Obstet Gynecol Scand. 2023;102(10):1359–70. 10.1111/aogs.14455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kjerulff KH, Langenberg PW, Rhodes JC, Harvey LA, Guzinski GM, Stolley PD. Effectiveness of hysterectomy. Obstet Gynecol. 2000;95(3):319–26. 10.1016/s0029-7844(99)00544-x. [DOI] [PubMed] [Google Scholar]
- 35.Stovall TG, Ling FW, Crawford DA. Hysterectomy for chronic pelvic pain of presumed uterine etiology. Obstet Gynecol. 1990;75:676–9. [PubMed] [Google Scholar]
- 36.Hillis SD, Marchbanks PA, Peterson HB. The effectiveness hysterectomy for chronic pelvic pain. Obstet Gynecol. 1995;86:941–5. 10.1016/0029-7844(95)00304-a. [DOI] [PubMed] [Google Scholar]
- 37.As-Sanie S, Till SR, Schrepf AD, Griffith KC, Tsodikov A, Missmer SA, et al. Incidence and predictors of persistent pelvic pain following hysterectomy in women with chronic pelvic pain. Am J Obstet Gynecol. 2021;225(5):568.e1–568.e11. 10.1016/j.ajog.2021.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hartmann KE, Ma C, Lamvu GM, Langenberg PW, Steege JF, Kjerulff KH. Quality of life and sexual function after hysterectomy in women with preoperative pain and depression. Obstet Gynecol. 2004;104(4):701–9. 10.1097/01.AOG.0000140684.37428.48. [DOI] [PubMed] [Google Scholar]
- 39.Grundström H, Gerdle B, Alehagen S, Berterö C, Arendt-Nielsen L, Kjølhede P. Reduced pain thresholds and signs of sensitization in women with persistent pelvic pain and suspected endometriosis. Acta Obstet Gynecol Scand. 2019;98(3):327–36. 10.1111/aogs.13508.E. PMID: 30472739. [DOI] [PubMed] [Google Scholar]
- 40.Raimondo D, Raffone A, Renzulli F, Sanna G, Raspollini A, Bertoldo L, et al. Prevalence and risk factors of central sensitization in women with endometriosis. J Minim Invasive Gynecol. 2023;30(1):73–80.e1. 10.1016/j.jmig.2022.10.007. PMID: 36441085. [DOI] [PubMed] [Google Scholar]
- 41.Orr NL, Huang AJ, Liu YD, Noga H, Bedaiwy MA, Williams C, et al. Association of Central Sensitization Inventory Scores with pain outcomes after endometriosis surgery. JAMA Netw Open. 2023;6(2):e230780. 10.1001/jamanetworkopen.2023.0780. PMID:36848090; PMCID:PMC9972194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harte SE, Harris RE, Clauw DJ. The neurobiology of central sensitization. J Appl Biobehav Res. 2018;23:e12137. 10.1111/jabr.12137. [Google Scholar]
- 43.Chapman CR, Vierck CJ. The transition of acute postoperative pain to chronic pain: An integrative overview of research on mechanisms. J Pain. 2017;18(4):359.e1–359.e38. 10.1016/j.jpain.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 44.Lamvu G, Carrillo J, Ouyang C, Rapkin A. Chronic Pelvic Pain in Women: A Review. JAMA. 2021;325(23):2381–91. 10.1001/jama.2021.2631. PMID: 34128995. [DOI] [PubMed] [Google Scholar]
- 45.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163(20):2433–45. 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 46.Lamé IE, Peters ML, Vlaeyen JW, Kleef Mv, Patijn J. Quality of life in chronic pain is more associated with beliefs about pain, than with pain intensity. Eur J Pain. 2005;9(1):15–24. 10.1016/j.ejpain.2004.02.006 [DOI] [PubMed]
- 47.Müller M, Bütikofer L, Andersen OK, Heini P, Arendt-Nielsen L, Jüni P, et al. Cold pain hypersensitivity predicts trajectories of pain and disability after low back surgery: a prospective cohort study. Pain. 2021;162(1):184–94. 10.1097/j.pain.0000000000002006. [DOI] [PubMed] [Google Scholar]
- 48.Ehrström S. Hysterektomi på benign indikation. Årsrapport från GynOp-registret avseende operationer utförda år 2022. [In Swedish]. https://www.gynop.se/wp-content/uploads/2023/05/Arsrapport-Hysterektomi-utford-ar-2022.pdf. [Last accessed: 06/26/2024]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to study participant privacy but are available from the corresponding author (Peter.Lukas@liu.se) on reasonable request and in accordance with Swedish legislation.