Abstract
Background
Adverse birth outcomes (preterm birth, low birth weight, small for gestational age, and stillbirth) seem to persist in infants born to people with HIV, even in the context of maternal antiretroviral therapy. However, findings have been disparate, inconclusive, and difficult to compare directly across settings, partly owing to variable outcome definitions. We aimed to collate, compare, and map existing adverse birth outcome definitions to inform a harmonized approach to universally measure these outcomes in studies including pregnant people with HIV.
Methods
We conducted a scoping review of studies that reported adverse birth outcomes associated with maternal HIV and antiretroviral use in pregnancy, specifically those that included definitions of ‘preterm birth’, ‘low birth weight’, ‘small for gestational age’, and ‘stillbirth’. Five databases were searched from 01 January 2011 to 15 August 2022. Title, abstract and full-text screening was conducted independently in duplicate. A comparative quantitative analysis was conducted to compare study characteristics by period of study (< 2013; 2013–2015; > 2016) and country income group. A qualitative content analysis was conducted to compare and map deviations from the WHO definitions as a reference.
Results
Of the 294 articles that included at least one adverse birth outcome, 214 (73%) studies started before 2013, 268 (91%) were published as primary research articles, and 137 (47%) were conducted in Eastern and Southern Africa. Among the 283 studies included in the country income group analysis, 178 (63%) were conducted in low- and middle-income countries. Studies reporting low birth weight, preterm birth, small for gestational age and stillbirth deviated from the WHO definitions in n = 11/169 (7%), n = 93/246 (39%), n = 40/112 (36%) and n = 85/108 (79%) instances, respectively. The variations included the use of different thresholds and the addition of new terminology.
Conclusion
The current WHO definitions are valuable tools for population-level monitoring; however, through consensus, these definitions need to be optimized for research data collection, analysis, and presentation. In conjunction with good reporting, variation in adverse birth outcome definitions can be decreased to facilitate comparability of studies as well as pooling of data for enhanced evidence synthesis.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12884-024-06939-5.
Keywords: Pregnancy, Low birth weight, Preterm birth, Stillborn, Small for gestational age, HIV
Background
According to current universal antiretroviral therapy (ART) guidelines, it is recommended that people who have acquired HIV commence ART upon diagnosis [1]. This recommendation led to 82% of pregnant people with HIV receiving ART globally during pregnancy in 2022 and a substantial proportion of those receiving ART at conception [2, 3]. Additionally, policies are in place for the administration of pre-exposure prophylaxis (PrEP) [2] to people at substantial risk of HIV acquisition as well as pregnant and breastfeeding people living without HIV and residing in high-HIV-incidence settings. These advances have resulted in a decline in the number of infants perinatally acquiring HIV [3, 4], an increase in the number of infants with fetal HIV and antiretroviral exposure, and the emergence of an infant population that is antiretroviral exposed without HIV [5].
The benefits of ART for pregnant people living with HIV and their children, who are at risk of vertical HIV acquisition, are undeniable. However, from ongoing research [6, 7], it seems that adverse birth outcomes occur more often in children born to pregnant people with HIV than in those without fetal HIV exposure. Furthermore, any child, irrespective of HIV and antiretroviral exposure, born preterm, with low birth weight (LBW) or restricted growth is at increased risk for morbidity and mortality in its early years [8]. The literature extensively details the adverse birth outcomes of preterm birth (PTB), LBW, small for gestational age (SGA) and stillbirth occurring in pregnancies of people with HIV. Despite the use of WHO definitions (Table 1) for all these outcomes, heterogeneity in how studies define the outcomes might contribute to disparate and inconclusive findings with limited generalizability8–10.
Table 1.
Published WHO definitions of adverse birth outcomes
| Adverse Birth Outcome | WHO Root Definition | WHO Subgroup Definitions |
|---|---|---|
| Low Birth Weight | Weight at birth less than 2500 g (5.5 lb) | Low birth weight may be subdivided into very low birth weight (less than 1500 g), and extremely low birth weight (less than 1 000 g) |
| Preterm Birth | Babies born alive before 37 weeks of pregnancy are completed | There are subcategories of preterm birth, based on gestational age: extremely preterm (less than 28 weeks), very preterm (28–32 weeks) a, moderate to late preterm (32–37 weeks) a |
| Small for Gestational Age | Infants below the 10th centile of birthweight-for-gestational-age, gender-specific reference population | None |
| Stillbirths | A baby born with no signs of life at or after 28 weeks' gestation | None |
aLack of clarity without mutually exclusive categories: 32 weeks and 0 days are included as both very preterm birth and moderate-to-late preterm birth
Agreement by researchers on definitions of these important outcomes represents a valuable step towards achieving more scientifically rigorous and directly comparable studies that have the potential to strengthen evidence-informed health policy development. Thus, we aimed to conduct a scoping review of studies evaluating adverse birth outcomes in pregnant people with HIV to i) compare study characteristics by temporal period and by country income group and ii) quantitatively and qualitatively map definition variation in reference to the WHO definitions for PTB, LBW, SGA, and stillbirth to inform a harmonized approach to defining these important outcomes for the purpose of research data collection, analysis, and presentation.
Methods
Scoping Review
We conducted a scoping review that sought to collate, compare, and map definitions for adverse birth outcomes associated with maternal HIV and antiretroviral use in pregnancy, including LBW, PTB, SGA and stillbirth. This scoping review was conducted according to the Joanna Briggs Institute guidelines and reported using the Preferred Reporting Items for Systematic Reviews and Meta-analysis Extension for Scoping Reviews (PRISMA-ScR) [11, 12]. The protocol has been published previously [13]. This review included definitions for the adverse birth outcomes LBW, PTB, SGA or stillbirth from case series and reports; primary experimental (e.g., controlled trials) and observational (e.g., cohorts) studies; and secondary studies (e.g., systematic reviews) presented in the article, abstract, poster or conference presentation formats. For inclusion, the specified adverse birth outcomes had to be evaluated as primary or secondary outcomes, and the definition of the outcome had to be provided in studies that examined HIV exposure or antiretroviral use during pregnancy or HIV exposure or antiretroviral use during pregnancy in conjunction with exposure to other communicable or noncommunicable diseases in the context of maternal and child health or pregnancy. The full text or digital recordings of the sources of evidence had to be retrievable, written or narrated in English. Five databases were searched: PubMed/MEDLINE, the Cochrane Library, Web of Science, Scopus, and CINHAL/EBSCOhost. The search strategy is detailed in the protocol [13]. This report focuses on the presentation of citations retrieved from the search of published literature. The search of gray and other unpublished literature will be presented in a separate report. To focus on the most recent decade of the HIV epidemic, the search was restricted to publications occurring from 01 January 2011 to 15 August 2022. Title and abstract screening as well as full-text screening were conducted independently and in duplicate (KRD and STdB) using eligibility criteria that were piloted and adjusted in consultation with a senior author (ALS). All disagreements were resolved through discussion with the ALS, who made the final decision if a consensus could not be reached.
Data extraction was conducted by one reviewer (KRD) via a standardized data extraction tool developed in REDCap. For the quantitative comparison of study characteristics, year of study, publication type, study design, country, setting and study sample size were collected. Additionally, the adverse birth outcome assessor (who measured the outcome), method of outcome assessment (what was used to measure the outcome) and definition of outcome (as per the WHO definition or deviation from the WHO definition) were collected. Outcome definitions that deviated from the WHO definitions, which are used as the reference definitions, were extracted verbatim for the qualitative content analysis.
Synthesis of Results
Quantitative comparison of study characteristics
The publication and study characteristics of the included studies were compared by temporal period (2011-2012; 2013–2015 and 2016-2022) and by country income group (high income, low- and middle-income, and mixed income). The temporal period categories corresponded with WHO ART guideline recommendations: < 2013 CD4, clinical or immunological criteria; 2013–2015 ART for all pregnant and breastfeeding people with HIV; and ≥ 2016 universal ART for all people with HIV [11–13]. The year of study commencement was used to categorize studies into temporal periods. The World Bank classification was used according to the year of study to classify the country income group (CIG) [14]. Patient characteristics are reported as frequencies and percentages. STATA 17 was used to conduct the analysis, and the results were tabulated and described narratively where applicable.
Qualitative content analysis and logic models of outcome definitions
For the content analysis [15], outcome definitions that deviated from the WHO definition were categorized by the position of the deviation on the basis of where the deviation occurred, at the root definition or at the subgroup definition. Each deviation category (root or subgroup deviation) was analysed for patterns of deviation and further classified according to the type of deviation from the reference definition (Table 2). Individual definitions could have more than one position of deviation (root or subgroup) and more than one type of deviation.
Table 2.
Types of deviation from the WHO reference outcome definitions and their descriptions
| Type of deviation from reference definition | Description |
|---|---|
| Minor jargon, formatting, and technicalities | Minor differences in format and terminology that did not meaningfully change the interpretation of the definition |
| Modified subgroup category thresholds | Thresholds for subgroup categories that deviated from those provided by the WHO |
| Specification of additional criteria in conjunction with WHO definition | Use of the reference WHO definition with additional criteria such as time of measurement or use of specific methods, formula, or tools to measure the outcome |
| Alternative criteria without WHO definition | Use of alternative criteria such as the use of a scoring system, formula or other criteria or measures to define the outcome instead of using the WHO definition |
| Introduction of new terminology | Emergence of subgroup classifications that are not defined by the WHO or common in literature |
The frequency of deviation types was counted to assist with interpretation and description, and deviations were further analysed qualitatively. The deviation types, such as key words, phrases, or thresholds used, are described in additional detail. The modification types were classified according to how frequently they occurred as high-frequency or low-frequency modifications. The definition for a high- or low-frequency modification was determined at analysis on the basis of the overall number of modifications observed for each definition. Modifications occurring at least twice for LBW and at least five times for PTB, SGA and stillbirth were considered high-frequency modifications. Studies that evaluated LBW had few modifications. Therefore, modifications occurring at least five times were considered high frequency for all outcomes except LBW.
The content analysis was reported graphically and descriptively as logic models. Briefly, logic models are graphical illustrations of complex concepts translated into simplified formats that show relationships between various elements [16]. For the logic models, the WHO root and subgroup definitions were labelled reference definitions, deviations from the WHO definitions as types of deviations from the reference and the key words, phrases or thresholds that frequently appeared in a deviation type as high-frequency modifications arising from deviations. For the logic models, only high-frequency modifications are illustrated.
Additionally, reasons for modifying definitions from the reference WHO definitions were abstracted and reported verbatim where available.
Results
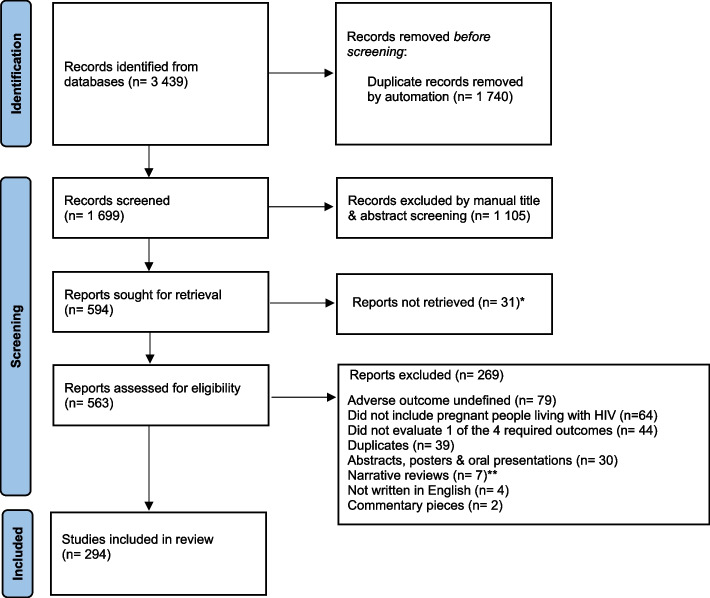
The search identified 3,439 citations, 294 of which were determined to be eligible and included in the review (Fig. 1).
Fig. 1.
PRISMA-ScR flow diagram of identification and inclusion of studies
Comparison of study characteristics by temporal period and country income group
Of the 294 studies that included a definition for at least one of the adverse birth outcomes, 214 (73%) were conducted and published prior to 2013, 56 (19%) during 2013–2015 and 24 (8%) in 2016 or later (Table 3).
Table 3.
Characteristics of studies including a definition for at least one of the four adverse birth outcomes, compared by temporal period of study (N = 294; data N (column %) unless otherwise specified)
| Total | 2011–2012 | 2013–2015 | 2016–2022 | |
|---|---|---|---|---|
| Total-N (row %) | 294 (100.0) | 214 (72.8) | 56 (19.0) | 24 (8.2) |
| Publication type – n (%) | ||||
| Primary original research | 268 (91.2) | 194 (90.7) | 52 (92.9) | 22 (91.7) |
| Secondary original research | 8 (2.7) | 8 (3.7) | 0 (0.0) | 0 (0.0) |
| Miscellaneous publications@ | 18 (6.1) | 12 (5.6) | 4 (7.1) | 2 (8.3) |
| Study design – n (%) | ||||
| Case control & case series | 13 (4.4) | 10 (4.7) | 2 (3.6) | 1 (4.2) |
| Cohort | 150 (51.0) | 112 (52.3) | 27 (48.2) | 11 (45.8) |
| Cross-sectional | 23 (7.8) | 13 (6.1) | 5 (8.9) | 5 (20.8) |
| Controlled trials* | 43 (14.6) | 31 (14.5) | 8 (14.3) | 4 (16.7) |
| Systematic reviews | 17 (5.8) | 17 (7.9) | 0 (0.0) | 0 (0.0) |
| Alternative designs$ | 28 (9.5) | 16 (7.5) | 11 (19.6) | 1 (4.2) |
| Unspecified | 20 (6.8) | 15 (7.0) | 3 (5.4) | 2 (8.3) |
| WHO region of study – n (%) | ||||
| Eastern and Southern Africa | 137 (46.6) | 74 (34.6) | 46 (82.1) | 17 (70.8) |
| Western and Central Africa | 22 (7.5) | 17 (8.0) | 2 (3.6) | 3 (12.5) |
| Americas | 46 (15.7) | 43 (20.1) | 3 (5.4) | 0 (0.0) |
| European | 35 (11.9) | 34 (15.9) | 1 (1.8) | 0 (0.0) |
| South‒east Asia & Western pacific | 15 (5.1) | 10 (4.7) | 3 (5.4) | 2 (8.3) |
| Multiregional/site | 31 (10.5) | 28 (13.1) | 1 (1.8) | 2 (8.3) |
| Unspecified | 4 (1.4) | 4 (1.9) | 0 (0.0) | 0 (0.0) |
| Not applicable | 4 (1.4) | 4 (1.9) | 0 (0.0) | 0 (0.0) |
| Study setting – n (%) | ||||
| Clinic | 76 (25.9) | 51 (23.8) | 18 (32.1) | 7 (29.2) |
| Hospital | 117 (39.8) | 74 (34.6) | 31 (55.4) | 12 (50.0) |
| Other% | 80 (27.2) | 70 (32.7) | 6 (10.7) | 4 (16.7) |
| Unspecified | 21 (7.1) | 19 (8.9) | 1 (1.8) | 1 (4.2) |
| Data collection source – n (%) | ||||
| Clinical records | 82 (27.9) | 55 (25.7) | 22 (39.3) | 5 (20.8) |
| Primary research data | 122 (41.5) | 75 (35.0) | 29 (51.8) | 18 (75.0) |
| Surveillance data | 29 (9.9) | 27 (12.6) | 2 (3.6) | 0 (0.0) |
| Other# | 55 (18.7) | 51 (23.8) | 3 (5.4) | 1 (4.2) |
| Unspecified | 6 (2.0) | 6 (2.8) | 0 (0.0) | 0 (0.0) |
| Unit of study: participants – n (%) | ||||
| Pregnancy (mother) | 245 (83.3) | 174 (81.3) | 49 (87.5) | 22 (91.7) |
| Newborn (child) | 15 (5.1) | 11 (5.1) | 3 (5.4) | 1 (4.2) |
| Pregnancy (mother) and newborn (child) | 25 (8.5) | 20 (9.4) | 4 (7.1) | 1 (4.2) |
| Other+ | 9 (3.1) | 9 (4.2) | 0 (0.0) | 0 (0.0) |
@Miscellaneous publications included published protocols with unpublished data; *Controlled trials included randomized and non-randomized trials; $Alternative designs included modelling studies; %Other study settings included departmental or organization centres; #Other data collection sources included region-specific patient registries; + Other unit of study included population data from households in a specific area. Most studies (n = 268, 91%) reported original research, with the highest proportion being cohort studies (n = 150, 51%), followed by controlled trials (n = 43, 15%), with minimal differences in study design across the temporal periods. Studies were pre-dominantly conducted in the Eastern and Southern Africa Region (n = 137, 47%), with an increase in studies in this region from 35% (n = 74) in < 2013 to 82% (n = 46) in 2013–2015, followed by a slight decrease in ≥ 2016 to 71% (n = 17). Hospital (n = 117, 40%) and clinic (n = 76, 26%) settings were used most often, and either prospective primary data collection (n = 122, 42%) or extraction of data from clinical records (n = 82, 28%) were the most frequent data sources, with pregnancy being the most common unit of study (n = 245, 83%)
Among the 294 studies that included a definition for at least one adverse birth outcome, 283 (95%) could be included in the CIG comparison [see Additional File 1]. Most studies occurred in low- and middle-income countries (n = 178, 63%). The predominant study types were cohort studies in the low- and middle-income groups (n = 84, 47%) and the high-income group (n = 61, 82%), whereas 19% (n = 33) of the studies in the low- and middle-income groups and 29% (n = 9) in the mixed income group were controlled trials. For setting, the data collection source and unit of study numeric comparison show no noteworthy differences across the CIG.
The comparisons by temporal period and CIG for individual adverse birth outcomes can be found in Additional File 2.
Comparison of adverse birth outcome definitions and their measurement by temporal period and country income group
Low birth weight
Among the 294 studies included, 169 (55%) evaluated LBW [see Additional file 2, Table 1]. The WHO reference definition was used without the subgroups of very LBW or extremely LBW by 68% (n = 115) of the studies overall, with decreasing use of the WHO definition with subgroups over time. The person who assessed the outcome and the outcome measurement methods for LBW were unspecified in 95% (n = 161) and 93% (n = 158) of the studies, respectively. Compared by CIG [see Additional file 2, Table 1], modified definitions occurred exclusively in the low- and middle-income group (2%, n = 4/162).
Preterm birth
Among the 294 included studies, 246 (84%) evaluated PTB [see Additional file 2, Table 1]. The use of the WHO definition without the subgroups increased over time. The use of the WHO definition with subgroups was infrequent (n = 3; 1%), whereas 30% (n = 73) utilized the WHO definition with modifications to the subgroup or used a completely modified definition (n = 20; 8%). The person who assessed the outcome of PTB was largely unspecified by studies (n = 218, 89%), whereas adverse birth outcome measurement methods were unspecified by 60% (n = 148) of studies, studies that did indicate measurement methods primarily used the last menstrual period (n = 41, 17%) to determine gestational age. There seems to be little difference in the use of the WHO definition for PTB without subgroups or with subgroup modifications between high and low- and middle-income group [see Additional file 2, Table 1].
Small for gestational age
SGA was evaluated by 112 (38%) of the 294 included studies [see Additional file 2, Table 1], and only 5 (5%) occurred in 2016-2022. The WHO definition was used by 60% (n = 67) of the studies, with modifications to the WHO definition occurring in 24% (n = 27) of the studies, and modified definitions emerged in 12% (n = 13) of the studies. The person who assessed the outcome as well as the method used to measure birth weight in SGA children remained largely unspecified in 98% (n = 110) of studies in both instances. The method of assessment for gestational age was unspecified in 65% (n = 73) of the studies, with 12% (n = 13) specifying the method used as the last menstrual period. The gender-specific reference standard used by studies was unspecified by 52% (n = 58) of the studies, with 15% (n = 17) indicating the use of a validated global standard. According to CIG comparisons [see Additional file 2, Table 1], 78% (n = 29/37) of high-income countries used the WHO SGA definition, whereas in low- and middle-income countries, 53% (n = 32/61) used the WHO SGA definition, and 36% (n = 22/61) used the WHO definition with modifications.
Stillbirth
Stillbirth was evaluated in 108 (37%) of the 294 included studies [see Additional file 2, Table 1]. Only 15% (n = 16) of the studies reporting stillbirth rates used the WHO definition for stillbirth, with 66% (n = 71) using a modified definition and 7% (n = 7) using the WHO definition with modifications. The person who assessed the adverse birth outcome and the adverse birth outcome measurement method were largely unspecified by 98% of the studies in both instances, with few differences noted across temporal periods. Low- and middle-income countries, primarily used the WHO definition with modification and the modified definition, accounted for 70% (n = 53/76) of the variation in stillbirth identification. [see Additional file 1, Table 2].
Content Analysis of Adverse Birth Outcome Definitions
Low birth weight
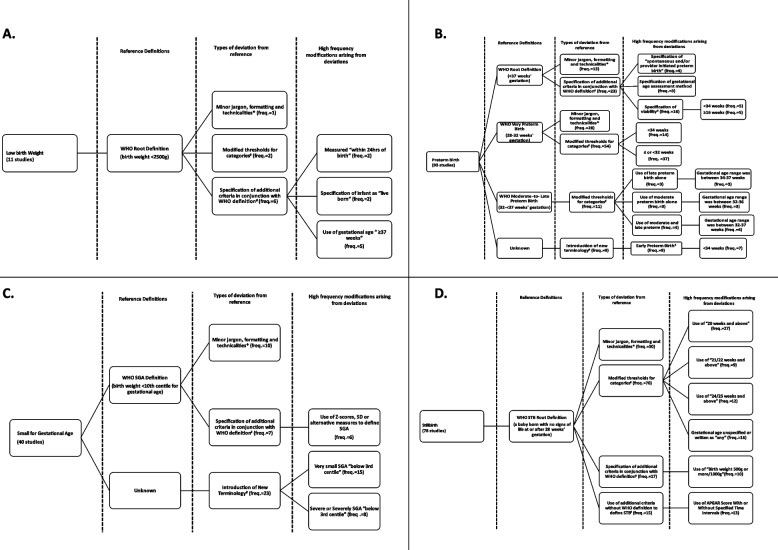
Among the 169 studies evaluating LBW, only 11 (7%) had modifications (Fig. 2, Panel A).
Fig. 2.
Logic Models of all adverse birth outcomes
Deviations from the reference definition were observed in the WHO LBW root definition. The highest deviation frequency (n = 6) was observed in the root definition with the specification of additional criteria in conjunction with the WHO definition. None of the authors provided justification for the modifications; thus, a thematic analysis of the reasons for additions was not performed.
Preterm birth
Among the 246 studies evaluating PTB, 93 (38%) deviated from the WHO definition (Fig. 2, Panel B). For the WHO root definition, the specification of additional criteria in conjunction with the WHO definition was the deviation of highest frequency (n = 23), with the specification of a viability threshold being observed 16 times. For viability, the lower limits of < 34 weeks and ≥ 16 weeks were determined five times. Additionally, the distinction between spontaneous and provider-initiated preterm birth was observed four times, and the specification of the gestational age assessment method (e.g., use of ultrasound) was observed three times.
For the subgroups, the WHO very PTB definition (28 to < 32 weeks) had the highest frequency of deviations. Modified thresholds were observed 54 times, 37 without the specification of the lower limit of 28 weeks as in the WHO very PTB definition and 14 instances of a threshold of < 34 weeks. The WHO moderate-to-late PTB subgroup had the second largest frequency of deviations observed, with modified threshold categories observed 11 times. The use of the terms moderate PTB alone and late PTB alone was observed three times while moderate and late PTB as per the WHO was observed four times. Various gestational age thresholds were used to define these subgroups; however, the thresholds remained within 32–37 weeks.
For PTB, the introduction of new terminology was observed nine times, with the term early PTB being observed nine times and the threshold of < 34 weeks being used seven times to define this subgroup.
The authors seldom provided reasons for deviation from the WHO definitions, but those that did cite the following considerations: i) clinical decision-making (related to using a 34-week threshold), ii) consistency with the Core Outcome Measures in Effectiveness Trials (COMET) initiative, and iii) limits of viability and the importance of distinguishing between spontaneous and provider-initiated PTB [17–20]. There were no themes that could be identified from the data; however, detailed reasons for modifications can be found in additional file 3.
Small for gestational age
Among the 112 studies that evaluated SGA, 40 (35%) deviated from the WHO definition (Fig. 2, Panel C). For this outcome, the specification of additional criteria was observed seven times. In six instances, the authors used the WHO definition with alternative measures such as the Z score or standard deviation to define SGA instead of centiles. New terminology was introduced 23 times, with authors using very SGA (n = 15) or severe/severely SGA (n = 8), defined by all 23 sources as having a weight at or below the 3rd centile for gestational age.
For the thematic analysis, no authors indicated why the specified additional criteria were used to define SGA. For very SGA and severe/severely SGA, two authors provided reasons related to identifying infants with the highest risk of adverse outcomes [21, 22]. No themes were identified from the justifications provided; however, detailed reasons for modifications can be found in additional file 3.
Stillbirth
Among the 108 studies that evaluated stillbirth, 78 (72%) deviated from the WHO definition (Fig. 2, Panel D). The modified threshold of viability categories as well as the minor jargon, formatting, and technicalities had the highest deviation type frequencies of 70 and 50, respectively. For the former, the use of 20 weeks or more as the threshold for stillbirth was observed 27 times. This was followed by unspecified gestational age or no consideration of gestation age (allowing for stillbirth at “any” gestational age) or written as “any”, which was observed 16 times, followed by 24/25 weeks and above (n = 12) or 21/22 weeks and above (n = 9). For minor jargon, formatting, and technicalities, the use of the phrases fetal/fetal death or fetal demise or intrauterine fetal demise in the definition was observed 39 times (not included in the logic model).
The use of additional criteria in conjunction with the WHO definition was observed 17 times, with the specification of birth weight at 500 g or more or 1000 g being used 10 times. The use of additional specifications alone to define the outcome was observed 15 times, with the use of the Apgar score with or without specified time intervals observed 13 times. A single author indicated the selection of a modified threshold of > 32 weeks for stillbirth with a rationale indicating “determines fetal viability in resource-limited settings” rather than the WHO definition for stillbirths needing to occur after 28 weeks gestation [23]. None of the other authors specified the reasons for the modifications.
Discussion
Systematic reviews and meta-analyses that have evaluated the association between maternal HIV and ART exposure with adverse birth outcomes have reported the exclusion of studies because i) adverse birth outcomes are defined differently than the protocol for the review, which is usually according to WHO definitions; ii) investigators do not differentiate between adverse birth outcome subgroups; and iii) methods used to assess adverse birth outcomes are omitted [24, 25]. This scoping review shows how these variations continue to persist over time and provides key insights into the type of variations that occur.
With respect to individual outcomes, LBW frequently uses the WHO root definition, with infrequent use of the WHO subgroups. While studies did not detail the reasons for the infrequent use of subgroups, not differentiating between subgroups means that a more nuanced understanding of severe outcomes is lost, which is a missed opportunity for evidence synthesis. When the LBW root definition was modified, studies incorporated additional criteria, such as the time at which weight was measured after birth or gestational age at birth, both of which contribute to improved specificity.
For PTB, the WHO definition without WHO subgroups was regularly used. Modifications to the WHO root definition predominantly include the addition of valuable criteria, including distinguishing between spontaneous and provider-initiated PTB, which could aid in understanding the potential mechanisms of preterm birth, detailing the gestational age assessment methods used, which is important for understanding the accuracy of preterm birth measurement, and specifying viability, all of which improve precision and specificity. There was irregular use of the WHO subgroup definitions, and when subgroups were used, there were substantial threshold modifications to very PTB and moderate-to-late PTB, as well as the introduction of a new subgroup, early PTB < 34 weeks. The overlap in the current WHO subgroups may have created the need for more clearly defined mutually exclusive subgroups that make classification precise and subsequent decision making for infant care more clinically relevant.
For SGA, there was frequent use of the WHO definition, with moderate use of the WHO definition with modifications and less use of modified definitions. When considering modifications, the WHO root definition was used in conjunction with additional criteria such as z scores and standard deviations. However, we speculate that the frequency at which these additions occur suggests study-specific modifications that seek to provide an additional measure for calculating SGA if the tools at hand in a low-resource setting, for example, could not allow for the calculation of centiles. While SGA does not have subgroups, we do see the frequent introduction and use of the terms very SGA, as well as severe or severely SGA, referencing infant birth weights at or below the third centile for gestational age. The addition of this subgroup may signal the need to look at the differences between infants below the 10th centile and those at or below the 3rd centile, as infants born at or below the 3rd centile are at increased risk for poorer outcomes, necessitating unique prevention or care approaches.
Compared with the other outcomes in this study, stillbirth had the highest frequency of modifications. The infrequent use of the WHO root definition suggests that applying the definition across various settings is challenging and that this has not improved over time. A closer look at the modified definitions reveals frequent use of the phrases fetal/fetal death, fetal demise or intrauterine fetal demise. The use of these phrases does not represent a clinically meaningful deviation from the WHO root definition but rather offers additional descriptive detail. Various modified thresholds of viability were observed, with 20 weeks and above being used frequently. The use of these lower limits of viability reflects progress in the survival of extremely preterm infants. Some definitions frequently include birth weight. This is because birth weight is a readily available measure for establishing limits of viability in resource-limited settings where clinical means of assessing gestational age are not always readily available. As with LBW, studies did not detail the reasons for modifications; however, both primary and secondary researchers should be encouraged to report the reasons for modifications to standardized definitions for transparency.
For all four outcomes, there was infrequent specification of both the adverse outcome assessor and the adverse outcome measurement methods, even in more recent studies. Investigators and authors should be encouraged to report these elements of outcome measurements, as this is consistent with the requirements of several reporting guidelines, such as STROBE, CONSORT and the Cochrane handbook for systematic reviews [26, 27]. This practice allows for improved study replication and comparability. Furthermore, modifications for all four outcomes frequently came from the low- and middle-income group. We suspect that modifications could have reflected the need to adapt to context-specific resource availability and efforts to improve precision.
While there are various existing and developing core outcome sets (COSs) related to pregnancy and childbirth, investigating adverse birth outcomes in relation to maternal HIV and antiretroviral exposure could be valuable for reaching a consensus on standardized definitions for use in this field[28]. It would also be valuable to consider participant involvement when developing or identifying the outcomes and associated definitions to ensure that studies evaluate adverse birth outcomes that are of interest to families affected by HIV [29]. The global alignment of immunization safety assessment in the pregnancy project of the Brighton collaboration has published several case definitions and guidelines for data collection, analysis, and presentation of immunization safety data, which could serve as a paradigm for birth outcome consensus definitions around pregnancy, HIV and the use of antiretroviral drugs [30–34]. The application of practices employed by the Brighton collaboration could decrease the amount of variation, enhance study comparability, and facilitate data pooling.
Our study has limitations as well as strengths. This study is the first comprehensive review of adverse birth outcome definitions involving persons with HIV. While a broad search strategy was implemented across five publication databases to identify all the relevant studies, the search resulted in a high yield and limited precision in the original search yield. Duplicate screening ensured that the inclusion criteria were applied consistently. However, being unable to conduct duplicate data extraction represents a potential limitation regarding the consistency of the data extraction. Additionally, the researcher (KRD) does not have experience in applying the definitions in clinical settings. While this may have reduced the degree of confirmation bias, observer bias may have been present. This observer bias, however, is likely to have been limited by the involvement of various stakeholders in the protocol development and conducting of this scoping review. The use of mixed methods assisted in developing a comprehensive understanding of the heterogeneity that is present in the application of adverse birth outcome definitions. Finally, this review did not evaluate the original validity or source material of definitions to determine if they were evidence informed.
Conclusion
This study quantifies and illustrates the heterogeneous use of the specified adverse birth outcome definitions in the published literature around pregnancies among persons with HIV. Of note is the persistent use of the WHO LBW and PTB definitions without accompanying subgroups, the modifications of thresholds to PTB subgroups, the introduction of new subgroups to both PTB and SGA, and the extensive use of modified stillbirth definitions. The evidence from future studies that evaluate adverse birth outcomes could be strengthened through i) a standardized taxonomy of outcomes and measurement, ii) the reporting of studies according to existing prescribed guidelines and iii) providing context for any modifications to standardized definitions or deviations from reporting guidelines. Considering that birth outcomes are critical in setting the life trajectory of infants, reducing adverse birth outcomes in children with HIV exposure and/or antiretroviral exposure is a critical step in the chain that will optimize their life course trajectory. Thus, research that focuses on the birth outcomes of children who are HIV- and/or antiretroviral-exposed needs to be scientifically rigorous and comparable. The WHO definitions for specified adverse birth outcomes are essential for population-level monitoring. However, a harmonized approach to defining these important outcomes for the purpose of research data collection, analysis, and presentation needs to be developed.
Supplementary Information
Acknowledgements
We would like to acknowledge Lynn Hendricks for providing specialist training and support in the content analysis and the DECIPHER team (members listed below in no order of significance) for their critical engagement. Marissa Vicari, Paige Williams, Andrew Edmonds, Ellen Chadwick, Tessa Goetghebuer, Claire Thorne, Elaine Abrams, Mary-Ann Davies, Lynne Mofenson, Mary Paul.
Abbreviations
- ART
Antiretroviral therapy
- CIG
Country income group
- CONSORT
Consolidated Standards of Reporting Trials
- COS
Core Outcome Sets
- LBW
Low birth weight
- PRISMA-ScR
Preferred Reporting Items for Systematic Reviews and Meta-analysis Extension for Scoping Reviews
- PTB
Preterm birth
- SGA
Small for Gestational Age
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
- WHO
World Health Organization
Authors’ contributions
ALS conceived the study. KRD designed and conducted the scoping review. The manuscript was written by KRD. ALS, MM, KMP and STdB extensively and critically reviewed the manuscript and provided scientific input. All the authors have read and approved the final manuscript.
Funding
This work was supported by the International AIDS Society (grant number 2020/400-DUB to KRD). The funders had no role in developing the study protocol or conducting the study. The authors were solely responsible for the preparation, review, and approval of the manuscript for submission.
Data availability
The data extracted and analysed from this study are available from the corresponding author upon request.
Declarations
Ethics approval and consent to participate
Ethics approval was obtained from the Human Research Ethics Committee of Stellenbosch University (S22/07/138). This study did not involve participants.
Consent for publication
Not applicable.
Competing interests
The authors have no competing interests to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection, recommendations for a public health approach second edition 2016. WHO Press. Published online 2016:1-432. 2023. https://www.who.int/publications/i/item/9789241549684. Accessed 15 Sept. [PubMed]
- 2.Evans C, Jones CE, Prendergast AJ. HIV-exposed, uninfected infants: new global challenges in the era of paediatric HIV elimination. Lancet Infect Dis. 2016;16(6):e92–107. 10.1016/S1473-3099(16)00055-4. [DOI] [PubMed] [Google Scholar]
- 3.UNAIDS. Miles to Go—Closing gaps, breaking barriers, righting injustices. 2018. https://www.unaids.org/sites/default/files/media_asset/miles-to-go_en.pdf. Accessed 11 May 2020.
- 4.WHO. Preventing HIV during pregnancy and breastfeeding. WHO Technical Brief. 2017. https://apps.who.int/iris/handle/10665/255866.
- 5.Slogrove AL, Powis KM, Johnson LF, Stover J, Mahy M. Estimates of the global population of children who are HIV-exposed and uninfected, 2000–18: a modelling study. Lancet Glob Health. 2020;8(1):e67–75. 10.1016/S2214-109X(19)30448-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eke AC, Mirochnick M, Lockman S. Antiretroviral therapy and adverse pregnancy outcomes in people living with HIV. N Engl J Med. 2023;388(4):344. 10.1056/NEJMRA2212877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slogrove AL, Bovu A, De Beer S, et al. Maternal and birth outcomes in pregnant people with and without HIV in the Western Cape. South Afr AIDS. 2024;38(1):59–67. 10.1097/QAD.0000000000003728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.United Nations Children’s Fund. Global and regional trends. UNICEF Data: Monitoring the situation of women and children. 2023. https://data.unicef.org/topic/hivaids/global-regional-trends/
- 9.Global U, Update A. The path that ends AIDS: UNAIDS Global AIDS Update 2023. Geneva: Joint United Nations Programme on HIV/AIDS. Published online; 2023. [Google Scholar]
- 10.Lawn JE, Ohuma EO, Ellen B, et al. Small babies, big risks: global estimates of prevalence and mortality for vulnerable newborns to accelerate change and improve counting. Infographic. 2023. https://www.thelancet.com/infographics-do/small-vulnerable-newborns-2023 [DOI] [PubMed]
- 11.Aromataris E, Munn Z, (Editors). JBI manual for evidence synthesis. 2020. 10.46658/jbimes-20-01
- 12.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med. 2018;169(7):467–73. 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 13.Dube KR, Powis KM, Mccaul M, Beer ST De, Slogrove AL. Adverse birth outcome case definitions associated with maternal HIV and antiretroviral drug use in pregnancy : a scoping review protocol. BMJ Open. Published online 2023:1–6. 10.1136/bmjopen-2023-072417. [DOI] [PMC free article] [PubMed]
- 14.The World Bank. World Bank Country and Lending Groups – World Bank Data Help Desk. 2023. 2023. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. Accessed 19 Oct 2023.
- 15.Erlingsson C, Brysiewicz P. A hands-on guide to doing content analysis. Afr J Emerg Med. 2017;7(3):93–9. 10.1016/j.afjem.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rohwer A, Pfadenhauer L, Burns J, et al. Logic models help make sense of complexity in systematic reviews and health technology assessments. J Clin Epidemiol. 2016;83:37–47. 10.1016/j.jclinepi.2016.06.012.This. [DOI] [PubMed] [Google Scholar]
- 17.Venkatesh KK, Edmonds A, Westreich D, et al. Associations between HIV, antiretroviral therapy and preterm birth in the US Women’s Interagency HIV Study, 1995–2018: a prospective cohort. HIV Med. 2022;23(4):406–16. 10.1111/hiv.13171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Venkatesh KK, Farhad M, Fenton T, et al. Association between HIV antiretroviral therapy and preterm birth based on antenatal ultrasound gestational age determination: A comparative analysis. AIDS. 2019;33(15):2403–13. 10.1097/QAD.0000000000002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smithmyer ME, Mabula-Bwalya CM, Mwape H, et al. Circulating angiogenic factors and HIV among pregnant women in Zambia: a nested case–control study. BMC Pregnancy Childbirth. 2021;21(1):1–9. 10.1186/s12884-021-03965-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madlala HP, Malaba TR, Newell ML, Myer L. Elevated body mass index during pregnancy and gestational weight gain in HIV-infected and HIV-uninfected women in Cape Town, South Africa: association with adverse birth outcomes. Tropical Med Int Health. 2020;25(6):702–13. 10.1111/tmi.13387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ndirangu J, Newell ML, Bland RM, Thorne C. Maternal HIV infection associated with small-for-gestational age infants but not preterm births: Evidence from rural South Africa. Hum Reprod. 2012;27(6):1846–56. 10.1093/humrep/des090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grignolo S, Agnello R, Gerbaldo D, et al. Pregnancy and neonatal outcomes among a cohort of HIV-infected women in a large Italian teaching hospital: A 30-year retrospective study. Epidemiol Infect. 2017;145(8):1658–69. 10.1017/S095026881700053X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moodley T, Moodley D, Sebitloane M, Maharaj N, Sartorius B. Improved pregnancy outcomes with increasing antiretroviral coverage in South Africa. BMC Pregnancy Childbirth. 2016;16(1):1–10. 10.1186/s12884-016-0821-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marazzi MC, Palombi L, Nielsen-Saines K, et al. Extended antenatal use of triple antiretroviral therapy for prevention of mother-to-child transmission of HIV-1 correlates with favorable pregnancy outcomes. AIDS. 2011;25(13):1611–8. 10.1097/QAD.0b013e3283493ed0. [DOI] [PubMed] [Google Scholar]
- 25.Wedi COO, Kirtley S, Hopewell S, Corrigan R, Kennedy SH, Hemelaar J. Perinatal outcomes associated with maternal HIV infection: A systematic review and meta-analysis. Lancet HIV. 2016;3(1):e33–48. 10.1016/S2352-3018(15)00207-6. [DOI] [PubMed] [Google Scholar]
- 26.EQUATOR Network. Reporting guidelines | EQUATOR Network. 2023. https://www.equator-network.org/reporting-guidelines/. Accessed 23 Oct 2023.
- 27.Peryer G, Golder S, Junqueira D, Vohra S, Loke Y. Adverse effects. In: Higgins J, Thomas J, Chandler J, et al., eds. Cochrane handbook for systematic reviews of interventions version 6.4 (Updated August 2023). 6th ed. Cochrane. 2023. www.training.cochrane.org/handbook. Accessed 17 May 2024.
- 28.Österberg M, Hellberg C, Jonsson AK, et al. Core Outcome Sets (COS) related to pregnancy and childbirth: a systematic review. BMC Pregnancy Childbirth. 2021;21(1): 691. 10.1186/s12884-021-04164-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bukasa LL, Namiba A, Brown M, et al. Setting the research agenda: involving parents in research on children who are HIV-free. J Int AIDS Soc. 2023;2023(S4):26150. 10.1002/jia2.26150/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonhoeffer J, Kochhar S, Hirschfeld S, et al. Global alignment of immunization safety assessment in pregnancy – The GAIA project. Vaccine. 2016;34(49):5993–7. 10.1016/j.vaccine.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Cutland CL, Lackritz EM, Mallett-Moore T, et al. Low birth weight: Case definition & guidelines for data collection, analysis, and presentation of maternal immunization safety data. Vaccine. 2017;35(48):6492–500. 10.1016/j.vaccine.2017.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Da Silva FT, Gonik B, McMillan M, et al. Stillbirth: Case definition and guidelines for data collection, analysis, and presentation of maternal immunization safety data. Vaccine. 2016;34(49):6057–68. 10.1016/j.vaccine.2016.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schlaudecker EP, Munoz FM, Bardají A, et al. Small for gestational age: Case definition & guidelines for data collection, analysis, and presentation of maternal immunisation safety data. Vaccine. 2017;35(48):6518–28. 10.1016/j.vaccine.2017.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quinn JA, Munoz FM, Gonik B, et al. Preterm birth: Case definition & guidelines for data collection, analysis, and presentation of immunisation safety data. Vaccine. 2016;34(49):6047–56. 10.1016/j.vaccine.2016.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data extracted and analysed from this study are available from the corresponding author upon request.