Abstract
Background
Upper limb impairment post-stroke often leads to a predominant use of the less affected arm and consequent learned disuse of the affected side, hindering upper limb outcome. Wearable sensors such as accelerometers, combined with smart reminders (i.e., based on the amount of arm activity), offer a potential approach to promote increased use of the affected arm to improve upper limb use during daily life. This study aimed to evaluate the efficacy of wrist vibratory reminders during a six-week home-based intervention in chronic stroke survivors.
Methods
We evaluated the impact of the home-based intervention on the primary outcome, the Motor Activity Log-14 Item Version scores Amount of Use (MAL-14 AOU), and the secondary outcomes MAL-14 Quality of Movement (QOM) and sensor-derived activity metrics from the affected arm. A randomized controlled trial design was used for the study: the intervention group received personalized reminders based on individualized arm activity goals, while the control group did not receive any feedback. Mixed linear models assessed the influence of the group, week of the intervention period, and initial impairment level on MAL-14 and arm activity metrics.
Results
Forty-two participants were enrolled in the study. Overall, participants exhibited modest but not clinically relevant increases in MAL-14 AOU (+ 0.2 points) and QOM (+ 0.2 points) after the intervention period, with no statistically significant differences between the intervention and control group. Feasibility challenges were noted, such as adherence to wearing the trackers and sensor data quality. However, in participants with sufficiently available sensor data (n = 23), the affected arm use extracted from the sensor data was significantly higher in the intervention group (p < 0.05). The initial impairment level strongly influenced affected arm use and both MAL-14 AOU and QOM (p < 0.01).
Conclusions
The study investigated the effectiveness of incorporating activity trackers with smart reminders to increase affected arm activity among stroke survivors during daily life. While the results regarding the increased arm use at home are promising, patient-reported outcomes remained below clinically meaningful thresholds and showed no group differences. Further, it is essential to acknowledge feasibility issues such as adherence to wearing the trackers during the intervention and missing sensor data.
Trial registration
Supplementary Information
The online version contains supplementary material available at 10.1186/s12984-024-01527-2.
Keywords: Stroke, Neurorehabilitation, Upper limb, Feedback, Affected arm use, Daily life
Introduction
Stroke affects approximately 13 million people annually and is a leading cause of upper limb impairment, which has a strong negative impact on quality of life [1, 2]. To overcome the limitations of a loss of upper limb sensorimotor function, stroke survivors may develop the tendency to use their less affected side predominately [3, 4]. This shift typically becomes more prominent following discharge from the rehabilitation clinic, where the need to perform any given activity more effectively intensifies, and available extrinsic support from the therapist diminishes. While using the less affected side helps to regain independence in performing activities of daily living, it often results in learned disuse of the affected side that can slow down and even reverse the recovery process [5–7]. Especially for individuals with severe impairments, keeping the motivation to perform everyday activities with their affected side is challenging due to limited functional capability [8]. To counteract the disuse, therapists often prescribe self-directed exercise programs [9]. While these programs are informative and initially beneficial, motivation and adherence decline over time due to insufficient supervision and a lack of engaging feedback during daily life [10]. The benefits of active and intensive arm movement in improving affected arm function are sustained even in the chronic phase of stroke survivors. Hence, the need for practical solutions that promote self-rehabilitation and re-integration of the affected arm during daily life, particularly after discharge, becomes essential [11–13].
Wearable sensors, such as inertial measurement units or accelerometers, are commonly used to measure physical activity in the healthy population by, for example, tracking steps throughout the day [14]. A positive effect on promoting physical activity has been observed when additional feedback is provided, such as movement reminders and a daily goal that should be reached [15–17]. A possible translation of this approach to stroke rehabilitation offers a promising tool to increase the dosage of affected arm use [18–20]. When deployed in the home environment, these sensors can monitor arm activity levels for several days [3, 21, 22]. Various metrics can be derived from the collected sensor data to quantify arm activity levels in stroke survivors. Established and well-understood metrics include activity counts [3, 21, 23–26], the activity ratio of the affected and less affected side [3, 24–27], as well as the duration of use during the day [23, 25–27]. Measuring continuous arm activity data allows for triggering and delivering personalized feedback even outside of a clinical setting. An example would be a combination of visual summary feedback on a mobile application and real-time vibratory feedback on current inactivity levels [20, 28, 29]. Smart reminders carry the potential to counteract the often observed decline in engagement in home-based interventions [30, 31].
Previous research has highlighted the positive effect of real-time feedback and daily summaries on the amount of arm use in stroke survivors to change their arm use behavior [28, 32–36]. However, the majority of research evaluating vibratory movement reminders in stroke survivors focused on short periods of only a few hours or days [32, 37], with a small number of participants [33], in a controlled clinical setting [37, 38], or with reminders triggered in a randomized manner [37, 39]. Therefore, it remains to be seen whether activity tracking in combination with smart reminders based on individual movement goals can influence the affected arm use behavior of stroke survivors over several weeks. Furthermore, there is an open question as to the extent to which any induced behavioral changes translate into improved upper limb function [40]. Finally, the feasibility of interacting with such a feedback device during a home-based intervention over several weeks remains to be investigated, particularly how different impairment levels may influence such an intervention.
In the present study, a randomized controlled trial was conducted during which chronic-stage stroke survivors were equipped with arm activity monitors on both wrists for six weeks at their homes [20]. In the intervention group, participants received smart reminders on their affected side, triggered based on the current daily arm activity level and an individually calculated, personalized arm activity goal. We first report on the effect on clinical outcome measures of such an intervention aiming at increasing arm use, primarily focusing on patient-reported affected arm movement quantity and quality. While a previous analysis of the collected data has already provided insights into the positive short-term effect of reminders on arm use [28], we here further aim to evaluate (1) the long-term effect of the reminders on affected arm use behavior and its ability to promote prolonged engagement over the six-week home-based intervention, and (2) how different upper limb impairment levels relate to arm activity behavior during daily life. We hypothesized that triggering smart reminders in the intervention group would increase perceived and objective (measured by activity counts) affected arm use, improve arm function, decrease the less affected-to-affected arm use ratio, and lead to a longer cumulative duration of active arm movement throughout the day. Moreover, we expected an influence of upper limb impairment level on the observed amount of arm activity, with higher levels of arm activity for less impaired participants and vice versa.
Methods
Participants
This study was conducted as a randomized controlled trial to determine the effect of wearing an activity tracking and feedback device on affected arm use during a 6-week intervention at home [20]. Forty-two participants were recruited, all of whom had a unilateral ischemic or hemorrhagic stroke with residual hemiparesis in the upper limb. Participants were included if they were older than 18 years, had no known intolerance to the material of the activity tracker, had no severely impaired sensation of the wrist (unable to feel a soft touch on the dorsal side of their paretic wrist with closed eyes), depression, major cognitive impairment, major comorbidities, or comprehensive aphasia. The study was approved by the Cantonal Ethics Committee Zurich and Cantonal Ethics Committee Northwest and Central Switzerland (BASEC-number 2017 − 00948) and was registered at https://clinicaltrials.gov, unique identifier NCT03294187, before recruitment. For more information on the study protocol, please refer to [20].
Experimental procedure
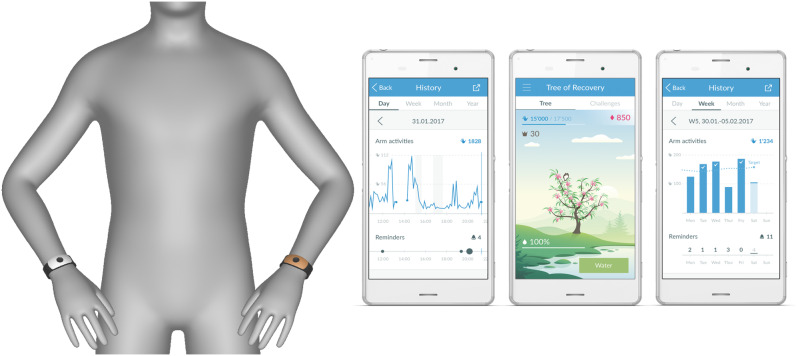
After obtaining a baseline assessment with a battery of clinical tests to assess the initial upper limb impairment, participants were randomly allocated to the intervention (n = 19) or control group (n = 23) [20]. Randomization was stratified based on the impairment level of the upper limb (< 32 and ≥ 32 of Fugl-Meyer Assessment – Upper Extremity Subscale (FMA-UE) score). To continuously monitor arm activity, participants in both groups were equipped with an “ARYS™ pro|tracker” (Tyromotion GmbH, Austria, formerly yband therapy AG, Switzerland) for their less affected wrist and the “ARYS™ me|tracker” on the affected wrist, which could trigger vibrotactile reminders [20] (Fig. 1). Participants were asked to wear both devices for as long as possible (i.e., ideally from waking up in the morning to bedtime in the evening) every day of the 6-week home-based intervention period. Additionally, the intervention group received two forms of feedback: participants received a phone application with visual feedback about arm use, allowing them to track their arm activity levels and whether arm activity deviated from the trajectory needed to achieve their daily goal by the end of the day (Fig. 1). Whenever participants were inactive and not on track to reach their arm activity goal, smart reminders such as vibrotactile (vibrating pulses) and visual cues (LED lights on the tracker) were triggered on the affected side. The tracker on the less affected side served solely as a reference and did not provide any feedback. The control group did not receive any feedback or reminders [20, 28].
Fig. 1.
The motion-tracking devices and phone application used for the measurements. The application was designed to allow the tracking of arm activity counts over the day, week, and month. Silver tracker: “ARYS™ me|tracker” for the paretic wrist; Golden tracker: “ARYS™ pro|tracker” for the non-paretic wrist
Outcome measures
The primary outcome to evaluate the effectiveness of the intervention was the patient-reported amount of paretic upper limb use in daily life, measured with the Motor Activity Log-14 Item Version, Amount of Use subscale (MAL-14 AOU) [41, 42]. Secondary outcomes were arm-use metrics derived from the sensor data collected during the 6-week intervention and the following clinical assessments: MAL-14 Quality of Movement subscale, evaluating the perceived quality of arm movement (MAL-14 QOM) [41, 42], FMA-UE [43], Action Research Arm Test (ARAT) [44], EuroQol Five Dimensions Five Levels questionnaire (EQ-5D-5 L) [45], modified Rankin Scale (mRS) [46]. Clinical assessments were recorded at baseline, after the 6-week post-intervention, and after the 6-week follow-up. The Global Rating of Perceived Changes (GRPC) assesses how the affected arm use and physical activity changes are perceived in everyday life at post-intervention and follow-up. For the EQ-5D-5 L, the index value and visual analog scale (VAS) results were extracted [47]. A more detailed description of the clinical outcome parameters and their properties can be found in the published clinical study protocol [20].
Raw sensor data were processed into arm activity counts from the affected and less affected sides using a threshold-based approach. Arm activity was considered true for each minute where the aggregated raw three-axis accelerometer data exceeded a threshold of 0.1 g acceleration after gravity subtraction [20, 28]. Three arm-use metrics were derived from the arm activity counts to characterize different aspects of affected arm activity. Affected Arm Use (AAU) represents the magnitude of total daily activity counts. It was calculated as the cumulative activity counts of the affected arm. Arm Use Ratio (AUR) represents the utilization ratio of the affected in relation to the less affected side. It was calculated as the ratio of cumulants between the affected and the less affected sides. Percentage of Time Active (PTA) represents the percentage of active time throughout the day. It was calculated as the cumulative duration that the affected arm activity was considered true within one day. These three metrics were computed for each day between 6:00 a.m. and 11:59 p.m., as this interval aligns with the assumed waking hours of participants, during which they were expected to be actively engaged in daily activities. An average of AAU, AUR, and PTA was taken each week to evaluate the change over the six-week study duration. A linear regression model was fitted on the available data to account for missing data and applied to intra- and extrapolate arm activity counts. Participants were excluded from the sensor data analysis if no data from the first week of data collection were available or if activity data on each side were collected of either the affected or less affected side on less than 70% of the study duration days, reducing the accuracy of intra- and extrapolation of activity counts.
Statistical analysis
All available clinical data from enrolled participants was analyzed using an intention-to-treat approach. Data was tested for normality using the Shapiro-Wilk test. Mixed linear models (MLM), with a random intercept, were used to identify differences between the intervention and control group and evaluate changes in clinical scores from baseline to post-intervention to follow-up. The scores of the clinical scales (MAL-14 AOM, MAL-14 QOM, FMA-UE, ARAT, EQ-5D-5 L, mRS) were used as input for the dependent variable. Timepoints (baseline, post-intervention, and follow-up) and group allocations (intervention and control) were set as covariates.
Subgroup analysis (per protocol analysis) was performed for the sensor data to account for incomplete datasets. The impact of the intervention on the amount of arm activity behavior over the six-week study duration was investigated with MLM. The MLM included (1) AAU, (2) AUR, (3) PTA, (4) MAL-14 AOM, and (5) MAL-14 QOM as the dependent variable. Group allocation (intervention and control), week of the study duration, and impairment (mild, moderate, and severe) were set as covariates for each model. Based on the baseline FMA-UE assessment, three impairment levels were defined: mild impairment with a score ranging from 43 to 66, moderate impairment ranging from 29 to 42, and severe impairment ranging from 0 to 28 [48]. A logarithmic transformation was applied in the case of inadequate model fits. The variance inflation factors were calculated to test for the multicollinearity of the predictor variables. Model performance was evaluated by calculating the R2. The Tukey’s honest significant difference (HSD) posthoc test was used to explore if there were differences concerning the metrics and the covariates. The distribution of the residuals was compared to the normal distribution using a kernel density estimate (KDE) and a Q-Q plot. Additionally, homoskedasticity was checked with a residual versus fitted values (RVF) plot.
Finally, the Pearson correlation coefficient was applied to examine the association between upper limb impairment level and daily AAU, AUR, and PTA averaged over the first week for the intervention and control group separately. A weak correlation was defined with a rho between 0.2 and 0.39, a moderate correlation between 0.40-0-59, a strong correlation between 0.6 and 0.79, and a very strong correlation between 0.80 and 1 [49]. Fisher’s z-transformation was applied to investigate differences in the correlation coefficients of the two groups. All analyses (post-processing and statistical) were performed using Python (Version 3.8, Python Software Foundation, packages: scipy.stats, sklearn, statsmodels). A two-sided significance level of alpha = 0.05 was used.
Results
The target goal of 62 participants, as outlined in the protocol paper [20], was not attained. Subject recruitment was prematurely terminated due to COVID-19 pandemic-related recruitment restrictions and a blinded interim analysis failing to detect changes greater than the minimal detectable change (MDC) of the primary outcome MAL-AOM. Of the 42 participants enrolled in the study between September 2017 and April 2021, 23 were allocated to the control and 19 to the intervention group.
Datasets had to be excluded from the sensor analysis due to feasibility issues, such as missing data on the affected side (n = 3), on the less affected side (n = 5), sparse data availability on either side (n = 4), early dropout (n = 2), or non-compliance with wearing the activity trackers (n = 5). Consequently, the subset for the longitudinal sensor data analysis over the six-week intervention consisted of 10 participants from the intervention group and 13 from the control group (Supplementary Material 1). Demographic details are presented in Table 1 for the recruited participants (n = 42) and the subset included in the sensor data analysis (n = 23).
Table 1.
Demographic data (mean ± standard deviation) of the recruited participants (n = 42) and the subset of participants included in the sensor data analysis (n = 23). Impairment level was defined based on the Fugl-Meyer Assessment- Upper Extremity subscale (FMA-UE) score
| Intervention Group | Control Group | |
|---|---|---|
| Recruited participants (n) | 19 | 23 |
| Age (years) | 62.1 (± 10.7) | 66.0 (± 11.6) |
| Weight (kilograms) | 75.7 (± 13.3) | 82.0 (± 20.0) |
| Gender (female/male) | 6/13 | 10/13 |
| Dominant side affected | 11 | 11 |
| Impairment level (mild/moderate/severe) | 8/6/5 | 8/6/9 |
| Subset included in sensor data analysis (n) | 10 | 13 |
| Age (years) | 59.9 (± 11.7) | 68.5 (± 10.4) |
| Weight (kilograms) | 74.7 (± 16.1) | 81.9 (± 21.9) |
| Gender (female/male) | 5/5 | 5/8 |
| Dominant side affected | 6 | 4 |
| Impairment level (mild/moderate/severe) | 4/4/2 | 4/4/5 |
According to intention-to-treat, all available clinical scores of the participants were included in the MLM to evaluate differences between the groups (intervention and control) and measurement timepoints (baseline, post-intervention, follow-up) (Table 2; Fig. 2). The MLM showed a significant influence of the measurement timepoints on the primary outcome MAL-14 AOU and secondary outcomes MAL-14 QOM, FMA-UE, and ARAT (p < 0.05, Table 2).
Table 2.
Primary (Motor Activity Log-14 Item Version amount of Use Subscale (MAL-14 AOU)) and secondary outcomes (MAL-14 Quality of Movement subscale (MAL-14 QOM)), Fugl-Meyer Assessment- Upper Extremity subscale (FMA-UE), Action Research Arm Test (ARAT), modified Rankin Scale (mRS) and EuroQol five dimensions five levels questionnaire (EQ-5D-5 L) index value and visual analogue scale (VAS) score and global rating of Perceived Changes (GRPC)) at baseline, after the 6-week intervention and after the 6-week follow-up for the total enrolled study population (n = 42) (mean ± standard deviation). * significant difference between timepoints (baseline, post-intervention, follow-up) (p < 0.05)
| Baseline | Post-Intervention | Follow-up | ||||
|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | |
| MAL-14 | ||||||
| AOU * | 2.0 (± 1.1) | 1.9 (± 1.2) | 2.1 (± 1.2) | 2.0 (± 1.2) | 2.3 (± 1.1) | 2.0 (± 1.2) |
| QOM * | 1.7 (± 0.9) | 1.6 (± 1.2) | 1.9 (± 1.0) | 1.8 (± 1.2) | 2.0 (± 1.0) | 1.8 (± 1.2) |
| FMA-UE * |
35.6 (± 14.8) |
34.7 (± 14.9) |
37.2 (± 16.2) |
35.8 (± 15.4) |
37.3 (± 15.4) |
35.6 (± 14.9) |
| ARAT * |
31.0 (± 18.1) |
29.5 (± 18.8) |
32.5 (± 19.3) |
31.6 (± 18.8) |
33.7 (± 19.4) |
32.0 (± 18.6) |
| mRS | 2.5 (± 0.5) | 2.7 (± 0.8) | 2.5 (± 0.5) | 2.7 (± 0.8) | 2.5 (± 0.5) | 2.7 (± 0.8) |
| EQ-5D-5 L | ||||||
| Index value | 0.8 (± 0.1) | 0.8 (± 0.2) | 0.8 (± 0.1) | 0.8 (± 0.2) | 0.8 (± 0.1) | 0.8 (± 0.2) |
| VAS |
66.5 (± 15.0) |
65.0 (± 7.0) |
65.6 (± 16.3) |
69.4 (± 16.7) |
67.8 (± 16.2) |
64.7 (± 20.5) |
| GRPC | ||||||
| Affected arm use | 4.7 (± 1.1) | 5.1 (± 1.2) | 4.7 (± 1.0) | 4.7 (± 1.3) | ||
| Physical activity | 4.9 (± 0.8) | 5.1 (± 0.9) | 4.9 (± 1.0) | 4.8 (± 1.2) | ||
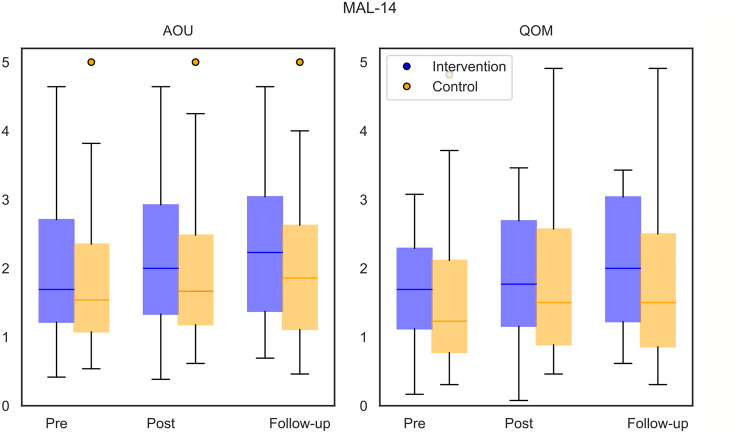
Fig. 2.
Primary outcome Motor Activity Log-14 Item Version (MAL-14) Amount of Use subscale (AOU) and secondary outcome Quality of Movement subscale (QOM) at baseline (pre), after the 6-week intervention (post) and after the 6-week follow-up of the recruited study population (n = 42) (intervention group in blue, n = 19) (control group in orange, n = 23)
Participants had a mean increase from baseline to post-intervention of 0.2 points for the MAL-14 AOU and MAL-14 QOM, 1.3 points for the FMA-UE, and 1.8 points for the ARAT. From post-intervention to follow-up, participants improved by 0.1 points for the MAL-14 AOU and 0.8 points for the ARAT, while the MAL-14 QOM and FMA-UE changes were below 0.1 points. No significant influence of the group allocation was identified. The results of the secondary outcome measures are reported in Table 2.
The data from the subset of 23 participants with sufficient available sensor data was analyzed with MLM to investigate the relationship between the average AAU/ AUR/ PTA of the affected side, MAL-14 AOM/ MAL-14 QOM, and the predictor variables group, week, and impairment (Table 3). Results of all collected secondary outcomes for the subset are reported in Supplementary Material 2. Model predictor variables demonstrated low multicollinearity as each variance inflation factor value was below 1.1. The group variable, i.e., whether a participant was in the intervention or control group, significantly influenced the AAU (p < 0.05, Table 3; Fig. 3). A more balanced AUR and a higher PTA were identified in the intervention group, but there was no significant difference between the two groups.
Table 3.
Mixed Linear Model Regression results for the primary (Motor Activity Log-14 Item Version Amount of Use Subscale (MAL-14 AOU)) and secondary outcomes (MAL-14 Quality of Movement subscale (MAL-14 QOM), sensor data) for the subset of the study population (n = 23). Logarithmic transformation was applied to the predictor variables (AAU, AUR, PTA). Intervention group = 1, control group = 0, mild impairment = 1, moderate impairment = 2, severe impairment = 3. Bold numbers indicate a significant predictor
| Mixed Linear Model Regression | |||||||
|---|---|---|---|---|---|---|---|
| Coefficient | Standard Error | P-Value | 95% Confidence interval | Model performance (R2) | |||
| Sensor Data | |||||||
| Affected Arm Use | 0.98 | ||||||
| Intercept | 8.12 | 0.37 | < 0.01 | 7.44 | 8.90 | ||
| Week | -0.04 | 0.01 | < 0.01 | -0.05 | -0.02 | ||
| Group | 0.55 | 0.26 | < 0.05 | 0.04 | 1.06 | ||
| Initial Impairment Level | 0.71 | 0.16 | < 0.01 | -1.04 | -0.40 | ||
| Arm Use Ratio | 0.92 | ||||||
| Intercept | -0.41 | 0.33 | 0.30 | -1.05 | 0.24 | ||
| Week | 0.001 | 0.01 | 0.99 | -0.02 | 0.02 | ||
| Group | -0.37 | 0.23 | 0.19 | -0.82 | 0.08 | ||
| Initial Impairment Level | 0.90 | 0.15 | < 0.01 | 0.62 | 1.18 | ||
| Percentage of Time Active | 0.90 | ||||||
| Intercept | 3.68 | 0.21 | < 0.01 | 3.27 | 4.08 | ||
| Week | 0.02 | 3.55 | < 0.01 | 0.01 | 0.03 | ||
| Group | -0.03 | -1.55 | 0.20 | -0.51 | 0.06 | ||
| Initial Impairment Level | 0.11 | 2.44 | < 0.05 | 0.04 | 0.40 | ||
| Motor Activity Log − 14 | |||||||
| Amount of Use | 0.97 | ||||||
| Intercept | 3.01 | 0.57 | < 0.01 | 1.95 | 4.20 | ||
| Timepoint | 0.09 | 0.04 | < 0.05 | 0.03 | 0.16 | ||
| Group | 0.35 | 0.40 | 0.38 | -0.43 | 1.13 | ||
| Initial Impairment Level | -0.71 | 0.24 | < 0.01 | -1.18 | -0.23 | ||
| Quality of Movement | 0.95 | ||||||
| Intercept | 3.02 | 0.42 | < 0.01 | 2.20 | 3.85 | ||
| Timepoint | 0.10 | 0.04 | < 0.05 | 0.03 | 0.17 | ||
| Group | 0.15 | 0.29 | 0.65 | -0.42 | 0.72 | ||
| Initial Impairment Level | -0.79 | 0.18 | < 0.01 | -1.14 | -0.44 | ||
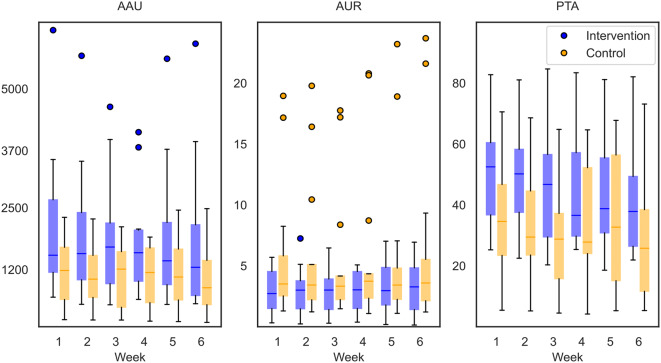
Fig. 3.
Data from the subset for the longitudinal sensor data analysis (n = 23). The boxplots illustrate the three metrics affected arm use (AAU, in arm activity counts), arm use ratio (AUR) and percentage of time active (PTA, in percentage), for the intervention (n = 10, blue) and control (n = 13, orange) groups averaged per day for each week over the six weeks
The week of the intervention was a significant predictor for AAU and PTA (p < 0.05), but the Tukey’s HSD posthoc results did not show significant differences between the single weeks. No significant change over the six weeks could be observed for AUR. The level of impairment (mild, moderate, severe) had a significant impact on AAU, AUR (p < 0.01), and PTA (p < 0.05, Table 3). Participants with a more severe impairment had overall less AAU and a smaller ratio – hence, more activity of the less affected compared to the affected arm – and were less time active than participants with a mild impairment. The Tukey’s HSD posthoc results highlighted a significant difference between all impairment levels for AAU and AUR and a significant difference between mild and severe as well as moderate and severe impairment for PTA (p < 0.01). The group variable did not significantly influence the MAL-14 AOU and QOM. The measurement timepoint (baseline, post-intervention, and follow-up) was a significant predictor for both MAL-14 AOU (p < 0.05, mean increase of 0.1 points) and QOM (p < 0.05, mean increase of 0.1 points), with a slight score increase over time. The impairment level assessed at baseline was a strong predictor for both MAL-14 AOU (p < 0.01) and QOM (p < 0.01). All MLM had a high model performance with R2 values ranging from 0.90 to 0.96 (Table 3).
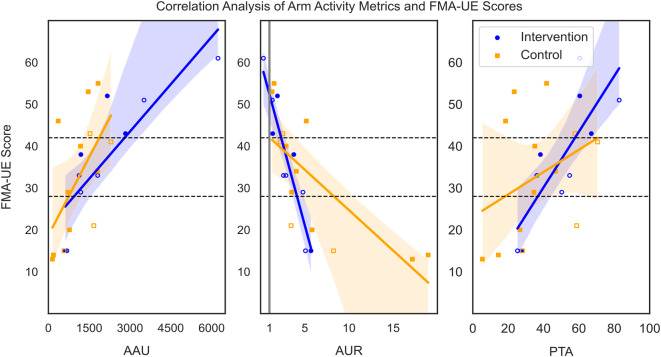
The baseline FMA-UE score was used as a proxy for the initial impairment level, and AAU showed a very strong correlation for the intervention group (r = 0.85, p < 0.01) and a strong correlation in the control group (r = 0.68, p = 0.02, Fig. 4). No significant difference in correlation coefficients between the intervention and control group was observed (z-score = 0.84, p = 0.41). A very strong negative correlation for both the intervention (r=-0.93, p < 0.01) and the control group (r=-0.80, p < 0.01) between the FMA-UE scores and the AUR was observed: the higher the FMA-UE score, the more balanced the AUR (Fig. 4). The two groups did not significantly differ in their correlation coefficients (z-score=-1.08, p = 0.28). A very strong correlation was observed between the PTA and FMA-UE scores for the intervention group (r = 0.80, p = 0.01). A moderate but not significant correlation (r = 0.47, p = 0.12) was identified for the control group. Again, there was no significant difference in the correlation between the intervention and control group (z-score = 1.18, p = 0.24).
Fig. 4.
Datapoints of the subset (n = 23) and linear regression models with 95% confidence intervals (shaded area) from the intervention (n = 10, blue, circle) and control (n = 13, orange, square) groups to visualize the relationship between the FMA-UE scores and affected arm use (AAU), arm use ratio (AUR) and percentage of time active (PTA) during the first week of the study, respectively. Full squares/ circles represent participants that have their non-dominant hand affected, empty squares/circles participants that have their dominant hand affected. The black dashed horizontal lines indicate the different impairment levels. The grey vertical line in the middle plot indicates an equal ratio of affected and less affected arm use (1:1). FMA-UE = Fugl-Meyer Assessment – Upper Extremity Subscale
Discussion
The present study explored how smart reminders triggered by monitoring arm use impact the affected arm use behavior in chronic stroke survivors over several weeks and whether behavioral changes translate into improved clinical outcome parameters. Specifically, we evaluated the long-term influence of reminders on arm use as a potential tool to counteract the non-use of the affected upper limb during a six-week intervention at home. We further investigated how initial arm impairment influenced the different aspects of affected arm activity.
According to intention-to-treat, the analysis of the clinical scales included all enrolled participants. Our results show a modest yet significant increase in the primary outcome MAL-AOU as well as for the secondary outcomes MAL-QOM and FMA-UE over the three measured timepoints, but they were below clinical effectiveness. Additionally, no significant differences between the intervention and control groups were detected. For both the perceived amount and quality of the affected arm use, a mean improvement of 0.2 was observed over the intervention period, which is far below the minimal clinically important difference (MCID) of 1.0 for QOM and below the MDC of 0.84 for AOM [50, 51]. In accordance with the small differences in MAL-14 scores, participants reported only a “slightly better, not relevant” change in their perceived change of using their affected arm in everyday life (GRPC). Further, although both groups demonstrated reduced impairment and improved FMA-UE scores by 1.3 points on average, this is far below the MCID for stroke survivors of 4.3–7.3 points (mild-moderate) and 13 points (severe) [52, 53]. These results align with previous research, where a modest but not clinically relevant improvement in clinical scores was observed, and no group difference was detected between stroke survivors receiving feedback on their performance and a control group [34].
As for the longitudinal sensor data analysis, almost half of the initially included participants were ineligible due to dropout, missing or improper data linked to technical issues, and/or non-compliance with wearing the activity trackers. Factors such as donning/ doffing, the robustness of the technology, and user-friendliness strongly influence whether stroke survivors will adhere to wearing such devices during daily life [54–56]. These feasibility issues resulted in a relatively small sample size, which weakens the statistical value of evaluating the intervention effectiveness and limits the number of covariates that could be included in the MLM. These observations are comparable with the work of Langerak et al. [57], in which sub-acute stroke survivors received activity trackers providing feedback, and more than 40% of the collected datasets had to be excluded for similar reasons. However, with a 29% dropout rate, they had a considerably higher number of participants who discontinued the study than in the results of our research and those of Da-Silva et al. [29]. This similarity certainly underlines the challenges linked to performing unsupervised rehabilitation interventions in the homes of stroke survivors and collecting quality data during daily life activities without the presence of a study investigator.
Interestingly, the subset of participants who adhered to wearing the trackers and received feedback on the affected side through smart vibrotactile movement reminders demonstrated significantly higher mean daily AAU compared to the control group. Further, they had a more balanced ratio of affected and less affected arm use and were more active with the affected arm throughout the day, although not statistically significant. This positive outcome aligns with a previous analysis of this dataset, where an immediate increase in affected arm activity (i.e., short-term effect within 5 and 15 min of a reminder) was visible right after a smart reminder was triggered [28]. Although no long-term increase in arm activity metrics was achieved, it should be taken into consideration that arm activity levels remained relatively steady throughout the six-week study period (Fig. 3). Further, the short-term effect of the reminders in immediately increasing activity was maintained throughout the six-week intervention (Mayrhuber et al., 2023). This contrasts with typical patterns often observed in other home-based interventions, where a drop in adherence commonly occurs over time [30, 31].
Although greater arm use could be observed in the intervention group over the study duration, the differences did not translate to the scores of the patient-reported AOU of the MAL-14. The mean increase of the AOU and QOM MAL-14 scores by 0.1 points for the subset included in the sensor analysis over the three timepoints was below the MCID and MDC, aligning with the results for the overall study population. Potential barriers to achieving a therapeutic benefit are whether it adequately addresses the clinical needs and targets the desired rehabilitation goals [56]. Given the absence of clinically relevant results, the benefit observed in affected arm use behavior may not have been dose-sufficient or lacked goal-directed exercises [13, 34]. Incorporating task-specific rehabilitation exercises whenever the reminder is triggered and adding information on the quality of movement may lead to more meaningful functional gains [34, 37, 58]. One could also question whether conventional clinical scores like the MAL-14 are sensitive enough to capture the potentially small changes resulting from the increase in arm use observed in this study. Further, the MAL-14 only assesses the amount and quality of movement for a specific set of ADLs, whereas the motion tracking devices recorded any movement based on the acceleration signal.
The distinct influence of the impairment level on affected arm use behavior is highlighted by the strong predictive power in the MLM for objective and subjective measures of affected arm activity. Further, a relationship between impairment level and affected arm activity levels for both groups is apparent. These results align with previous literature, where a strong correlation between the FMA-UE and affected arm movement (AAU; r = 0.60) and a very strong correlation with arm use ratio (AUR; r=-0.85) were reported [26]. In our study, participants with milder impairments generally had a greater AAU but also a higher variability between participants. This could reflect different lifestyles or behavioral factors that may influence how the impaired arm is involved in daily tasks despite the physical capacity to use it [59, 60]. Moreover, the group with a milder impairment had a more balanced AUR and a higher PTA during the day.
Despite some feasibility challenges, incorporating activity tracking devices that provide smart reminders effectively increased affected arm activity levels in the daily lives of chronic stroke survivors. To address the barriers to successful implementation, a robust device is needed that engages the participants to adhere to wearing the tracking and feedback device. Furthermore, additional features could be integrated, such as a mobile application with an artificial intelligence-based conversational agent to enhance compliance and motivation [61, 62]. Also, adding a specific set of rehabilitation exercises to increase arm activity that needs to be executed when a reminder is triggered might further improve the feasibility and effectiveness of such interventions [63].
Limitations and future research
Since no baseline sensor data was collected during this study, evaluating the actual difference and the potential increase in affected arm activity compared to pre-intervention was impossible [64]. This could lead to overestimating the possible effect of the smart reminders on arm use metrics. Additionally, it should be investigated whether a retention effect is maintained even if no further reminders are provided. The early termination of recruitment, along with exclusions due to missing data, dropout, or non-compliance resulted in a smaller sample size for the statistical analysis, which reduced the statistical power. While the threshold-based approach used in the software of the device to calculate activity counts includes information on when and how much the arm is used, it cannot provide insight into the functional relevance or content of performed activities and is prone to distortion caused by ballistic movements [65–67].
Conclusion
In conclusion, our study evaluated the effect of incorporating activity trackers with smart reminders to increase affected arm activity levels among stroke survivors during daily life. Despite positive results regarding arm use and increases in the MAL-14 AOU and QOM, no group difference was observed, and patient-reported outcomes remained below clinically meaningful thresholds. Interpretation of the results should further consider that only part of the study population could adhere to the intervention as feasibility challenges of such technology in an unsupervised setting were identified, such as the adherence to wearing the devices and quality of the sensor data.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank all the volunteers who participated in the study and Johannes Pohl and Simona Barzaghi for their valuable input. This research is supported by the National Research Foundation, Prime Minister’s Office, Singapore under its Campus for Research Excellence and Technological Enterprise (CREATE) programme.
Abbreviations
- AAU
Affected Arm Use
- AOU
Amount of Use Subscale
- ARAT
Action Research Arm Test
- AUR
Arm Use Ratio
- EQ-5D-5L
EuroQol Five Dimensions Five Levels questionnaire
- FMA-UE
Fugl-Meyer Assessment – Upper Extremity
- GRPC
Global Rating of Perceived Changes
- HSD
Honest Significant Difference
- KDE
Kernel Density Estimate
- MAL-14
Motor Activity Log-14 Item Version
- MCID
Minimal Clinically Important Difference
- MDC
Minimal Detectable Change
- MLM
Mixed Linear Model
- mRS
modified Rankin Scale
- PTA
Percentage of Time Active
- QOM
Quality of Movement subscale
- RVF
Residual Versus Fitted Values
Author contributions
JH, AL, SA, and JMV designed the experimental protocol. JH, SA, JMV, and AS took part in data collection. LM and SA analyzed the data and wrote the manuscript. OL, CAE, JH, and ML supervised the work and critically revised the manuscript. FR, RG, JMV, AS, and JvD supported data management and analysis of the sensor data. KF, CR, and ES supervised the project. All authors read and approved the final manuscript.
Funding
This work was supported by the Commission for Technology and Innovation (grant number 18156.2 PFLS-LS) and the P&K Pühringer Foundation. Yband Therapy AG provided the medical devices.
Data availability
The datasets generated and analyzed during the current study are not publicly available due to sensitive participant information but are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Cantonal Ethics Committee Zurich and Cantonal Ethics Committee Northwest and Central Switzerland (BASEC-number 2017 − 00948) and was registered at https://clinicaltrials.gov, unique identifier NCT03294187. The study was carried out in compliance with the Declaration of Helsinki, ICH-GCP, ISO 14155:2011, and Swiss legal and regulatory requirements.
Consent for publication
Not applicable.
Competing interests
OL is a member of the Editorial Board of the Journal of NeuroEngineering and Rehabilitation. OL was not involved in the journal’s peer review process or decisions related to this manuscript.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Laura Mayrhuber and Sebastian D. Andres contributed equally to this work.
Jeremia P. O. Held, Chris Awai Easthope and Olivier Lambercy share last authorship.
References
- 1.Markus HS, Brainin M, Fisher M. Tracking the global burden of stoke and dementia: World Stroke Day 2020., Oct. 2020, United States. 10.1177/1747493020959186 [DOI] [PubMed]
- 2.Feigin VL, et al. World Stroke Organization (WSO): global stroke fact sheet 2022. No X. 2022;17. 10.1177/17474930211065917.
- 3.Uswatte G, Giuliani C, Winstein C, Zeringue A, Hobbs L, Wolf SL. Validity of Accelerometry for Monitoring Real-World arm activity in patients with Subacute Stroke: evidence from the Extremity Constraint-Induced therapy evaluation trial. Arch Phys Med Rehabil. 2006;87(10):1340–5. 10.1016/j.apmr.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Gebruers N, Vanroy C, Truijen S, Engelborghs S, De Deyn PP. Monitoring of physical activity after stroke: a systematic review of Accelerometry-based measures. Arch Phys Med Rehabil. 2010;91(2):288–97. 10.1016/j.apmr.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 5.Taub E, Uswatte G, Mark VW, Morris DMM. The learned nonuse phenomenon: implications for rehabilitation., Eura. Medicophys., vol. 42, no. 3, pp. 241–256, Sep. 2006. [PubMed]
- 6.Andrews K, Steward J. Stroke recovery: he can but does he? Rheumatology. Feb. 1979;18(1):43–8. 10.1093/rheumatology/18.1.43. [DOI] [PubMed]
- 7.Pomeroy V et al. Neurological principles and rehabilitation of action disorders: rehabilitation interventions., Neurorehabil. Neural Repair, vol. 25, no. 5 Suppl, pp. 33S-43S, Jun. 2011, 10.1177/1545968311410942 [DOI] [PMC free article] [PubMed]
- 8.Barker RN, Brauer SG. Upper limb recovery after stroke: the stroke survivors’ perspective., Disabil. Rehabil.x, vol. 27, no. 20, pp. 1213–1223, Oct. 2005, 10.1080/09638280500075717 [DOI] [PubMed]
- 9.Miller KK, Porter RE, DeBaun-Sprague E, Van Puymbroeck M, Schmid AA. Exercise after Stroke: Patient Adherence and beliefs after Discharge from Rehabilitation. Top Stroke Rehabil. Feb. 2017;24(2):142–8. 10.1080/10749357.2016.1200292. [DOI] [PubMed]
- 10.Chin LF, Rosbergen ICM, Hayward KS, Brauer SG. A self-directed upper limb program during early post-stroke rehabilitation: a qualitative study of the perspective of nurses, therapists and stroke survivors. PLoS ONE. 2022;17(2):e0263413. 10.1371/journal.pone.0263413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daly JJ, McCabe JP, Holcomb J, Monkiewicz M, Gansen J, Pundik S. Long-Dose Intensive Therapy Is Necessary for Strong, Clinically Significant, Upper Limb Functional Gains and Retained Gains in Severe/Moderate Chronic Stroke., Neurorehabil. Neural Repair, vol. 33, no. 7, pp. 523–537, Jul. 2019, 10.1177/1545968319846120 [DOI] [PMC free article] [PubMed]
- 12.Winstein C, Kim B, Kim S, Martinez C, Schweighofer N. Dosage Matters., Stroke, vol. 50, no. 7, pp. 1831–1837, Jul. 2019, 10.1161/STROKEAHA.118.023603 [DOI] [PubMed]
- 13.Ballester BR, Winstein C, Schweighofer N. Virtuous and vicious cycles of arm use and function post-stroke. Front Neurol. March, 2022;13. 10.3389/fneur.2022.804211. [DOI] [PMC free article] [PubMed]
- 14.Kristoffersson A, Lindén M. A systematic review of wearable sensors for monitoring physical activity. Sensors. 2022;22(2):573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bravata DM, et al. Using pedometers to increase physical activity and improve HealthA systematic review. JAMA. 2007;298:2296–304. 10.1001/jama.298.19.2296. [DOI] [PubMed] [Google Scholar]
- 16.Cara CBC, Sidman L, Masurier GL. Promoting physical activity among sedentary women using pedometers. Res Q Exerc Sport. 2004;75(2):122–9. 10.1080/02701367.2004.10609143. [DOI] [PubMed] [Google Scholar]
- 17.Fry JP, Neff RA. Periodic prompts and reminders in health promotion and health behavior interventions: systematic review. J Med Internet Res. May 2009;11(2):e16. 10.2196/jmir.1138. [DOI] [PMC free article] [PubMed]
- 18.Sloan RA, Kim Y, Sahasranaman A, Müller-Riemenschneider F, Biddle SJH, Finkelstein EA. The influence of a consumer-wearable activity tracker on sedentary time and prolonged sedentary bouts: secondary analysis of a randomized controlled trial. BMC Res Notes. 2018;11(1):189. 10.1186/s13104-018-3306-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leuenberger K, Gonzenbach R, Wachter S, Luft A, Gassert R. A method to qualitatively assess arm use in stroke survivors in the home environment. Med Biol Eng Comput. 2017;55(1):141–50. 10.1007/s11517-016-1496-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Held JPO, Luft AR, Veerbeek JM. Encouragement-induced real-world upper limb use after stroke by a tracking and feedback device: A study protocol for a multi-center, assessor-blinded, randomized controlled trial, Front. Neurol., vol. 9, no. JAN, pp. 1–11, 2018, 10.3389/fneur.2018.00013 [DOI] [PMC free article] [PubMed]
- 21.Subash T, et al. Comparing algorithms for assessing upper limb use with inertial measurement units. Front Physiol. 2022;13:1–13. 10.3389/fphys.2022.1023589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim GJ, Parnandi A, Eva S, Schambra H. The use of wearable sensors to assess and treat the upper extremity after stroke: a scoping review. Disabil Rehabil. 2022;44(20):6119–38. 10.1080/09638288.2021.1957027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michielsen ME, Selles RW, Stam HJ, Ribbers GM, Bussmann JB. Quantifying nonuse in chronic stroke patients: a study into Paretic, Nonparetic, and Bimanual Upper-Limb Use in Daily Life. Arch Phys Med Rehabil. 2012;93(11):1975–81. 10.1016/j.apmr.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Langerak AJ, et al. Requirements for home-based upper extremity rehabilitation using wearable motion sensors for stroke patients: a user-centred approach. Disabil Rehabil Assist Technol. 2023;0(0):1–13. 10.1080/17483107.2023.2183993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bailey RR, Klaesner JW, Lang CE. Quantifying Real-World Upper-Limb Activity in Nondisabled Adults and Adults With Chronic Stroke, Neurorehabil. Neural Repair, vol. 29, no. 10, pp. 969–978, Apr. 2015, 10.1177/1545968315583720 [DOI] [PMC free article] [PubMed]
- 26.Thrane G, Emaus N, Askim T, Anke A. Arm use in patients with subacute stroke monitored by accelerometry: association with motor impairment and influence on self-dependence., J. Rehabil. Med., vol. 43, no. 4, pp. 299–304, Mar. 2011, 10.2340/16501977-0676 [DOI] [PubMed]
- 27.Demers M et al. Sep., Wearable technology to capture arm use of stroke survivors in home and community settings: feasibility and early insights on motor performance., 2023, United States. 10.1101/2023.01.25.23284790 [DOI] [PMC free article] [PubMed]
- 28.Mayrhuber L et al. Movement Reminders to Encourage Arm Use During Daily Life in Stroke Patients, in., 2023 International Conference on Rehabilitation Robotics (ICORR), 2023, pp. 1–6. 10.1109/ICORR58425.2023.10304727 [DOI] [PubMed]
- 29.Da-Silva RH, et al. Wristband accelerometers to motiVate arm exercises after stroke (WAVES): a pilot randomized controlled trial. Clin Rehabil. 2019;33(8):1391–403. 10.1177/0269215519834720. [DOI] [PubMed] [Google Scholar]
- 30.Argent R, Daly A, Caulfield B. Patient involvement with home-based Exercise Programs: can Connected Health interventions Influence Adherence? JMIR Mhealth Uhealth. 2018;6(3):e47. 10.2196/mhealth.8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jack K, McLean SM, Moffett JK, Gardiner E. Barriers to treatment adherence in physiotherapy outpatient clinics: a systematic review. Man Ther. 2010;15(3):220–8. 10.1016/j.math.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Demers M, et al. Understanding stroke survivors’ preferences regarding wearable sensor feedback on functional movement: a mixed-methods study. J Neuroeng Rehabil. 2023;20(1):146. 10.1186/s12984-023-01271-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Da-Silva RH, et al. Prompting arm activity after stroke: a clinical proof of concept study of wrist-worn accelerometers with a vibrating alert function. J Rehabil Assist Technol Eng. 2018;5:205566831876152. 10.1177/2055668318761524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwerz de Lucena D, Rowe JB, Okita S, Chan V, Cramer SC, Reinkensmeyer DJ. Providing real-time wearable feedback to increase Hand Use after Stroke: a Randomized, Controlled Trial. Sensors. 2022;22(18):1–17. 10.3390/s22186938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bai Z, Fong KNK. Remind-to-move’ treatment enhanced activation of the Primary Motor Cortex in patients with stroke. Brain Topogr. 2020;33(2):275–83. 10.1007/s10548-020-00756-7. [DOI] [PubMed] [Google Scholar]
- 36.Fong KN, et al. Effects of sensory cueing on voluntary arm use for patients with chronic stroke: a preliminary study. Arch Phys Med Rehabil. Jan. 2011;92(1):15–23. 10.1016/j.apmr.2010.09.014. [DOI] [PubMed]
- 37.Signal N et al. Haptic nudging using a wearable device to promote Upper Limb Activity during Stroke Rehabilitation: exploring diurnal variation, repetition, and duration of Effect, 2023. 10.3390/bs13120995 [DOI] [PMC free article] [PubMed]
- 38.Luster EL et al. Vibrotactile cueing using wearable computers for overcoming learned non-use in chronic stroke, in., 2013 7th International Conference on Pervasive Computing Technologies for Healthcare and Workshops, 2013, pp. 378–381. 10.4108/icst.pervasivehealth.2013.252351
- 39.Toh SFM, et al. Usability of a wearable device for home-based upper limb telerehabilitation in persons with stroke: a mixed-methods study. Digit Heal. Jan. 2023;9:20552076231153736. 10.1177/20552076231153737. [DOI] [PMC free article] [PubMed]
- 40.Wei WXJ, Fong KNK, Chung RCK, Cheung HKY, Chow ESL. Remind-to-move’ for promoting Upper Extremity Recovery using Wearable devices in Subacute Stroke: a Multi-center Randomized Controlled Study. IEEE Trans Neural Syst Rehabil Eng Publ IEEE Eng Med Biol Soc. Jan. 2019;27(1):51–9. 10.1109/TNSRE.2018.2882235. [DOI] [PubMed]
- 41.Taub E, Morris D, Bowman M, Delgado A, Uswatte G. Upper-extremity motor activity log [manual], Available from Edward Taub, Psychol. Dep. UAB, CH415, vol. 1530, 2000.
- 42.Taub E, et al. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. 1993;74(4):347–54. [PubMed] [Google Scholar]
- 43.Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7(1):13–31. [PubMed] [Google Scholar]
- 44.Lyle RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int J Rehabil Res Int Z fur Rehabil Rev Int Rech Readapt. 1981;4(4):483–92. 10.1097/00004356-198112000-00001. [DOI] [PubMed] [Google Scholar]
- 45.Herdman M et al. Dec., Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L)., Qual. life Res. an Int. J. Qual. life Asp. Treat. care Rehabil., vol. 20, no. 10, pp. 1727–1736, 2011, 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed]
- 46.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients., Stroke, vol. 19, no. 5, pp. 604–607, May 1988, 10.1161/01.str.19.5.604 [DOI] [PubMed]
- 47.Devlin N, Pickard S, Busschbach J. The development of the EQ-5D-5L and its value sets, Value sets eq-5d-5l a Compend. Comp. Rev. user Guid., pp. 1–12, 2022.
- 48.Woytowicz EJ et al. Mar., Determining Levels of Upper Extremity Movement Impairment by Applying a Cluster Analysis to the Fugl-Meyer Assessment of the Upper Extremity in Chronic Stroke., Arch. Phys. Med. Rehabil., vol. 98, no. 3, pp. 456–462, 2017, 10.1016/j.apmr.2016.06.023 [DOI] [PMC free article] [PubMed]
- 49.Edwards R. Statistics at Square one. J Epidemiol Community Heal. Feb. 1997;51. 10.1136/jech.51.1.104.
- 50.Lang CE, Edwards DF, Birkenmeier RL, Dromerick AW. Estimating minimal clinically important differences of Upper-Extremity measures Early after Stroke. Arch Phys Med Rehabil. 2008;89(9):1693–700. 10.1016/j.apmr.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen S, Wolf SL, Zhang Q, Thompson PA, Winstein CJ. Minimal detectable change of the actual amount of use test and the motor activity log: the EXCITE Trial., Neurorehabil. Neural Repair, vol. 26, no. 5, pp. 507–514, Jun. 2012, 10.1177/1545968311425048 [DOI] [PMC free article] [PubMed]
- 52.Page SJ, Fulk GD, Boyne P. Clinically Important Differences for the Upper-Extremity Fugl-Meyer Scale in People With Minimal to Moderate Impairment Due to Chronic Stroke, Phys. Ther., vol. 92, no. 6, pp. 791–798, Jun. 2012, 10.2522/ptj.20110009 [DOI] [PubMed]
- 53.Huynh BP et al. Jul., Sensitivity to Change and Responsiveness of the Upper Extremity Fugl-Meyer Assessment in Individuals With Moderate to Severe Acute Stroke, Neurorehabil. Neural Repair, vol. 37, no. 8, pp. 545–553, 2023, 10.1177/15459683231186985 [DOI] [PubMed]
- 54.Gupta AS, Luddy AC, Khan NC, Reiling S, Thornton JK. Real-life Wrist Movement Patterns Capture Motor Impairment in individuals with Ataxia-telangiectasia. Cerebellum. 2023;22(2):261–71. 10.1007/s12311-022-01385-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schreiweis B, Pobiruchin M, Strotbaum V, Suleder J, Wiesner M, Bergh B. Barriers and Facilitators to the Implementation of eHealth Services: Systematic Literature Analysis., J. Med. Internet Res., vol. 21, no. 11, p. e14197, Nov. 2019, 10.2196/14197 [DOI] [PMC free article] [PubMed]
- 56.Braakhuis HEM, Bussmann JBJ, Ribbers GM, Berger MAM. Wearable activity monitoring in day-to-day stroke care: a Promising Tool but not widely used, 2021. 10.3390/s21124066 [DOI] [PMC free article] [PubMed]
- 57.Langerak AJ et al. A sensor-based feedback device stimulating Daily Life Upper Extremity Activity in Stroke patients: a feasibility study, 2023. 10.3390/s23135868 [DOI] [PMC free article] [PubMed]
- 58.Okita S, De Lucena DS, Reinkensmeyer DJ. Movement diversity and complexity increase as arm impairment decreases after stroke: quality of movement experience as a possible target for wearable feedback, 2023. [DOI] [PMC free article] [PubMed]
- 59.Hall J, et al. Factors influencing sedentary behaviours after stroke: findings from qualitative observations and interviews with stroke survivors and their caregivers. BMC Public Health. 2020;20(1):967. 10.1186/s12889-020-09113-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rajagopalan V, Natarajan M, Alex J, Solomon JM. How does context influence arm use after stroke? A qualitative content analysis among rural community-dwelling stroke survivors. Brazilian J Phys Ther. 2020;24(1):61–8. 10.1016/j.bjpt.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hocking J, Oster C, Maeder A. Use of conversational agents in rehabilitation following brain injury, disease, or stroke: a scoping review protocol., JBI Evid. Synth., vol. 19, no. 6, pp. 1369–1381, Jun. 2021, 10.11124/JBIES-20-00225 [DOI] [PubMed]
- 62.Ding H, Simmich J, Vaezipour A, Andrews N, Russell T. Evaluation framework for conversational agents with artificial intelligence in health interventions: a systematic scoping review., J. Am. Med. Inform. Assoc., vol. 31, no. 3, pp. 746–761, Feb. 2024, 10.1093/jamia/ocad222 [DOI] [PMC free article] [PubMed]
- 63.Whitford M, Schearer E, Rowlett M. Effects of in home high dose accelerometer-based feedback on perceived and actual use in participants chronic post-stroke., Physiother. Theory Pract., vol. 36, no. 7, pp. 799–809, Jul. 2020, 10.1080/09593985.2018.1493759 [DOI] [PubMed]
- 64.Toh FM, Lam WWT, Cruz P, Gonzalez, Fong KNK. Effects of a wearable-based intervention on the Hemiparetic Upper Limb in persons with stroke: a Randomized Controlled Trial. Neurorehabil Neural Repair. Sep. 2024;15459683241283412. 10.1177/15459683241283412. [DOI] [PubMed]
- 65.Balasubramanian S, GMAC. A simple measure to quantify upper limb use from wrist-worn accelerometers, medRxiv, p. 2023.11.26.23299036, Jan 2023, 10.1101/2023.11.26.23299036 [DOI] [PubMed]
- 66.Pohl J et al. Classification of functional and non-functional arm use by inertial measurement units in individuals with upper limb impairment after stroke, Front. Physiol., vol. 13, 2022, [Online]. Available: https://www.frontiersin.org/journals/physiology/articles/10.3389/fphys.2022.952757 [DOI] [PMC free article] [PubMed]
- 67.Pohl J, Verheyden G, Held J, Luft A, Awai-Easthope C, Awai-Easthope J. Concurrent Validity and Responsiveness of Clinical Upper Limb Outcome Measures and Sensor-based Arm Use Metrics within the First Year after Stroke: A Longitudinal Cohort Study. 2024. 10.21203/rs.3.rs-4103325/v1
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available due to sensitive participant information but are available from the corresponding author upon reasonable request.