Abstract
Transplantation of induced pluripotent stem cell-derived neural cells represents a promising strategy for treating neurodegenerative diseases. However, reprogramming of somatic cells and their subsequent neural differentiation is complex and time-consuming, thereby impeding autologous applications. Recently, direct transcription factor-based conversion of blood cells into induced neural stem cells (iNSCs) has emerged as a potential alternative. However, little is known about the functionality of iNSC-derived neurons upon in vivo transplantation. Here, we grafted human iNSCs derived from adult peripheral blood by temporary overexpression of the transcription factors SOX2 and cMYC into the hippocampus or striatum of adult unlesioned immunodeficient Rag2tm1FwaIl2rgtm1Wjl mice of both sexes. Engrafted cells gave rise to stable transplants composed of mature neurons displaying extensive neurite outgrowth and dendritic spine formation. Functional analyses of acute slices using patch clamp recordings revealed that already after 12 weeks of in vivo maturation, most of iNSC-derived cells possess unique properties exclusive to neurons and exhibit voltage-dependent ion channel currents as well as action potential firing. Moreover, the formation of spontaneous inhibitory and excitatory postsynaptic currents, along with Rabies virus-based retrograde monosynaptic tracing data, strongly supports the structural and functional integration of graft-derived neurons. Taken together, our data demonstrate that iNSCs directly derived from peripheral blood cells have the inherent capacity to achieve full functional maturation in vivo, qualifying them as an alternative potential donor source for restorative applications and deserving further investigation.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13287-024-04110-7.
Keywords: Cell programming, Direct conversion, Induced neural stem cells, Transplantation, Neural network integration
Letter
Transplantation of human neural precursors represents a promising strategy for neurorestoration. The advent of cell reprogramming and the generation of induced pluripotent stem cell (iPSC)-derived neural precursors even provide prospects for autologous grafts [1]. However, the generation of iPSCs and their subsequent neural differentiation is laborious and time-consuming. More recently, transcription factor-based generation of induced neural stem cells (iNSC) from fibroblasts or blood cells has emerged as an exciting alternative approach [for review see 2, 3]. This direct route of donor cell generation from patient cells offers several attractive advantages. First, the accelerated cell generation, which eliminates the need to first produce and validate iPSCs, offers a realistic avenue for generating autologous donor cells in relative short time frames. Second, bypassing the pluripotent stage should eliminate risks associated with the use of pluripotent cells such as in vivo teratoma formation. Third, similar to iPSCs, iNSCs have been shown to undergo epigenetic de-aging [4, 5], which could be beneficial for generating donor cell populations from older age patients.
Despite a growing body of work on iNSCs, only few studies have addressed the electrophysiological functionality of iNSC-derived neurons after transplantation [6–8]. In particular, data on synaptic maturation and integration are still scarce or missing. Our previous study has shown that iNSCs generated directly from peripheral blood cells by temporary overexpression of the transcription factors SOX2 and cMYC exhibit neurogenic and gliogenic differentiation potential both in vitro and in vivo [5]. Here, we now focus on the ability of these cells to undergo functional maturation and integration upon transplantation.
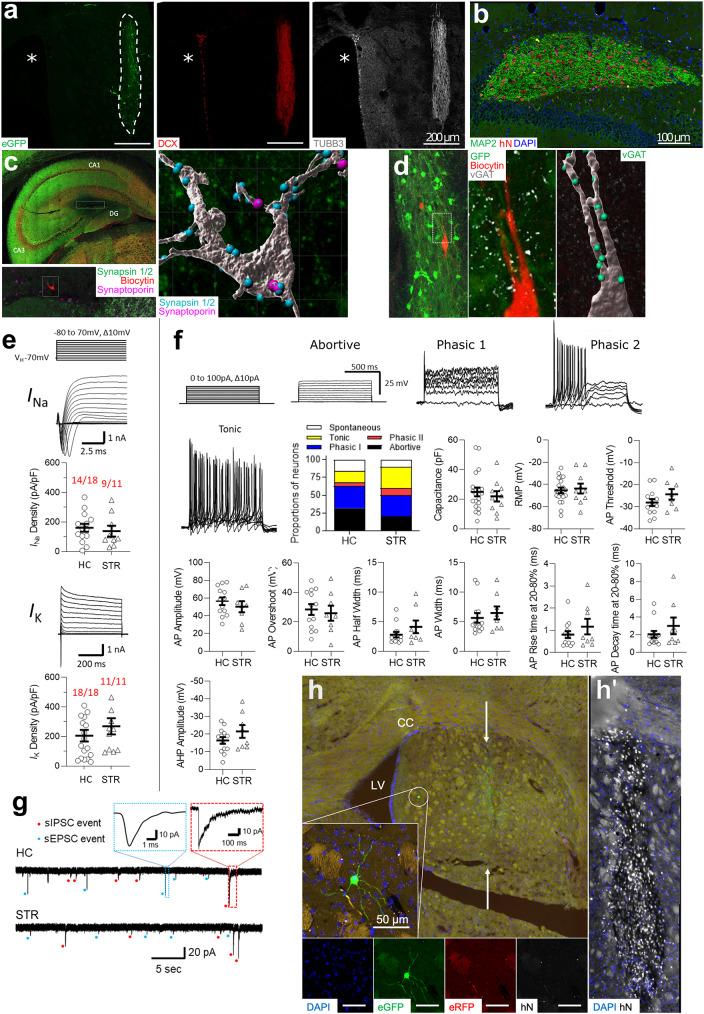
Following stereotaxic engraftment into the hippocampus (HC) or striatum (STR) of deeply anesthetized, unlesioned adult immunodeficient mice, we observed compact grafts containing numerous DCX-, TUBB3- and MAP2-positive human neurons (Fig. 1a, b). iNSC-derived neurons remained detectable until the end of the longest post-operative follow-up period of 24 weeks. In addition, GFAP-positive ramified human cells with the morphology of astrocytes were observed inside the grafts (Supplementary Fig. 1a). Engrafted neurons extended numerous processes into the adjacent host brain tissue. In dentate gyrus grafts, axonal projections seemed to follow endogenous trajectories toward pyramidal cell layer, entorhinal cortex and septum (Supplementary Fig. 1b). A closer inspection of the GFP-labeled engrafted neurons revealed dendritic protrusions decorated with juxtaposed VGLUT1 and SHANK2-positive dots at 24 weeks post transplantation, suggesting the establishment of both pre- and post-synaptic compartments, being indicative of synapse formation (Supplementary Fig. 2). At 12 weeks after transplantation, the engrafted donor cells already displayed various dendritic spine morphologies, such as filopodia, long thin, thin stubby and mushroom-shaped spines (Supplementary Fig. 3; Supplementary Table 1).
Fig. 1.
Differentiation, functional maturation and integration of engrafted iNSC-derived neurons. (a) STR iNSC graft (dotted line) at 12 weeks after transplantation stained for neuronal markers DCX and TUBB3. Asterisks: lateral ventricle. (b) Ten-week-old iNSC graft in the dentate gyrus labeled with antibodies to human nuclei (hN) and MAP2. (c-d) Staining of presynaptic markers in human neurons, which were identified by their GFP labelling and biocytin content (after biocytin loading during patch clamping). (c) iNSC-derived neuron in a 12-week-old HC graft, which is decorated with synapsin- and synaptoporin-positive puncta, some of which are co-localized. (d) Donor neuron in a 12-week-old STR graft, which is covered with VGAT-positive puncta. The digital reconstruction depicts only puncta in close proximity to the biocytin-labelled neuron (< 1 µm from the surface). (e) Detection of voltage-dependent sodium and potassium currents in engrafted GFP-positive iNSC-derived neurons under voltage clamp condition. Currents were evoked by 500 ms depolarizing voltage steps from − 80 to + 70 mV in 10 mV steps from a holding potential of -70 mV. Potassium currents were measured in the presence of 300 nM tetrodotoxin (TTX). Recordings are from 11 to 24-week-old HC and STR grafts. Red: Number of cells exhibiting sodium and potassium currents vs. number of all measured cells. (f) AP classification in iNSC-derived neurons engrafted into HC (n = 19) and STR (n = 10) at 11–24 weeks after transplantation. (g) Representative spontaneous IPSC and EPSC events that were measured in the presence of TTX in neurons from a 12-week-old HC and a 12-week-old STR graft. These two types of events can be distinguished based on their different release kinetics. (h) RABV-based mono-transsynaptic tracing after infection of a 12-week-old STR graft, depicting a GFP-labeled donor neuron remote from the compact graft core. Note that this cell is negative for the human marker hN and RFP (encoded by donor cells). Insert to the right (h’) depicts the graft core in a consecutive section, labeled with an antibody to hN
We next performed electrophysiological recordings in GFP-labeled donor cells, within or in close proximity to the graft area, from acute brain slices, while simultaneously filling the measured neurons with biocytin for post hoc verification of their human origin and further analyses of synaptic inputs by immunohistochemistry. In the HC, we noted abutting structures positive for the presynaptic marker synapsin, some of which showed co-localization with synaptoporin that is predominantly expressed in the mossy fiber bouton of dentate gyrus neurons [9], suggesting innervation by murine mossy fibers (Fig. 1c). Within the STR, VGAT-positive puncta were identified on biocytin-filled neurons, likely representing input from the host’s abundant medium spiny neurons (Fig. 1d).
At 11–24 weeks after transplantation, neurons engrafted into the mouse HC and STR generated functional voltage-gated Na+/K+ channels with similar current densities across both regions (Fig. 1e; Supplementary Table 2). The iNSC-derived neurons were further able to generate action potentials (APs) upon current injection. This finding is consistent with what has already been reported for grafted cord blood-derived iNSCs [7] and transplanted induced neural plate border stem cells [8]. However, in these preceding studies, human iNSCs were pre-differentiated before implantation into adult mice, and the electrophysiological properties of iNSC-derived neurons were not assessed in further detail. Here, detailed analysis of the recorded APs revealed five unique firing patterns, ranging from phasic (HC: 36.8%, STR: 40%) to tonic (HC: 15.8%, STR: 30%) firing. In addition, a smaller fraction of spontaneously firing human neurons was observed (HC: 15.8%, STR: 10%; Fig. 1f). All five firing patterns were found in both implant sites.
Interestingly, for the hippocampus, we have additionally produced anecdotal data recording from mouse hippocampal neurons in the same brain slice containing the transplanted human neurons. The mouse neurons had a resting membrane potential of approximately − 55 mV and showed spontaneous and tonic action potential firing patterns similar to those observed in human iNSC-derived neurons. Interpreting the biological significance of these firing patterns in this in vivo context is complex, as it likely reflects multiple factors, including (i) neuronal maturity, (ii) intrinsic neuronal phenotype, and (iii) network integration. However, this observation suggests that human neurons may acquire electrophysiological properties similar to those of their murine counterpart following in vivo maturation, although a systematic side-by-side comparison would be required to confirm this notion.
We further measured the passive and active membrane properties of human neurons including resting membrane potential (RMP), capacitance, AP threshold, AP overshoot, AP amplitude, AP rise time, AP decay time and afterhyperpolarization (AHP) amplitude. These membrane properties, too, were comparable in both transplantation sites (Fig. 1f; Supplementary Table 3). Finally, we assessed the presence of postsynaptic currents in iNSC-derived neurons. Since in vitro models have demonstrated that spontaneous firing can occur even without synaptic input [10], spontaneous action potential firing in vivo could arise from intrinsic neuronal properties. Yet, it can also be triggered and modulated by various synaptic inputs. Consistent with the immunohistochemical evidence for synapse formation, we recorded bicuculline-sensitive spontaneous inhibitory and NBQX-sensitive spontaneous excitatory postsynaptic currents (sIPSC and sEPSC events, respectively) from engrafted neurons (Fig. 1g; Supplementary Table 4). So far, EPSCs and IPSCs have only been described in grafted murine iNSCs derived from mouse fibroblasts [6]. Our data extend these observations for the first time to transplanted human iNSCs generated from peripheral blood and demonstrate that human iNSC-derived neurons can fire APs and receive synaptic inputs; properties we also confirmed in a small series of neonatal grafts (Supplementary Fig. 4; Supplementary Table 5).
To corroborate synaptic integration of our transplanted iNSC-derived neurons, we employed Rabies virus (RABV)-based mono-transsynaptic retrograde tracing to visualize input neurons [11]. We engineered grafts to express the ASLA receptor TVA and the RABV B19 glycoprotein, and infected them at ≥ 10 weeks after transplantation with a replication-deficient pseudotyped RABV encoding GFP. This allowed us to detect first-order input neurons [11]. Ten days after infection, mice were sacrificed by transcardial perfusion and cleared recipient mouse brains were analyzed with light sheet fluorescence microscopy (LSFM). This experiment indeed revealed a small number of GFP-positive neurons inside the host brain tissue and well outside the graft core, of which some were found in regions known to project toward the respective transplantation site (Supplementary Fig. 5). In order to reliably characterize the species identity of GFP-positive cells, we next performed immunostaining following tissue rehydration and cryo-sectioning. Due to this methodological constraint, however, only some of the cells that were originally identified in 3D LSFM data could be retrieved. Yet, these exemplary GFP-positive cells remote from the graft were found to be negative for the human nuclei antigen (hN), suggesting that these are mouse neurons providing synaptic input to iNSC-derived human neurons (Fig. 1h).
Our data demonstrate that iNSCs, generated by direct conversion from human adult peripheral blood cells and transplanted into the adult unlesioned mouse brain, undergo functional maturation, form synapses and integrate into the host central nervous system in both HC and STR. While we assume that the observed cell-intrinsic morphological and functional maturation of iNSCs is generally translatable to a human host brain, our xenograft model is limited by comparably short follow-up times, which restricts generalization of our findings especially concerning the cells’ capacity to undergo long-term circuit integration in a human-in-human context. Furthermore, our study was limited by the fact that we encountered highly heterogeneous reporter fluorescence during electrophysiological recordings, potentially resulting from varying degrees of transgene silencing in vivo, and very occasional direct labeling of host neurons by RABV in control experiments. We also realize that our data are mainly restricted to donor neuron functionality and connectivity, and that further work is required to assess full graft composition over time, along with a fine-grained analysis of potentially evolving neuronal subtypes. Still, considering the advantages of direct derivation of neural cells from patient blood samples, our findings provide an important and promising basis for further exploration of this alternative donor source in regenerative applications – also in comparison to iPSC-derived neurons.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Additional file 1: Supplement to this Letter to the Editor (Description of data: Contains Material and Methods section as well as five Supplementary Figures and four Supplementary Tables along with their respective legends)
Acknowledgements
We would like to acknowledge Lydia Fischer (Functional Neuroconnectomics, Institute of Experimental Epileptology and Cognition Research, University of Bonn Medical Faculty and University Hospital Bonn) for technical support producing RABV∆G-EGFP(EnvA). Additionally, we want to sincerely thank Florian Schloen, Anne Stockhausen, Alexander Harder and Ulrich Kubitscheck (all previous or current members of the Biophysical Chemistry Workgroup, University of Bonn) for their continuous and kind support in building, maintaining and improving the LSFM platform-associated hard- and software settings. Anja Guenther (Department of Molecular Neurobiology, Max-Planck-Institute for Multidisciplinary Sciences, City Campus, Göttingen) provided perfect technical support. We acknowledge Taconic Biosciences, Rensselaer, for providing mice. The authors declare that artificial intelligence is not used in this study.
Abbreviations
- AP
Action potential
- AHP
Afterhyperpolarization
- EPSC
Spontaneous excitatory postsynaptic current
- HC
Hippocampus
- iNSC
Induced neural stem cell
- iPSC
Induced pluripotent stem cell
- IPSC
Spontaneous inhibitory postsynaptic current
- LSFM
Light sheet fluorescence microscopy
- PSC
Pluripotent stem cell
- RABV
Rabies virus
- RMP
Resting membrane potential
- STR
Striatum
- TTX
Tetrodotoxin
Author contributions
LJB cultivated and engineered the iNSC line iLB-82bf-3-SC to express the vectors relevant for RABV-based monosynaptic tracing. LJB, AL and JS performed intracerebral transplantations. LJB and AL performed RABV injections, brain tissue clearing and LSFM; these experiments were mainly supervised by MKS. LJB, CKL, AL, RK and AHS performed histological and immunohistochemical stainings. CKL and AHS performed dendrite, spine and synapse reconstructions. CKL and JSR performed and analyzed electrophysiological recordings in acute brain slices of adult mice; PR performed respective analyses in acute brain slices after neonatal transplantation. CS established the iNSC wildtype line iLB-82bf-3-SC and its GFP-expressing derivative used for transplantation experiments. HB, MKS, NB, JSR and OB interpreted the data, supervised the work and provided financial support. LJB, CKL, NB, JSR and OB conceived and designed the study. LJB, CKL, HM, JSR and OB were major contributors in writing the manuscript. All authors contributed to the final editing of the paper, reading and approving its final version.
Funding
This project has been supported by the European Union’s Horizon 2020 research and innovation program, grant agreement no. 874758 (NSC-Reconstruct) to O.B. and LIFE & BRAIN GmbH, the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), SFB 1089, Project-ID 227953431 to MKS and HB, as well as the European Commission (H2020), Comorbidity and Synapse Biology in Clinically Overlapping Psychiatric Disorders Project ID 66730 to CKL and AHS. HM was supported by stipends from Japan Society for the Promotion of Science Overseas Research Fellowship, Uehara Memorial Foundation research fellowship, and Ibaraki Prefecture Global Human Resource Development Program. The funders had no specific role in the conceptualization, design, data collection, analysis, decision to publish, or preparation of the manuscript.
Data availability
Representative selections of all datasets are included in this published letter or are supplied within the additional file. The full datasets are not publicly available due to their file sizes, especially applying to LSFM data, but are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The collection of human blood for direct conversion was approved by the ethics committee of the University of Bonn Medical Centre (title: ‘Gewinnung autologer pluripotenter Zellinien für Krankheits- und Therapieforschung’; approval number 275/08; granted April 20, 2015). The donated material was pseudonymized before being processed. All subjects gave written informed consent.
Animal experiments were approved by the Agency for Nature, Environment and Consumer Protection of the state North Rhine Westphalia (LANUV; title: ‘Transplantation humaner ES-Zell-abgeleiteter neuraler Zellen in das Nervensystem von Labornagetieren mit nachfolgender viraler Transduktion des Transplantats nach dessen Ausreifung’, approval number 84-02.04.2013.A368, granted December 5, 2013; title: ‘Remyelinisierungspotential induzierter neuraler Vorläuferzellen’, approval number 84-02.04.2016.A179, granted July 26, 2016; and title: ‘Transplantation induzierter neuraler Vorläuferzellen in das Nervengewebe von Mäusen mit nachfolgender viraler Transduktion des Transplantats nach dessen Ausreifung’, approval number 81.02.04.2019.A054, granted June 24, 2019) as well as the Lower Saxony State Office for Consumer Protection and Food Safety (LAVES; title: ‘Transplantation induzierter neuraler Vorläuferzellen in das Nervengewebe von Mäusen mit nachfolgender viraler Transduktion des Transplantats nach dessen Ausreifung’; approval number 33.19-42502-04-20/3457; granted June 10, 2020). The non-technical summary of this animal experiment was registered a priori at the German data base https://www.animaltestinfo.de.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lea Jessica Berg and Chung Ku Lee contributed equally to this work.
References
- 1.Barker RA, Götz M, Parmar M. New approaches for brain repair - from rescue to reprogramming. Nature. 2018;557:329–34. [DOI] [PubMed] [Google Scholar]
- 2.Tian Z, Zhao Q, Biswas S, Deng W. Methods of reactivation and reprogramming of neural stem cells for neural repair. Methods. 2018;133:3–20. [DOI] [PubMed] [Google Scholar]
- 3.Flitsch LJ, Brüstle O. Evolving principles underlying neural lineage conversion and their relevance for biomedical translation. F1000Research. 2019; 8:F1000 Faculty Rev-1548. [DOI] [PMC free article] [PubMed]
- 4.Lo Sardo V, Ferguson W, Erikson GA, Topol EJ, Baldwin KK, Torkamani A. Influence of donor age on induced pluripotent stem cells. Nat Biotechnol. 2017;35:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheng C, Jungverdorben J, Wiethoff H, Lin Q, Flitsch LJ, Eckert D, et al. A stably self-renewing adult blood-derived induced neural stem cell exhibiting patternability and epigenetic rejuvenation. Nat Commun. 2018;9:4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hemmer K, Zhang M, Van Wüllen T, Sakalem M, Tapia N, Baumuratov A, et al. Induced neural stem cells achieve long-term survival and functional integration in the adult mouse brain. Stem Cell Rep. 2014;3:423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giorgetti A, Marchetto MCN, Li M, Yu D, Fazzina R, Mu Y, et al. Cord blood-derived neuronal cells by ectopic expression of Sox2 and c-Myc. Proc Natl Acad Sci U S A. 2012;109:12556–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thier MC, Hommerding O, Panten J, Pinna R, García-González D, Berger T, et al. Identification of embryonic neural plate border stem cells and their generation by direct reprogramming from adult human blood cells. Cell Stem Cell. 2019;24:166–82. [DOI] [PubMed] [Google Scholar]
- 9.Singec I, Knoth R, Ditter M, Hagemeyer CE, Rosenbrock H, Frotscher M, et al. Synaptic vesicle protein synaptoporin is differently expressed by subpopulations of mouse hippocampal neurons. J Comp Neurol. 2002;452:139–53. [DOI] [PubMed] [Google Scholar]
- 10.Rhee HJ, Shaib AH, Rehbach K, Lee CK, Seif P, Thomas C, et al. An Autaptic Culture System for Standardized Analyses of iPSC-Derived Human Neurons. Cell Rep. 2019;27:2212–e22287. [DOI] [PubMed] [Google Scholar]
- 11.Doerr J, Schwarz MK, Wiedermann D, Leinhaas A, Jakobs A, Schloen F, et al. Whole-brain 3D mapping of human neural transplant innervation. Nat Commun. 2017;8:14162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplement to this Letter to the Editor (Description of data: Contains Material and Methods section as well as five Supplementary Figures and four Supplementary Tables along with their respective legends)
Data Availability Statement
Representative selections of all datasets are included in this published letter or are supplied within the additional file. The full datasets are not publicly available due to their file sizes, especially applying to LSFM data, but are available from the corresponding author upon reasonable request.