Abstract
Background
Data on the relationship between potassium intake and major cardiovascular events (MACE) in patients with diabetes are scarce. We aim to study the association between estimated potassium intake and risk of MACE in individuals with type 2 diabetes.
Methods
The discovery cohort consisted of 1572 participants with type 2 diabetes from a secondary hospital. The validation cohort consisted of 1430 participants with diabetes from a multicenter study (Chronic Renal Insufficiency Cohort, CRIC). Potassium intake was estimated from potassium in spot urine using Kawasaki formula and in 24-h urine collection in two cohorts, respectively. The primary outcome was MACE defined as a composite of myocardial infarction, stroke and cardiovascular death.
Results
During a median of 8.2 years of follow-up, 341 MACE events were identified in discovery cohort. Compared to the lowest tertile, participants with potassium intake in the top tertile had 34% lower risk for MACE after adjustment for cardio-renal risk factors (adjusted hazard ratio, aHR [95% CI], 0.66 [0.49–0.89]). This inverse association was more pronounced in participants with normal or moderately elevated albuminuria as compared to those with severely elevated albuminuria (urine albumin-to-creatinine ratio > 300 mg/g, p for interaction < 0.05). In consistence, a higher potassium intake was independently associated with a lower risk of MACE in CRIC participants with diabetes and moderately elevated albuminuria (aHR 0.61 [0.42–0.90], top vs. lowest tertile).
Conclusions
A high level potassium intake estimated from urine potassium excretion was independently associated with a low risk of MACE in patients with type 2 diabetes. Increasing potassium intake may be a potential effective strategy for cardiovascular risk reduction beyond controlling traditional risk factors.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-024-02546-y.
Keywords: Potassium intake, Urine potassium excretion, Major adverse cardiovascular event, All-cause mortality, Type 2 diabetes
Background
The incidence rate of cardiovascular events has declined substantially in patients with diabetes in developed countries over the past two decades, largely attributable to improved control of traditional risk factors including hypertension and dyslipidaemia [1, 2]. Nevertheless, cardiovascular disease (CVD) is still the leading cause of morbidity and mortality in diabetic population [2, 3]. Compared to their counterparts with no diabetes, individuals with type 2 diabetes have approximately two-fold increased risk for CVD even after adjustment for traditional risk factors [2, 4, 5]. This unmet clinical need may require interventional strategies targeting non-traditional risk factors to further reduce the risk of CVD.
Despite the large number of observational studies that have associated inadequate potassium intake with an elevated blood pressure and a high risk of cardiovascular events in general population [6–11], increasing potassium intake as an approach for CVD risk reduction has been less emphasized in current guidelines in contrast with the focus on controlling excessive sodium intake [12, 13]. Data from two recent large-scale randomized controlled trials suggest that the role of adequate potassium intake in CVD risk management should be revisited [14]. Specifically, both the SSaSS (Salt Substitute and Stroke Study) study and the DECIDE-Salt (The Diet, ExerCIse and carDiovascular hEalth-Salt Reduction Strategies for Seniors in Residential Facilities) study showed that potassium supplementation significantly reduced the risk of CVD even without an overt reduction in sodium intake [15, 16]. Noteworthy, subgroup analysis in SSaSS study suggested that potassium supplementation had a similar beneficial effect on stroke prevention in both diabetic and non-diabetic subpopulations [16].
The majority of the previous clinical studies on the relationship between potassium intake and CVD outcome have focused on the general population whereas data from the patients with diabetes are scarce. To our knowledge, only one prospective study reported an inverse association between potassium intake and a composite cardio-renal outcome in patients with type 2 diabetes and preserved kidney function. Nevertheless, the authors did not specifically report the CVD outcome [17]. To fill this knowledge gap, we sought to study whether the level of potassium intake may predict the risk of major cardiovascular events (MACE) and all-cause mortality in individuals with type 2 diabetes. We hypothesize that a higher level of potassium intake estimated from urine excretion is associated with a lower risk of MACE in patients with diabetes.
Methods
We adopted a discovery and trans-ethnic validation approach for this prospective cohort study.
Participants
The discovery study was nested in Singapore KTPH-DKD (Khoo Teck Puat Hospital Diabetic Kidney Disease) cohort, an observational study aimed at characterizing the risk factors for CVD and CKD in South East Asian patients with diabetes [3, 18]. Briefly, 2061 patients with type 2 diabetes and age above 21 years old were recruited consecutively from outpatient clinics in a regional hospital in Singapore between March 2004 and December 2015. Type 2 diabetes was diagnosed by attending physicians after excluding type 1 diabetes and diabetes attributable to specific causes. Exclusion criteria were pregnancy, cancer, autoimmune disease and overt infection on active treatments, end stage kidney disease (sustained eGFR below 15 ml/min/1.73 m2 or dialysis) and kidney disease attributable to known genetic or immune causes. We excluded 82 participants with no urine samples available and 240 participants with missing values of either weight or height which prevented the estimation of 24-h potassium intake from fasting spot urine. We further excluded 167 participants with baseline eGFR < 30 ml/min/1.73 m2 because patients with advanced kidney disease are often advised to reduce intake of high potassium foods due to the perceived risk of hyperkalemia.
The validation study is nested in the Chronic Renal Insufficiency Cohort (CRIC), a multicenter observational study aimed at characterizing the risk factors for CVD and CKD progression in patients with CKD in the United States. Baseline demographic and clinical characteristics of the cohort have been described previously [19]. The data used for the current study were provided by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Central Repository. Of 3766 CRIC participants with 24-h urine potassium measured at baseline visit, 1818 had diabetes at cohort enrolment. We included all 1430 participants with diabetes and baseline eGFR ≥ 30 ml/min/1.73 m2 in this analysis. It was estimated that type 1 diabetes might account for 10% of CRIC participants with diabetes. Participant selection has been illustrated in (Additional File 1, Figure S1).
Both discovery and validation cohort studies are observational. Participants were treated by their attending physicians in usual clinical care. No protocol-based dietary advice was given to participants.
Identification of MACE and mortality events
The primary outcome was 3-point MACE, i.e. a composite of myocardial infarction, stroke and cardiovascular death, whichever occurred first. The secondary outcome was the individual component of the composite MACE and all-cause mortality.
In discovery cohort, participant baseline clinical data were linked with the Singapore myocardial infarction registry, stroke registry and death registry, respectively, by the unique national registration identification number. The myocardial infarction registry captures fatal and non-fatal cases diagnosed in all public and private hospitals based on symptoms, cardiac biomarkers and/or abnormal electrocardiogram. Similarly, stroke was diagnosed by certified doctors based on clinical signs of cerebral function disturbance lasting more than 24 h with no apparent cause other than a vascular origin. Cardiovascular death was identified by the primary cause of death on the death certificate. As the notification of all myocardial infarction cases and death events to the national registry is mandated by law, the capture rate may be considered 100%. The stroke registry captures cases diagnosed in all public hospitals and covers about 94% of the stroke cases in the country [20]. Event identification for this study was censored at December 31, 2018.
In validation cohort, myocardial infarction, stroke, cardiovascular death and all-cause death events were ascertained by CRIC investigators and transferred through NIDDK central repository.
Estimation of 24-h potassium intake
Urinary potassium excretion is an established surrogate for dietary potassium intake because dietary potassium is primarily excreted in urine [21]. In discovery study, spot urine specimen was collected after overnight fasting. The 24-h urine potassium excretion was estimated by the formula described by Kawasaki (Additional File 1, supplementary method) [22]. This method was derived in Asian population and has been widely used in observational studies [8, 23]. In the validation study in CRIC participants, 24-h urine potassium excretion was measured in 24-h urine collection during the baseline visit.
Clinical and biochemical variables
In the discovery cohort, age, sex, ethnicity and smoking status were self-reported. History of cardiovascular disease which included non-fatal myocardial infarction and stroke were queried at cohort recruitment and validated by reviewing electronic medical records after enrolment. Blood pressure was measured by a semi-auto sphygmomanometer. Body weight was measured by a calibrated scale and height was measured by stadiometer. Body mass index (BMI) was calculated as weight in kg divided by the square of height in meter. HbA1c was measured by immunoturbidimetric method (Cobas Integra 800 Analyzer; Roche, Basel, Switzerland). High-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol and triacylglycerol were measured by enzymatic methods (Cobas C System; Roche Diagnostic GmbH, Mannheim, Germany). Serum and urine creatinine was quantified by an enzymatic method traceable to an isotope dilution mass spectrometry reference. The estimated glomerular filtration rate (eGFR) was calculated based on serum creatinine using 2009 CKD-EPI (Chronic Kidney Disease-Epidemiology Collaboration) formula. Urine albumin was quantified using an immunoturbidimetric assay (Roche Cobas c; Roche Diagnostics, Mannheim, Germany). Albuminuria was presented as urine albumin-to-creatinine ratio (ACR).
In the validation cohort, baseline clinical and biochemical variables were collected by CRIC investigators and have been described in details elsewhere [19]. Consistent with the discovery study, we also used eGFR estimated by CKD-EPI formula based on serum creatinine in this analysis. Information on diabetes duration was not collected in CRIC study.
Statistical analysis
Clinical and biochemical variables were presented as mean (SD), median (interquartile range, IQR) or percentage where appropriate. Urine ACR and serum triacylglycerol were natural log-transformed due to the skewed distribution. Differences in baseline characteristics across tertiles of 24-h urine potassium excretion were compared using one-way ANOVA or χ2 test for continuous and categorical variables respectively, whilst differences between participants with and without MACE events were compared by Student t test or χ2 test. We handled missing values by listwise deletion because of the low rate of missingness (< 0.5% for each variable in both cohorts).
We plotted the cumulative risk of MACE across tertiles of 24-h potassium excretion using the Kaplan-Meier method. We fitted multivariable Cox proportional hazard (PH) regression models to study the associations between the 24-h potassium excretion at baseline and MACE outcome during follow-up. Based on biological plausibility, we adjusted age, sex, ethnicity (Chinese or White as reference, respectively), CVD history (yes or no), active smoking (yes or no), BMI, diabetes duration (discovery cohort only), HbA1c, SBP, HDL cholesterol, LDL cholesterol, triacylglycerol (log-transformed), baseline eGFR, urine ACR (log-transformed), usage of renin-angiotensin system (RAS) blocker (yes or no) and statin (yes or no). We adjusted usage of diuretics (yes or no) only in validation cohort because this variable was not available in discovery cohort. We assessed the associations between 24-h urine potassium excretion and individual cardiovascular outcome as well as all-cause mortality using the same approach. PH assumption was assessed by Schoenfeld residuals and by modelling the product of urine potassium X time as a covariate in the Cox regression models. We did not identify violation of PH assumption in Cox regression models.
We included the multiplicative interaction term (urine potassium tertile X subgroup) as a covariate in the Cox regression models to assess whether sex, CKD status (eGFR ≥ 60 vs. < 60 ml/min/1.73 m2), albuminuria severity (urine ACR ≥ 300 vs. < 300 mg/g), HbA1c level (≥ 8% vs. < 8%) and CVD history (yes or no) modified the association between 24-h urine potassium excretion and MACE outcome, respectively.
We performed two sensitivity analyses to assess the robustness of the main findings. One, we treated non-CVD death as a competing risk in the analysis on MACE outcome (Fine and Gray subdistribution). Two, we re-defined the tertile stratification of 24-hour urine potassium excretion in validation cohort using the same cut-off value in the discovery cohort.
Data analyses were performed using STATA 18 (StataCorp LP, Texas) and SPSS (version 27, IBM, Chicago, IL). A two-sided p value < 0.05 was considered as statistically significant.
Results
Participant characteristics
A total of 1572 participants were included in the discovery study. Mean age was 57 +/- 12 years old and diabetes duration was 11 +/- 9 years. The proportion of Chinese, Malay and Asian Indian was 62%, 20% and 18%, respectively. The mean eGFR at baseline was 83 +/- 26 ml/min/1.73 m2 (Table 1). The median of potassium intake estimated from spot urine was 2.09 (IQR 1.78–2.44) g/day while only 24 (1.5%) participants had potassium intake above 3.5 g/day as recommended by the guideline [24]. Compare to those in the lower tertile, participants with potassium intake in the top tertile were younger, had a lower systolic blood pressure, higher BMI, higher eGFR and lower urine ACR. They were more likely to be of male sex, Chinese ethnicity and active smoker (Additional File 1, Table S1).
Table 1.
Participant baseline clinical and biochemical characteristics
| KTPH-DKD cohort | CRIC cohort | |
|---|---|---|
| N = 1572 | N = 1430 | |
| Estimated potassium intake (g/24 h)* | 2.09 (1.78–2.44) | 2.02 (1.52–2.73) |
| Age (years) | 57.1 ± 11.8 | 58.5 ± 9.8 |
| Male sex (%) | 61.9 | 57.1 |
| Ethnicity (%) | ||
| Chinese | 62.1 | – |
| Malay | 20.4 | – |
| Asian Indian | 17.5 | – |
| White | – | 42.8 |
| Black | – | 44.3 |
| Others | – | 12.9 |
| Active smoking (%) | 13.9 | 12.0 |
| CVD history (%) | 8.6 | 41.3 |
| Body mass index (kg/m2) | 26.8 ± 5.2 | 34.2 ± 8.2 |
| Diabetes duration (years)** | 11.3 ± 8.5 | – |
| HbA1c (%) | 8.5 ± 2.0 | 7.7 ± 1.7 |
| HbA1c(mmol/mol) | 69 ± 16 | 61 ± 13 |
| Blood pressure (mmHg) | ||
| Systolic | 135 ± 19 | 132 ± 22 |
| Diastolic | 77 ± 11 | 70 ± 13 |
| Lipid profile (mM) | ||
| HDL cholesterol | 1.26 ± 0.38 | 1.16 ± 0.36 |
| LDL cholesterol | 2.80 ± 0.90 | 2.48 ± 0.88 |
| Triacylglycerol | 1.49 (1.08–2.17) | 1.51 (1.04–2.20) |
| eGFR (ml/min/1.73 m2) | 83 ± 26 | 47 ± 12 |
| Urine ACR (mg/g) | 37 (11–176) | 102 (15–691) |
| Medication usage (%) | ||
| RAS blocker | 63.0 | 81.5 |
| Diuretics** | – | 68.0 |
| Statin | 75.4 | 68.6 |
| Insulin | 33.9 | 49.8 |
ACR albumin-to-creatinine ratio, CVD cardiovascular disease, RAS renin-angiotensin system
*Potassium intake was estimated from 24-h urine potassium excretion. 24-h urine potassium excretion was estimated by Kawasaki formula in fasting spot urine in Singapore KTPH-DKD cohort whilst it was measured in 24-h urine collection in CRIC cohort
**Data on diuretics usage in KTPH-DKD cohort and diabetes duration in CRIC cohort were not collected. Normally distributed continuous variables were presented as mean ± SD. Serum triacylglycerol, urine ACR and 24-h urine potassium excretion were presented as median (interquartile range)
A total of 1430 CRIC participants with diabetes were included in the validation study. Their mean age was 59 +/- 10 years old. The proportion of the White, Black and other ethnicity was 43%, 44% and 13%, respectively. The mean eGFR at baseline was 47 +/- 12 ml/min/1.73 m2 (Table 1). The median of potassium intake estimated from potassium in 24-hour urine collection was 2.02 (IQR 1.52–2.73) g/day whilst 133 (9.3%) participants had potassium intake above 3.5 g/day. Compare to those in the lower tertile, participants with potassium intake in the top tertile had lower SBP, HbA1c, higher BMI and eGFR. They were more likely to be male sex, White race and less likely to be active smokers (Additional File 1, Table S2).
Association between estimated potassium intake and risk of MACE in discovery cohort
During a median of 8.2 (IQR 4.1–10.1) years of follow-up (11,520 patient-years), we identified 341 MACE events in the discovery cohort (crude incidence 2.96 per 100 patient-years). Compared to counterparts without the events, participants with MACE occurrence were older, had a longer diabetes duration, higher HbA1c, SBP, lower eGFR and higher urine ACR. Also, they were more likely to have CVD history and be active smokers (Additional File 1, supplementary Table S3).
Compared to those in the lowest tertile, participants with potassium intake in the top tertile had a 34% lower risk for the MACE outcome after adjustment for known demographic and cardio-renal risk factors including eGFR and urine ACR (adjusted HR 0.66 [0.49–0.89], top vs. lowest tertile, Table 2).
Table 2.
Associations between potassium intake estimated from 24-h urine potassium excretion and risk of major adverse cardiovascular events (MACE)
| All participants | Urine ACR < 300 mg/g | Urine ACR ≥ 300 mg/g | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Discovery (KTPH-DKD) | ||||||
| Event/N | 341/1572 | 228/1269 | 113/303 | |||
| Tertile 1 | Reference | Reference | Reference | |||
| Tertile 2 | 0.84 (0.65–1.09) | 0.19 | 0.91 (0.66–1.24) | 0.54 | 0.61 (0.37–1.00) | 0.05 |
| Tertile 3 | 0.66 (0.49–0.89) | 0.007 | 0.63 (0.43–0.93) | 0.020 | 0.65 (0.38–1.11) | 0.11 |
| Validation (CRIC) | ||||||
| Event/N | 381/1393 | 198/883 | 183/510 | |||
| Tertile 1 | Reference | Reference | Reference | |||
| Tertile 2 | 0.88 (0.68–1.13) | 0.31 | 0.75 (0.53–1.05) | 0.09 | 1.02 (0.70–1.50) | 0.92 |
| Tertile 3 | 0.84 (0.64–1.10) | 0.20 | 0.61 (0.42–0.90) | 0.012 | 1.14 (0.77–1.69) | 0.51 |
Multivariable Cox PH regression model: time to first MACE event was outcome. The estimated potassium intake was exposure (tertile 1 with the lowest potassium intake as reference). Multivariable model adjusted age, sex, ethnicity (Chinese and White as reference, respectively), CVD history (yes or no), active smoking (yes or no), body mass index, diabetes duration (Singapore cohort only), HbA1c, SBP, HDL cholesterol, LDL cholesterol, triacylglycerol (log-transformed), eGFR, urine ACR (log-transformed), usage of statin (yes or no), RAS blocker (yes or no) and diuretics (yes or no, CRIC only). HR (95% CI) that reach statistical significance are bold
We identified 181 events of myocardial infarction, 109 events of stroke and 141 events of death attributable to CVD causes. Compared to the lowest tertile, participants with potassium intake in the top tertile had a 34% lower risk for myocardial infarction (adjusted HR 0.66 [0.43–1.00]) and 51% lower risk for stroke (adjusted HR 0.49 [0.29–0.84]) after adjustment for known cardio-renal risk factors. The association between potassium intake and CVD death was not statistically significant in the multivariable model (Additional File 1, supplementary Table S4).
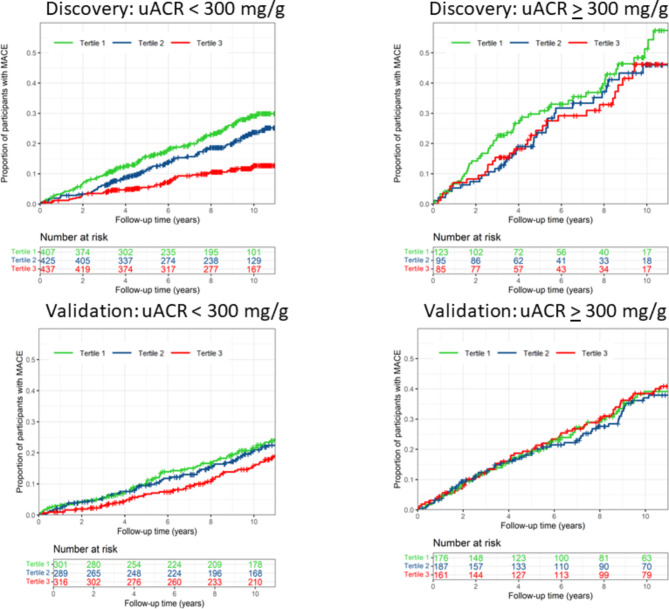
Urine albuminuria level modified the association between potassium intake and the risk of MACE (p for interaction < 0.05). Subgroup analysis showed that the inverse association between potassium intake and the risk of MACE was more pronounced in participants with normal to moderately elevated albuminuria (ACR < 300 mg/g), while the association was not statistically significant in those with severely elevated albuminuria (ACR ≥ 300 mg/g, Table 2; Fig. 1).
Fig. 1.
Cumulative of risk of MACE stratified by level of potassium intake estimated from 24-h urine potassium excretion in participants with normal to moderately elevated albuminuria (urine ACR < 300 mg) and those with severely elevated albuminuria (urine ACR ≥ 300 mg/g)
The level of estimated potassium intake did not interact with sex, CVD history (yes vs. no), HbA1c level (≥ 8% vs. < 8%) and CKD status (eGFR ≥ 60 vs. < 60 ml/min/1.73 m2, Additional File 1, supplementary Table S5) in association with MACE outcome (all p values for interaction terms > 0.05).
Validation study in CRIC cohort
During a median of 10.9 (IQR 5.4–14.4) years of follow-up (14,124 patient-years), 390 MACE events were registered in validation cohort (crude incidence 2.76 per 100 patient-years). Similar to that in discovery study, CRIC participants with MACE occurrence were older, had a higher HbA1c, SBP, a lower eGFR and a higher urine ACR. They were more likely to have CVD history, be of active smoker and male sex (Additional File 1, supplementary Table S3).
Consistent with findings in the discovery study, urine albuminuria level also modified the association between the estimated potassium intake and the risk of MACE in CRIC participants with diabetes (p for interaction < 0.05). In the subgroup with normal to moderately elevated albuminuria, participants with estimated potassium intake in the top tertile had 39% lower risk for MACE after adjustment for clinical risk factors (adjusted HR 0.61 [0.42–0.90]) as compared to the lowest tertile. In contrast, potassium intake level was not associated with the risk of MACE in those with severely elevated albuminuria (Table 2; Fig. 1). Further analysis showed that the associations between estimated potassium intake and myocardial infarction and stroke were more pronounced in participants with normal to moderately elevated albuminuria as compared to those with severely elevated albuminuria. Estimated potassium intake was not significantly associated with risk of CVD death (Additional File 1, Table S6).
Sensitivity analysis
The associations between estimated potassium intake and risk of MACE were minimally changed when non-CVD death was treated as a competing risk for MACE in both discovery and validation cohorts (Additional File 1, supplementary Table S7). Similar outcome was obtained when the tertiles of estimated potassium intake in CRIC cohort were re-defined using the cutoff value in KTPH-DKD cohort (Additional File 1, supplementary Table S8).
Association of estimated potassium intake with risk of all-cause mortality
We identified 315 death events during follow-up in the discovery cohort. Compared to the lowest tertile, participants with estimated potassium intake in the top tertile had a significantly lower risk for all-cause death in univariable analysis (HR 0.42 [0.32–0.56]). However, the strength of association was non-significant after adjustment for cardio-renal risk factors (adjusted HR 0.77 [0.56–1.06], Table 3).
Table 3.
Associations between potassium intake estimated from 24-h urine potassium excretion and risk of all-cause mortality
| Discovery (KTPH-DKD) | Validation (CRIC) | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Event/N | 315/1572 | 704/1393 | ||
| Tertile 1 | Reference | Reference | ||
| Tertile 2 | 0.81 (0.62–1.07) | 0.13 | 0.87 (0.72–1.04) | 0.12 |
| Tertile 3 | 0.77 (0.56–1.06) | 0.12 | 0.69 (0.57–0.84) | < 0.001 |
Multivariable Cox PH regression: time to all-cause death event was outcome. Potassium intake estimated from 24-h urine potassium excretion was exposure (tertile 1 with lowest potassium intake as reference). Multivariable model adjusted age, sex, ethnicity (Chinese and White as reference, respectively), CVD history (yes or no), active smoking (yes or no), BMI, diabetes duration (Singapore cohort only), HbA1c, SBP, HDL cholesterol, LDL cholesterol, triacylglycerol (log-transformed), eGFR, urine ACR (log-transformed), usage of statin (yes or no), RAS blocker (yes or no) and diuretics (yes or no, CRIC only). HR (95% CI) that reach statistical significance are bold
In the validation cohort, 704 death events were registered during follow-up. Compared to those in the lowest tertile, CRIC participants with estimated potassium intake in the top tertile had a 31% lower risk for all-cause death after adjustment for known clinical risk factors (adjusted HR 0.69 [0.57–0.84], Table 3).
Albuminuria level, HbA1c level and CVD history did not modify the association between the level of potassium intake and risk of all-cause death in either the discovery or the validation cohort (all p value for interaction terms > 0.05).
Discussion
In this prospective study which included two cohorts of patients with vast differences in genetic background, environmental exposure and a broad spectrum of kidney function, we found that a high level of potassium intake estimated from potassium excretion in spot urine and 24-hour urine collection, respectively, was associated with a low risk for the adverse cardiovascular outcome in patients with diabetes, especially in those with normal to moderately elevated albuminuria. Also, a high level of estimated potassium intake was potentially associated with a relatively low risk of all-cause death regardless of albuminuria status. The inverse associations between estimated potassium intake and the risk of MACE and all-cause death were independent of traditional cardio-renal risk factors including blood pressure, lipid profile and baseline kidney filtration function. These data suggest that increasing potassium intake may be a potential effective strategy for CVD risk reduction in patients with diabetes, if this hypothesis can be tested in future interventional studies.
Previous observational studies have associated a high level of potassium intake estimated from urine potassium with a low risk for cardiovascular events in the general population [6, 9, 10, 25, 26], although inconsistent outcomes have also been reported. For example, Groenland et al. found that a high 24-hour urine potassium excretion estimated using the Kawasaki formula was associated with an increased risk of recurrent MACE and mortality in participants with prevalent vascular disease [27], whilst Kieneker et al. reported that measured 24-hour urinary potassium excretion was not independently associated with cardiovascular events in participants oversampled with albuminuria [28]. We postulate that these discrepancies may be partly accounted by the differences in population characteristics and comorbidities. Noteworthy, two recently reported cluster randomized controlled trials showed that potassium supplementation by replacing 25% of regular salt with potassium chloride significantly reduced the risk of CVD independent of sodium intake [15, 16], supporting that increasing potassium intake may be effective for CVD risk reduction at the population level. To our knowledge, data on the relationship between potassium intake and major cardiovascular events in diabetic population are scarce. Our data fills this knowledge gap and may hopefully prompt future interventional studies to determine the efficacy of increasing potassium intake as an approach for CVD risk control in patients with diabetes.
We reasoned that a high potassium intake may reduce the risk of cardiovascular disease through blood pressure—lowering effect and blood pressure—independent mechanisms in patients with diabetes. The blood pressure-lowering effect of potassium has been well established [6]. However, the association of potassium intake with CVD outcome was independent of blood pressure in the current and previous studies, suggesting that the blood pressure lowering effect only partly mediates the CVD risk reduction conferred by an increased amount of potassium intake. High potassium intake may also improve endothelial function and prevent atherosclerosis by a blood pressure-independent mechanism [29]. More importantly, potassium-rich foods like fresh fruits, vegetables and whole grains are major components of a healthy diet [30, 31]. They contain a high amount of vitamins, fiber, high quality protein and lipids which have beneficial effects on cardiovascular health [32]. We could not address whether the inverse association of potassium intake with risk of CVD was mediated by healthy food in our study. Nevertheless, the SSaSS and DECIDE-Salt studies provide strong evidence to support that increasing potassium intake alone may reduce CVD events independent of foods rich in potassium.
Under-consumption of potassium has been a public health concern for decades [33]. Both World Health Organization and the Europe Food Safety Authority recommend 3.5 g potassium intake per day [24, 34]. Other than among those with advanced CKD and high risk of hyperkalemia, current guideline does not recommend a restriction on potassium intake [35]. The estimated ~ 2.1 g/day potassium intake in participants of our cohorts was close to the 2.4 g/day intake in the general population in Singapore [36], and 2.2 g/day in the general population in US [37, 38]. Of note, only less than 10% participants have potassium intake reaching the recommended level, highlighting the existence of under-consumption of potassium in patients with diabetes in both Asia and the United States [14].
There are two approaches to increase potassium intake, i.e. through consuming a high amount of potassium-rich foods or by direct potassium supplementation such as salt substitution [14]. The latter approach does not require a dramatic change in dietary pattern in individuals [39]. Given that there are no robust data from clinical trials demonstrating the efficacy and safety of potassium supplementation in patients with diabetes, clinical practitioners may encourage patients consume a high amount of potassium-rich foods which are in line with current guidelines [12, 13].
The finding that potassium intake interacted with albuminuria level, but not the eGFR-based CKD status, in association with MACE is novel and intriguing. Albuminuria and a low eGFR represent overlapping but distinct pathophysiology associated with kidney disease [40]. Albuminuria may reflect systemic endothelial dysfunction that alters inflammatory and thrombotic cascades, leading to atherothrombotic events [41]. Compared to a low eGFR, albuminuria is a more sensitive biomarker for incident cardiovascular events in patients with diabetes mellitus [42]. In an early study in CRIC cohort, an elevated albuminuria but not a low eGFR was independently associated with risk of stroke [43]. We postulate that patients with diabetes and severely elevated albuminuria may represent the subpopulation with severe endothelial dysfunction and a greater atherosclerotic burden. Therefore, a high level of potassium intake may not significantly alter the disease course of CVD in this subpopulation. Nevertheless, an increased potassium intake was associated with a low risk of all-cause mortality regardless of albuminuria levels, suggesting that an increased potassium intake may confer beneficial effects beyond CVD risk reduction in patients with diabetes.
Strengths of the current work include a relatively large sample size and a long-term follow-up. We have considered the main cardiovascular risk factors including smoking, CVD history, dyslipidemia and blood pressure in our study. Notably, we adjusted eGFR and urine ACR, two important determinants of CVD and mortality in diabetic population, in our data analysis. The main findings in two cohorts of patients with vast differences in CKD status, genetic background and environmental exposure were generally in agreement despite that we used different approaches to estimate potassium intake, suggesting our findings are potentially generalizable.
Several important weaknesses should be highlighted. First, we do not have data on dietary intake and food sources of potassium. A high intake of potassium-rich foods is often associated with choice of other healthier lifestyles such as increased physical activity which cannot be accounted for in this study [30, 31]. Second, although widely used in epidemiology studies, the 24-hour urine potassium excretion estimated by Kawasaki formula based on fasting spot urine lacks accuracy for individuals [37]. This may introduce random error, reduce statistical precision and likely bias the association toward the null, i.e. false negative, although this weakness of methodology may not be a major concern for the statistically significant findings in the discovery cohort. On the other hand, we would highlight that urine potassium excretion might have been altered in the presence of kidney impairment, comorbidity and medication usage in patients with diabetes. Further studies are warranted to ascertain to what extent the potentially altered kidney potassium metabolism may affect the relationship between 24-hour urine potassium excretion and dietary potassium intake specifically in patients with diabetes and moderately impaired kidney function. Third, due to nature of the study, we cannot infer causality. Neither are we able to elucidate the pathophysiologic mechanisms underlying the association between potassium intake and CVD risk. Fourth, like all observational studies, unmeasured or residual confounding is inevitable. For example, we do not have information on diuretics usage in discovery cohort. Some important risk factors for CVD such as socioeconomic status are not available in this study either.
Conclusion
A high level of potassium intake estimated from urine potassium excretion was associated with a low risk for cardiovascular events and all-cause mortality independent of clinical cardio-renal risk factors in patients with diabetes. Our findings may lay the foundation for, and prompt future interventional studies to explore whether increasing potassium intake is an effective approach for cardiovascular prevention in patients with diabetes, especially in those with normal to moderately elevated albuminuria.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Material 1. Supplementary method: Kawasaki formula for estimation of 24-h urine potassium excretion using morning spot urine; Fig. S1: participant selection flow chart. Table S1: Clinical and biochemical characteristics of participants in Singapore KTPH-DKD cohort (discovery) stratified by potassium intake tertile; Table S2: Clinical and biochemical characteristics of CRIC participants with diabetes (validation cohort) stratified by tertiles of estimated potassium intake; Table S3: Participant baseline clinical and biochemical characteristics stratified by MACE occurrence during follow-up; Table S4: Associations of estimated potassium intake with myocardial infarction, stroke and CVD death in Singapore KTPH-DKD cohort (discovery); Table S5: Association of estimated 24-h potassium intake with risk of MACE stratified by CKD status in discovery cohort; Table S6: Association between estimated potassium intake and myocardial infarction, stroke and CVD death in CRIC participants with diabetes (validation); Table S7: Associations of estimated potassium intake and risk of MACE after taking non-CVD death as a competing risk (Fine and Gray subdistribution); Table S8: Associations of estimated potassium intake with risk of MACE in CRIC cohort (re-define tertile of estimated potassium intake in CRIC using the cut-off values from KTPH-DKD cohort).
Acknowledgements
We thank the Singapore National Registry of Disease Office (NRDO) for providing information on myocardial infarction, stroke and death outcomes by data linkage. We thank all staff from the Clinical Research Unit in Khoo Teck Puat hospital in Singapore for their assistance with participant recruitment, biobanking and data storage. We thank CRIC participants and investigators for their contribution to our work by sharing their data through NIDDK data repository. The CRIC study was conducted by the CRIC Investigators and supported by NIDDK. This manuscript was not prepared in collaboration with investigators of the CRIC study and does not necessarily reflect the opinions or views of the CRIC study, the NIDDK Central Repository, or NIDDK.
Abbreviations
- ACR
Albumin-to-creatinine ratio
- CKD
Chronic kidney disease
- CKD-EPI
Chronic kidney disease epidemiology collaboration
- CRIC
Chronic renal insufficiency cohort
- CVD
Cardiovascular disease
- DECIDE-Salt
The Diet, ExerCIse and carDiovascular hEalth-salt reduction strategies for seniors in residential facilities
- eGFR
Estimated glomerular filtration rate
- HDL
High density lipoprotein
- IQR
Interquartile range
- LDL
Low density lipoprotein
- MACE
Major adverse cardiovascular event
- NIDDK
National institute of diabetes and digestive and kidney
- SSaSS
Salt Substitute and Stroke Study
Author contributions
JJL designed the study. JJL, SL, TKK, CC, RLG, KA, JL and SCL acquired clinical data. HZ, SL and JJL performed data analysis. JJL wrote the manuscript. All co-authors contributed important intellectual knowledge, critically revised the manuscript and approved publication of the work.
Funding
This work was supported by Khoo Teck Puat hospital STAR Grant (23201) and Singapore National Medical Research Council Grant (000714-01). The funders have no roles in study design, data collection, analysis, writing and decision for publication.
Data availability
The dataset generated/analysed during the study could not be shared publicly. However, anonymized data may be shared upon reasonable request from corresponding authors after obtaining approval from Singapore National Healthcare Group Ethic Committee.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the principles laid by Declaration of Helsinki. KTPH-DKD study was approved by the Singapore National Healthcare Group Domain Specific Review Committee (DSRB 2017/00662, May 2019). All participants gave written informed consent. Consent from CRIC participants for the current study was waived by NIDDK.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, Williams DE, Geiss L. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med. 2014;370(16):1514–23. [DOI] [PubMed] [Google Scholar]
- 2.Sattar N, McMurray J, Boren J, Rawshani A, Omerovic E, Berg N, Halminen J, Skoglund K, Eliasson B, Gerstein HC, et al. Twenty years of cardiovascular complications and risk factors in patients with type 2 diabetes: a nationwide Swedish cohort study. Circulation. 2023;147(25):1872–86. [DOI] [PubMed] [Google Scholar]
- 3.Liu JJ, Choo RWM, Liu S, Gurung RL, Wee SL, Lim SC. Cause-specific mortality in multiethnic South East Asians with type 2 diabetes mellitus. Asia Pac J Public Health. 2019;31(4):306–14. [DOI] [PubMed] [Google Scholar]
- 4.Gregg EW, Cheng YJ, Srinivasan M, Lin J, Geiss LS, Albright AL, Imperatore G. Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: an epidemiological analysis of linked national survey and vital statistics data. Lancet. 2018;391(10138):2430–40. [DOI] [PubMed] [Google Scholar]
- 5.Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aburto NJ, Hanson S, Gutierrez H, Hooper L, Elliott P, Cappuccio FP. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta-analyses. BMJ. 2013;346: f1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kieneker LM, Gansevoort RT, Mukamal KJ, de Boer RA, Navis G, Bakker SJ, Joosten MM. Urinary potassium excretion and risk of developing hypertension: the prevention of renal and vascular end-stage disease study. Hypertension. 2014;64(4):769–76. [DOI] [PubMed] [Google Scholar]
- 8.Mente A, O’Donnell MJ, Rangarajan S, McQueen MJ, Poirier P, Wielgosz A, Morrison H, Li W, Wang X, Di C, et al. Association of urinary sodium and potassium excretion with blood pressure. N Engl J Med. 2014;371(7):601–11. [DOI] [PubMed] [Google Scholar]
- 9.O’Donnell M, Mente A, Rangarajan S, McQueen MJ, Wang X, Liu L, Yan H, Lee SF, Mony P, Devanath A, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med. 2014;371(7):612–23. [DOI] [PubMed] [Google Scholar]
- 10.O’Donnell MJ, Yusuf S, Mente A, Gao P, Mann JF, Teo K, McQueen M, Sleight P, Sharma AM, Dans A, et al. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA. 2011;306(20):2229–38. [DOI] [PubMed] [Google Scholar]
- 11.Whelton PK, He J, Cutler JA, Brancati FL, Appel LJ, Follmann D, Klag MJ. Effects of oral potassium on blood pressure. Meta-analysis of randomized controlled clinical trials. JAMA. 1997;277(20):1624–32. [DOI] [PubMed] [Google Scholar]
- 12.Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Back M, Benetos A, Biffi A, Boavida JM, Capodanno D, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42(34):3227–337. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association. 10. Cardiovascular disease and risk management: standards of care in diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S179–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Donnell M, Yusuf S, Vogt L, Mente A, Messerli FH. Potassium intake: the Cinderella electrolyte. Eur Heart J. 2023;44(47):4925–34. [DOI] [PubMed] [Google Scholar]
- 15.Yuan Y, Jin A, Neal B, Feng X, Qiao Q, Wang H, Zhang R, Li J, Duan P, Cao L, et al. Salt substitution and salt-supply restriction for lowering blood pressure in elderly care facilities: a cluster-randomized trial. Nat Med. 2023;29(4):973–81. [DOI] [PubMed] [Google Scholar]
- 16.Neal B, Wu Y, Feng X, Zhang R, Zhang Y, Shi J, Zhang J, Tian M, Huang L, Li Z, et al. Effect of salt substitution on cardiovascular events and death. N Engl J Med. 2021;385(12):1067–77. [DOI] [PubMed] [Google Scholar]
- 17.Araki S, Haneda M, Koya D, Kondo K, Tanaka S, Arima H, Kume S, Nakazawa J, Chin-Kanasaki M, Ugi S, et al. Urinary potassium excretion and renal and cardiovascular complications in patients with type 2 diabetes and normal renal function. Clin J Am Soc Nephrol. 2015;10(12):2152–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu JJ, Liu S, Saulnier PJ, Gand E, Choo RWM, Gurung RL, Hadjadj S, Lim SC. Association of urine haptoglobin with risk of all-cause and cause-specific mortality in individuals with type 2 diabetes: a transethnic collaborative work. Diabetes Care. 2020;43(3):625–33. [DOI] [PubMed] [Google Scholar]
- 19.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, et al. Chronic renal insufficiency cohort (CRIC) study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol CJASN. 2009;4(8):1302–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng SH, Wong AWK, Chen CH, Tan CS, Muller-Riemenschneider F, Chan BPL, Baum MC, Lee JM, Venketasubramanian N, Koh GC. Stroke factors associated with thrombolysis use in hospitals in Singapore and US: a cross-registry comparative study. Cerebrovasc Dis. 2019;47(5–6):291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holbrook JT, Patterson KY, Bodner JE, Douglas LW, Veillon C, Kelsay JL, Mertz W, Smith JC Jr. Sodium and potassium intake and balance in adults consuming self-selected diets. Am J Clin Nutr. 1984;40(4):786–93. [DOI] [PubMed] [Google Scholar]
- 22.Kawasaki T, Itoh K, Uezono K, Sasaki H. A simple method for estimating 24 h urinary sodium and potassium excretion from second morning voiding urine specimen in adults. Clin Exp Pharmacol Physiol. 1993;20(1):7–14. [DOI] [PubMed] [Google Scholar]
- 23.Smyth A, Dunkler D, Gao P, Teo KK, Yusuf S, O’Donnell MJ, Mann JF, Clase CM. The relationship between estimated sodium and potassium excretion and subsequent renal outcomes. Kidney Int. 2014;86(6):1205–12. [DOI] [PubMed] [Google Scholar]
- 24.Turck D, Bresson J-L, Burlingame B, Dean T, Fairweather-Tait S, Heinonen M, Hirsch-Ernst KI, Mangelsdorf I, McArdle H, Neuhäuser-Berthold M, et al. Dietary reference values for potassium. EFSA J. 2016;14(10): e04592. [Google Scholar]
- 25.Ma Y, He FJ, Sun Q, Yuan C, Kieneker LM, Curhan GC, MacGregor GA, Bakker SJL, Campbell NRC, Wang M, et al. 24-Hour urinary sodium and potassium excretion and cardiovascular risk. N Engl J Med. 2022;386(3):252–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aaron KJ, Sanders PW. Role of dietary salt and potassium intake in cardiovascular health and disease: a review of the evidence. Mayo Clin Proc. 2013;88(9):987–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Groenland EH, Vendeville JP, Bots ML, de Borst GJ, Nathoe HM, Ruigrok YM, Blankestijn PJ, Visseren FLJ, Spiering W. The relation between urinary sodium and potassium excretion and risk of cardiovascular events and mortality in patients with cardiovascular disease. PLoS ONE. 2022;17(3): e0265429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kieneker LM, Gansevoort RT, de Boer RA, Brouwers FP, Feskens EJ, Geleijnse JM, Navis G, Bakker SJ, Joosten MM. Urinary potassium excretion and risk of cardiovascular events. Am J Clin Nutr. 2016;103(5):1204–12. [DOI] [PubMed] [Google Scholar]
- 29.He FJ, Marciniak M, Carney C, Markandu ND, Anand V, Fraser WD, Dalton RN, Kaski JC, MacGregor GA. Effects of potassium chloride and potassium bicarbonate on endothelial function, cardiovascular risk factors, and bone turnover in mild hypertensives. Hypertension. 2010;55(3):681–8. [DOI] [PubMed] [Google Scholar]
- 30.Mente A, Irvine EJ, Honey RJ, Logan AG. Urinary potassium is a clinically useful test to detect a poor quality diet. J Nutr. 2009;139(4):743–9. [DOI] [PubMed] [Google Scholar]
- 31.Ikizler TA, Burrowes JD, Byham-Gray LD, Campbell KL, Carrero JJ, Chan W, Fouque D, Friedman AN, Ghaddar S, Goldstein-Fuchs DJ, et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am J Kidney Dis. 2020;76(3 Suppl 1):S1–107. [DOI] [PubMed] [Google Scholar]
- 32.Loftfield E, Yi S, Curtis CJ, Bartley K, Kansagra SM. Potassium and fruit and vegetable intakes in relation to social determinants and access to produce in New York City. Am J Clin Nutr. 2013;98(5):1282–8. [DOI] [PubMed] [Google Scholar]
- 33.Ix JH, Anderson CAM. Measurements of 24-hour urinary sodium and potassium excretion: importance and implications. JAMA. 2018;319(12):1201–2. [DOI] [PubMed] [Google Scholar]
- 34.WHO Guideline Approval Committee. Guideline: potassium intake for adults and children. Geneva: WHO; 2012. [Google Scholar]
- 35.KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 2024;105(4S):S117–314. [DOI] [PubMed]
- 36.Talaei M, Koh WP, Yuan JM, van Dam RM. DASH dietary pattern, mediation by mineral intakes, and the risk of coronary artery disease and stroke mortality. J Am Heart Assoc. 2019;8(5): e011054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cogswell ME, Loria CM, Terry AL, Zhao L, Wang CY, Chen TC, Wright JD, Pfeiffer CM, Merritt R, Moy CS, et al. Estimated 24-hour urinary sodium and potassium excretion in US adults. JAMA. 2018;319(12):1209–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reddin C, Ferguson J, Murphy R, Clarke A, Judge C, Griffith V, Alvarez A, Smyth A, Mente A, Yusuf S, et al. Global mean potassium intake: a systematic review and Bayesian meta-analysis. Eur J Nutr. 2023;62(5):2027–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruzicka M, Hiremath S. Salt and hypertension: ’switch’ing the focus to potassium. Am J Kidney Dis. 2024;83(4):546–8. [DOI] [PubMed] [Google Scholar]
- 40.de Boer IH, Steffes MW. Glomerular filtration rate and albuminuria: twin manifestations of nephropathy in diabetes. J Am Soc Nephrol. 2007;18(4):1036–7. [DOI] [PubMed] [Google Scholar]
- 41.Pedrinelli R, Giampietro O, Carmassi F, Melillo E, Dell’Omo G, Catapano G, Matteucci E, Talarico L, Morale M, De Negri F, et al. Microalbuminuria and endothelial dysfunction in essential hypertension. Lancet. 1994;344(8914):14–8. [DOI] [PubMed] [Google Scholar]
- 42.Bouchi R, Babazono T, Yoshida N, Nyumura I, Toya K, Hayashi T, Hanai K, Tanaka N, Ishii A, Iwamoto Y. Association of albuminuria and reduced estimated glomerular filtration rate with incident stroke and coronary artery disease in patients with type 2 diabetes. Hypertens Res. 2010;33(12):1298–304. [DOI] [PubMed] [Google Scholar]
- 43.Sandsmark DK, Messe SR, Zhang X, Roy J, Nessel L, Lee Hamm L, He J, Horwitz EJ, Jaar BG, Kallem RR, et al. Proteinuria, but Not eGFR, predicts stroke risk in chronic kidney disease: chronic renal insufficiency cohort study. Stroke. 2015;46(8):2075–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1. Supplementary method: Kawasaki formula for estimation of 24-h urine potassium excretion using morning spot urine; Fig. S1: participant selection flow chart. Table S1: Clinical and biochemical characteristics of participants in Singapore KTPH-DKD cohort (discovery) stratified by potassium intake tertile; Table S2: Clinical and biochemical characteristics of CRIC participants with diabetes (validation cohort) stratified by tertiles of estimated potassium intake; Table S3: Participant baseline clinical and biochemical characteristics stratified by MACE occurrence during follow-up; Table S4: Associations of estimated potassium intake with myocardial infarction, stroke and CVD death in Singapore KTPH-DKD cohort (discovery); Table S5: Association of estimated 24-h potassium intake with risk of MACE stratified by CKD status in discovery cohort; Table S6: Association between estimated potassium intake and myocardial infarction, stroke and CVD death in CRIC participants with diabetes (validation); Table S7: Associations of estimated potassium intake and risk of MACE after taking non-CVD death as a competing risk (Fine and Gray subdistribution); Table S8: Associations of estimated potassium intake with risk of MACE in CRIC cohort (re-define tertile of estimated potassium intake in CRIC using the cut-off values from KTPH-DKD cohort).
Data Availability Statement
The dataset generated/analysed during the study could not be shared publicly. However, anonymized data may be shared upon reasonable request from corresponding authors after obtaining approval from Singapore National Healthcare Group Ethic Committee.