Abstract
Background
The elderly population is a high-risk group for tuberculosis, and increasing evidence demonstrates a comparatively high proportion of asymptomatic tuberculosis in this group. This study aimed to determine the proportion of asymptomatic tuberculosis among patients with active tuberculosis through active case finding in the elderly population.
Materials and methods
We searched for relevant articles published from the establishment of each database to December 31, 2023 in Web of Science, PubMed, VIP database, Chinese National Knowledge Infrastructure, and Wanfang database. The studies’ quality was assessed using the Agency for Healthcare Research and Quality’s criteria. We used the I² statistic and Q test to evaluate heterogeneity across the included studies. We employed subgroup analysis, sensitivity analysis, and meta-regression to pinpoint sources of heterogeneity. Moreover, Begg’s and Egger’s tests were employed to detect any potential publication biases.
Results
Nine studies involving 364,260 elderly individuals met the criteria for the analysis. In active case finding, the proportion of asymptomatic tuberculosis in the elderly population was 67.7% (95% CI: 54.7–79.5%, I2random effects model = 90.197, P < 0.001). The subgroup analysis revealed that the proportion of asymptomatic tuberculosis in high-burden countries was high, at 66.3% (95%CI: 52.5–78.9%, P < 0.001). Studies using multiple screening strategies including chest X-ray showed a higher percentage of asymptomatic patients, at 67.6% (95% CI: 51.1–82.1%, P < 0.001). However, in studies conducted after 2019 and studies with large sample sizes (≥ 15,000), the proportion of asymptomatic tuberculosis decreased (54.3%, 95%CI: 48.6–60.1%; and 62.3%, 95%CI: 45.9–77.4%, respectively).
Conclusions
The latest results revealed a significantly high percentage of elderly individuals with asymptomatic tuberculosis. This study highlighted the importance of mass screening to identify active tuberculosis cases in this specific group which could help health policymakers develop better strategies to reduce the burden of tuberculosis in the elderly population.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-024-21019-1.
Keywords: Asymptomatic tuberculosis, Active tuberculosis, Active case finding, Elderly
Background
Tuberculosis (TB) is a major global health concern. According to the Global Tuberculosis Report 2023, the estimated number of TB cases globally in 2022 is 10.6 million, equivalent to that in 2021, but showing an increasing trend compared with 2020 [1]. In some countries, such as South Korea, the elderly population accounts for a relatively large proportion, showing that between 2001 and 2018, the number of TB cases in the elderly population aged ≥ 65 years was consistently high as well [2]. Additionally, as a country with a high TB burden, China’s data from the fifth national TB epidemiological sampling survey showed that the prevalence rate of active TB among the elderly aged ≥ 65 years was 1,270 per 100,000, significantly higher than that in other age groups [3]. Factors such as a weakened immune system due to aging, inadequate nutrition, low socio-economic status, lack of healthcare access, diabetes, and other adverse health conditions raise the likelihood of TB in this specific group. Consequently, as the global population ages, TB has become an increasingly critical concern requiring heightened focus [4–11]. Addressing the prevention and control of TB in older adults is essential to mitigate the broader impact of this disease.
The elderly, with generally weaker health and declining physiological functions, often experience reduced resistance and compromised respiratory function. This can result in a diminished cough reflex and a muted response to pneumonia, thereby leading to fewer noticeable respiratory symptoms compared to younger patients, making the condition more difficult to detect [12]. For TB, avaiable findings showed that approximately 2/5 of elderly patients have no symptoms, in which some may excrete a large amount of Mycobacterium tuberculosis (MTB) in their sputum [3]. That is to say, although these patients do not show symptoms, they can become hidden sources of transmission in their daily lives and pose risks to social communication.
Active case finding (ACF), provided by health institutions using methods such as symptom screening, chest radiography, and/or pathogen identification, was crucial for identifying active TB. It could also provide opportunities for early detection, intervention, and treatment, thereby reducing TB spread at a household and community level. Increasing asymptomatic active TB cases were identified through the ACF strategy using mass screening in community level. The purpose of this study was to conduct a systematic review and meta-analysis to determine the proportion of asymptomatic TB.
Materials and methods
This systematic review and meta-analysis was carried out following the guidelines set by the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) standards (Table S1). The study has been officially recorded with PROSPERO and can be identified by the registration number CRD42024557826.
Literature search strategy
We performed a computer-assisted literature search across several databases, including PubMed, Web of Science, VIP database, Chinese National Knowledge Infrastructure (CNKI), and Wanfang database - spanning from the date of database inception until December 2023. This literature search aimed to identify articles reporting the proportion of asymptomatic TB in the elderly population using ACF. The search terms used included (“active case finding” OR “ACF” OR “active case screening” OR “mass screening” OR “active screening”) AND (“pulmonary tuberculosis” OR “PTB” OR “tuberculosis” OR “TB”) AND (“elderly” OR “aged”).
Eligibility criteria
The inclusion criteria were as follows: (1) Studies reporting the number of active TB cases, including both clinically diagnosed and bacteriologically confirmed cases that based on diagnostic criteria in different countries among the elderly population through active case finding. For instance, in China, a clinical diagnosis of TB is determined by chest imaging showing lesions indicative of active pulmonary TB (PTB), provided that other lung conditions are ruled out. Additionally, at least one of the following criteria must be met: The patient reports TB-related symptoms such as cough, sputum production, or hemoptysis; a tuberculin skin test yields moderate or greater reactivity, a gamma-interferon release assay is positive; an MTB antibody test is positive; extrapulmonary tissue pathology confirms TB; bronchoscopy shows TB-like changes; or exudative pleural effusion with increased adenosine deaminase levels is present. Bacteriologically diagnosed TB is characterized by a positive result from a TB smear, culture, or other rapid diagnostic tests approved by the WHO, such as Xpert MTB/RIF; (2) studies reporting the number of asymptomatic TB cases among the elderly population. Asymptomatic TB was defined as disease caused by viable MTB bacteria without clinical signs of TB, such as coughing up sputum for more than 2 weeks, night sweats, emaciation, hemoptysis, fever, malaise, chest tightness, chest pain, and anorexia, but with abnormalities detectable through radiographic imaging or microbiological assays; (3) studies that specifically mention the screening approach and the year screening was initiated.
The exclusion criteria were as follows: (1) Meta-analyses, reviews, case reports, meeting abstracts, or editorials; (2) studies not published in English or Chinese.
Data extraction
Y. Z and F. W evaluated the eligibility of the articles for inclusion. When disagreements arose, K. L, the third reviewer, made the final decision. Y. Z and F. W independently extracted data from the articles, including information such as the first author’s name and the publication year, country, study area, study design, study year, sample size, the number of active TB and asymptomatic TB cases in the elderly population, definition of symptomatic TB, and screening strategy. A provisional data extraction form was provided in Table S2. The initial data extraction tool was adjusted as required while extracting data from each article.
Risk of bias assessment and assessment of study quality
We used an Agency for Healthcare Research and Quality (AHRQ) tool for the quality assessment of cross-sectional studies, with a maximum score of 11 [17]. The AHRQ tool consisted of an 11-item checklist. If a question was answered “NO” or “UNCLEAR”, the item received a score of “0”; and if the reply was “YES”, the item received a score of “1”. Article were graded low quality if the score was 0–3, moderate quality if the score was 4–7, and high-quality if the score was 8–11.
Definition
30 high-burden countries include 20 nations with the highest absolute number of new TB cases and the top 10 countries with the highest TB incidence rates that are not among the former top 20 [18].
Statistical analysis
Data processing was performed using Stata version 18. The original studies utilized the number of active and asymptomatic TB cases screened by the ACF among the elderly population to calculate the proportion of asymptomatic TB among active TB patients. Statistical heterogeneity was assessed using Q and I2 statistics. During heterogeneity tests, a random-effects model was applied when P < 0.1, while a fixed-effects model was used when P > 0.1. Heterogeneity detection employed three methods: subgroup analysis encompassing the screening start year (2019 or before vs. after 2019), study country (high-burden countries vs. non-high-burden countries), screening strategy (chest X-ray (CXR) only vs. CXR and others), and sample size (< 15,000 vs. ≥ 15,000); sensitivity analysis was performed by progressively excluding each study and recalculating the pooled effect. Additionally, meta-regression analysis was used to pinpoint sources of heterogeneity. To assess publication bias, we employed Begg’s test and Egger’s linear regression test.
Results
Selection and characteristics of study publication
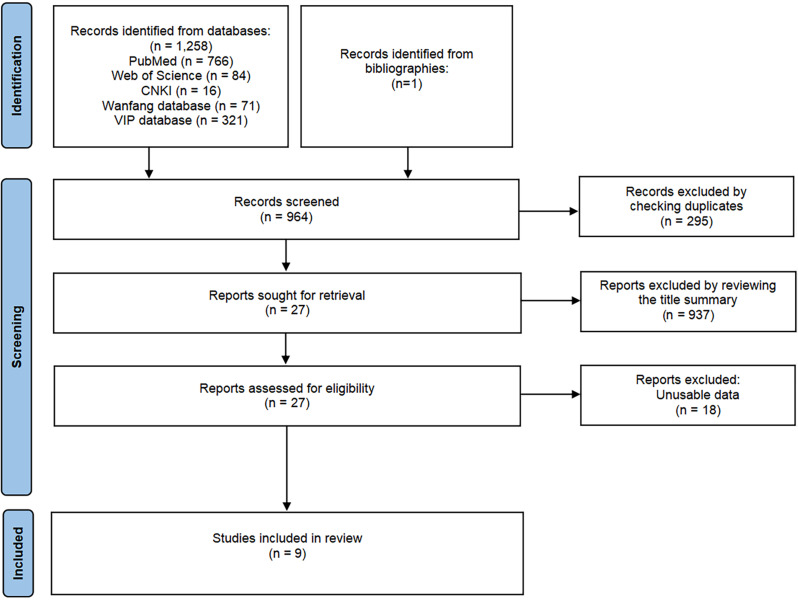
We identified a total of 1259 potential articles through searches in the PubMed, Web of Science, VIP, CNKI, and Wanfang databases. Following a thorough screening process, we selected nine articles for inclusion (Fig. 1). These articles were published from the inception of the databases up until December 2023 [19–27]. Table 1 illustrated that the studies were commenced between 2010 and 2021, with participant sample sizes varying from 453 to 186,096. Among the nine included studies, eight (88.89%) were conducted in countries with a high TB burden. Overall, 364,260 elderly individuals, 644 patients with active TB, and 428 asymptomatic TB patients were included in this analysis. Among them, two studies used only one screening strategy (CXR) to screen the population, while the other seven studies used multiple screening strategies.
Fig. 1.
Flow diagram of the study selection
Table 1.
Characteristics of the studies related to asymptomatic tuberculosis proportion in the elderly population
| Study | Study year | Study design | Country | Area | Sample size | No. of Asymptomatic TB | No. of active TB | Screening strategy | Symptom |
|---|---|---|---|---|---|---|---|---|---|
| Zhang [23] | 2013 | Cross-sectional study | China | Shandong Province | 93,094 | 65 | 82 | Questionnaire survey, and CXR. | / |
| Hu [19] | 2021 | Cross-sectional study | China | Zhejiang Province | 49,399 | 57 | 115 | Questionnaire survey, physical examinations and CXR. | Cough, expectoration, hemoptysis, fever, fatigue. |
| Kim [21] | 2017 | Cross-sectional study | Korea | South Korean Province | 12,402 | 13 | 16 | CXR. | / |
| Chen [26] | 2017 | Cross-sectional study | China | Guizhou Province | 1119 | 23 | 38 | Questionnaire survey and CXR. | Cough, expectoration, hemoptysis or bloody sputum, fever, chest pain, night sweats, etc. |
| Zhao [25] | 2010–2014 | Cross-sectional study | China | Guangxi Province | 10,325 | 73 | 80 | Questionnaire survey, CXR, and sputum examination. | / |
| Zhang [24] | 2016 | Cross-sectional study | China | Shanxi Province | 2053 | 9 | 27 | Suspicious symptom screening and CXR. | Cough, expectoration, etc. |
| Jiang [20] | 2019 | Cross-sectional study | China | Chongqing Municipality | 453 | 10 | 16 | Suspicious symptom screening and CXR. | Cough, expectoration, etc. |
| Wang [22] | 2020 | Cross-sectional study | China | Zhejiang Province | 186,096 | 100 | 174 | CXR. | Cough, expectoration, production, fatigue, loss of appetite, reduced body weight, chest pain, hemoptysis, night sweating. |
| Chen [27] | 2011–2012 | Cross-sectional study | China | Zhejiang Province; Henan Province; Yunnan Province | 9319 | 78 | 96 | CXR, sputum smear and sputum culture. | / |
Quality of the included studies
After the studies’ quality assessment, the nine cross-sectional studies were rated moderate to high (Table S3). The deduction items were concentrated in 5, 7, 9, and 11.
Asymptomatic TB proportion among the elderly population
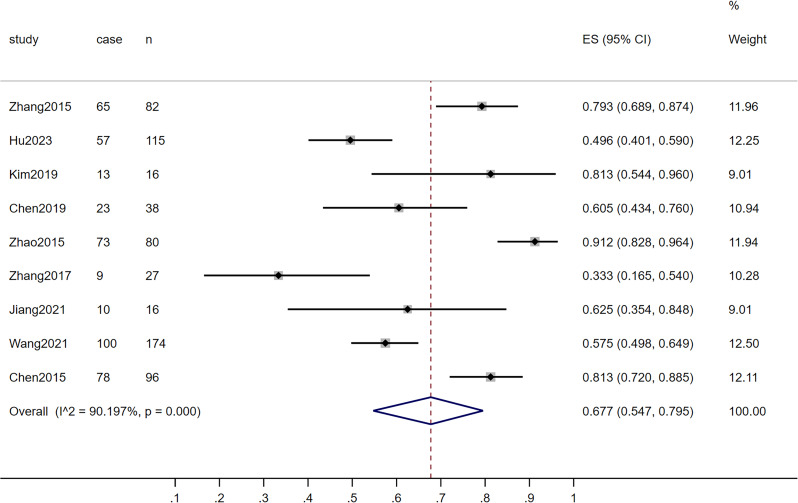
The pooled results showed that the proportion of asymptomatic TB among elderly patients with active TB was 0.677 (95% CI: 0.547–0.795, I2random effects model = 90.197, P < 0.001) (Fig. 2). The subgroup analyses results were shown in Table 2. Among these, the combined results of seven studies conducted in or before 2019 indicated that the proportion of asymptomatic TB was 0.723 (95% CI: 0.581–0.847, P < 0.001) [20, 21, 23–27]. Two other studies conducted after 2019 showed a proportion of 0.543 (95% CI: 0.486–0.601, P < 0.001) [19, 22]. The percentage of asymptomatic patients when active screening was performed in high-burden countries was 0.663 (95% CI: 0.525–0.789, P < 0.001). Two studies used only one screening strategy, while seven studies used multiple strategies including CXR and others. Studies using multiple screening strategies including CXR revealed a higher percentage of asymptomatic patients at 0.676 (95% CI: 0.511–0.821, P < 0.001). Regarding sample size, when it was < 15,000, the combined study results showed a proportion of 0.706 (95% CI: 0.523–0.862, P < 0.001) [20, 21, 24–27]. When the sample size was ≥ 15,000, the proportion was 0.623 (95% CI: 0.459–0.774, P < 0.001) [19, 22, 23].
Fig. 2.
Asymptomatic tuberculosis proportion in the elderly population analyzed by the Forest plot
Table 2.
Subgroup analysis of asymptomatic tuberculosis proportion in the elderly population
| Subgroup analysis type | No. of studies | Effect size | M | Heterogeneity | ||
|---|---|---|---|---|---|---|
| ES [95%CI] | PES | I2 (%) | PH | |||
| All studies | 9 | 0.677[0.547,0.795] | < 0.001 | R | 90.197 | < 0.001 |
| Subgroup analysis by sample size | ||||||
| < 15,000 | 4 | 0.706[0.523,0.862] | < 0.001 | R | 88.033 | < 0.001 |
| ≥ 15,000 | 5 | 0.623[0.459,0.774] | < 0.001 | NA | NA | NA |
| Subgroup analysis by study country | ||||||
| High-burden countries | 8 | 0.663[0.525,0.789] | < 0.001 | R | 91.285 | < 0.001 |
| Non-high-burden countries | 1 | 0.813[0.544,0.960] | < 0.001 | NA | NA | NA |
| Subgroup analysis by screening strategy | ||||||
| CXR only | 2 | 0.598[0.525,0.669] | < 0.001 | NA | NA | NA |
| CXR and others | 7 | 0.676[0.511,0.821] | < 0.001 | R | 91.526 | < 0.001 |
| Subgroup analysis by study start year | ||||||
| Before 2019 | 7 | 0.723[0.581,0.847] | < 0.001 | R | 85.711 | < 0.001 |
| After 2019 | 2 | 0.543[0.486,0.601] | < 0.001 | NA | NA | NA |
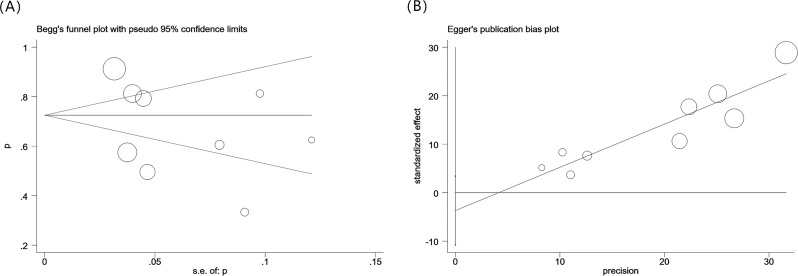
Potential publication bias
We used Begg’s funnel plots and conducted an Egger’s linear regression test (Fig. 3) to assess possible publication bias. Both Begg’s and Egger’s tests yielded consistent results, indicating the absence of any substantial publication bias (PB = 0.466, PE = 0.259). Additionally, the aggregated data from previous studies revealed a significant difference in the prevalence of asymptomatic pulmonary tuberculosis among the elderly. We conducted a subgroup analysis using four variables: sample size, study country, screening strategy, and study start year. We also conducted a meta-regression analysis incorporating covariates, such as sample size (P = 0.005), study country (P = 0.051), screening strategy (P = 0.048), and study start year (P = 0.002). A sensitivity analysis was conducted for the specified group (Figure S1).
Fig. 3.
Begg’s funnel plot and Egger’s linear regression test of asymptomatic tuberculosis proportion in the elderly population. No publication bias was detected
Discussion
Still, the effective implementations for TB prevention and control were to identify TB cases more efficiently with sufficient treatment. Increasing evidence indicated that asymptomatic TB had accounted for a certain proportion in the general population [15]. Because these groups had no obvious symptoms related to TB, they generally did not seek healthcare, thereby causing unwitting transmission in the household and community. Meanwhile, previous studies had demonstrated that poor adherence to TB treatment commonly occurred among mild to moderate patients, implying a possible lower treatment compliance among asymptomatic group, especially in the elderly population along with complications such as diabetes and renal insufficiency [28, 29]. In order to achieve the goal of “End TB”, it was urgent to implement more comprehensive countermeasures and actions to improve the findings of asymptomatic patients, accompanied by adequate health education, nutrition support, and psychological interventions [30].
ACF based on CXR for TB detection provided additional possibilities for identifying asymptomatic elderly patients. Several studies found that ACF could shorten the patient’s delay compared to passive case finding, thereby avoiding disease deterioration, improving treatment success rate of treatment, and reducing the occurrence of complications [31–33]. Unlike screening for non-communicable diseases, carrying out ACF for TB in the elderly could promote timely treatment and also provide additional benefits for the whole community, with significant public health benefits. Additionally, ACF among the elderly for TB detection could provide more opportunities for early disease detection in people with insufficient medical security, thereby improving medical equity [19].
In the nine studies we included, ACF was performed based on CXR, and seven of them added other screening tools, such as suspicious symptoms screening and sputum examination. To our knowledge, only symptom screening was easy to perform but had low sensitivity and specificity, while ACF with CXR had higher sensitivity and specificity than symptom screening [34, 35]. Furthermore, our results showed that the combined screening strategy based on CXR identified a higher percentage of asymptomatic patients than the single screening strategy suggesting that the former might result in a higher detection rate of asymptomatic active TB. Simple CXR screening might ignore active patients with TB who had normal chest radiographs but are bacterialized [35, 36]. Consequently, with the development of tongue swabs and mixed test technologies, new methods with more sensitivity and specificity needed to be applied to the community screening scenario to improve the detection of active TB [37].
The studies we included were conducted in various areas, with the majority being community-based. Some articles targeted the entire elderly population in selected areas, while others used random samples of people within selected areas. When ACF was performed in smaller sample size (< 15,000), a higher proportion of asymptomatic TB cases was observed compared to all studies we included. This may because these studies were carried out in smaller scale or sampled populations, potentially implying a more rigorous design and a higher quality control during the screening, thereby increasing the number of symptomatic TB cases and consequently lowering the proportion of asymptomatic patients. Additionally, in studies with smaller sample sizes, sampling bias among certain individuals or groups might have influenced the outcomes.
In 2022, 30 high-burden TB countries accounted for 87% of all global TB cases [1]. Most of these countries had incidence rates between 150 and 400 per 100,000, with rates above 500 per 100,000 in countries such as the Central African Republic and the Democratic People’s Republic of Korea. Some studies have shown that insidious TB, such as that in asymptomatic patients, was challenging to control [38]. Our findings revealed that eight of the studies included in this research were carried out in China, a nation facing a significant burden of TB. The proportion of asymptomatic patients was as high as 66.3%, while one study was conducted in Korea [21]. Given the limited research outside China, conducting more active screening in other countries providing details of asymptomatic cases in the elderly was necessary to obtain a more reliable estimation. Furthermore, high-burden countries should conduct pertinent studies to examine the proportion of asymptomatic TB cases among elderly individuals across diverse genetic and socioeconomic contexts.
Our study showed there was a decreasing trend in the proportion of asymptomatic TB cases detected through ACF after 2019, compared to before 2019. More than half of TB patients with COVID-19 exhibit symptoms, as indicated by a study [39]. Therefore, we hypothesized that the decrease could be linked to the COVID-19 pandemic, possibly resulting in a higher prevalence of symptoms in certain elderly TB patients co-infected with COVID-19. As previously mentioned, TB symptoms were often not obvious in the elderly population. With the ongoing COVID-19 pandemic before 2023, a significant proportion of patients had contracted the virus; the common symptoms overlapped significantly with those of TB, and distinguishing between the two was difficult [40]. Therefore, when co-infection with COVID-19 and TB occurred in the elderly population, the rate of symptom detection in the elderly population might increase, leading to a temporary decline in the proportion of asymptomatic TB cases after 2019.
Overall, our pooled research indicated that the proportion of asymptomatic patients detected through active screening in the elderly population was relatively high, almost 70%. With advancements in technology and improvements in artificial intelligence (AI), we recommended the use of AI for active screening of the elderly population in the community. Moreover, several studies have demonstrated its feasibility and simple accessibility [41, 42]. Furthermore, it was also suggested that individuals with suspected TB symptoms but no active TB should receive regular follow-up visits for at least 6 months to enhance early patient detection and ultimately achieve the goal of “End TB”.
Our study had some limitations. First, research conducted in high-burden countries, such as India, was not included in our study, resulting in some bias in representativeness. Second, because most of the literature did not provide detailed information on the number of asymptomatic individuals or corresponding authors of eligible articles did not give feedback about details, we included a relatively small number of articles, limiting the extrapolation of our results to the global level. Third, some articles used questionnaire survey as a screening tool, but the included screening symptoms may vary. Finally, owing to the different strategies for implementing active screening in different studies, there may be some differences in the final research results. However, this study had some advantages. First, it filled a key gap in current research and provided valuable insights into the field of active TB screening. Secondly, we thoroughly analyzed all accessible research data to unveil, for the first time, the proportion of asymptomatic elderly TB patients, offering deeper insights for advancing TB prevention and management strategies.
Conclusion
Our results showed that the proportion of asymptomatic TB cases could reach as high as almost 70% among the elderly population, indicating a significant potential impact on community transmission. Given that the elderly were at high risk for TB, more advanced technologies should be implemented in ACF to increase the sensitivity and specificity of active TB detection. These technologies included combined ACF strategy based on CXR, AI-assisted film reading, and innovative tests such as tongue swabs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- TB
Tuberculosis
- MTB
Mycobacterium tuberculosis
- PTB
Pulmonary tuberculosis
- CNKI
Chinese National Knowledge Infrastructure
- ACF
Active case finding
- COVID-19
Coronavirus disease 2019
- CXR
Chest X-ray
- AI
Artificial intelligence
- AHRQ
Agency for Healthcare Research and Quality
Author contributions
Yiqing Zhou, Qian Wu, and Kui Liu were involved in the conception and design. Yiqing Zhou, Dan Luo, and Yuxiao Ling searched literature, selected the studies, and completed the extraction of data. Yiqing Zhou was involved in analysis and interpretation of the data. Yiqing Zhou wrote the draft of the manuscription. Qian Wu, Fei Wang, Songhua Chen, Yu Zhang, Wei Wang, Yang Li, Luyu Wang, Jingru Wei, and Kui Liu contributed to revising it critically for intellectual content. Bin Chen, Canyou Zhang, Kui Liu, and Yiqing Zhou were involved in the final approval of the version to be published. All authors agree to be accountable for all aspects of the work.
Funding
This work was supported by the Policy Advocacy Project of Major Infectious Disease Prevention and Control in the New Era, Bill & Melinda Gates Foundation [INV-035022], Zhejiang Science and Technology Plan for Disease Prevention and Control [Project No.2025JK178], and Zhejiang Provincial Medical and Health Project [2025KY774].
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors gave final approval for this study to be published.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yiqing Zhou and Fei Wang contributed equally to this work.
Contributor Information
Bin Chen, Email: bchen@cdc.zj.cn.
Canyou Zhang, Email: zhangcy@chinacdc.cn.
Kui Liu, Email: kliu@cdc.zj.cn.
References
- 1.Organization WH. Global tuberculosis report 2023. Geneva: World Health Organization 2023 [ https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2023
- 2.Lee SH. Active case finding in the Elderly Tuberculosis in South Korea. Tuberc Respir Dis. 2019;82(3):261–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang L, Zhang H, Ruan Y, Chin DP, Xia Y, Cheng S, et al. Tuberculosis prevalence in China, 1990–2010; a longitudinal analysis of national survey data. Lancet. 2014;383(9934):2057–64. [DOI] [PubMed] [Google Scholar]
- 4.Caraux-Paz P, Diamantis S, de Wazieres B, Gallien S. Tuberculosis in the Elderly. J Clin Med. 2021;10(24):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donald PR, Marais BJ, Barry CE. Age and the epidemiology and pathogenesis of tuberculosis. Lancet. 2010;375(9729):1852–4. [DOI] [PubMed] [Google Scholar]
- 6.Pratt RH, Winston CA, Kammerer JS, Armstrong LR. Tuberculosis in older adults in the United States, 1993–2008. J Am Geriatr Soc. 2011;59(5):851–7. [DOI] [PubMed] [Google Scholar]
- 7.Tatar D, Senol G, Alptekin S, Anar C, Aydin M, Arslangiray SS. Tuberculosis in older adults. Eur Geriatr Med. 2013;4(1):15–9. [Google Scholar]
- 8.Velayutham BR, Nair D, Chandrasekaran V, Raman B, Sekar G, Watson B, et al. Profile and response to anti-tuberculosis treatment among elderly tuberculosis patients treated under the TB Control programme in South India. PLoS ONE. 2014;9(3):e88045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Negin J, Abimbola S, Marais BJ. Tuberculosis among older adults–time to take notice. Int J Infect Dis. 2015;32:135–7. [DOI] [PubMed] [Google Scholar]
- 10.Di Gennaro F, Vittozzi P, Gualano G, Musso M, Mosti S, Mencarini P et al. Active pulmonary tuberculosis in Elderly patients: a 2016–2019 retrospective analysis from an Italian Referral Hospital. Antibiot (Basel). 2020;9(8). [DOI] [PMC free article] [PubMed]
- 11.Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology. 2007;120(4):435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J. Nursing experience of Pneumonia in 50 Elderly patients without Respiratory Symptom. Guide China Med. 2020;18(11).
- 13.Onozaki I, Law I, Sismanidis C, Zignol M, Glaziou P, Floyd K. National Tuberculosis prevalence surveys in Asia, 1990–2012: an overview of results and lessons learned. Trop Med Int Health. 2015;20(9):1128–45. [DOI] [PubMed] [Google Scholar]
- 14.Vynnycky E, Borgdorff MW, Leung CC, Tam CM, Fine PE. Limited impact of tuberculosis control in Hong Kong: attributable to high risks of reactivation disease. Epidemiol Infect. 2008;136(7):943–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frascella B, Richards AS, Sossen B, Emery JC, Odone A, Law I, et al. Subclinical Tuberculosis Disease-A Review and analysis of prevalence surveys to inform definitions, Burden, associations, and Screening Methodology. Clin Infect Dis. 2021;73(3):E830–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuen CM, Amanullah F, Dharmadhikari A, Nardell EA, Seddon JA, Vasilyeva I, et al. Turning off the tap: stopping Tuberculosis transmission through active case-finding and prompt effective treatment. Lancet. 2015;386(10010):2334–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newman L. AHRQ’s evidence-based practice centres prove viable. Agency for Healthcare Research and Quality. Lancet. 2000;356(9246):1990. [DOI] [PubMed] [Google Scholar]
- 18.WHO. WHO global lists of high burden countries for tuberculosis (TB), TB/HIV and multidrug/rifampicin-resistant TB (MDR/RR-TB), 2021–2025: background document. Geneva. World Health Organization 2021 [ https://apps.who.int/iris/handle/10665/341980
- 19.Hu ZF, Liu K, Zhou M, Jiang XN, Feng YL, Yu ZC, et al. Mass Tuberculosis Screening among the Elderly: a Population-based study in a Well-Confined, Rural County in Eastern China. Clin Infect Dis. 2023;77(10):1468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang M, Zheng Y, Liu X, Luo Y. Screening methods for tuberculosis in the elderly aged 65 years and above in Qianjiang district. Chin Sci Technol J Database Med. 2021(7).
- 21.Kim H, Kim HJ, Oh KH, Oh HW, Choi H. A Pilot Project of systematic tuberculosis screening in the Elderly in a South Korean Province. Tuberc Respir Dis. 2019;82(3):194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang W, Hao X, Zhu P. Characteristics of tuberculosis cases detected by large-scale active screening in the elderly in rural area of Quzhou,Zhejiang. Disease Surveillance. 2021;36(12):1308–11. [Google Scholar]
- 23.Zhang XL, Li SG, Li HT, Li GX, Guo XY, Wang Y, et al. Integrating tuberculosis screening into annual health examinations for the rural elderly improves case detection. Int J Tuberc Lung Dis. 2015;19(7):787–91. [DOI] [PubMed] [Google Scholar]
- 24.Zhang L, Li X, Xian X. Effect analysis of tuberculosis screening for aged population over 65 years of age in Baqiao District, Xian City in 2016. Chin J Antituberculosis. 2017;39(4).
- 25.Zhao J. The implemention effect of active case finding strategy for tuberculosis in Pingguo County Guangxi. Guangxi Medical University; 2015.
- 26.Chen H, Li J, Yuan W, Zhang R, Mou Y. Active detection of tuberculosis in high epidemic areas, Guizhou. Mod Prev Med. 2019;46(19):3540–3. [Google Scholar]
- 27.Chen Wei LY, Jun C, Xiaomeng XananW, Jin X, Lin X. Wang Lixia. Survey and analysis on TB prevalence of the elderly people in three counties. Chin J Antituberculosis. 2015;37(10).
- 28.Huangfu B, Dou M, Chen X, Sun F. Research progress in the treatment of elderly patients with pulmonary tuberculosis. CHINA Mod MEDCINE |CHIN MOD MED. 2022;29(36):27–30. 5. [Google Scholar]
- 29.Schaaf HS, Collins A, Bekker A, Davies PD. Tuberculosis at extremes of age. Respirology. 2010;15(5):747–63. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Chung PH, Leung CLK, Nishikiori N, Chan EYY, Yeoh EK. The strategic framework of tuberculosis control and prevention in the elderly: a scoping review towards end TB targets. Infect Dis Poverty. 2017;6(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Golub JE, Mohan CI, Comstock GW, Chaisson RE. Active case finding of tuberculosis: historical perspective and future prospects. Int J Tuberc Lung Dis. 2005;9(11):1183–203. [PMC free article] [PubMed] [Google Scholar]
- 32.Bothamley GH, Ditiu L, Migliori GB, Lange C, Contributors T. Active case finding of tuberculosis in Europe: a Tuberculosis Network European Trials Group (TBNET) survey. Eur Resp J. 2008;32(4):1023–30. [DOI] [PubMed] [Google Scholar]
- 33.Chen JO, Qiu YB, Rueda ZV, Hou JL, Lu KY, Chen LP, et al. Role of community-based active case finding in screening tuberculosis in Yunnan Province of China. Infect Dis Poverty. 2019;8(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Camelique O, Scholtissen S, Dousset JP, Bonnet M, Bastard M, Hewison C. Mobile community-based active case-finding for tuberculosis among older populations in rural Cambodia. Int J Tuberc Lung Dis. 2019;23(10):1107–14. [DOI] [PubMed] [Google Scholar]
- 35.Zhang H, Cheng J, Yu Y, Shen X, Lu W, Wang X, et al. Evaluation of the effectiveness of community-based pulmonary tuberculosis active case-finding among key populations:a multicenter prospective cohort study. Chin J Antituberculosis | Chin J Antitubercul. 2021;43(12):1248–59. [Google Scholar]
- 36.Zhang C, Wang L, Zhang H, Cheng J. Cost-effectiveness study of different strategies for pulmonary tuberculosis case finding in elderly people. Chin J Antituberculosis | Chin J Antitubercul. 2013;35(10):793–8. [Google Scholar]
- 37.Fan M, Zhang H. Interpretation of the evidence-based guidelines for active screening of Pulmonary Tuberculosis in Chinese communities. J Tuberculosis Lung Disease. 2023;4(01):1–4. [Google Scholar]
- 38.Wong EB. It is time to focus on asymptomatic tuberculosis. Clin Infect Dis. 2021;72(12):e1044–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Groff D, Sun A, Ssentongo AE, Ba DM, Parsons N, Poudel GR, et al. Short-term and long-term rates of Postacute Sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open. 2021;4(10):e2128568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee B, Ashcroft T, Agyei-Manu E, Farfan de los Godos E, Leow A, Krishan P, et al. Clinical features of COVID-19 for integration of COVID-19 into influenza surveillance: a systematic review. J Glob Health. 2022;12:05012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liao Q, Feng H, Li Y, Lai X, Pan J, Zhou F, et al. Evaluation of an artificial intelligence (AI) system to detect tuberculosis on chest X-ray at a pilot active screening project in Guangdong, China in 2019. J Xray Sci Technol. 2022;30(2):221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okada K, Yamada N, Takayanagi K, Hiasa Y, Kitamura Y, Hoshino Y, et al. Applicability of artificial intelligence-based computer-aided detection (AI-CAD) for pulmonary tuberculosis to community-based active case finding. Trop Med Health. 2024;52(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.