Abstract
Background
Cardiovascular diseases are among the most common and clinically significant comorbidities of chronic obstructive pulmonary disease (COPD). Exercise has been shown to reduce the risk of cardiovascular diseases, and high-intensity inspiratory muscle training (H-IMT) has emerged as a promising intervention for improving arterial stiffness in individuals with COPD. Yet, there is limited evidence from randomized controlled trials (RCTs) regarding the impact of H-IMT alone or in combination with exercise on reducing arterial stiffness in COPD. We designed a three-arm RCT to evaluate the effectiveness of H-IMT, both alone and in combination with exercise, in reducing brachial-ankle pulse wave velocity (baPWV) in individuals with stable COPD within a community setting.
Methods
This is a three-arm, parallel-group, assessor-blinded, randomized controlled trial with an eight-week intervention period and a 24-week follow-up. The trial will recruit a total of at least 162 participants with stable COPD. All participants will undergo arterial stiffness assessment using an atherosclerosis detector. Eligible participants will then be randomized into either a control group or one of two intervention groups: an H-IMT group combined with aerobic and resistance trainin, or an H-IMT group alone. The primary outcome is the baPWV at eight weeks. Secondary outcomes include baPWV at 4, 16, and 32 weeks, along with self-reported lifestyle factors, sleep quality, mental health outcomes, self-efficacy, implicit health attitudes, quality of life, and clinical outcomes at 4, 8, 16, and 32 weeks. The main analysis will follow the intention-to-treat principle, with the difference in outcome between groups analyzed using multi-level regression at eight weeks.
Discussion
This study will provide evidence on the effects of H-IMT and combined exercise interventions for individuals with COPD in a community setting, offering insights into the use of integrated approaches to enhance cardiovascular health among community-dwelling residents.
Trial registration number
ChiCTR2400085483. Date of registration: June 7, 2024. https://www.chictr.org.cn/index.aspx.
Keywords: Arterial stiffness, chronic obstructive pulmonary disease, Aerobic training, Resistance training, High-intensity inspiratory muscle training, Randomized controlled trial
Introduction
Cardiovascular diseases are the most frequent and clinically relevant comorbidities of chronic obstructive pulmonary disease (COPD), affecting up to 70% of individuals with COPD [1–3]. Despite a reduction in cardiovascular mortality in the general population, mortality in individuals with COPD continues to rise [4]. Cardiovascular risk, independent of the effects of smoking and factors such as physical fitness, accounts for approximately 50% of deaths among those with COPD [5]. Although the mechanisms underlying the increased cardiovascular risk in COPD remain unclear, atherosclerosis and the loss of large artery elasticity are implicated [6]. Epidemiological evidence has indicated that individuals with COPD exhibit greater arterial stiffness compared to healthy controls [7, 8]. Arterial stiffness has emerged as a valid predictor of cardiovascular risk [9] and a promising outcome to study therapeutic interventions at the early and potentially reversible stages of atherogenesis [10]. Arterial stiffness can be assessed non-invasively through measurements of brachial-ankle pulse wave velocity (baPWV), which is recognized as an essential biomarker of increased cardiovascular risk [11–13]. Consequently, there is a pressing need for effective intervention strategies to mitigate elevated baPWV in individuals with COPD.
It is well-established that exercise can reduce the risk of cardiovascular diseases [14]. Specifically, exercise enhances muscle function, alleviates symptoms, and may offer anti-inflammatory and antioxidant effects [15], all of which may contribute to the prevention and management of cardiovascular diseases in COPD patients. For example, an RCT in COPD patients demonstrated that exercise training can improve arterial stiffness [16]. Moreover, high-intensity inspiratory muscle training (H-IMT), an intervention designed to enhance inspiratory muscles and reduce dyspnea for pulmonary rehabilitation [17, 18], has emerged as a promising intervention for improving arterial stiffness and cardiovascular function, as it promotes benefits for cardiac autonomic control [19, 20]. Notably, the combination of H-IMT and exercise, as non-invasive and non-pharmacological interventions, is increasingly recognized as an effective strategy to improve muscle function, reduce dyspnea, and enhance cardiopulmonary fitness in individuals with cardiovascular diseases, often outperforming each intervention alone [21–23]. These findings suggest that H-IMT and exercise may serve as a promising strategy to reduce baPWV among individuals with COPD and hold substantial potential for application in stable COPD patients within the community setting. For individuals with COPD, only a recent RCT using H-IMT combined with endurance training reported an improvement in functional balance [24]. To date, only a few observational studies have shown significant reductions in baPWV with exercise and/or pulmonary rehabilitation [16, 25]. Yet, limited evidence exists from RCTs regarding the effect of H-IMT alone or in combination with exercise on lowering baPWV in individuals with COPD.
Therefore, we designed a three-arm randomized-controlled trial, named the High-intensity Inspiratory Muscle Training, and Resistance and Aerobic Exercise for Cardiovascular Health in COPD (HIRAC-COPD) trial. The HIRAC-COPD trial aims to evaluate the effectiveness of H-IMT, and H-IMT combined with exercise, in reducing baPWV in COPD patients within a community setting. A secondary aim is to determine whether one intervention is more effective than the other.
Methods
The protocol has been meticulously crafted in alignment with the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) statement [26] and the interventions are described in accordance with the Template for the Intervention Description and Replication (TIDieR) framework [27].
Study design overview
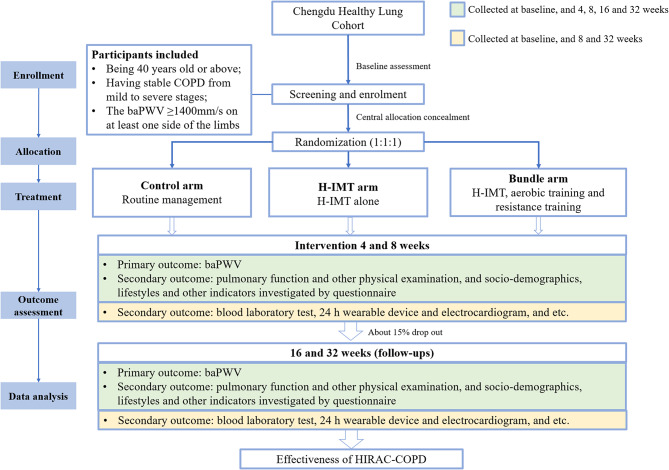
This study is a three-arm, parallel, assessor-blinded randomized controlled trial aimed at evaluating baPWV among individuals with COPD in China. The study includes an 8-week intervention and a 24-week follow-up phase. The primary hypothesize is that participants in the two intervention groups will exhibit greater arterial stiffness reduction compared to the control group. The study design and participant flow are illustrated in Fig. 1.
Fig. 1.
Flow of study design. baPWV, brachial-ankle pulse wave velocity; COPD, chronic obstructive pulmonary disease; H-IMT, high-intensity inspiratory muscle training; HRAC-COPD, High-intensity Inspiratory Muscle Training, and Resistance and Aerobic Exercise for Cardiovascular Health in COPD trial
Settings and participants
This study is nested in the Chengdu Healthy Lung Cohort (CHLC), a comprehensive initiative aimed at identifying risk factors of COPD among community-dwelling adults living with COPD in Chengdu Eastern New Area. Potential participants with COPD were recruited from CHLC, and all will be invited for arterial stiffness examination using an atherosclerosis detector (Beijing CHIOY Medical Technology Co.,Ltd). Eligible participants will be randomized into either a control group or one of two intervention groups featuring H-IMT with exercise training, or H-IMT alone.
Eligibility criteria of participants
Inclusion criteria
Being 40 years old or above;
Having stable COPD from mild to severe stages: FEV1/FVC < 0.70 after bronchodilator inhalation [28];
The baPWV ≥ 1400 mm/s on at least one side of the limbs [29].
Exclusion criteria
Having underwent pulmonary resection or lobectomy within the past 6 months;
Having pneumothorax or rib fracture;
Severe movement disorders (not caused by chronic obstructive pulmonary disease), or other neurological, muscular, skeletal, or cardiovascular diseases that seriously affect the safety of the subject’s training;
Having severe heart disease (acute heart failure, myocardial infarction, unstable angina, uncontrolled arrhythmia, patients with third degree atrioventricular block, unknown causes of chest pain and palpitations);
Having pulmonary bulla > 1 cm in distended diameter;
Having acute exacerbation of COPD, according to the GOLD 2023 definition [28];
Being pregnancy or breastfeeding;
Cognitive impairment or unable to communicate, or other inability to cooperate with training;
Individuals who have not signed informed consent forms;
Participate in other similar experiments.
Termination criteria
Withdrawing informed consent and/or withdrawing from the trial;
Development of new serious diseases (e.g., cancer) that makes it inappropriate to continue the interventions as specified in the trial protocol;
Experiencing serious adverse events during the intervention phase.
Randomization and allocation concealment
After recruitment and baseline data collection, eligible participants will be randomized using a computer-based random number generator (www.random.org) and assigned to one of the three arms: (1) the bundle arm, provided with H-IMT and aerobic training and resistance training of global muscles, (2) the H-IMT arm, provided with H-IMT alone, and (3) the control arm, provided with routine management (e.g., medical examination, consultation, and health literacy education).
Independent statisticians will generate a randomization list using a computer-generated random number table, assigning potential participants labeled with unique random numbers in a 1:1:1 ratio to the three study arms. In accordance with central randomization [30], the generated randomization list will be kept confidential and securely transmitted to the central study center responsible for participant enrollment. The allocation labels will only be disclosed after all participants have been confirmed as eligible and have provided informed consent.
Blinding
Due to the nature of the study, participants and intervention providers cannot be blinded to group allocation. However, statisticians and assessors will remain blinded throughout the trial period. Statisticians will be assisted by a research assistant who is responsible for group assignment, assigning a number to each group (e.g., A, B, C) instead of using specific group names. Statisticians will receive data with anonymized group identifiers, and the true allocation will be disclosed only after the statistical analysis has been completed and the results are ready for interpretation. Assessors, who are independent research assistants not involved in the intervention delivery process, will evaluate the outcomes.
Sample size
The primary outcome is the arterial stiffness measured by baPWV. Due to the innovative nature of our intervention, there was insufficient prior data to perform formal a priori calculations for effect size and sample size estimation for the primary outcome, baPWV, among people living with COPD. According to previous studies [31] and our internal data, baPWV was normally distributed with an standard deviation (SD) of 180 mm/s. We considered the minimal clinically important difference of the continuous measure baPWV over an 8-week period. Assuming a minimal clinical difference of 120 mm/s for H-IMT arm and bundle arm, we would need to enroll 111 participants (37 per arm) to reject the null hypothesis with a power of 0.8. Moreover, we considered a binary measure, defined as a mean PWV reduction of 200 mm/s at eight weeks, as a favorable outcome based on previous studies [32, 33]. Assuming that 10% of individuals in the control arm will achieve favorable outcome, improvement targets of 15% and 35% were set for H-IMT arm and bundle arm, respectively. To achieve a power of 0.80 to obtain a statistically significant at P < 0.05, p1 = 0.10 for control arm, p2 = 0.15 for H-IMT arm, p3 = 0.35 for bundle arm, the sample size is estimated as 129 (43 per group) [34]. To account for an anticipated attrition rate of 20%, we set a recruitment target of 54 participants per group, resulting in a total sample size of 162 participants.
Intervention design and strategy
The two intervention groups will receive intervention for eight weeks after participants’ recruitment into the study. Participants will undergo assessments at baseline, 4, 8, 16, and 32 weeks to observe changes in their arterial stiffness. This three-arm randomized controlled trial will compare the reduction of baPWV in the bundle arm and the H-IMT arm with that in the control arm. The study will be conducted at the pulmonary rehabilitation center of the local town hospital. A total of 16 training sessions will be carried out over 8 weeks (2× per week), supervised and monitored by two experienced physiotherapists specialized in pulmonary rehabilitation. The detailed intervention strategy is summarized in Table 1.
Table 1.
Overview of training parameters of the three trial arms
| H-IMT | Aerobic Training | Resistance Training | |
|---|---|---|---|
| Bundle arm |
• Tapered flow resistance device • 50% MIP • 10 × 3, 1-minute interval • 2 times/week • 5% MIP increment per week |
• Ergometer • Borg 4–6/10 • 20 min • Consistent training including 2-minute warm-up and 2-minute cool-down • 2 times/week • Workload progression according to Borg scale (4–6/10) |
• Elastic band, free body weight • Upper extremities: arm vertical extension, arm horizontal extension • Lower extremities: half-squat • 60% 1-RM • 10 × 4, 1-minute interval • 2 times/week • Re-assessment of 1-RM at 4th week for progression |
| H-IMT arm |
• Tapered flow resistance device • 50% MIP • 10 × 3, 1-minute interval • 2 times/week • 5% MIP increment per week |
None | None |
| Control arm | None | None | None |
H-IMT, high-intensity inspiratory muscle training; MIP, maximal inspiratory pressure; RM, repetition maximum
The bundle arm consists of three components of exercise training: H-IMT, aerobic training, and resistance training for global muscles. After recruitment, participants will initially start the H-IMT program at 50% of their oral maximal inspiratory pressure (MIP), assessed at baseline. The assessment and training procedures will be implemented using a hand-held digital device (Blue Whale™, Xeek, Xiamen, China) with a tapered flow resistance in sitting position. A total of 30 repetitions will be performed, divided into three sets with a one-minute recovery interval between sets. An increment of 5% MIP will be set each week after two sessions for progression. Aerobic training will be conducted using an ergometer. Instead of adjusting the intensity by watts, participants will be asked to maintain their Borg scale at 4–6/10 through the training. The duration of the aerobic training will be 20 min, including two-minute warm-up and two-minute cool-down. Physiotherapists will continuously and closely monitor patients’ peripheral saturation and heart rate to avoid adverse events. Resistance training will target global muscles of the upper and lower extremities using three functional movements: arm vertical extension (shoulder abduction with elbow extension at frontal plane), arm horizontal extension (shoulder extension with elbow extension at horizontal plane), and half-squat (leaning on a swiss-ball against the wall with a 60-degree knee flexion). The one-repetition maximum (1-RM) will be firstly assessed at baseline, and the intensity of resistance training will be conducted at 60% 1-RM (10 × 4/d), considering the deconditioned fitness of all participants. After four weeks, a re-assessment of 1-RM will be conducted to reset the intensity. The intervention will be guided by the self-efficacy theory to improve participants’ adherence to intervention and behavior maintenance [35]. We will assist participants in setting realistic training goals and continuously monitor their performance throughout the training process. Those who successfully complete the training will be recognized and rewarded for their achievements. For participants facing obstacles during training, we will provide encouragement and support to help them overcome challenges and complete the program.
In contrast, the H-IMT arm will solely undergo H-IMT program without aerobic and resistance training. The H-IMT training parameters will follow the same criteria as those in the bundle arm. The training schedules between the bundle and H-IMT arms will be allocated to different two days each week to avoid potential influence on patients’ compliance with the study and contamination of different trainings. The intervention will also be guided by self-efficacy theory.
In the controlled arm, no training will be administered throughout the 8-week duration. In all three arms, physiotherapists will routinely and equally offer regular medical consultations, health literacy education, and answer any questions participants may have about their condition.
Data collection and outcomes
Data will be collected onsite at baseline, 4, 8, 16, and 32 weeks. Information such as basic demographics and lifestyles will be collected using online questionnaires. Physical examinations will be conducted by doctors with at least 5 years of clinical experience, and blood samples will be collected by trained nurses. The stool samples will be collected by laboratory technicians and nurses. All procedures for questionnaire survey, physical examinations, and sample collection and storage will be in strict accordance with the Standard Operating Procedures.
Primary outcomes
The primary outcome is the baPWV after the intervention at 8 weeks. baPWV will be measured using an atherosclerosis detector, with patients in the supine position after at least 5 min of rest, as previously reported [36, 37]. In detail, four oscillometric cuffs are utilized, each featuring a plethysmographic sensor to ascertain the volume pulse, which is correlated with an oscillometric pressure sensor designed to measure blood pressure. These cuffs were positioned on both the brachial regions and the ankles. After measurement, two simultaneous measurements of baPWV will be recorded on both the right and left sides.
Secondary outcomes
Secondary outcomes include baPWV at 4, 16, and 32 weeks, as well as lifestyle factors, sleep quality, mental health outcomes, self-efficacy, implicit health attitudes, quality of life, and clinical outcomes at 4, 8, 16, and 32 weeks, with further details as follows:
Scales
Lifestyles
Lifestyles will be measured by questionnaire, including smoking status, alcohol intake, physical activity measured by International Physical Activity Questionnaire [38], dietary habits measured by Food Frequency Questionnaire [39].
Sleep quality
Sleep quality will be measured with the Pittsburgh Sleep Quality Index (PSQI) [40].
Mental health outcomes
Anxiety and depression will be evaluated with the 7-item scale for General Anxiety Disorder (GAD-7) [41] and the Patient Health Questionnaire Depression Module (PHQ-9) [42], respectively.
Health-related self-efficacy
Self-efficacy will be measured with reference to the General Self-Efficacy Scale (GSES) [43].
Implicit health attitudes
Implicit health attitudes will be measured using the lay theories of healthy behavior intentions scale [44].
Quality of life
Quality of life will be measured by 36-Item Short Form Health Survey (SF-36) [45].
Clinical outcomes
The study will evaluate clinical outcomes associated with arterial stiffness and COPD, encompassing conditions such as general obesity, central obesity, hypertension, diabetes mellitus, dyslipidemia, sarcopenia, arteriosclerosis, and syndromes such as metabolic syndrome. These outcomes will be correlated with patients’ medical histories, complemented by physical examinations and laboratory test results. Venous blood samples, obtained after a minimum of 8 h of overnight fasting, will be utilized for clinical laboratory assessments, which include complete blood counts, fasting blood glucose levels, lipid profiles, and hepatic function tests. Besides, cardiovascular biomarkers such as apolipoproteins and serum creatine kinase isoenzymes will also be measured. Body composition will be analyzed using Inbody 370. The measurements of body circumference, blood pressure, grip strength, and physiological indicators will be conducted using medical examination tools or equipment of standard specifications.
Multi-omics data
Gut microbiota, along with its metabolites and RNA, will be detected through transcriptomic, metagenomic, and metabolomic analyses. Venous blood will be collected from participants following an overnight fast of at least 8 h, utilizing vacuum blood collection tubes and blood lancets. Concurrently, blood samples and stool samples will be obtained and store at biobank in West China School of Sichuan University through cold chain transportation within two hours, and delivered to a Third-Party Medical Laboratory for multi-omics analysis.
Timeline of data collection
Demographic characteristics and medical record data will be surveyed or extracted at baseline. The primary and secondary outcomes, assessed by physical examinations, will be collected at five measurement points: baseline, 4 weeks, primary end-point (8 weeks), the 16-week follow-up, and the 32-week follow-up. Secondary outcomes assessed through questionnaires will be collected at three measurement points: baseline, the primary end-point (8 weeks), the 16-week follow-up, and the 32-week follow-up, and the laboratory indicators will be collected at three measurement points: baseline, the primary end-point (8 weeks), and the 32-week follow-up (Table 2).
Table 2.
Schedule for events and measurements of enrolment, interventions, and assessments
| Study Period | |||||||
|---|---|---|---|---|---|---|---|
| Enrolment | Allocation | Post-allocation | Close-out | ||||
| Timepoint | -t1 | 0 | t1 (Baseline) |
t2 (4 weeks) |
t3 (8 weeks) |
t4 (16 weeks) |
tx (32 weeks) |
| ENROLMENT: | |||||||
| Eligibility screen | X | ||||||
| Informed consent | X | ||||||
| Randomization | X | ||||||
| Allocation | X | ||||||
| Blinding | X | ||||||
| INTERVENTIONS: | |||||||
| Bundle arm | X | X | X | ||||
| IMT arm | X | X | X | ||||
| Control arm | X | X | X | ||||
| ASSESSMENTS: | |||||||
| Demographics | X | X | X | X | X | ||
| Medical records | X | X | X | X | |||
| Questionnaire | |||||||
| Smoking | X | X | X | X | |||
| Drinking | X | X | X | X | |||
| FFQ | X | X | X | X | |||
| IPAQ | X | X | X | X | |||
| PSQI | X | X | X | X | |||
| GAD-7 | X | X | X | X | |||
| PHQ-9 | X | X | X | X | |||
| mGSES | X | X | X | X | |||
| Implicit health attitude | X | X | X | X | |||
| SF-36 | X | X | X | X | |||
| Satisfaction and adherence evaluation | X | ||||||
| Physical examination | |||||||
| Height | X | ||||||
| Weight | X | X | X | X | X | ||
| Body circumference a | X | X | X | X | X | ||
| Pulmonary function b | X | X | X | X | X | ||
| Pulse rate | X | X | X | X | X | ||
| Finger pulse oxygen | X | X | X | X | X | ||
| Body composition | X | X | X | X | X | ||
| Blood pressure | X | X | X | X | X | ||
| Grip strength | X | X | X | X | X | ||
| SPPB c | X | X | X | X | X | ||
| PWV | X | X | X | X | X | ||
| 24 h wearable device | X | X | X | ||||
| Electrocardiogram | X | X | X | ||||
| Laboratory test | |||||||
| Blood laboratory tests d | X | X | X | ||||
| Stool laboratory tests e | X | X | X | ||||
FFQ, Food Frequency Questionnaire; IPAQ, short version of the International Physical Activity Questionnaire; PSQI, Pittsburgh Sleep Quality Index; GAD-7, 7-item scale for General Anxiety Disorder; PHQ-9, patient health questionnaire depression module; mGSES, modified General Self-Efficacy Scale; SF-36, 36-item short form survey; SPPB, Short Physical Performance Battery; PWV, pulse wave velocity
aBody circumference included neck, waist, belly, hip, arm and leg circumference
bPulmonary function assessment includes Forced Expiratory Volume in 1 s (FEV1), Forced Vital Capacity (FVC), FEV1% predicted (FEV1%Pre), Maximum Inspiratory Pressure (MIP), and Maximum Expiratory Pressure (MEP)
cSPPB included feet together test, semi-tandem stand test, tandem stand test, 4-meter walk test, and five times sit-to-stand test
dBlood tests include complete blood count (CBC) testing and biochemical analysis. The former encompasses indicators such as total white blood cell count (WBC), red blood cells (RBC), hemoglobin (HGB), platelets (PLT), lymphocytes, monocytes, neutrophils, and eosinophils. The latter includes indicators such as fasting blood glucose (FBG), triglycerides (TG), cholesterol (CHOL), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), total protein (TP), albumin, globulin, alanine transaminase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transpeptidase (GGT), creatinine (Cr), urea, uric acid (UA), C-reactive protein (CRP), high-sensitivity C-reactive protein (hsCRP), serum iron, alkaline phosphatase (ALP), total bilirubin (TBIL), apolipoprotein A-I (ApoA-I), apolipoprotein B (ApoB), creatine kinase-isoenzymes (CK-MB), hemoglobin A1c, interleukin 6 (IL-6), interleukin 10 (IL-10), insulin (INS), and C-peptide (C-P)
eStool laboratory tests include transcriptomic, metagenomic, and metabolomic analyses
X The item will be implemented or on relevant time point
Data analysis
Data analyses will be conducted using R software on Windows. Categorical variables will be summarized as frequencies and percentage, both overall and by intervention group. Continues variables will be summarized as mean ± standard deviation or median (interquartile range), also reported overall and by intervention group.
The main analysis will be conducted based on the intention-to-treat (ITT) population. The primary outcome will utilize both continuous outcomes (baPWV values, or change in baPWV values) and categorical outcomes (favorable outcome vs. not, or percentage change in favorable outcome), which will be analyzed using generalized linear mixed effects regression at eight weeks [46]. Categorical variables for randomization groups will be included in the model as the explanatory variable (with the control group as the reference group). The model will include a random intercept for participants and fixed effects for demographics and regions (i.e., communities), as well as an interaction term for time point and intervention group. The treatment effect at eight weeks (immediately after intervention) will be presented as odds ratio. For secondary outcomes, we will use a similar analysis strategy to that used for the primary outcome. For continuous outcomes, longitudinal analysis of covariance (ANCOVA) models can be estimated with time and time-group interaction, adjusted for the respective outcome at baseline and incorporating random intercepts for participants as well as a fixed effect for demographics and regions (i.e., communities) [47]. Estimates of differences in continuous outcomes between groups at different time points will be analyzed post hoc using a group×time repeated measures ANCOVA, with between and within arms. For two-period data, t-tests or chi-square tests can also be used as analytical strategies.
Missing values
The attrition and dropout rates will be presented as frequencies and percentages. Additionally, patterns of missing data will be analyzed, and corresponding tables and illustrations will be provided. For the main analysis, missing data will not be imputed (complete case analysis). Multiple imputation on 100 sets using chained equations, with models according to variables’ scale levels, will be performed for all sensitivity analyses [48]. Other imputation methods, such as predictive mean matching and regression imputation, will be used alternatively if applicable.
Subgroup analysis
We will perform subgroup analyses based on sex and age. To investigate the heterogeneity of intervention effects across subgroups, we will incorporate an interaction term between intervention assignment and subgroup in the regression models, with statistical significance assessed using Wald tests.
Safety and harms
The Independent Supervisory Group of the Second People’s Hospital of Eastern New Area will monitor the trial’s proper conduct and ensure participants’ safety. During the RCT, participants’ interests will be closely monitored and safeguarded. The overall conduct of the trial will be supervised, and participant safety will be ensured through a systematic review of adverse events and timely responses to any severe distress. In the event of any adverse event or emergency, participants will be strongly encouraged to seek additional help and counseling, ensuring their well-being throughout the trial.
An adverse event is defined as any unfavorable or unintended medical occurrence experienced by a participant following an intervention, irrespective of whether it is directly related to the intervention. Should a participant’s condition be linked to a pre-existing medical issue documented in their medical history, it will not be classified as an adverse event unless there is a notable change in its nature, severity, or relation to the intervention. All adverse events will be carefully recorded, detailing the timing, symptoms, and treatments administered, and will be reported to the Supervisory Group and the Ethics Committee. Each adverse event will be monitored until it is medically resolved. Given that this study does not entail invasive procedures or pharmacological interventions, any anticipated adverse events are likely to arise from the participant’s underlying health condition or the study’s operational circumstances. Participants have the right to discontinue or withdraw from the study at any point and are encouraged to communicate any concerns to the research team.
Quality control
All assessors will receive offline training on the study protocol before group assignment, including ethical norms and detailed instructions with respect to the evaluation process. Health professionals from local health community hospitals will help schedule participants for the intervention and be responsible for addressing any issues that may arise during the implementation of the intervention. During the intervention period, a series of forms will be designed to document implementation and monitor participants’ safety and compliance, including a form for MIP adjustment for participants, diaries detailing their adherence to the intervention measures. Through these forms, we will be able to identify individuals at risk and take timely action to improve or enhance interventions.
Quality control (QC) measures for questionnaire investigations will involve data oversight by inspectors who will evaluate for outliers and human error during data collection. For medical examinations, QC procedures will include verifying the functionality of measurement instruments, and ensuring adherence to Standard Operating Procedures. In terms of clinical laboratory tests, QC will be maintained by sending blinded sample panels to independent third-party laboratories for validation. Additionally, data QC will involve two independent assistants who will cross-verify electronically entered data against trial record sheets, excluding any data identified as unqualified from the final analysis.
Data monitoring and confidentiality
Questionnaires survey, physical examination, and sample collection will be conducted by medical staff, with strict adherence to confidentiality protocols. The responsible investigator and other research team members will use de-identified names during the intervention and data analysis, employing unique ID numbers to match results. No individual information will be disclosed in the publication of the study’s findings. During the intervention phase, the intervention will be carried out using these ID numbers. All research team members are required to sign a confidentiality agreement to ensure data security.
Ethics approval, protocol amendments and consent
This trial will be conducted in accordance with the study protocol, the Declaration of Helsinki, and good clinical practice. Ethical approval for this study has been granted by the Ethics Committee of the West China School of Public Health and West China Fourth Hospital (Gwll2024146). The trial has been registered with the Chinese Clinical Trials Registry (ChiCTR2400085483, https://www.chictr.org.cn/index.aspx). The design and implementation of this RCT adhere to the extension of the SPIRIT 2013 statement [26], and reporting of the trial will follow the Consolidated Standards of Reporting Trials (CONSORT) statement [49]. Any significant protocol modifications will be communicated to all relevant parties, including the supervisory committee, trial registries, the ethical committee, and researchers.
Before participation, interested participants will be provided with a written informed consent form that outlines the details of the trial, explains the potential risks and benefits, and includes contact information for the study team. Participants have right to decline participation in this study or to request withdrawal at any time by informing the investigator, without any adverse impact on their rights. The investigator may discontinue a subject’s participation in the study if the subject fails to adhere to the study protocol, or if an injury related to intervention.
Discussion
COPD is highly prevalent in community populations, particularly in China, where recent epidemiological studies have reported a prevalence rate of approximately 13.7% among adults aged 40 years and above [50]. Concurrently, comorbidities of atherosclerosis, as indicated by PWV, are frequently observed in patients with COPD, leading to a substantial health burden [1]. Physical exercise and pulmonary rehabilitation have emerged as potential strategies for managing both PWV and COPD comorbidities, as suggested by existing literature [51, 52]. However, there is a paucity of studies investigating the combined effects of exercise and pulmonary rehabilitation on improving atherosclerosis and COPD comorbidities. The intervention proposed in this protocol aims to fill this gap by providing an integrated approach that may offer a novel solution for managing the complex comorbidities.
Our study has several strengths. First, our intervention targets a community population characterized by a high prevalence of COPD, predominantly in the stable stage of the disease rather than acute exacerbations. This population is especially well-suited for non-pharmacological interventions, such as physical exercise, due to the high cost-effectiveness and the potential for broader dissemination [50]. Second, we implemented a three-arm randomized controlled trial design, which enables us to compare differences across intervention arms and better evaluate the effects of individual versus combined interventions (i.e., H-IMT, aerobic training, and resistance training). Third, our exercise intervention incorporates both aerobic and anaerobic exercise, as previous studies have demonstrated that combined models are more effective in lowering the baPWV in COPD than single-mode exercise interventions like aerobic, resistance, or endurance training alone [53]. This design maximizes the potential benefits of exercise interventions. Moreover, our intervention is guided by self-efficacy theory and conducted with support from family members and healthcare professionals to enhance participants’ confidence in managing their condition. Finally, we utilized diverse approaches for data collection, including questionnaires, medical examination, and wearable devices, allowing us to capture multidimensional indicators. With estimates of the short-, medium-, and long-term intervention’s effects on changes in indicators of interest, we can identify key points where the intervention begins to have an effect.
Our protocol provides valuable insights into potential applications in routine practice, translating research findings into real-world settings and assessing strategies for COPD management and comorbidity prevention. In the context of Chinese model of physical and medical integration [54], implementing H-IMT and exercise interventions presents a community-based case for reducing arterial stiffness in COPD, which may also improve the lung capacity, and overall quality of life for COPD patients. If this intervention proves to be effective, it has potential for widespread validation and dissemination throughout China and internationally.
Several limitations should be cautious. First, the study findings may have limited representativeness due to volunteer bias from community recruitment. Second, for some secondary outcomes (e.g., lifestyle factors), we primarily relied on questionnaire indicators, which could be influenced by recall bias or tendencies to provide socially desirable responses. Third, there may be challenges for participant’s adherence, as the intervention requires supervised and regular training (i.e., training twice a week). However, we incorporated self-efficacy theory and offered incentives, such as gifts (e.g., household goods), to motivate participants. Additionally, family members can provide encouragement to support the intervention. Despite these limitations, the trial represents a pioneering initiative aimed at providing important measures and strategies for interventions in cardiovascular health in COPD within a community setting.
Acknowledgements
We appreciate all the participants, local medical workers in the study sites for their collaboration and assistance.
Author contributions
SY, BY and YKH conceived the study idea and designed the study. SY, BY, YKH, WBL, YLH, PJ and HJZ drafted the initial manuscript and critically reviewed the draft. BY, SY, YKH, WBL, HJZ, QD, YTY, CMF, ZZ, LYL, BY, ZYL, YZF, YCL, JW, JXZ, PY and JQY will provide critical input into the data collection and data analysis. All authors revised and approved the final manuscript version for submission.
Funding
This work was supported by Chengdu Eastern New Area’s Health Field “Leader Selection by Posting Challenges” Project (H231227). This study protocol was peer reviewed as part of the funding process. The funder did not take part in the design of the study and preparation of the manuscript.
Data availability
All investigators and implementation staff will have unrestricted access to the full data set for verification interpretation purposes. Data will be made available to the public after publication of study findings upon request from the corresponding author (Dr. Shujuan Yang, yangsj@scu.edu.cn, rekiny@126.com).
Declarations
Ethics approval and consent to participate
The protocol was approved by the Ethics Committee of the West China School of Public Health and the West China Fourth Hospital, Sichuan University (Gwll2024146). All individuals will voluntarily participate in this study and provide informed consent before enrollment. Participants will be informed that they have the right to withdraw from the study without any repercussion. The study will adhere to the Declaration of Helsinki.
Consent for publication
All authors reviewed the final protocol and approved submission for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bin Yu, Wenbin Liu, Yuekong Hu and Yuling Huang contributed equally to this work.
Contributor Information
Haojiang Zuo, Email: zuohaojiang@scu.edu.cn.
Peng Jia, Email: jiapengff@hotmail.com.
Shujuan Yang, Email: yangsj@scu.edu.cn, Email: rekiny@126.com.
References
- 1.Trinkmann F, Saur J, Borggrefe M, Akin I. Cardiovascular comorbidities in Chronic Obstructive Pulmonary Disease (COPD)-Current considerations for clinical practice. J Clin Med 2019, 8(1). [DOI] [PMC free article] [PubMed]
- 2.Polman R, Hurst JR, Uysal OF, Mandal S, Linz D, Simons S. Cardiovascular disease and risk in COPD: a state of the art review. Expert Rev Cardiovasc Ther. 2024;22(4–5):177–91. [DOI] [PubMed] [Google Scholar]
- 3.Divo M, Cote C, de Torres JP, Casanova C, Marin JM, Pinto-Plata V, Zulueta J, Cabrera C, Zagaceta J, Hunninghake G, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(2):155–61. [DOI] [PubMed] [Google Scholar]
- 4.Yang HM, Ryu MH, Carey VJ, Kinney GL, Hokanson JE, Dransfield MT, Hersh CP, Silverman EK. Chronic obstructive pulmonary disease exacerbations increase the risk of subsequent Cardiovascular events: a longitudinal analysis of the COPDGene Study. J Am Heart Association. 2024;13(11):e033882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camilli AE, Robbins DR, Lebowitz MD. Death certificate reporting of confirmed airways obstructive disease. Am J Epidemiol. 1991;133(8):795–800. [DOI] [PubMed] [Google Scholar]
- 6.Stavem K, Aaser E, Sandvik L, Bjørnholt JV, Erikssen G, Thaulow E, Erikssen J. Lung function, smoking and mortality in a 26-year follow-up of healthy middle-aged males. Eur Respir J. 2005;25(4):618–25. [DOI] [PubMed] [Google Scholar]
- 7.Fisk M, McEniery CM, Gale N, Mäki-Petäjä K, Forman JR, Munnery M, Woodcock-Smith J, Cheriyan J, Mohan D, Fuld J, et al. Surrogate markers of Cardiovascular Risk and Chronic Obstructive Pulmonary Disease: a large case-controlled study. Hypertens (Dallas Tex: 1979). 2018;71(3):499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maclay JD, McAllister DA, Mills NL, Paterson FP, Ludlam CA, Drost EM, Newby DE, Macnee W. Vascular dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180(6):513–20. [DOI] [PubMed] [Google Scholar]
- 9.Weber T, Auer J, O’Rourke MF, Kvas E, Lassnig E, Berent R, Eber B. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation. 2004;109(2):184–9. [DOI] [PubMed] [Google Scholar]
- 10.Tam LS, Li EK, Shang Q, Tomlinson B, Lee VW, Lee KK, Li M, Kuan WP, Li TK, Tseung L, et al. Effects of rosuvastatin on subclinical atherosclerosis and arterial stiffness in rheumatoid arthritis: a randomized controlled pilot trial. Scand J Rheumatol. 2011;40(6):411–21. [DOI] [PubMed] [Google Scholar]
- 11.Sugawara J, Tanaka H. Brachial-ankle pulse Wave Velocity: myths, misconceptions, and realities. Pulse (Basel Switzerland). 2015;3(2):106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement D, Coca A, De Simone G, Dominiczak A, et al. 2018 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC Task Force for the management of arterial hypertension. J Hypertens. 2018;36(12):2284–309. [DOI] [PubMed] [Google Scholar]
- 13.Collaboration RVAS. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J. 2010;31(19):2338–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oliveira NL, Ribeiro F, Alves AJ, Campos L, Oliveira J. The effects of exercise training on arterial stiffness in coronary artery disease patients: a state-of-the-art review. Clin Physiol Funct Imaging. 2014;34(4):254–62. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Anto JM. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax. 2006;61(9):772–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vivodtzev I, Minet C, Wuyam B, Borel JC, Vottero G, Monneret D, Baguet JP, Lévy P, Pépin JL. Significant improvement in arterial stiffness after endurance training in patients with COPD. Chest. 2010;137(3):585–92. [DOI] [PubMed] [Google Scholar]
- 17.Troosters T, Janssens W, Demeyer H, Rabinovich RA. Pulmonary rehabilitation and physical interventions. Eur Respiratory Review: Official J Eur Respiratory Soc 2023, 32(168). [DOI] [PMC free article] [PubMed]
- 18.Rehder-Santos P, Abreu RM, Signini ÉF, da Silva CD, Sakaguchi CA, Dato CC, Catai AM. Moderate- and high-intensity inspiratory muscle training equally improves Inspiratory muscle strength and Endurance-A double-blind randomized controlled trial. Int J Sports Physiol Perform. 2021;16(8):1111–9. [DOI] [PubMed] [Google Scholar]
- 19.Cipriano GF, Cipriano G Jr., Santos FV, Güntzel Chiappa AM, Pires L, Cahalin LP, Chiappa GR. Current insights of inspiratory muscle training on the cardiovascular system: a systematic review with meta-analysis. Integr Blood Press Control. 2019;12:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Abreu RM, Rehder-Santos P, Minatel V, Dos Santos GL, Catai AM. Effects of inspiratory muscle training on cardiovascular autonomic control: a systematic review. Auton Neuroscience: Basic Clin. 2017;208:29–35. [DOI] [PubMed] [Google Scholar]
- 21.Laoutaris ID, Adamopoulos S, Manginas A, Panagiotakos DB, Kallistratos MS, Doulaptsis C, Kouloubinis A, Voudris V, Pavlides G, Cokkinos DV, et al. Benefits of combined aerobic/resistance/inspiratory training in patients with chronic heart failure. A complete exercise model? A prospective randomised study. Int J Cardiol. 2013;167(5):1967–72. [DOI] [PubMed] [Google Scholar]
- 22.Laoutaris ID. The ‘aerobic/resistance/inspiratory muscle training hypothesis in heart failure’. Eur J Prev Cardiol. 2018;25(12):1257–62. [DOI] [PubMed] [Google Scholar]
- 23.Dos Santos TD, Pereira SN, Portela LOC, Cardoso DM, Lago PD, Dos Santos Guarda N, Moresco RN, Pereira MB, de Albuquerque IM. Moderate-to-high intensity inspiratory muscle training improves the effects of combined training on exercise capacity in patients after coronary artery bypass graft surgery: a randomized clinical trial. Int J Cardiol. 2019;279:40–6. [DOI] [PubMed] [Google Scholar]
- 24.Tounsi B, Acheche A, Lelard T, Tabka Z, Trabelsi Y, Ahmaidi S. Effects of specific inspiratory muscle training combined with whole-body endurance training program on balance in COPD patients: Randomized controlled trial. PLoS ONE. 2021;16(9):e0257595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore LE, Byers BW, Fuhr DP, Wong E, Bhutani M, Stickland MK. Cardiovascular benefits from standard pulmonary rehabilitation are related to baseline exercise tolerance levels in chronic obstructive pulmonary disease. Respir Med. 2017;132:56–61. [DOI] [PubMed] [Google Scholar]
- 26.Chan A-W, TJ, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, Altman DG, Barbour V, Macdonald H, Johnston M, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
- 28.Dransfield M, Kalhan R, Stolz D. Pushing (for) GOLD. Eur Respir J 2023, 61(4). [DOI] [PubMed]
- 29.Committee ESH-ESCG. 2003 European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. Journal of hypertension 2003, 21(6):1011–1053. [DOI] [PubMed]
- 30.Pildal J, Chan A-W, Hróbjartsson A, Forfang E, Altman DG, Gøtzsche PC. Comparison of descriptions of allocation concealment in trial protocols and the published reports: cohort study. BMJ 2005, 330(7499). [DOI] [PMC free article] [PubMed]
- 31.Sabit R, Bolton CE, Edwards PH, Pettit RJ, Evans WD, McEniery CM, Wilkinson IB, Cockcroft JR, Shale DJ. Arterial stiffness and osteoporosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175(12):1259–65. [DOI] [PubMed] [Google Scholar]
- 32.Lopes S, Afreixo V, Teixeira M, Garcia C, Leitão C, Gouveia M, Figueiredo D, Alves AJ, Polonia J, Oliveira J, et al. Exercise training reduces arterial stiffness in adults with hypertension: a systematic review and meta-analysis. J Hypertens. 2021;39(2):214–22. [DOI] [PubMed] [Google Scholar]
- 33.Liu H, Shivgulam ME, Schwartz BD, Kimmerly DS, O’Brien MW. Impact of exercise training on pulse wave velocity in healthy and clinical populations: a systematic review of systematic reviews. Am J Physiol Heart Circ Physiol. 2023;325(5):H933–48. [DOI] [PubMed] [Google Scholar]
- 34.Grayling MJ, Wason JM. A web application for the design of multi-arm clinical trials. BMC Cancer. 2020;20(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selzler AM, Rodgers WM, Berry TR, Stickland MK. Coping Versus Mastery modeling intervention to enhance self-efficacy for Exercise in patients with COPD. Behav Med (Washington DC). 2020;46(1):63–74. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki E, Kashiwagi A, Nishio Y, Egawa K, Shimizu S, Maegawa H, Haneda M, Yasuda H, Morikawa S, Inubushi T, et al. Increased arterial wall stiffness limits flow volume in the lower extremities in type 2 diabetic patients. Diabetes Care. 2001;24(12):2107–14. [DOI] [PubMed] [Google Scholar]
- 37.Katakami N, Osonoi T, Takahara M, Saitou M, Matsuoka TA, Yamasaki Y, Shimomura I. Clinical utility of brachial-ankle pulse wave velocity in the prediction of cardiovascular events in diabetic patients. Cardiovasc Diabetol. 2014;13:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95. [DOI] [PubMed] [Google Scholar]
- 39.Buckland G, Gonzalez CA, Agudo A, Vilardell M, Berenguer A, Amiano P, Ardanaz E, Arriola L, Barricarte A, Basterretxea M, et al. Adherence to the Mediterranean diet and risk of coronary heart disease in the Spanish EPIC Cohort Study. Am J Epidemiol. 2009;170(12):1518–29. [DOI] [PubMed] [Google Scholar]
- 40.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 41.Spitzer RL, Kroenke K, Williams JB. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–7. [DOI] [PubMed] [Google Scholar]
- 42.Kroenke K, Spitzer Rl Fau -, Williams JB, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luszczynska A, Scholz U, Schwarzer R. The general self-efficacy scale: multicultural validation studies. J Psychol. 2005;139(5):439–57. [DOI] [PubMed] [Google Scholar]
- 44.Bunda K, Busseri MA. Lay theories of health, self-rated health, and health behavior intentions. J Health Psychol. 2019;24(7):979–88. [DOI] [PubMed] [Google Scholar]
- 45.Brazier JE, Harper R, Jones NM, O’Cathain A, Thomas KJ, Usherwood T, Westlake L. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305(6846):160–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zou GY, Donner A. Extension of the modified Poisson regression model to prospective studies with correlated binary data. Stat Methods Med Res. 2013;22(6):661–70. [DOI] [PubMed] [Google Scholar]
- 47.M W JTLBTHJR. Different ways to estimate treatment effects in randomised controlled trials. Contemp Clin Trials Commun. 2018;10:80–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.White IR, Royston P, Fau - Wood AM, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377–99. [DOI] [PubMed] [Google Scholar]
- 49.Calvert M, Blazeby J, Fau - Altman DG, Altman Dg Fau - Revicki DA, Revicki Da Fau - Moher D, Moher D Fau - Brundage MD, Brundage MD. Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. JAMA 2013, 309(8):814–822. [DOI] [PubMed]
- 50.Wang C, Xu J, Yang L, Xu Y, Zhang X, Bai C, Kang J, Ran P, Shen H, Wen F, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet (London England). 2018;391(10131):1706–17. [DOI] [PubMed] [Google Scholar]
- 51.Gloeckl R, Marinov B, Pitta F. Practical recommendations for exercise training in patients with COPD. Eur Respiratory Review: Official J Eur Respiratory Soc. 2013;22(128):178–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, Hill K, Holland AE, Lareau SC, Man WD, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13–64. [DOI] [PubMed] [Google Scholar]
- 53.Jian C, Peng X, Yang Y, Xu Y, Wang L, Cai D. A Network Meta-Analysis on the effects of different Exercise types in patients with COPD. Respir Care. 2024;69(9):1189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tu SJ, Jin C, Chen BT, Xu AY, Luo C, Wang XH. Study on the fusion of sports and medicine in China from 2012 to 2021: a bibliometric analysis via CiteSpace. Front Public Health. 2022;10:939557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All investigators and implementation staff will have unrestricted access to the full data set for verification interpretation purposes. Data will be made available to the public after publication of study findings upon request from the corresponding author (Dr. Shujuan Yang, yangsj@scu.edu.cn, rekiny@126.com).