Abstract
Background
Psoriasis is an immune-mediated chronic inflammatory disease associated with multiple factors. To evaluate the extent to which C-reactive protein (CRP) and genetic predisposition affect the incidence of psoriasis.
Methods
The cohort study retrieved 420,040 participants without psoriasis at baseline from the UK Biobank. Serum CRP was categorized into two levels: < 2 mg/L (normal) and ≥ 2 mg/L (elevated). The polygenic risk score (PRS) was used to estimate genetic predisposition, and was characterized as low, moderate and high PRS. The possible interaction and joint associations between CRP and PRS were assessed using Cox proportional hazards models.
Results
Participants with high CRP levels had an increased risk of incident psoriasis compared to those with low CRP levels (HR: 1.26, 95% CI: 1.18–1.34). Participants with high CRP levels and high PRS had the highest risk of incident psoriasis [2.24 (95% CI: 2.01, 2.49)], compared with those had low CRP levels and low PRS. Significant additive and multiplicative interaction were found between CRP and PRS in relation to the incidence of psoriasis.
Conclusions
Our results suggest that higher CRP concentration may be associated with higher psoriasis incidence, with a more pronounced association observed in individuals with high PRS for psoriasis. So, clinicians should be aware that the risk of incident psoriasis may increase in general population with high CRP levels and high PRS, so that early investigation and intervention can be initiated.
Supplementary Information
The online version contains supplementary material available at 10.1186/s41927-024-00450-2.
Keywords: C-reactive protein, Genetic predisposition, Psoriasis
Background
Psoriasis, a chronic inflammatory skin disorder, is mediated by the immune system and impacts an estimated 2–4% of individuals globally [1–3]. This condition poses significant challenges for individuals, often leading to disfigurement, functional impairment, and comorbidities that profoundly impact physical, emotional, and psychosocial well-being [4, 5]. Furthermore, psoriasis places a substantial burden on healthcare systems and societal resources worldwide [6]. Characterized by its recalcitrant nature and propensity for relapse, psoriasis remains a formidable challenge in dermatology [7]. Therefore, elucidating the key determinants of psoriasis development and identifying high-risk individuals are crucial steps toward effective disease prevention and management.
Currently, the etiology of psoriasis remains incompletely understood, but involves a complex interplay of genetic, environmental, and immunological factors [8]. Genetic predisposition is considered a major contributor, with approximately 40% of individuals with psoriasis reporting a family history of the disease [9]. To date, nearly 100 genetic loci associated with psoriasis susceptibility have been identified through candidate gene and genome-wide association studies [10]. Polygenic risk scores (PRSs), which aggregate information from multiple risk alleles, are increasingly used to estimate an individual’s genetic predisposition to complex diseases, including psoriasis [11]. However, while genetic factors are clearly important, the development and progression of psoriasis are also influenced by a variety of environmental and immunological triggers [12]. Of particular interest are systemic pro-inflammatory cytokines, which have been implicated in both the initiation and exacerbation of psoriasis [13, 14].
C-reactive protein (CRP), a widely recognized marker of systemic inflammation, has been inconsistently linked to psoriasis severity. While some studies suggest a positive association between CRP levels and psoriasis severity [15], others have reported contradictory findings [16]. This discrepancy highlights the need for further research to clarify the complex relationship between CRP and psoriasis. Moreover, to date, there is a paucity of research examining the potential interactions or joint effects of CRP and genetic predisposition on psoriasis risk in the general population. Prospective studies are warranted to systematically evaluate these associations and elucidate the interplay between inflammatory markers and genetic susceptibility in psoriasis development.
To address these research gaps, we conducted a large-scale prospective study with the primary aim of evaluating the extent to which CRP and genetic predisposition would contribute to the incidence of psoriasis in the general population. The secondary aim was to investigate the possible interaction and joint associations of CRP and genetic predisposition with adverse outcomes.
Methods
Study design and study population
This study utilized data obtained from the UK Biobank database (Application Number: 69,550). This extensive, population-based cohort study facilitates in-depth investigations into the genetic and environmental determinants of various diseases [17]. The cohort study encompassed approximately 500,000 participants aged 40 to 69 years at recruitment. Baseline data was collected between 2006 and 2010 across 22 assessment centers throughout the United Kingdom. The study design incorporates multiple ongoing follow-up assessments. Biological samples were collected from all participants at baseline.
Of the initial 502,366 participants in the UK Biobank, this study excluded individuals based on the following criteria: a pre-existing diagnosis of psoriasis (n = 11,022), withdrawal from the study (n = 4,302), missing C-reactive protein data (n = 32,052), missing data for quality-controlled genotyping (n = 3,964), and missing covariate data (n = 30,986). This resulted in a final analytic sample of 420,040 participants (Supplemental Fig. 1).
Ascertainment of C-reactive protein
serum C-reactive protein (CRP) concentrations (mg/L) were measured using a high-sensitivity immunoturbidimetric assay conducted on a Beckman Coulter AU5800. Based on established thresholds from previous research, serum CRP levels were dichotomized into two categories: normal (< 2 mg/L) and elevated (≥ 2 mg/L) [18]. For trend analysis, participants were further stratified into four groups based on quartiles of serum CRP levels. Detailed protocols for blood sample collection and processing have been previously described elsewhere [19].
Follow-up and outcome ascertainment
Participants without psoriasis were followed from their baseline assessment until the earliest occurrence of one of the following events: (1) the study outcome (incident psoriasis or mortality), (2) death, (3) withdrawal from the study, or (4) the end of the follow-up period (July 31, 2021, for Scotland and September 30, 2021, for England and Wales).
The primary outcome of this study was incident psoriasis. Diagnosis of psoriasis was primarily determined through hospital inpatient records obtained from the Hospital Episode Statistics (England), Scottish Morbidity Record (Scotland), and the Patient Episode Database (Wales). These diagnoses were supplemented by linkage with primary care records, death register data, and self-reported diagnoses. For this study, psoriasis was defined using the International Classification of Diseases, 10th Edition (ICD-10) code L40.
Definition of genetic predisposition
Genetic predisposition to psoriasis was quantified for each participant by calculating their Polygenic Risk Score (PRS). These scores were derived from the standard PRS (Category 301) available within the UK Biobank’s PRS database. Participants were then categorized into low, moderate, and high genetic risk groups based on tertiles of the calculated PRS distribution.
Covariates
Several demographic and clinical covariates were considered in the analysis. These included age (< 65, ≥ 65 years), sex (male, female), race/ethnicity (White, Mixed, Asian or Asian British, Black or Black British, Chinese, Other), smoking status (never, previous, current), alcohol consumption status (never, previous, current), body mass index (BMI) (< 25, 25 to < 30, ≥ 30 kg/m2), self-reported average annual household income (< £18,000, £18,000–30,999, £31,000–51,999, £52,000–100,000, > £100,000, Unknown), diabetes mellitus (yes, no), dyslipidemia (yes, no), and hypertension (yes, no).
Statistical analyses
Baseline characteristics of the participants, including sociodemographic factors, socioeconomic status, disease history, and polygenic risk scores, were summarized. Chi-square tests and analysis of variance (ANOVA) were used to assess differences in categorical and continuous variables, respectively. Kaplan-Meier curves were generated to depict the cumulative incidence of the study outcome over the follow-up period, with between-group differences assessed using the log-rank test. Incidence rates were calculated by dividing the number of incident events by the total person-years of follow-up.
Multivariable Cox proportional hazards regression models were employed to examine the associations between CRP (as a continuous variable [log-transformed, per 1-SD increment], dichotomized [< 2 vs. ≥ 2 mg/dL], and quartiles), PRS, and incident psoriasis. Models were adjusted for potential confounders, including age, sex, ethnicity, annual income, smoking status, alcohol consumption status, BMI, and history of diabetes mellitus, dyslipidemia, and hypertension. The proportional hazards assumption was assessed using Schoenfeld residuals. Population attributable fractions (PAFs) were calculated to estimate the proportion of incident psoriasis cases theoretically attributable to exposure to elevated CRP levels (relative to the low-risk group).
Stratified analyses were performed to investigate whether the association between CRP levels and psoriasis risk varied across strata of genetic risk (PRS). A combined CRP-PRS variable was created to facilitate this assessment, with six categories representing all possible combinations of CRP levels (dichotomized: low vs. high) and PRS tertile (low, medium, high). Hazard ratios (HRs) and 95% confidence intervals (CIs) for incident psoriasis were estimated for each of the six groups, with the reference group defined as low CRP and low PRS.
To quantify additive interactions, the relative excess risk due to interaction (RERI) and corresponding 95% CIs were calculated. This metric represents the excess risk attributable to the joint effect of elevated CRP and higher PRS, beyond the risk expected based on their independent effects. RERI was calculated using the delta method. Multiplicative interaction was assessed using likelihood ratio tests.
The consistency of the association between CRP and incident psoriasis across strata of PRS over time was assessed by examining the effect of CRP on psoriasis risk within consecutive 5-year time intervals. Subgroup analyses were conducted to explore potential effect modification by age, sex, ethnicity, BMI, smoking status, alcohol consumption status, PRS, diabetes mellitus, dyslipidemia, and hypertension history.
Results
In this study, a total of 420,040 participants without psoriasis at baseline were enrolled. During a median follow-up of 12.6 (IQR 11.9–13.3) years, 4160 individuals experienced psoriasis. The mean age was 56.5 ± 8.1 years, 54.4% were women, and 95.1% were White. A total of 147,028 (35.0%) were of high CRP levels, and 273,012 (65.0%) were of low CRP levels. A total of 139,876 (33.3%) were of low PRS, 139,870 (33.3%) were of intermediate PRS, and 140,294 (33.4%) were of high PRS. Baseline characteristics stratified by CRP levels were presented in Table 1.
Table 1.
Baseline characteristics comparisons according to CRP levels
| Variables | Total (N = 420040) |
Low (N = 273012) |
High (N = 147028) |
P Value |
|---|---|---|---|---|
| Age | 56.5 ± 8.1 | 56.1 ± 8.1 | 57.5 ± 7.9 | < 0.0001 |
| Female | 228,649 (54.4) | 144,038 (52.8) | 84,611 (57.5) | < 0.0001 |
| White | 399,546 (95.1) | 260,022 (95.2) | 139,524 (94.9) | < 0.0001 |
| Smoking | < 0.0001 | |||
| Never | 230,775 (54.9) | 157,001 (57.5) | 73,774 (50.2) | |
| Previous | 145,720 (34.7) | 92,232 (33.8) | 53,488 (36.4) | |
| Current | 43,545 (10.4) | 23,779 (8.7) | 19,766 (13.4) | |
| Drinking | < 0.0001 | |||
| Never | 18,043 (4.3) | 10,281 (3.8) | 7762 (5.3) | |
| Previous | 14,739 (3.5) | 8345 (3.1) | 6394 (4.3) | |
| Current | 387,258 (92.2) | 254,386 (93.2) | 132,872 (90.4) | |
| Body mass index | < 0.0001 | |||
| <25 | 139,899 (33.3) | 114,151 (41.8) | 25,748 (17.5) | |
| >=25 & <30 | 179,025 (42.6) | 118,695 (43.5) | 60,330 (41.0) | |
| >=30 | 101,116 (24.1) | 40,166 (14.7) | 60,950 (41.5) | |
| Income | < 0.0001 | |||
| <18,000 | 81,141 (19.3) | 45,698 (16.7) | 35,443 (24.1) | |
| 18,000–30,999 | 91,979 (21.9) | 58,573 (21.5) | 33,406 (22.7) | |
| 31,000–51,999 | 94,443 (22.5) | 64,524 (23.6) | 29,919 (20.3) | |
| 52,000-100,000 | 73,991 (17.6) | 53,325 (19.5) | 20,666 (14.1) | |
| >100,000 | 19,664 (4.7) | 15,046 (5.5) | 4618 (3.1) | |
| Unknown | 58,822 (14.0) | 35,846 (13.1) | 22,976 (15.6) | |
| Diabetes mellitus | < 0.0001 | |||
| No | 395,322 (94.1) | 260,541 (95.4) | 134,781 (91.7) | |
| Yes | 24,718 (5.9) | 12,471 (4.6) | 12,247 (8.3) | |
| Dyslipidemia | < 0.0001 | |||
| No | 217,461 (51.8) | 145,917 (53.4) | 71,544 (48.7) | |
| Yes | 202,579 (48.2) | 127,095 (46.6) | 75,484 (51.3) | |
| Hypertension | < 0.0001 | |||
| No | 187,608 (44.7) | 135,067 (49.5) | 52,541 (35.7) | |
| Yes | 232,432 (55.3) | 137,945 (50.5) | 94,487 (64.3) | |
| Polygenic risk score | < 0.0001 | |||
| Low | 139,876 (33.3) | 91,914 (33.7) | 47,962 (32.6) | |
| Intermediate | 139,870 (33.3) | 90,626 (33.2) | 49,244 (33.5) | |
| High | 140,294 (33.4) | 90,472 (33.1) | 49,822 (33.9) |
CRP, C-reactive protein
Associations of CRP with the incidence of psoriasis
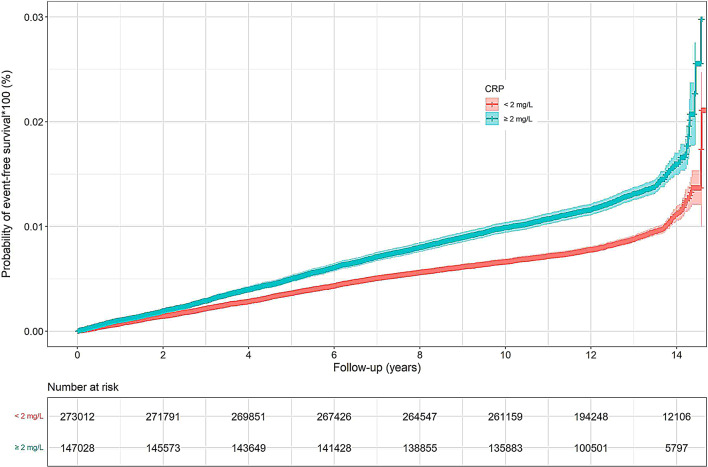
Cumulative rates of psoriasis were higher in those with CRP ≥ 2 mg/L vs. CRP < 2 mg/L (Log-rank P-value < 0.0001, Fig. 1). As shown in Table 2; Fig. 2, participants with high CRP group (≥ 2 mg/dL) had an increased risk of incident psoriasis compared to those with low CRP group (< 2 mg/dL) after adjusting for confounding factors (IR: 1.03, 95% CI, per 1000 person-years: 0.98–1.08 and HR: 1.26, 95% CI: 1.18–1.34 for high CRP). A relationship was also observed between CRP concentration in quartiles and incident psoriasis. Participants with the second, third, fourth quartile of CRP concentration had a gradually increased risk of incident psoriasis compared to those with the first quartile of CRP concentration (HR: 1.11, 95% CI: 1.01–1.22 for the second quartile of CRP concentration; HR: 1.24, 95% CI: 1.13–1.37 for the third quartile of CRP concentration; HR: 1.45, 95% CI: 1.31–1.59 for the fourth quartile of CRP concentration). Moreover, each 1-SD increase in CRP concentration was associated with a 16% increased risk of incident psoriasis (HR: 1.16, 95% CI: 1.12–1.20). Compared with those had low CRP levels, individuals with high CRP had adjusted PAF of 7.65 (95% CI: 5.70, 9.60) for psoriasis incidence.
Fig. 1.
Survival curve of psoriasis affected by CRP
Table 2.
Crude incidence rate, and cox proportional hazard analysis for psoriasis
| Event, n (%) | IR (95% CI) per 1000 person-years |
Adjusted HR (95% CI) |
P Value | PAF (%) (95% CI) |
|
|---|---|---|---|---|---|
| CRP | |||||
| Log-CRP per 1-SD increase | 4160/420,040 (0.99%) | 0.81 (0.78, 0.83) | 1.16 (1.12, 1.20) | < 0.0001 | - |
| < 2 mg/dL | 2327/273,012 (0.85%) | 0.69 (0.66, 0.72) | Reference | Reference | |
| ≥ 2 mg/dL | 1833/147,028 (1.25%) | 1.03 (0.98, 1.08) | 1.26 (1.18, 1.34) | < 0.0001 | 7.65 (5.70, 9.60) |
| Q1 | 748/103,526 (0.72%) | 0.58 (0.54, 0.62) | Reference | Reference | |
| Q2 | 933/106,014 (0.88%) | 0.71 (0.67, 0.76) | 1.11 (1.01, 1.22) | 0.0355 | 2.00 (0.28, 3.72) |
| Q3 | 1106/105,216 (1.05%) | 0.86 (0.81, 0.91) | 1.24 (1.13, 1.37) | < 0.0001 | 4.57 (2.74, 6.40) |
| Q4 | 1373/105,284 (1.30%) | 1.08 (1.02, 1.14) | 1.45 (1.31, 1.59) | < 0.0001 | 8.68 (6.37, 11.00) |
| P-trend < 0.0001 | |||||
| PRS | |||||
| Per 1 score increase | 4160/420,040 (0.99%) | 0.81 (0.78, 0.83) | 1.32 (1.29, 1.36) | < 0.0001 | - |
| [0%, 33.3%] | 999/139,876 (0.71%) | 0.58 (0.55, 0.62) | Reference | Reference | |
| (33.3%, 66.6%] | 1291/139,870 (0.92%) | 0.75 (0.71, 0.79) | 1.29 (1.19, 1.40) | < 0.0001 | 6.64 (4.63, 8.65) |
| (66.6%, 100%] | 1870/140,294 (1.33%) | 1.09 (1.04, 1.14) | 1.87 (1.73, 2.02) | < 0.0001 | 19.97 (17.56, 22.39) |
| P-trend < 0.0001 |
CRP, C-reactive protein; PRS: polygenic risk score; IR, incidence rate; CI, confidence interval; HR, hazard ratio; PAF, population attributable fraction
Adjustment for age, sex, ethnicity, annual income, smoking status, drinking status, Body mass index (BMI), diabetes mellitus, dyslipidemia, and hypertension history
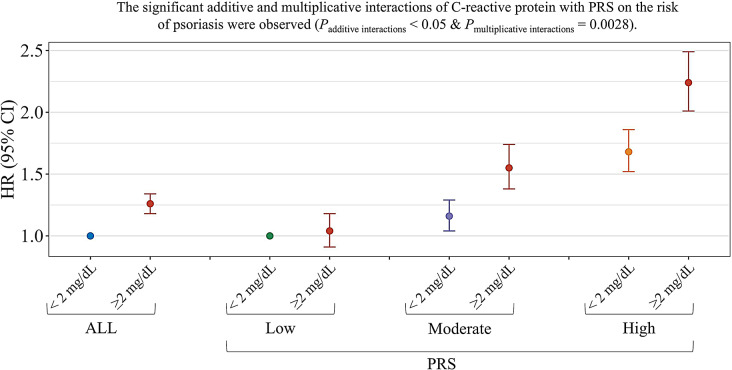
Fig. 2.
Associations and interactions of CRP with PRS and incident Psoriasis
Associations of PRS with the incidence of psoriasis
Table 2 shows adjusted HRs (95% CIs) for psoriasis incidence by PRS. After adjusting for confounding factors, the HR for psoriasis incidence increased monotonically following an increase in the PRS (HR: 1.29, 95% CI: 1.19–1.40 for PRS in (33.3%, 66.6%]; HR: 1.87, 95% CI: 1.73–2.02 for PRS in (66.6%, 100%]). Additionally, A 1-score increase in PRS was associated with a 32% increased risk of incident psoriasis (HR: 1.32, 95% CI: 1.29–1.36). Compared with those had low PRS, individuals with moderate CRP and high CRP had adjusted PAF of 6.64 (95% CI: 4.63, 8.65) and 19.97 (95% CI: 17.56, 22.39) for psoriasis incidence, respectively.
Interactions between CRP and PRS on the incidence of psoriasis
Table 3; Fig. 2 show the combined effects between CRP and PRS on the risk for incident psoriasis. Generally, participants with higher levels of CRP or higher PRS had higher risks of psoriasis. Participants with high CRP levels and high PRS had the highest risk of incident psoriasis [2.24 (95% CI: 2.01, 2.49)], compared with those had low CRP levels and low PRS. On an additive scale, positive interactions were observed between CRP (low and high levels) and PRS (low and high levels) on the incidence of psoriasis. For individuals with low CRP levels and low PRS, the RERI and AP were 0.51 and 0.23, suggesting that a 0.51 relative excess risk was due to the additive interaction. This accounted for 23% of the risk of psoriasis in those with low CRP levels and low PRS. And a multiplicative interaction also was found between CRP and PRS in relation to the incidence of psoriasis (Pmultiplicative interactions < 0.05).
Table 3.
Combined effects of CRP, PRS and the risk of Psoriasis
| Outcomes | PRS levels (HR, 95% CI) | RERI ‡ |
P for interaction § |
||||
|---|---|---|---|---|---|---|---|
| Low PRS | Moderate PRS | High PRS | Moderate PRS | High PRS | |||
| CRP | |||||||
| < 2 mg/dL | 1.00 | 1.16 (1.04, 1.29) | 1.68 (1.52, 1.86) | 0.0028 | |||
| ≥ 2 mg/dL | 1.04 (0.91, 1.18) | 1.55 (1.38, 1.74) | 2.24 (2.01, 2.49) | 0.37 (0.17, 0.56) | 0.51 (0.30, 0.73) | ||
Adjustment for age, sex, ethnicity, annual income, smoking status, drinking status, Body mass index (BMI), diabetes mellitus, dyslipidemia, and hypertension history
HR, hazard ratio; CI, confidence interval; CRP, C-reactive protein; PRS: polygenic risk score; RERI, relative excess risk due to interaction
‡: The estimates of RERI were calculated based on the reference group with low CRP and low PRS
§: Likelihood tests was applied to test the significance of interaction term by comparing the model with and without the interaction term
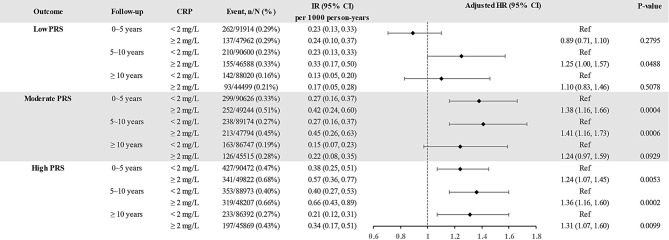
The association between CRP and incident psoriasis was consistent over time, and higher baseline CRP remained significantly associated with an increased risk of incident psoriasis even between 5 and 10 years after the initial measurement (Fig. 3), and this phenomenon was more pronounced with moderate and high PRS.
Fig. 3.
Association between CRP and outcomes stratified by PRS over time
The accumulative incidence rates of psoriasis, stratified by PRS, were observed to be higher in individuals with CRP levels of ≥ 2 mg/L compared to those with CRP levels of < 2 mg/L, a difference that was statistically significant (Log-rank P-value < 0.0001, Supplemental Fig. 2).
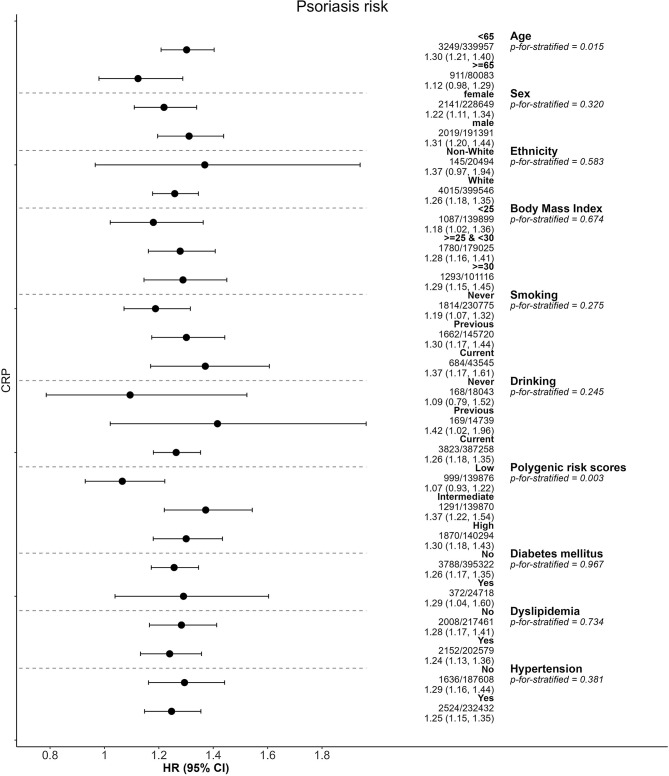
Subgroup analyses
Several subgroup analyses were conducted and presented in Fig. 4, the magnitude of the associations between CRP and the incidence of psoriasis were greater in young individuals (< 65 years; Paddi < 0.05), and participants with higher PRS (Paddi < 0.05). The results were not substantially different in other factors.
Fig. 4.
Subgroup analysis
Discussion
So far as we know, this is the largest prospective cohort study to evaluate both the independent and combined effects of adherence to CRP and genetic predisposition on the risk of incident psoriasis in general population. Our study reveals that high CRP levels and high PRS may be related to higher risk of incident psoriasis. Furthermore, significant additive interactions were observed between CRP and PRS on the morbidity of psoriasis. Our findings suggested high CRP levels may increase the risk of incident psoriasis more greatly in individuals with high PRS than in those with low PRS.
There are three important clinical implications to be drawn from this study. First, approximately 8% of psoriasis cases could be avoided if those maintained in low CRP levels. Second, individuals with a high genetic risk, if they also maintain high levels of CRP, may face a 23% increased risk for psoriasis. Third, individuals younger than 65 should pay enhanced attention to the escalated psoriasis risk influenced by elevated levels of CRP. These findings suggest that in clinical practice, clinicians need to pay more attention to individuals with high CRP levels, high PRS, and those under 65 years old. This is beneficial for clinicians to assess the risk and intervene the occurrence of psoriasis in advance, which may reduce the occurrence of psoriasis.
Comparison with other studies
Previous studies have shown that C-reactive protein had been widely studied as a marker of systemic inflammation [20, 21]. Currently, studies have shown that psoriasis is a chronic and systemic inflammatory disease [22]. It is interesting that the available evidence in favor of higher CRP levels in psoriatic patients seems to outweigh the evidence against that, numerous literature indicates that the elevated levels of CRP among psoriatic patients with medium to severe [23]. Despite its systemic nature, the quantification of the burden of systemic inflammation in psoriatic disease is challenging [24]. Currently, the vast majority of studies were based on psoriasis patients to investigate the relationship between CRP and the severity of psoriasis [15, 16, 23, 25]. Besides, some studies described significant relationships between CRP and cardiovascular risk in persons with psoriasis [25]. However, no associations have been reported for the associations between CRP and risk of psoriasis in general population until now. Excitingly, our study further revealed that higher CRP levels had a increased risk of incident psoriasis compared to those with low CRP levels, and each 1-SD increase in CRP concentration was associated with a 16% increased risk of incident psoriasis. Importantly, several studies have found that biological agents play a role in regulating inflammatory responses in the treatment and improvement of psoriasis, especially the decrease in CRP levels during the treatment of different biological agents. This suggests that CRP levels may play an important role in the occurrence and development of psoriasis, which supports our research results [26, 27]. Furthermore, our study further revealed CRP had a relatively larger effect on the risk of psoriasis among younger participants. These findings indicate greater significance in strengthening CRP interventions among participants with older and lower income.
Numerous investigations have suggested a strong genetic correlation in the etiology and pathogenesis of psoriatic disease [28]. In addition, A larger-scale prospective cohort study 331,631 participants results imply that an elevated PRS is linked to a heightened risk of psoriasis [29], which was consistent with our research findings. In our study, we found that we demonstrated a positive association between PRS and psoriasis risk. Many people might consider gene-based risk estimates as deterministic, lack of control over the ability to improve outcomes [11]. However, based on our study, we also observed significant synergistic interactions for CRP and PRS in relation to the risk of incident psoriasis even between 5 and 10 years after the initial measurement, and this phenomenon was more pronounced among CRP concentration with moderate and high PRS. Consequently, in an effort to reduce the risk of incident psoriasis, these findings would encourage the individuals with high CRP levels to control inflammatory levels by strengthening CRP interventions, especially among those with high PRS that they might benefit more from it.
Strengths and limitations
This study has several key strengths. The prospective cohort design, leveraging the extensive UK Biobank database, provided data from over 420,000 individuals with long-term follow-up. The robust baseline assessment of potential confounders and the study’s substantial statistical power further strengthen the findings. Importantly, this study addresses a gap in the literature by comprehensively evaluating the complex interplay between CRP, genetic predisposition, and incident psoriasis, an area that has been insufficiently explored.
Despite the aforementioned strengths, this study has limitations that warrant consideration. First, the observational design precludes definitive causal inferences. Second, while a substantial number of participants were excluded due to missing covariate data, potentially introducing selection bias, the lack of significant differences between included and excluded individuals suggests a minimal impact on the findings. Third, the inherent genetic correlations between CRP and certain diseases limit the ability to fully disentangle genetic confounding [30]. Fourth, relying on a single CRP measurement may not fully capture potential fluctuations in CRP levels over time, potentially influencing psoriasis risk assessment [31, 32]. Lastly, the generalizability of the findings to other racial or ethnic groups is uncertain given the predominantly White British composition of the UK Biobank. Future studies in more diverse populations are needed to confirm these findings.
Conclusions
Our study indicates a clear association between increased CRP concentration and a higher incidence of psoriasis, particularly in those with a high PRS for psoriasis. Therefore, clinicians need to be aware of this heightened risk among the general population with heightened CRP and PRS levels, enabling early investigation and intervention.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
We wish to acknowledge the UK Biobank Resource under Application Number 69,550 and also gratefully acknowledge many participants who provided medical data to the UK Biobank.
Abbreviations
- CRP
C-reactive protein
- PRS
Polygenic risk score
- ICD
International Classification of Diseases
- BMI
Body mass index
- HR
Hazard ratio
- CI
Confidence interval
- IR
Incidence rate
- PAF
Population attributable fraction
- RERI
Relative excess risk due to interaction
Author contributions
HL and ZL designed research and generated study plan; HZ, XZ and JH conducted the analysis and drafted the manuscript; HL and JZ analyzed the data; JZ, JW and SQ revised the manuscript. ZL, JW and SQ had primary responsibility for final content. All gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Funding
This study was supported by the Science and Technology Projects in Guangzhou (grant number 2023A03J0269).
Data availability
Data are available upon reasonable request to the UK Biobank management team (https://www.ukbiobank.ac.uk/media/px5gbq4q/access_019-access-management-system-user-guide-v4-1.pdf).
Declarations
Ethics approval and consent to participate
The project has approval from the North West Multi-centre Research Ethics Committee (MREC) (REC reference: 21/NW/0157), and informed written consent was obtained from each participant.
Consent for publication
Not applicable.
Clinical trial number
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhaoyan Liu, Email: liuzhy235@mail.sysu.edu.cn.
Ju Wen, Email: wenju3139@163.com.
Si Qin, Email: vanness1988@163.com.
References
- 1.Wang Y, Han D, Huang Y, Dai Y, Wang Y, Liu M, et al. Oral administration of punicalagin attenuates imiquimod-induced psoriasis by reducing ROS generation and inflammation via MAPK/ERK and NF-κB signaling pathways. Phytother Res. 2024;38(2):713–26. [DOI] [PubMed] [Google Scholar]
- 2.Ma F, Plazyo O, Billi AC, Tsoi LC, Xing X, Wasikowski R, et al. Single cell and spatial sequencing define processes by which keratinocytes and fibroblasts amplify inflammatory responses in psoriasis. Nat Commun. 2023;14(1):3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watanabe T, Watanabe Y, Asai C, Asami M, Watanabe Y, Saigusa Y, et al. Risks of malignancies among patients with psoriasis: A cohort study of 360 patients. J Dermatol. 2023;50(5):615–21. [DOI] [PubMed] [Google Scholar]
- 4.Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(Suppl 2):ii18–23. discussion ii24-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker JNWN. Psoriasis Lancet. 2021;397(10281):1301–15. [DOI] [PubMed] [Google Scholar]
- 6.Guo J, Zhang H, Lin W, Lu L, Su J, Chen X. Signaling pathways and targeted therapies for psoriasis. Signal Transduct Target Ther. 2023;8(1):437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Musumeci ML, Trecarichi AC, Caruso G, Aleo A, Platania H, Micali G. Long lasting response to anti-tumor necrosis factor α agents in psoriasis: A real life experience. Dermatol Ther. 2022;35(12):e15956. [DOI] [PubMed] [Google Scholar]
- 8.Nedoszytko B, Szczerkowska-Dobosz A, Stawczyk-Macieja M, Owczarczyk-Saczonek A, Reich A, Bartosiñska J, et al. Pathogenesis of psoriasis in the omic era. Part II. Genetic, genomic and epigenetic changes in psoriasis. Postepy Dermatol Alergol. 2020;37(3):283–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang CY, Wang CW, Chen CB, Chen WT, Chang YC, Hui RC, et al. Pharmacogenomics on the Treatment Response in Patients with Psoriasis: An Updated Review. Int J Mol Sci. 2023;24(8):7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan D, Gudjonsson JE, Le S, Maverakis E, Plazyo O, Ritchlin C, et al. New frontiers in psoriatic disease research, Part I: Genetics, environmental triggers, immunology, pathophysiology, and precision medicine. J Invest Dermatol. 2021;141(9):2112–e21223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J, Chen G, Habudele Z, Wang X, Cai M, Li H, et al. Relation of Life’s Essential 8 to the genetic predisposition for cardiovascular outcomes and all-cause mortality: results from a national prospective cohort. Eur J Prev Cardiol. 2023;30(15):1676–85. [DOI] [PubMed] [Google Scholar]
- 12.Lowe ME, Akhtari FS, Potter TA, Fargo DC, Schmitt CP, Schurman SH, et al. The skin is no barrier to mixtures: Air pollutant mixtures and reported psoriasis or eczema in the Personalized Environment and Genes Study (PEGS). J Expo Sci Environ Epidemiol. 2023;33(3):474–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735–41. [DOI] [PubMed] [Google Scholar]
- 14.Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263–71. [DOI] [PubMed] [Google Scholar]
- 15.Boehncke S, Salgo R, Garbaraviciene J, Beschmann H, Hardt K, Diehl S, et al. Effective continuous systemic therapy of severe plaque-type psoriasis is accompanied by amelioration of biomarkers of cardiovascular risk: results of a prospective longitudinal observational study. J Eur Acad Dermatol Venereol. 2011;25:1187–93. [DOI] [PubMed] [Google Scholar]
- 16.Kommoss KS, Bieler T, Ringen J, Lehmann A, Mihalceanu S, Hobohm L, et al. A simple tool for evaluation of inflammation in psoriasis: Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio as markers in psoriasis patients and related murine models of psoriasis-like skin disease. J Mol Med (Berl). 2023 Dec 21. [DOI] [PMC free article] [PubMed]
- 17.Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS /APhA et al. /ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082-e1143. [DOI] [PMC free article] [PubMed]
- 19.Elliott P, Peakman TC, UK Biobank. The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int J Epidemiol. 2008;37(2):234–44. [DOI] [PubMed] [Google Scholar]
- 20.Said S, Pazoki R, Karhunen V, Võsa U, Ligthart S, Bodinier B, et al. Genetic analysis of over half a million people characterises C-reactive protein loci. Nat Commun. 2022;13(1):2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luan YY, Yao YM. The clinical significance and potential role of C-reactive protein in chronic inflammatory and neurodegenerative diseases. Front Immunol. 2018;9:1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann JHO, Enk AH. Evaluation of psoriasis area and severity index thresholds as proxies for systemic inflammation on an individual patient level. Dermatology. 2022;238(4):609–14. [DOI] [PubMed] [Google Scholar]
- 23.Beygi S, Lajevardi V, Abedini R. C-reactive protein in psoriasis: a review of the literature. J Eur Acad Dermatol Venereol. 2014;28(6):700–11. [DOI] [PubMed] [Google Scholar]
- 24.Sokolova MV, Simon D, Nas K, Zaiss MM, Luo Y, Zhao Y, et al. A set of serum markers detecting systemic inflammation in psoriatic skin, entheseal, and joint disease in the absence of C-reactive protein and its link to clinical disease manifestations. Arthritis Res Ther. 2020;22(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karas A, Holmannova D, Borsky P, Fiala Z, Andrys C, Hamakova K, et al. Significantly altered serum levels of NAD, AGE, RAGE, CRP, and Elastin as potential biomarkers of psoriasis and aging-A case-control study. Biomedicines. 2022;10(5):1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.An I, Ucmak D, Ozturk M. The effect of biological agent treatment on neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, mean platelet volume, and C-reactive protein in psoriasis patients. Postepy Dermatol Alergol. 2020;37(2):202–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esen M. The effect of IL17 and IL23 inhibitors on hematological parameters and C-reactive protein in psoriasis patients. Cutan Ocul Toxicol. 2024;43(1):38–45. [DOI] [PubMed] [Google Scholar]
- 28.Babaie F, Omraninava M, Gorabi AM, Khosrojerdi A, Aslani S, Yazdchi A, et al. Etiopathogenesis of psoriasis from genetic perspective: An updated review. Curr Genomics. 2022;23(3):163–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tian R, He Q, Yang Y, Nong X, Wang S. Associations of polysocial risk score, lifestyle and genetic factors with incident psoriasis: a larger-scale prospective cohort study. Public Health. 2023;225:320–6. [DOI] [PubMed] [Google Scholar]
- 30.Si S, Li J, Tewara MA, Xue F. Genetically Determined Chronic Low-Grade Inflammation and Hundreds of Health Outcomes in the UK Biobank and the FinnGen Population: A Phenome-Wide Mendelian Randomization Study. Front Immunol. 2021;12:720876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burger PM, Koudstaal S, Mosterd A, Fiolet ATL, Teraa M, van der Meer MG, et al. C-reactive protein and risk of incident heart failure in patients with cardiovascular disease. J Am Coll Cardiol. 2023;82(5):414–26. [DOI] [PubMed] [Google Scholar]
- 32.Zhu M, Ma Z, Zhang X, Hang D, Yin R, Feng J, et al. C-reactive protein and cancer risk: a pan-cancer study of prospective cohort and Mendelian randomization analysis. BMC Med. 2022;20(1):301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request to the UK Biobank management team (https://www.ukbiobank.ac.uk/media/px5gbq4q/access_019-access-management-system-user-guide-v4-1.pdf).