Abstract
Intraoperative bleeding is a common issue in various surgical procedures, and the extent of bleeding significantly impacts the safety, efficacy, and prognosis of surgery. Therefore, accurate assessment of intraoperative blood loss and timely intervention are essential for the smooth progression of surgery and favorable clinical outcomes. Currently, clinical methods for estimating blood loss are generally classified into two main categories: visual and calculation methods. Calculation methods are further delineated into weighing techniques and concentration-based approaches. Although the visual method is criticized for its subjectivity and inaccuracy, it remains the most widely used approach in clinical practice for assessing intraoperative blood loss. This article reviews different methods for assessing blood loss during surgery and compares their respective advantages and disadvantages, aiming to provide surgeons with a more reliable foundation for intraoperative blood loss evaluation.
Keywords: Surgical operation, Blood loss, Calculation method
Intraoperative bleeding is an inevitable issue in surgical operations, and the amount of blood loss during surgery is critical to surgical safety and patient prognosis [1, 2]. Therefore, accurately assessing intraoperative blood loss is essential for the smooth progression of the operation and the patient’s postoperative recovery. Currently, methods for estimating blood loss are mainly divided into visual estimation and calculation methods. Although visual method can significantly deviate from the actual amount of blood loss [3], it remains a commonly used technique in clinical practice. Consequently, there is a pressing need for more accurate methods to estimate intraoperative blood loss, enabling surgeons to determine the extent of bleeding and intervene promptly. Additionally, precise blood loss assessment is crucial for making appropriate transfusion decisions, as inappropriate transfusions can adversely affect patient outcomes. A study showed that intraoperative blood transfusions represented an independent risk factors for intra- and post-operative complications and adversely affected outcomes in colorectal surgery [4]. Among various methods, the hematocrit (Hct) and hemoglobin (Hb) calculation methods may offer superior precision and accuracy [5]. However, when using these methods, it is important to consider the patient’s blood volume and changes in blood volume postoperatively. Factors such as postoperative blood loss and fluid replacement can influence blood volume, thereby affecting Hb concentration and the accuracy of blood loss calculations [6]. Therefore, further research is needed to determine whether Hct and Hb calculation methods can reliably assess intraoperative blood loss in clinical practice. This article reviews various methods for assessing intraoperative blood loss and compares their advantages and disadvantages to provide a reference for clinical practice.
Visual method
Visual method is the most commonly used method for clinically assessing intraoperative blood loss [7–9]. This method involves estimating blood loss based on the amount of blood in the suction bucket, blood on the surgical gauze, and visible intraoperative blood loss. It is favored for its simplicity, ease, and speed, allowing surgical personnel to make quick assessments based on personal experience and clinical observations. However, visual method is highly subjective and often fails to accurately reflect actual blood loss due to the influence of personal bias [5]. Despite these limitations, visual method remains widely used in various surgical settings, particularly in obstetrics for assessing postpartum blood loss. Studies have consistently shown that visual estimation tends to underestimate actual blood loss. Anya et al. [10] compared blood loss estimates from the visual method with blood loss calculated based on Hb levels in 60 patients undergoing cesarean delivery. They found that the visual estimates were consistently lower than Hb-based calculations, with the discrepancy increasing significantly when blood loss exceeded 500 mL. Similarly, in a study of intraoperative blood loss during arthroplasty, Ram et al. [11] observed that the visual method consistently underestimated blood loss. Budair et al. [12] also examined the accuracy of blood loss estimation by orthopedic surgeons and anesthesiologists during hip fracture surgery, finding that both groups significantly underestimated intraoperative blood loss. A study by Kollberg et al. [13] further demonstrated that visual method is not sufficiently accurate and often underestimates actual blood loss. Additionally, as intraoperative blood loss increases, the discrepancy between estimated and actual blood loss widens [14]. Conversely, Guinn et al. [15] included 60 patients undergoing posterior spine surgery and compared anesthesiologists’ estimates of intraoperative blood loss with measurements based on Hb loss. They found that the mean estimated blood loss exceeded the measured blood loss by an average of 246 mL. Similarly, Howe et al. [16] used visual estimates of blood loss during orthopedic surgery to predict postoperative Hb levels. Retrospective analyses of estimated blood loss versus the Hb difference in 198 patients showed a very low correlation, suggesting that the visual method still has significant error. In addition, the presence of other body fluids, such as flushing fluid, lymph, and bile, during surgery can further complicate accurate blood loss estimation [17]. We reviewed extensive literature, and nearly all studies concluded that the visual method of estimating blood loss was notably inaccurate when compared to reference methods.
Although the visual method has its inaccuracies, it remains irreplaceable in clinical practice due to its speed and simplicity, especially in emergency situations such as massive intraoperative bleeding or significant amounts of bloody fluid in postoperative abdominal drainage. In such cases, the physician visually estimates the amount of blood in the gauze and suction devices, and combines this assessment with the patient’s vital signs, including blood pressure, heart rate, and central venous pressure. Based on this evaluation, therapeutic measures such as pressor drug therapy, rehydration, and blood transfusion are implemented to rapidly resuscitate the patient. Therefore, the effectiveness of this method heavily depends on the experience of doctors and anesthesiologists and is significantly influenced by their training and education. Toledo et al. [18] conducted a study assessing the impact of didactic training on the accuracy of blood loss estimation using the visual method. The study found that estimation accuracy improved by 34% after training, compared to the accuracy levels before training. Kreutziger et al. [19] conducted a prospective, paired, single-blind trial involving 44 doctors specializing in anesthesia or emergency medicine. Participants were trained on the use of a professionally designed visual inspection tool and then estimated blood volumes on four test surfaces of different materials. The blood used for testing was a concentrated mixture of human red blood cells, with Hct levels ranging from 0.33 to 0.42. Fourteen days later, participants re-estimated the same blood volumes. The results showed a significant improvement in estimation accuracy following training, suggesting that professionally designed visual inspection tools can enhance the accuracy of visual methods. In clinical practice, visual blood loss estimates are usually agreed upon through joint discussions between the surgeon and anesthesiologist. Therefore, the accuracy of blood loss estimation can be improved by training surgical personnel in the visual method. Since the attending surgeon has a thorough understanding of the patient’s intraoperative and postoperative condition, an initial assessment using the visual method is essential in emergency situations. This approach helps guide clinical decisions, such as timely blood transfusions, to benefit patient outcomes.
Calculation method
Weighing calculation method
The weighing method was first proposed by Wangensteen [20]. This method estimates the amount of blood loss by comparing the weight difference of materials used before and after surgery. Specifically, all gauze used during the operation is weighed both before and after use, and the difference in weight is calculated. This difference is then added to the amount of blood collected in the suction bucket, and the weight of any flushing fluid is subtracted to obtain the total intraoperative blood loss [21].
 |
Vitello et al. [22] found through experimental research that the density of blood in intraoperative bleeding is similar to that of water, meaning that 1 g of weight obtained by weighing is equivalent to 1 mL of blood loss. Lee et al. [23] compared the weighing method and the laboratory method for estimating blood loss during animal surgery. They quantified blood loss by measuring weight changes in irrigation fluid and surgical gauze during the procedure and found that the weighing method was superior to the laboratory method, showing a significant correlation between the two. While Zajak et al. [24] analyzed 61 patients undergoing liver or pancreas surgery to compare intraoperative blood loss assessment methods—visual, weighing, and spectrophotometric. They found that the weighing calculation method was significantly less accurate than the spectrophotometric method in actual surgical settings. Rain [25] compared several blood loss measurement methods, including two that involved weighing. One method involved weighing all blood-stained gauze and pads after surgery to calculate blood loss, similar to the method used by Lee et al. The other method involved weighing the patient before and after surgery to account for factors such as infusion, resected tissue, dressings, ligations, and invisible water loss from the skin. The difference in weight was used to determine intraoperative blood loss. In addition, Atukunda et al. [26] assessed the clinical value of the weighing method as a cost-effective approach for diagnosing postpartum hemorrhage in resource-limited settings.
The weighing method is more accurate than the visual method for assessing blood loss; However, it still has a certain degree of error. Studies have indicated that the commonly used approximation of 1 g equaling 1 mL of blood is not entirely accurate, as the actual density of blood can vary depending on changes in Hct [7]. Additionally, the presence of other fluids, such as flushing fluid, bile, and amniotic fluid, can lead to an overestimation of blood loss [27]. Conversely, if all blood-soaked materials are not thoroughly weighed, blood loss may be underestimated. The main disadvantage of the weighing method is its complexity and cumbersome nature, making routine clinical application challenging. But in resource-limited areas, combining weighing with visual inspection can enhance the accuracy of blood loss quantification to some extent.
Concentration calculation method
The concentration calculation method estimates blood loss by measuring Hb concentration or Hct levels. When using this method, it is essential to account for changes in the patient’s blood volume before and after surgery, making accurate calculation of the patient’s blood volume crucial. Various formulas are available for calculating blood volume, and the choice of formula may depend on specific patient characteristics and clinical circumstances.
Blood volume calculation formula
Nadler’s formula [28]:
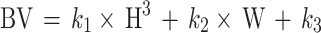 |
BV is the preoperative blood volume (L), H is the patient’s height (m), W is the patient’s weight (kg). Male patient k1 = 0.3669, k2 = 0.03219, k3 = 0.6041, while female patients k1 = 0.3561, k2 = 0.03308, k3 = 0.1833.
-
(2)
Choi’s formula [29]:
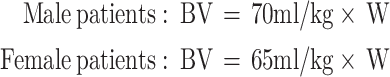
BV is the patient’s preoperative blood volume (mL) and W is the patient’s body weight (kg).
-
(3)
Rosencher’s formula [30]:
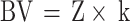 |
BV is the patient’s blood volume (mL), Z refers to body surface area, and k is a gender-specific constant: for females, k = 2430, for males, k = 2530.
The formula of body surface area:
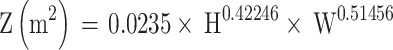 |
H is the patient’s height (m) and W is the patient’s weight (kg).
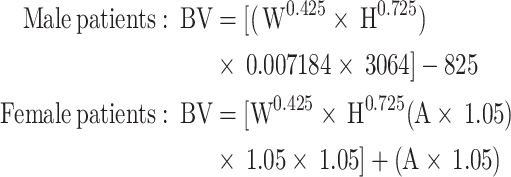 |
BV is the patient’s blood volume (mL), W is the patient’s weight (kg), H is the patient’s height (cm), and A is the patient’s age.
-
(5)
Fluorescein labeling [33]:
Marx et al. applied this method to a piglet model of septic shock to measure changes in blood volume using a fiber optic detection system. In this study, indocyanine green (ICG) was dissolved in a 5% glucose solution at a concentration of 2 mg/mL and injected into the circulatory system via a central venous catheter. ICG rapidly binds to Hb, and its concentration in the blood was measured by the fiber optic detection system after five circulatory cycles or 80 s post-injection. Using the mass of ICG injected and its concentration in the blood, the total blood volume can be calculated with the following formula:
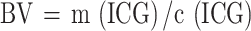 |
BV is the patient’s blood volume (mL), m(ICG) is the mass of ICG, and c(ICG) is the concentration of ICG in the blood. Since the liver is the only organ that metabolizes ICG, this method assumes no hepatic metabolism of ICG during the five circulatory cycles. This assumption allows for the total mass of ICG to be used in the calculation, yielding more accurate results.
Different formulas for calculating blood volume can yield varying results. The Nadler formula, Rosencher formula, and ICSH formula consider factors such as the patient’s gender, height, and weight. A study by Lopez-picado et al. [34] compared blood volumes calculated by different formulas and found that the difference between the Nadler formula and the ICSH formula was not significant. However, Schwaiger et al. [35] found statistically significant differences between blood volumes calculated using the Nadler formula and Choi’s formula. Although ICG can be used in humans, the process of injecting it through the central vein is invasive, making its clinical application complex and difficult to widely implement. A review of the literature revealed that the Nadler formula is the most widely used method for calculating blood volume in various studies. Therefore, choosing an appropriate blood volume calculation formula is crucial for accurately assessing blood loss.
Colorimetric method
Colorimetry is an analytical method used to determine the concentration of a substance by comparing the color intensity of a solution. The basic principle involves comparing the color of the test solution with a series of standard solutions of known concentrations in colorimetric tubes to estimate the Hb concentration in the test solution. This concentration is then multiplied by the total volume of the aspirated solution to calculate the patient’s blood loss [36]. The specific calculation formula is as follows:
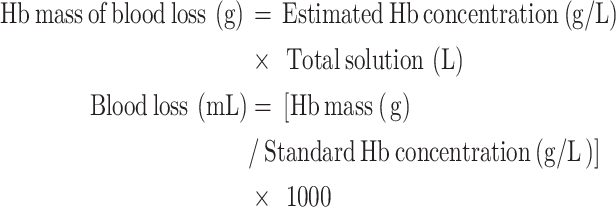 |
The colorimetric method calculates blood loss by measuring the Hb concentration in the lost blood, and the results are relatively accurate. Brant [37] and Wallace [38] reported an early method for using machine extraction to determine the optical density of blood, followed by spectrometric measurement of oxygenated Hb to estimate blood loss during vaginal delivery. The procedure involved placing collected blood-soaked materials, such as gauze and pads, into a machine containing a preset volume of water, ammonium hydroxide, and a surfactant to accelerate Hb release. After thorough mixing, the sample was centrifuged and filtered, and the concentration of Hb in the resulting solution was measured using colorimetry, enabling an accurate calculation of blood loss. Gerdessen et al. [7] conducted a meta-analysis to evaluate the accuracy of various commonly used methods for measuring blood loss. The study compared colorimetry, weighing, and visual method, and the results showed that the colorimetric method has a relative advantage in estimating blood loss. Li et al. [39] utilized the colorimetric method to quantify blood present in the liposuction solution of patients undergoing liposuction, developing a standard colorimetric card that allows for quick, accurate, and convenient calculation of blood loss, which holds clinical significance. Spectrophotometry has been recognized as the most accurate method for measuring blood loss, yet it is also the most costly and complex [40]. Additionally, this technique is influenced by factors such as light source stability and the observer’s subjective judgment. Its lengthy, multi-step process further restricts its application in routine clinical practice.
In recent years, advancements in science and technology have led to new applications of colorimetry, such as the Triton system [41]. The Triton system, developed based on the principles of colorimetry, is an iPad application approved by the U.S. Food and Drug Administration (FDA) for quantitatively estimating blood loss. The system connects to a foot pedal that controls the iPad via Bluetooth and can continuously take photos during surgery to capture bleeding in surgical gauze and suction buckets in real time. These photos are wirelessly transmitted to a remote server and analyzed using colorimetric techniques to calculate the Hb content in the gauze or suction bucket, while also recording and calculating cumulative blood loss. The Triton system offers the advantage of quickly and continuously analyzing the amount of bleeding. Because its measurement of Hb is based on image colorimetric analysis, it effectively eliminates the influence of other liquid components on the gauze. A study using the Triton system continuously scanned 709 surgical gauze from 50 surgical operations, including orthopedics, urology, and obstetrics. Compared with visual inspection and weighing methods, the Triton system proved to be more feasible and accurate for real-time measurement of Hb loss during surgery [27]. Rubenstein et al. [21] conducted a study using the Triton system to assess blood loss in 274 patients undergoing transvaginal deliveries. The results were compared to blood loss estimates from the visual method, revealing that the Triton system calculated higher blood loss volumes and more accurately identified patients experiencing excessive blood loss. Additionally, a prospective cohort study of obstetric cesarean sections used both visual estimation and the Triton system to assess blood loss and monitor the difference in Hb(ΔHb) before and 24 h after cesarean section. The results showed that the Triton system had a strong predictive value for ΔHb > 20 g/L, demonstrating its sensitivity to detecting increased postpartum hemorrhage [42].
From the above, it is evident that the colorimetric method offers greater accuracy in assessing blood loss compared to visual and weighing methods. However, its cumbersome and costly operation limits its widespread clinical application, and the Triton system faces similar challenges with broad implementation. Therefore, we believe that this method is not suitable for routine use in clinical practice unless its procedure is simplified—an area that warrants further exploration.
Hct calculation method
The Hct calculation method was first proposed by Ward in 1980 for calculating circulating blood volume using Hct values [43]. Later, in 1983, Gross further developed this method and introduced a linear equation for calculating circulating blood volume based on the perioperative average Hct. This method is also known as the Gross formula [44].
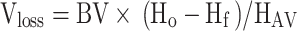 |
Vloss(mL) is the patient’s calculated blood loss, and the patient’s total blood volume, BV (mL), is calculated using the Nadler formula, Ho is the patient’s preoperative Hct value, Hf is the patient’s postoperative Hct value, HAV was the mean value of preoperative and postoperative Hct.
Gross verified the accuracy of this method in practice and found that the blood loss calculated by this method is very close to the actual amount, except when the patient experiences extremely large or rapid blood loss, which can cause the equation to deviate from the normal curve. Hct calculation method is mainly used in major orthopedic surgeries, such as joint replacement. For example, Cai et al. [45] applied this method to calculate postoperative hidden blood loss in 707 patients undergoing total hip arthroplasty and found that the actual postoperative hidden blood loss exceeded physicians’ expectations. Lopez-picado et al. [34] used this method to study the effect of tranexamic acid on blood loss in patients undergoing total hip replacement, comparing it with other calculation methods. They found no significant difference in blood loss between the treatment and placebo groups on postoperative day 2, possibly because the method does not account for transfusions that may have occurred. Gao et al. [46] also used this method to evaluate blood loss in total knee arthroplasty. They applied four different calculation methods to 245 patients who underwent total knee replacement and established the Pearson correlation coefficient of blood loss between these methods. The correlation coefficient indicates the strength of the relationship between the methods, with the results showing that the Hct calculation method had the lowest correlation coefficient, suggesting that its results may be less reliable.
When using this method to calculate blood loss, it is important to consider changes in the patient’s blood volume before and after surgery, as well as the Hct levels selected on the days following surgery, which can affect the calculation results. Additionally, this method does not account for blood transfusions. After a transfusion, the Hct level increases compared to pre-transfusion levels, resulting in calculated blood loss being lower than the actual amount. Therefore, the actual blood loss needs to include the amount of blood transfused [47]. Since the Hct calculation method does not account for Hb-related factors or the changes in Hct due to blood transfusions, it has certain limitations and is not routinely recommended.
Hb calculation method
Hb mass method
The Hb mass method was proposed by Good et al. [48] in their study on the effect of tranexamic acid on total blood loss in patients undergoing total knee arthroplasty. This method estimates total perioperative blood loss by calculating the amount of Hb lost, using both preoperative and postoperative Hb concentrations.
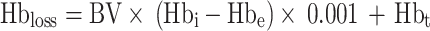
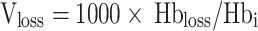
Hbloss(g) represents the Hb mass of blood loss. The patient’s blood volume BV (mL) is calculated using the Nadler formula. Hbi(g/L) is the preoperative Hb concentration of the patient, Hbe(g/L) is the patient’s Hb concentration on the fifth day after surgery. Hbt(g) refers to the Hb mass transfused to the patient, one unit (1U) of stored blood contains 52 ± 5.4 g Hb [49]. Vloss(mL) is the calculated blood loss of the patient.
When using this method to calculate blood loss, it is important to consider changes in the patient’s blood volume before and after surgery. Good et al. assumed that the patient’s blood volume returned to preoperative levels by the fifth postoperative day, so the Hb concentration on the fifth day was used for calculations. However, factors such as postoperative hemoconcentration, fluid replacement, and blood transfusion can affect the patient’s blood volume, thereby impacting Hb concentration. Barrachina et al. [32] found that a patient’s total blood volume can return to normal preoperative levels 2 ~ 4 days after surgery. George’s study [50] observed that Hb concentration remained stable 2 ~ 4 days after surgery and did not change significantly within 6 ~ 8 days after stabilization. The research by Meunier et al. [6] showed that the average Hb level of volunteers gradually decreased over six days after blood loss, reaching its lowest value on the sixth day before beginning to gradually recover. Adamson et al. [51] studied blood volume changes in six healthy volunteers after extracting 15 ~ 20% of their blood volume. They measured Hct levels before and after surgery and used I131-labeled plasma albumin to measure blood loss and subsequent changes in plasma proteins. Their studies found that it takes at least 72 h to mobilize enough protein to return intravascular blood volume to normal. It can be seen that the patient’s blood volume can be basically restored to the normal level 72 h after blood loss, and making calculations based on Hb concentration at this time relatively accurate.
Literature searches reveal that the Hb mass method is primarily used in orthopedic surgery [52]. For instance, Chechik et al. [53] investigated the effects of the anticoagulants clopidogrel and aspirin on perioperative bleeding in patients with hip fractures. They used Hb concentration on the seventh day post-surgery to calculate the amount of Hb lost during the perioperative period, thereby estimating blood loss. Foss and Kehlet [54] also calculated hidden blood loss in various hip fracture surgeries based on Hb concentration. Additionally, Jaramillo et al. [5] used the Hb mass loss formula to estimate blood loss in 100 consecutive patients undergoing laparoscopic urological surgery. The study highlighted that hematological parameters in commonly used formulas may not accurately reflect actual blood volume loss and therefore may not provide precise estimates of blood loss. In contrast, estimating Hb mass loss is based solely on the actual amount of blood lost, avoiding potential errors related to the degree of blood dilution. Therefore, using Hb mass to estimate blood loss may be more reliable. Yu et al. [55] also applied the Hb mass method to calculate intraoperative blood loss during pancreaticoduodenectomy and compared it with estimates based on visual method. The calculated blood loss was approximately 743.2 mL, significantly higher than visually estimated values. However, this discrepancy suggests that the Hb mass method may provide a more accurate assessment of actual blood loss. Gao et al. [46] calculated blood loss during total knee arthroplasty using four different methods and determined the Pearson correlation coefficients for each. They found considerable variation in blood loss estimates across methods, with the Hb mass calculation method emerging as potentially the most reliable. Additionally, Hahn-Klimroth et al. [56] verified the accuracy of the Hb loss formula through linear and ridge regression models using clinical and laboratory data from healthy individuals. This method can be used to guide transfusion and blood management.
The Hb mass method is straightforward to use, requiring only the patient’s preoperative and postoperative Hb concentrations to calculate blood loss. This method also accounts for the effects of blood transfusions, making it applicable in cases where patients have received transfusions. The blood loss calculated using this method reflects the total blood loss intraoperative and 72-hour postoperative, encompassing both overt intraoperative blood loss and postoperative occult blood loss. When combined with the patient’s postoperative Hb concentration, this calculated blood loss can guide therapeutic decision-making, including the need for blood transfusion, rehydration, haemostatic therapy, and other interventions. However, this method assumes that the patient’s blood volume returns to preoperative levels after surgery. Generally, the Hb concentration of patients stabilizes around 72 h post-surgery, leading to minimal calculation error. But in cases of significant hemorrhage, the visual method is more intuitive and rapid, making it more suitable for urgent situations. In non-emergency situations, this method is simple, objective, and accurate, allowing for precise calculation of blood loss throughout the entire perioperative period. It is highly significant for the management of postoperative patients and is therefore recommended for clinical use.
Hb dilution method
Studies have shown that after a patient loses a significant amount of blood, the body mobilizes plasma proteins into the blood vessels to draw fluids from outside the blood vessels into the bloodstream, thereby replenishing the lost blood volume [51]. As a result, Hb concentration is diluted before and after acute blood loss. In theory, the loss of blood volume can be estimated by the degree of Hb dilution. Meunier [9] used this calculation method to test whether Hb dilution can effectively measure blood loss.
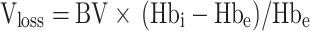 |
Vloss(mL) represents the patient’s calculated blood loss, and the patient’s total blood volume, BV (mL), is still calculated using the Nadler formula, Hbi(g/L) is the patient’s preoperative Hb concentration, while Hbe(g/L) is the patient’s postoperative Hb concentration. The Hb dilution factor, K, is defined as (Hbi−Hbe)/Hbe.
This method of calculating blood loss is primarily based on the concept of Hb dilution, estimating the lost blood volume by multiplying the patient’s total blood volume by the Hb dilution factor (K). Meunier conducted a study with 39 volunteers in an experiment similar to blood donation. The results showed that the actual blood loss of each volunteer was 442 ± 10 mL. The study measured Hb concentration from the first to the 14th day after blood loss, showing that Hb concentration reached its lowest level on the sixth day post-blood loss. Using the Hb dilution formula, the estimated theoretical blood loss on the first day was 152 ± 214 mL, which was approximately 60% less than the actual blood loss of 442 ± 10 mL. On the sixth day, the theoretical blood loss was calculated as 301 ± 145 mL, about 32% less than the actual loss. As Hb concentration continued to recover, the accuracy of the theoretical calculations further decreased. This method was validated in healthy volunteers with a blood loss of approximately 400 mL, which differs from the physiological conditions of surgical patients and presents certain limitations. However, similar to the Hb mass method, it calculates blood loss by measuring the difference in Hb concentration before and after surgery, while also factoring in the impact of blood transfusions on Hb levels. Gaya et al. [57] introduced the concept of ΔHb, the difference between the preoperative Hb concentration and the lowest postoperative Hb concentration. They analyzed 4,669 patients undergoing major gastrointestinal surgery and found that a postoperative ΔHb ≥ 50% was associated with a higher rate of postoperative complications and could potentially be used to guide transfusion practices. Farinha et al. [58] analyzed 270 patients undergoing colorectal surgery, comparing blood loss estimated by doctors and anesthesiologists with the difference in Hb concentration before and after surgery (ΔHb). They found a correlation between the two, suggesting that ΔHb can be used to assess blood loss. Similarly, in a study on the use of tranexamic acid to prevent obstetric bleeding after cesarean section, Pacheco et al. [59] used the difference in Hb concentration before surgery and 48 h post-surgery to estimate blood loss. Therefore, in non-emergency situations, the Hb dilution method, similar to the Hb mass method, offers advantages such as ease of operation and relatively accurate results, particularly when accounting for transfusion factors. This method also shows promise for broader clinical application.
Discussion
Accurate quantification of intraoperative blood loss is crucial, as it serves as a key indicator of surgical mass. Numerous studies have shown a strong link between intraoperative blood loss and both postoperative morbidity and mortality [60, 61]. In hepatobiliary and pancreatic surgery specifically, intraoperative blood loss is recognized as a major risk factor for postoperative pancreatic fistula (POPF)—a significant cause of morbidity after pancreatic surgery—and a predictor of postoperative liver failure [61–63]. Furthermore, prssential for transfusion decision-making; inaccurate estimations can lead to unnecessary transfusions, which have been linked to postoperative complications and adverse patient outcomes [64]. Perri et al. [65] conducted a systematic review of studies published between 2006 and 2021 that reported on blood loss in patients undergoing pancreatic or hepatic resection. Their findings highlight an urgent need for standardized methods to quantify intraoperative blood loss in order to enhance surgical safety and improve patient outcomes. Consequently, there is a pressing clinical demand for accurate methods to calculate intraoperative blood loss.
Our literature review of methods for calculating surgical blood loss revealed a variety of approaches, yet no single method is universally accepted as the “gold standard.” This lack of consensus underscores the need for continued research to identify or develop an optimal, standardized method for accurately assessing blood loss in surgical settings. Visual method is the most commonly used method in clinical practice. We note that visual method tended to provide lower blood loss volumes than formula-based or other methods, a finding that was consistently found across most studies. Although visual method is relatively subjective and often inaccurate, it remains valuable in emergency situations, such as massive intraoperative bleeding or large volumes of bloody fluid in postoperative peritoneal drains. In these cases, visual method allows for a rapid assessment of blood loss, enabling timely treatment and guiding transfusion decisions, ultimately improving patient outcomes and prognosis. Studies have shown that the accuracy of visual assessment is influenced by the clinician’s experience and skill. After simulated training, the accuracy of blood loss estimation can improve. Therefore, training personnel in visual blood loss assessment is essential for enhancing its reliability [41, 66].
The accuracy of calculating blood loss using the weighing method is better than visual method, but it is still prone to significant errors. This method can be easily influenced by other fluids such as irrigation water, bile, and amniotic fluid, potentially leading to an overestimation of blood loss. Conversely, if all blood-soaked materials are not fully weighed, blood loss may be underestimated. Additionally, the weighing method is complex and cumbersome, making it difficult to apply routinely in clinical practice. The colorimetric method estimates blood loss by measuring the Hb concentration in the lost blood. Studies have demonstrated that colorimetry provides a more accurate assessment of blood loss compared to visual and weighing method [7]. Notably, the latest Triton system [41] can quickly and accurately analyze intraoperative bleeding and facilitate timely intervention and treatment. However, this system remains costly and complex, limiting its clinical application. Further research is needed to assess its suitability across various surgical procedures and to evaluate its accuracy in calculating blood loss reliably. Therefore, the weighing method and colorimetric method are not suitable for clinical promotion due to their operational complexity.
The fundamental principles of the Hb mass method and the Hb dilution method are similar. Both methods estimate the patient’s total blood volume using individualized parameters and calculate blood loss by comparing preoperative and postoperative Hb concentrations. Various methods exist for calculating a patient’s blood volume. According to literature, the Nadler formula [28] is the most widely used due to its high accuracy, as it incorporates the patient’s gender, height, weight, and other parameters. However, both methods have inherent inaccuracies when estimating actual blood loss. The primary sources of error include: estimating total blood volume, assessing intraoperative blood loss, the effects of perioperative fluid replacement and blood transfusion on Hb concentration, and the body’s compensatory mechanisms for Hb. Even though there is some deviation in calculation, it is still much more accurate and objective compared to the visual method. Oba et al. [67] compared blood loss estimations in partial hepatectomy using both Hb-based calculations and traditional weighing methods. They found that the weighing method tended to underestimate blood loss, whereas Hb formula calculations provided more accurate estimations. In addition, Tran et al. [68] conducted a meta-analysis of blood loss calculation methods in non-cardiac major surgeries, showing that the visual method tends to significantly underestimate blood loss compared to other methods. While the formulaic method cannot be considered the definitive gold standard, its reliance on inter-observer consistency provides potential advantages. Therefore, it is recommended to prioritize the formula-based calculation method as the preferred approach for estimating blood loss. In situations where the patient’s abdominal drainage tube shows little to no bloody fluid after surgery, or hematomas occur during orthopedic procedures, the visual method is limited. In contrast, the Hb concentration method, which calculates blood loss by comparing preoperative and postoperative Hb levels while accounting for blood transfusion, offers a more objective and accurate reflection of blood loss within 72 h postoperatively. Routine postoperative blood tests make it easy to obtain Hb concentrations, and this difference provides an intuitive reflection of blood loss, aiding in treatment decisions such as blood transfusion. International transfusion guidelines recommend using Hb concentration as a threshold for determining blood transfusion needs, generally indicating transfusion at Hb levels of 7 ~ 8 g/dL. However, the actual amount of blood to be transfused should be based on the precise calculation of blood loss [69]. The Hb concentration calculation method, by analyzing postoperative Hb levels, can efficiently establish whether the transfusion threshold has been met. Its ability to accurately estimate blood loss, when combined with postoperative Hb concentration, plays a key role in guiding therapeutic decisions such as blood transfusion, rehydration, hemostasis, and other interventions.
The Hb concentration at 72 h postoperatively was selected because, by this time, it has generally stabilized and is minimally affected by postoperative fluid resuscitation and transfusion interventions. In addition to intraoperative bleeding, hidden blood loss within 72 h after surgery—such as from wounds, anastomoses, or stress ulcers—should not be underestimated. The Hb concentration method accounts for both intraoperative and hidden blood loss within this timeframe, making it more useful for guiding patient treatment. While every method has its limitations, and the Hb calculation method is no exception, and the postoperative condition of each patient varies, so it cannot be invariably applied to all patients to calculate blood loss, but is limited to those whose general condition is more stable after surgery and whose blood volume is basically restored to the preoperative level after surgery. Nonetheless, we found that the formulaic method of Hb concentration calculation is objective, accurate, and easy to manipulate, interpret, and replicate compared with other methods currently available, and it is worthwhile to promote it in the clinical setting to verify its accuracy.
Conclusion and outlook
Our analysis of intraoperative blood loss calculation methods in surgical operations reveals that each method has its own advantages and disadvantages, leading to varying results. In emergency situations, such as acute bleeding, we cannot overlook the irreplaceable role of visual method in quickly initiating the evaluation and treatment process. However, in non-emergency cases, the Hb concentration difference method offers significant advantages. It relies on easily obtainable parameters, produces intuitive results, and can be replicated across various surgical procedures. While there is no universally accepted gold standard for estimating blood loss, we recommend the promotion and application of the Hb concentration calculation method in clinical practice, as it is more reliable compared to other techniques. Finally, we hope future studies will explore more objective, accurate, and practical methods for calculating intraoperative blood loss to improve surgical outcomes.
Acknowledgements
Not applicable.
Author contributions
Yi-Min Lin designed the study, participated in the collection and analysis of the literature, and wrote the manuscript; Chao Yu participated in the study’s conception, literature collection; Guo-Zhe Xian participated in study design and provided guidance; All authors read and approved the final manuscript.
Funding
The paper is supported by Shandong Provincial Natural Science Foundation General Project, No. ZR2020MH248.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nakamura H, et al. Impact of intraoperative blood loss on long-term survival after lung cancer resection. Annals of Thoracic and Cardiovascular Surgery. Official J Association Thorac Cardiovasc Surg Asia. 2015;21(1):18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stenberg E, et al. Bleeding during laparoscopic gastric bypass surgery as a risk factor for less favorable outcome. A cohort study from the Scandinavian Obesity Surgery Registry. Surg Obes Relat Diseases: Official J Am Soc Bariatr Surg. 2017;13(10):1735–40. [DOI] [PubMed] [Google Scholar]
- 3.Meiser A, et al. [Quantification of blood loss. How precise is visual estimation and what does its accuracy depend on?]. Anaesthesist. 2001;50(1):13–20. [DOI] [PubMed] [Google Scholar]
- 4.Kirchhoff P, Clavien P-A, Hahnloser D. Complications in colorectal surgery: risk factors and preventive strategies. Patient Saf Surg. 2010;4(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaramillo S, et al. Perioperative blood loss: estimation of blood volume loss or haemoglobin mass loss? Blood Transfus. 2020;18(1):20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meunier A, et al. Validation of a haemoglobin dilution method for estimation of blood loss. Vox Sang. 2008;95(2):120–4. [DOI] [PubMed] [Google Scholar]
- 7.Gerdessen L, et al. Comparison of common perioperative blood loss estimation techniques: a systematic review and meta-analysis. J Clin Monit Comput. 2021;35(2):245–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Athar MW et al. Accuracy of visual estimation of blood loss in obstetrics using clinical reconstructions: an observational simulation cohort study. Int J Obstet Anesth. 2022;50. [DOI] [PubMed]
- 9.Lemée J et al. Visual estimation of postpartum blood loss during a simulation training: a prospective study. J Gynecol Obstet Hum Reprod. 2020;49(4). [DOI] [PubMed]
- 10.Anya SU, Onyekwulu FA, Onuora EC. Comparison of visual estimation of intra-operative blood loss with haemoglobin estimation in patients undergoing caesarean section. Niger Postgrd Med J. 2019;26(1):25–30. [DOI] [PubMed] [Google Scholar]
- 11.Ram G-G, Suresh P, Vijayaraghavan P-V. Surgeons often underestimate the amount of blood loss in replacement surgeries. Chin J Traumatol = Zhonghua Chuang Shang Za Zhi. 2014;17(4):225–8. [PubMed] [Google Scholar]
- 12.Budair B, et al. Are we all guilty of under-estimating intra-operative blood loss during hip fracture surgery? J Orthop. 2017;14(1):81–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kollberg SE, et al. Accuracy of visually estimated blood loss in surgical sponges by members of the surgical team. AANA J. 2019;87(4):277–84. [PubMed]
- 14.Sosa CG, et al. Risk factors for postpartum hemorrhage in vaginal deliveries in a latin-American population. Obstetrics Gynecol. 2009;113(6):1313–9. [DOI] [PMC free article] [PubMed]
- 15.Guinn NR, et al. Comparison of visually estimated blood loss with direct hemoglobin measurement in multilevel spine surgery. Transfusion. 2013;53(11):2790–4. [DOI] [PubMed] [Google Scholar]
- 16.Howe C, et al. A model for clinical estimation of perioperative hemorrhage. Off J Int Acad Clin Appl Thrombosis/Hemostasis. 2003;9(2):131–5. [DOI] [PubMed] [Google Scholar]
- 17.Samantha Thomas LG, Sill AM. Measured versus estimated blood loss interim analysis of a prospective quality improvement study. American Surgeon. 2020. [PubMed]
- 18.Toledo P, et al. The effect of live and web-based education on the accuracy of blood-loss estimation in simulated obstetric scenarios. Am J Obstet Gynecol. 2010;202(4):e4001–5. [DOI] [PubMed] [Google Scholar]
- 19.Kreutziger J, et al. Accuracy of training blood volume quantification using a visual estimation tool. World J Emerg Med. 2021;12(3):174–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wangensteen OH. The controlled administration of fluid to surgical patients. Minn Med. 1942;25:783. [Google Scholar]
- 21.Rubenstein AF, et al. Automated Quantification of Blood Loss versus Visual Estimation in 274 Vaginal Deliveries. Am J Perinatol. 2020;38(10):1031–5. [DOI] [PubMed] [Google Scholar]
- 22.Vitello DJ, et al. Blood Density Is Nearly Equal to Water Density: A Validation Study of the Gravimetric Method of Measuring Intraoperative Blood Loss. J Veterinary Med. 2015;2015:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee MH, et al. Quantification of surgical blood loss. Volume 35. Veterinary Surgery: VS; 2006. pp. 388–93. 4. [DOI] [PubMed] [Google Scholar]
- 24.Zajak J, et al. Blood loss quantification during major abdominal surgery: prospective observational cohort study. BMC Surg. 2024;24(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rains AJ. Experience in the measurement of blood-and fluid-loss at operation. Br J Surg. 1955;43(178):191–6. [DOI] [PubMed] [Google Scholar]
- 26.Atukunda EC, et al. Measuring Post-Partum Haemorrhage in Low-Resource Settings: The Diagnostic Validity of Weighed Blood Loss versus Quantitative Changes in Hemoglobin. PLoS ONE. 2016;11(4):e0152408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konig G, et al. Real-time evaluation of an image analysis system for monitoring surgical hemoglobin loss. J Clin Monit Comput. 2017;32(2):303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224–32. [PubMed] [Google Scholar]
- 29.Choi WS, Irwin MG, Samman N. The Effect of Tranexamic Acid on Blood Loss During Orthognathic Surgery: A Randomized Controlled Trial. J Oral Maxillofac Surg. 2009;67(1):125–33. [DOI] [PubMed] [Google Scholar]
- 30.Rosencher N, et al. Orthopedic Surgery Transfusion Hemoglobin European Overview (OSTHEO) study: blood management in elective knee and hip arthroplasty in Europe. Transfusion. 2003;43(4):459–69. [DOI] [PubMed] [Google Scholar]
- 31.Pearson TC, et al. Interpretation of measured red cell mass and plasma volume in adults: Expert Panel on Radionuclides of the International Council for Standardization in Haematology. Br J Haematol. 1995;89(4):748–56. [DOI] [PubMed] [Google Scholar]
- 32.Barrachina B, et al. Analysis of the estimation of bleeding using several proposed haematometric equations. Ir J Med Sci (1971 -). 2022;192(1):327–33. [DOI] [PubMed] [Google Scholar]
- 33.Marx G, et al. Blood volume measurements using an integrated fiberoptic monitoring system in a porcine septic shock model. Crit Care Med. 2006;34(5):1483–8. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Picado A, Albinarrate A, Barrachina B. Determination of Perioperative Blood Loss: Accuracy or Approximation? Anesth Analg. 2017;125(1):280–6. [DOI] [PubMed] [Google Scholar]
- 35.Schwaiger M, et al. Determination of blood loss in bimaxillary surgery: does the formula and the time point affect results? Int J Oral Maxillofac Surg. 2022;51(4):493–500. [DOI] [PubMed] [Google Scholar]
- 36.Doctorvaladan S, et al. Accuracy of Blood Loss Measurement during Cesarean Delivery. Am J Perinatol Rep. 2017;07(02):e93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brant HA. Precise estimation of postpartum haemorrhage: difficulties and importance. BMJ. 1967;1(5537):398–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wallace G. Blood loss in obstetrics using a haemoglobin dilution technique. J Obstet Gynecol Br Commonw. 1967;74(1):64–7. [DOI] [PubMed] [Google Scholar]
- 39.Li X-B, et al. Assessment of Blood Volume in Liposuction Fluids Using Colorimetry. Aesthetic Plast Surg. 2024;48(7):1457–64. [DOI] [PubMed] [Google Scholar]
- 40.Schorn MN. Measurement of blood loss: review of the literature. J Midwifery Womens Health. 2010;55(1):20–7. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, et al. Assessing the accuracy of visual blood loss estimation in postpartum hemorrhage in Shanghai hospitals: A web-based survey for nurses and midwives. J Clin Nurs. 2021;30(23–24):3556–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saoud F, et al. Validation of a new method to assess estimated blood loss in the obstetric population undergoing cesarean delivery. Am J Obstet Gynecol. 2019;221(3):e2671–6. [DOI] [PubMed] [Google Scholar]
- 43.Ward CF, et al. A Computer Nomogram for Blood Loss Replacement. Anesthesiology. 1980;53(3 Suppl):S126–126. [Google Scholar]
- 44.Gross JB. Estimating allowable blood loss: corrected for dilution. Anesthesiology. 1983;58(3):277–80. [DOI] [PubMed] [Google Scholar]
- 45.Cai L, et al. Influencing factors of hidden blood loss after primary total hip arthroplasty through the posterior approach: a retrospective study. BMC Musculoskelet Disord. 2023;24(1):582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao F-Q, et al. Four Methods for Calculating Blood-loss after Total Knee Arthroplasty. Chin Med J. 2015;128(21):2856–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li B, et al. The effect of tourniquet use on hidden blood loss in total knee arthroplasty. Int Orthop. 2009;33(5):1263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003;90(5):596–9. [DOI] [PubMed] [Google Scholar]
- 49.Lisander B, Ivarsson I, Jacobsson SA. Intraoperative autotransfusion is associated with modest reduction of allogeneic transfusion in prosthetic hip surgery. Acta Anaesthesiol Scand. 1998;42(6):707–12. [DOI] [PubMed] [Google Scholar]
- 50.George TJ, et al. Hemoglobin drift after cardiac surgery. Ann Thorac Surg. 2012;94(3):703–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adamson J, Hillman RS. Blood volume and plasma protein replacement following acute blood loss in normal man. JAMA. 1968;205(9):609–12. [PubMed] [Google Scholar]
- 52.Sehat KR, Evans R, Newman JH. How much blood is really lost in total knee arthroplasty? Correct blood loss management should take hidden loss into account. Knee. 2000;7(3):151–5. [DOI] [PubMed] [Google Scholar]
- 53.Chechik O, et al. The effect of clopidogrel and aspirin on blood loss in hip fracture surgery. Injury. 2011;42(11):1277–82. [DOI] [PubMed] [Google Scholar]
- 54.Foss NB, Kehlet H. Hidden blood loss after surgery for hip fracture. J Bone Joint Surg Br Vol. 2006;88(8):1053–9. [DOI] [PubMed] [Google Scholar]
- 55.Yu C, Lin Y-M, Xian G-Z. Hemoglobin loss method calculates blood loss during pancreaticoduodenectomy and predicts bleeding-related risk factors. World J Gastrointest Surg. 2024;16(2):419–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hahn-Klimroth M, et al. Generation and validation of a formula to calculate hemoglobin loss on a cohort of healthy adults subjected to controlled blood loss. J Translational Med. 2021;19(1):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spolverato G et al. Effect of Relative Decrease in Blood Hemoglobin Concentrations on postoperative morbidity in patients who undergo major gastrointestinal surgery. JAMA Surg. 2015;150(10). [DOI] [PubMed]
- 58.Teixeira Farinha H, et al. Proposition of a simple binary grading of estimated blood loss during colon surgery. Int J Colorectal Dis. 2021;36(10):2111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pacheco LD, et al. Tranexamic Acid to Prevent Obstetrical Hemorrhage after Cesarean Delivery. N Engl J Med. 2023;388(15):1365–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seykora TF, et al. The Beneficial Effects of Minimizing Blood Loss in Pancreatoduodenectomy. Ann Surg. 2019;270(1):147–57. [DOI] [PubMed] [Google Scholar]
- 61.Trudeau MT, et al. The Influence of Intraoperative Blood Loss on Fistula Development Following Pancreatoduodenectomy. Ann Surg. 2022;276(5):e527–35. [DOI] [PubMed] [Google Scholar]
- 62.Casciani F, et al. The effect of high intraoperative blood loss on pancreatic fistula development after pancreatoduodenectomy: An international, multi-institutional propensity score matched analysis. Surgery. 2021;170(4):1195–204. [DOI] [PubMed] [Google Scholar]
- 63.Fukushima K, et al. Assessment of ISGLS definition of posthepatectomy liver failure and its effect on outcome in patients with hepatocellular carcinoma. J Gastrointest Surgery: Official J Soc Surg Aliment Tract. 2014;18(4):729–36. [DOI] [PubMed] [Google Scholar]
- 64.Saporito A, et al. Perioperative inappropriate red blood cell transfusions significantly increase total costs in elective surgical patients, representing an important economic burden for hospitals. Front Med. 2022;9:956128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perri G, et al. Intraoperative Blood Loss Estimation in Hepato-pancreato-biliary Surgery- Relevant, Not Reported, Not Standardized: Results From a Systematic Review and a Worldwide Snapshot Survey. Ann Surg. 2023;277(4):e849–55. [DOI] [PubMed] [Google Scholar]
- 66.Dildy GA, et al. Estimating blood loss: can teaching significantly improve visual estimation? Obstet Gynecol. 2004;104(3):601–6. [DOI] [PubMed] [Google Scholar]
- 67.Oba A, et al. Possible underestimation of blood loss during laparoscopic hepatectomy. BJS Open. 2019;3(3):336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tran A, et al. Techniques for blood loss estimation in major non-cardiac surgery: a systematic review and meta-analysis. Can J Anaesth = J Canadien D’anesthesie. 2021;68(2):245–55. [DOI] [PubMed] [Google Scholar]
- 69.Carson JL, et al. Clinical Practice Guidelines From the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA. 2016;316(19):2025–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.


