Abstract
Purpose
The routine perioperative use of opioids has recently been questioned due to opioid-related side effects, which can be potentially harmful in geriatric patients. This study aimed to evaluate the effects of opioid-free anesthesia in geriatric patients undergoing hip surgery.
Patients and Methods
A total of 121 patients, aged 60 years or older, undergoing elective hip surgery were randomized to receive either opioid-free anesthesia with dexmedetomidine and esketamine (OFA group) or balanced anesthesia with opioids (CON group). All patients received a preoperative fascia iliaca block and postoperative patient-controlled analgesia using tramadol. The primary outcome was the incidence of a composite of anesthetic-related complications (nausea and vomiting, hypoxemia, ileus, urinary retention and delirium) within 48 hours postoperatively. The hemodynamics, postoperative pain and quality of life were also assessed.
Results
The incidence of composite adverse events was significantly reduced in the OFA group compared with the CON group (35.0% vs 62.3%, estimated difference: 27.3%, 95% confidence interval: 10.2%–44.4%, P = 0.003). Notably, patients in the OFA group experienced less postoperative nausea and vomiting (P = 0.040), and hypoxemia (P = 0.025) compared with those in the CON group. However, the incidences of postoperative ileus, urinary retention and delirium were comparable between the two groups. Also, patients in the OFA group had less pain in motion at 24 h postoperatively, as well as less risks of intraoperative hypotension and bradycardia (P <0.05). No significant differences in the postoperative quality of life were observed between the two groups.
Conclusion
Opioid-free anesthesia with dexmedetomidine and esketamine reduced postoperative anesthetic-related complications and provided improved hemodynamic stability in geriatric patients undergoing hip surgery.
Keywords: nonopioid, dexmedetomidine, esketamine, geriatric, hip injuries
Introduction
Approximately 2.6 million hip fractures occur worldwide each year and the incidence is estimated to increase to 6.26 million by 2050.1 The majority of hip fractures occur in patients aged 60 or over and most cases are treated with surgery that requires some form of anesthesia.2 Despite improvements in perioperative care, about 20% suffer from severe postoperative complications.3 Therefore, there is an urgent unmet need to improve the quality and outcomes of anesthesia care for patients at high risk of developing postoperative complications.
Opioids are widely used in clinical anesthesia to relieve acute pain and prevent chronic pain. However, opioid-related adverse effects, such as hypoxemia, delirium, nausea and vomiting may delay recovery and can be potentially harmful in geriatric patients.4 Previous studies have shown that the intraoperative hemodynamic stability and analgesia obtained with conventional opioids can be achieved with non-opioid drugs such as N-methyl-D-aspartate antagonists (NMDA), local anesthetics, and alpha2-agonists for multimodal administration.5 Meanwhile, numerous studies have demonstrated that opioid-free anesthesia can mitigate the incidence of postoperative adverse events6 and improve the quality of recovery7 after major surgery. However, there is limited evidence demonstrating the benefits or harms of opioid-free anesthesia in geriatric patients.
Here, we report on a randomized controlled trial (RCT) in geriatric patients at high risk of developing postoperative complications following hip surgery. Specifically, we test the hypothesis that opioid-free anesthesia can improve outcomes by decreasing the incidence of postoperative complications in the elderly undergoing hip surgery. The primary outcome was the incidence of a composite of postoperative anesthetic-related complications (postoperative nausea and vomiting, hypoxemia, ileus, urinary retention and delirium) within 48 hours after surgery.
Material and Methods
Study Design and Ethics
This prospective RCT was conducted after approval by the Ethics Committee of our hospital (YX2021-124) on December 30, 2021. The study was prospectively registered in the Chinese Clinical Trial Registry (http://www.chictr.org.cn, ChiCTR2200056421) on 05/02/2022. The study was conducted in accordance with the Consolidated Standards of Reporting Trials (CONSORT) criteria8 and in compliance with the Helsinki Declaration. Written informed consent was obtained from all participants.
Participants
All patients with fractures were scheduled for hip surgery, including close reduction and internal fixation, hemiarthroplasty, and total hip arthroplasty, and screened to assess their eligibility. The inclusion criteria were patients aged ≥60 years with physical status grades from I–III based on the American Society of Anesthesiologists (ASA). The exclusion criteria were known allergies to any of the anesthesia drugs used in this study, contraindications for regional anesthesia, severe hepatic or renal dysfunction, atrioventricular, intra-ventricular or sinoatrial block, bradyarrhythmia with a baseline rate <50 beats/min, uncontrolled hypertension, severe pulmonary hypertension, preoperative oxygen saturation measured by pulse oximetry (SpO2) <90%, a history of mental disorders, severe visual or auditory disorders and in-ability to consent to the study.
Randomization and Blinding
Patients were randomized to receive opioid-free anesthesia (Group OFA) or opioid-balanced anesthesia (Group CON) at a ratio of 1:1 using computer software. An assistant who was not involved in the study prepared the randomization list and concealed group assignments in consecutively numbered, sealed, opaque envelopes. A nurse unaffiliated with patient care opened the envelopes shortly before surgery and prepared the study medication. The anesthesiologist in charge of the patient administered the medication and adjusted the depth of anesthesia intraoperatively. The nurse and the anesthesiologist did not participate in the following assessment at any time. Thereafter, the nurses who provided postoperative care, the surgeons, investigators, and outcome assessors were blinded to the group allocations and did not have access to randomization until data analysis was complete.
Procedures
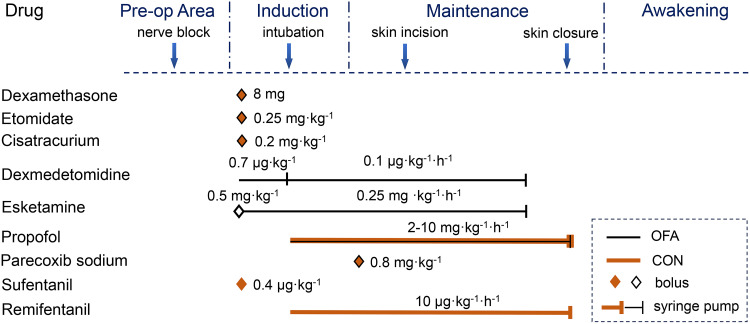
After arrival in the preoperative holding area with standard monitoring, intravenous access was established and premedication (midazolam 0.02 mg kg−1 and sufentanil 0.1 μg kg−1) was administered to every patient. Radial artery catheterization was performed for invasive arterial blood pressure monitoring. All patients received a preoperative fascia iliaca block with 25 mL of 0.25% ropivacaine. The sensory blockade was tested using a cold test, in comparison with the contralateral dermatomes along the distribution of the lateral femoral cutaneous nerve and femoral nerve 30 min after block performance. Thereafter, the patients were transferred to the operating room and received general anesthesia. All patients received intravenous dexamethasone 8 mg to prevent postoperative nausea and vomiting (PONV). Parecoxib sodium (0.8 mg∙kg−1) was administered after induction as prophylactic analgesia.
Patients in the OFA group received dexmedetomidine at a loading dose of 0.7 μg∙kg−1 over 10 min before induction. Anesthesia induction and tracheal intubation were performed using intravenous esketamine 0.5 mg∙kg−1, etomidate 0.25 mg∙kg−1, and cisatracurium 0.2 mg∙kg−1. Maintenance of anesthesia was achieved by continuous infusion of dexmedetomidine 0.1 μg∙kg−1∙h−1 and esketamine 0.25 mg∙kg−1∙h−1 until the beginning of wound closure, and propofol 2–10 mg∙kg−1∙h−1 was terminated at the end of surgery. Patients in the CON group received intravenous sufentanil 0.4 μg∙kg−1, etomidate 0.25 mg∙kg−1, and cisatracurium 0.2 mg∙kg−1 for anesthesia induction and tracheal intubation, and continuous infusion of remifentanil 10 μg∙kg−1∙h−1 combined with propofol 2–10 mg∙kg−1∙h−1 for anesthesia maintenance which was terminated at the end of surgery (Figure 1). When intraoperative HR or MAP increases by more than 20%, esketamine 0.1 mg∙kg−1 in the OFA group or sufentanil 0.1 μg∙kg−1 in the CON group was administered as topup. The depth of anesthesia was adjusted to maintain a bispectral index target in the range of 40–60.
Figure 1.
The timings and dosing of multimodal analgesia during surgery.
At a proper depth of anesthesia, an intermittent bolus of urapidil was administered if the MAP was >90 mm Hg or >20% of the baseline values. Esmolol was administered if the HR was >120 bpm. In hypotensive cases, defined as a MAP <60 mmHg or a reduction of >20% of baseline values, additional fluid and an intermittent bolus of ephedrine or phenylephrine were administered. Atropine was given in cases of severe bradycardia (HR <50 bpm). The dosage of vasoactive agent was at the discretion of the anesthesiologist.
All patients were transferred to the anesthesia intensive care unit (AICU) for recovery after extubation. A nurse, blinded to the protocols, evaluated the level of sedation using the Richmond Agitation Sedation Scale (RASS)9 and pain intensity using the visual analogue scale (VAS) score, which ranges from “0” (meaning no pain) to “100” (meaning worst pain imaginable). Postoperative analgesia was maintained with a tramadol infusion using a patient–controlled intravenous analgesia (PCIA) device. The PCIA device administered a 30 mg bolus dose with a 15–minute lock-time and the basal infusion rate was set to 6 mg∙h−1. If three boluses of tramadol did not alleviate the pain or the VAS score was >3, a fascia iliaca compartment block with 20 mL of ropivacaine 0.25% was performed as rescue analgesia. When the SpO2 was <90%, oxygen therapy was performed using a nasal cannula with an oxygen inflow of 2 L∙min−1.
Patients were followed during the first 48 h after the operation. Adverse events including PONV, hypoxemia (defined as a SpO2 level <90% with a need for oxygen supplementation),10 postoperative ileus (defined as an absence of flatus or stools), urinary retention (defined as the inability to void with bladder distention and need of catheterization), delirium (measured twice daily using the Confusion Assessment Method)11 were recorded. Sleep quality during the first postoperative night was evaluated using the Richards-Campbell Sleep Questionnaire.12 Quality of life was assessed using the VAS score of the EuroQol 5 Dimension 5 Level (EQ-5D-5L) 30 days after surgery by a telephone interview.13
Outcomes
The primary outcome was the incidence of composite adverse events occurring within 48 hours after surgery. The secondary endpoints included pain scores at rest and in motion during the first 48 h postoperatively, the number of patients who received nerve block for rescue analgesia, the RASS scores during the first 30 min in the AICU, the time to extubation (defined as the interval between the end of the operation and extubation), unplanned intensive care unit (ICU) admissions, sleep quality and the length of postoperative hospital stay. Safety elements included intraoperative cardiovascular events and severe postoperative complications such as cerebrovascular events, myocardial infarction, heart failure, and acute kidney injury until hospital discharge. The incidence of readmission and all-cause mortality were also assessed.
Statistical Analysis
The primary outcome of this study was the incidence of postoperative anesthetic drug-related complications. We assumed a 40% incident rate for the primary outcome (10% rates of postoperative hypoxemia,14 nausea and vomiting15 and delirium;16 5% rate of postoperative ileus17 and urinary retention18). Our pilot data indicated a 43% incidence of adverse events in the control group (n=21) which was close to the observations, whilst the incidence of adverse events in the opioid-free group was 19% (n=21). A 2-sampled t-test was used for the power analysis. The required sample size was 55 patients per group with an alpha error of 0.05 and a power of 0.8 calculated using the PASS software version 15.0 (NCSS LLC). Assuming a 10% dropout rate, 60 subjects per group were considered for recruitment in this study.
All statistical analyses were performed using SPSS Statistics (version 24.0; IBM, Armonk, NY, USA). Kolmogorov–Smirnov test was performed to assess data normality. Continuous variables were expressed as means (SD) or medians (interquartile range, IQR), and inter-group differences were assessed for significance using Student’s t-test for normally distributed data or Wilcoxon rank sum test for nonparametric data. For categorical variables, data are presented as counts with percentages and differences were compared using a chi-square or Fisher exact test if necessary with a constructed 95% confidence interval (CI).
The effects of interventions on the outcomes of interest (pain scores, hemodynamic parameters) over time were assessed using the interaction of group and time in a repeated measures analysis of variance. The Greenhouse-Geisser correction was applied if the data violated the assumption of sphericity as determined by the Mauchly test. For measures that showed significant group by time interaction effects, a post hoc analysis was performed using Bonferroni correction. Assessments were 2-tailed, and statistical significance was set at P <0.05.
Results
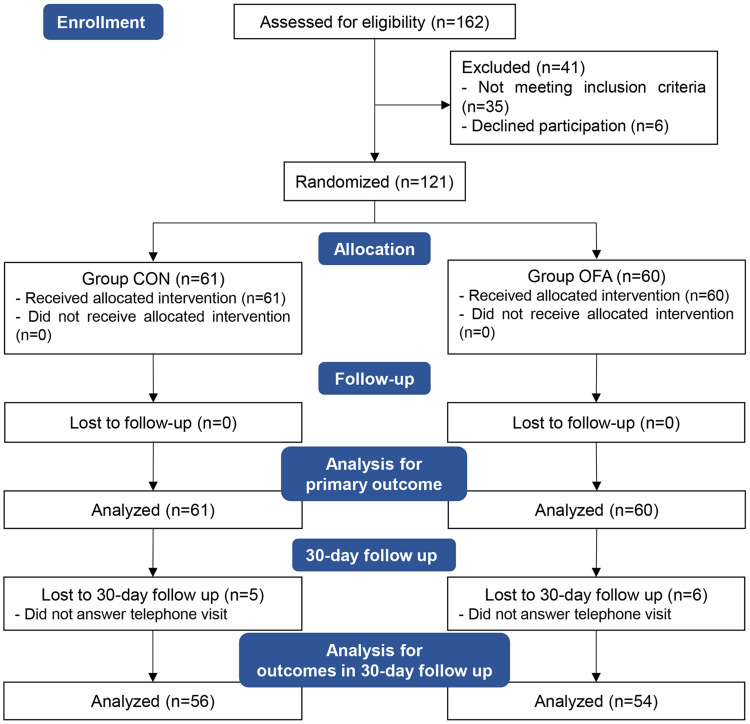
Patients were recruited and completed the study from February 10, 2022 to September 30, 2022. A total of 162 were initially screened for suitability. Thirty-five patients did not meet the inclusion criteria and six patients declined to participate in the study. In total, 121 patients were enrolled and randomized. However, 11 patients lost contact during the 30-day follow-up, thus 110 patients were analyzed for the outcome of 30-day follow up (Figure 2).
Figure 2.
Subject recruitment and workflow.
Abbreviations: CON, control; OFA, opioid-free anesthesia.
The general and surgical characteristics of the patients are summarized in Table 1. No significant differences were observed in the demographic characteristics, medical histories, types of surgery, duration of anesthesia, total intraoperative infusion or estimated blood loss between the two groups (Table 1).
Table 1.
Summary of the Demographic Characteristics, Medical Histories and Types of Surgery in the Patient Cohort
| CON | OFA | P Value | |
|---|---|---|---|
| Characteristic | (n=61) | (n=60) | |
| Age, y | 71[66–80] | 73[66–82] | 0.554 |
| Sex, n (%) | 0.770 | ||
| Female | 35(57.4) | 36(60.0) | |
| Male | 26(42.6) | 24(40.0) | |
| BMI, kg∙m–2 | 23[20–25] | 21[20–24] | 0.099 |
| ASA physical classification status, n (%) | 0.138 | ||
| II | 37(60.7) | 44(73.3) | |
| III | 24(39.3) | 16(26.7) | |
| NYHA classification, n (%) | 0.182 | ||
| I | 14(23.0) | 23(38.3) | |
| II | 44(72.1) | 35(58.3) | |
| III | 3(4.9) | 2(3.3) | |
| Surgical history, n (%) | 0.498 | ||
| Abdominal surgery | 7(11.5) | 4(6.7) | |
| Orthopedic surgery | 20(32.8) | 17(28.3) | |
| Comorbid diseases, n (%) | 0.522 | ||
| Hypertension | 25(41.0) | 26(43.3) | |
| Coronary artery disease | 4(6.6) | 4(6.7) | |
| Diabetes mellitus | 8(13.1) | 9(15.0) | |
| Asthma | 4(6.6) | 4(6.7) | |
| Type of surgery, n (%) | 0.893 | ||
| Close reduction and internal fixation | 22(36.1) | 24(40.0) | |
| Total hip arthroplasty | 32(52.5) | 29(48.3) | |
| Hemiarthroplasty | 7(11.5) | 7(11.7) | |
| Duration of anesthesia, min | 125[111–136] | 115[98.5–134.5] | 0.156 |
| Total infusion, mL | 700[600–800] | 600[500–800] | 0145 |
| Estimated blood loss, mL | 50[50–50] | 50[50–50] | 0.446 |
Notes: Data are expressed as median [interquartile range] or number (%).
Abbreviations: CON, control; OFA, opioid-free anesthesia; BMI, body mass index; ASA, American Society of Anesthesiologists; NYHA, New York Heart Association.
The incidence of composite adverse events occurred in 38 of 61 (62.3%) patients in the CON group, which was significantly reduced in the OFA group (21 of 60 [35.0%] patients); the estimated difference was 27.3% (95% CI: 10.2% to 44.4%, P = 0.003). Notably, PONV occurred in 20 of 61 (32.8%) patients in the CON group and in 10 of 60 (16.7%) patients in the OFA group (P = 0.040). Postoperative hypoxemia occurred in 17 of 61 (27.9%) patients in the CON group and in 7 of 60 (11.7%) patients in the OFA group (P = 0.025). The incidence of postoperative ileus was 3.3% in the CON group whilst no case was reported in the OFA group. The incidence of postoperative urinary retention (8.2% in the CON group and 3.3% in the OFA group) and postoperative delirium (14.8% in the CON group and 11.7% in the OFA group) were not significantly different between the groups (Table 2).
Table 2.
Summary of the Primary Outcomes
| Variable | CON | OFA | Rate Difference (95% CI) (%) | P Value |
|---|---|---|---|---|
| (n=61) | (n=60) | |||
| Composite primary outcome | 38(62.3) | 21(35.0) | 27.3 (10.2–44.4) | 0.003 |
| Postoperative nausea and vomiting | 20(32.8) | 10(16.7) | 16.1 (1.0–31.2) | 0.040 |
| Postoperative hypoxemia | 17(27.9) | 7(11.7) | 16.2 (2.3–30.1) | 0.025 |
| Postoperative ileus | 2(3.3) | 0(0) | 3.3 (−1.2–7.7) | 0.496 |
| Postoperative urinary retention | 5(8.2) | 2(3.3) | 4.9 (−3.4–13.1) | 0.449 |
| Postoperative delirium | 9(14.8) | 7(11.7) | 3.1 (−9.0–15.1) | 0.616 |
Notes: Data are expressed as number (%).
Abbreviations: CON, control; OFA, opioid-free anesthesia; CI, confidence interval.
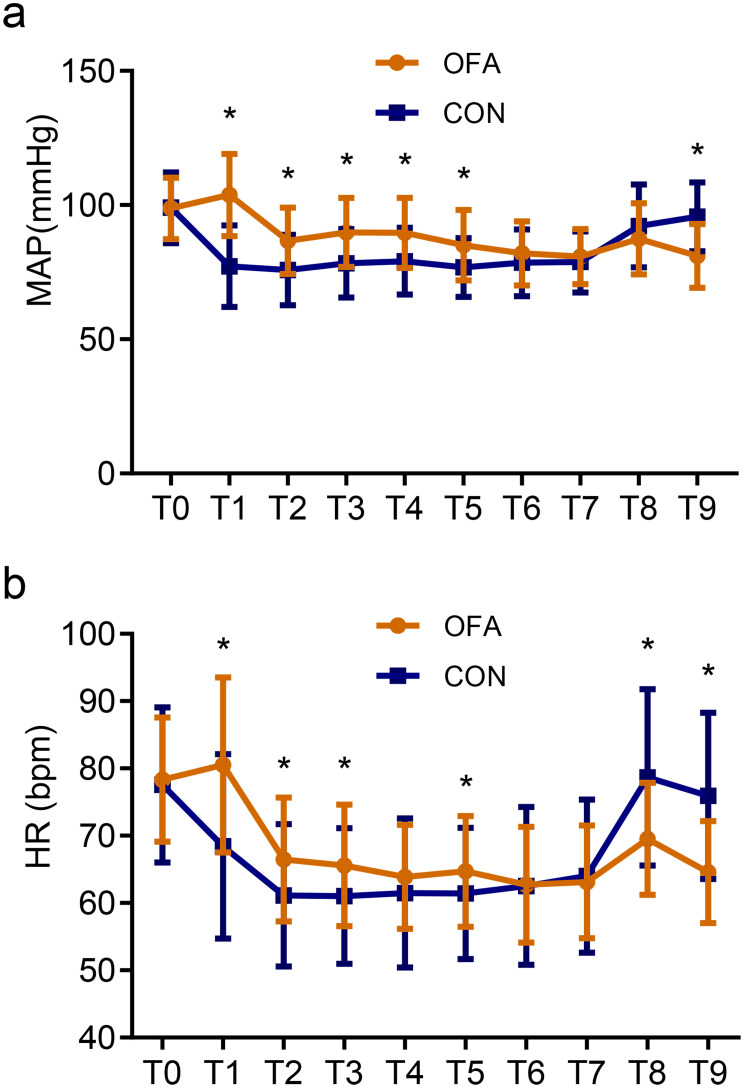
During surgery, the MAP and HR were significantly higher in the OFA group (Figure 3). Patients receiving opioid-anesthesia had higher incidences of hypotension and bradycardia compared with those receiving opioid-free anesthesia (hypotension: 32.8% vs 15.0%, P = 0.022; bradycardia: 95.1% vs 61.7%, P <0.001). There was no difference in the propofol requirement between the groups (P = 0.067). The use of phenylephrine and ephedrine was significantly higher in patients in the CON group compared with those in the OFA group (both P <0.001). No significant difference was observed in the incidence of hypertension between groups (P = 0.065) whilst the use of urapidil was significantly higher in the OFA group (P = 0.022) (Table 3).
Figure 3.
The perioperative MAP (a) and HR (b) values.
Notes: *P <0.05 indicates statistically significant differences between groups. T0: Baseline; T1: Immediately at induction; T2: 15 min after induction; T3: Incision; T4: 15 min after incision; T5: 30 min after incision; T6: 45 min after incision; T7: Skin closure; T8: Extubation; T9: AICU admission.
Abbreviations: CON, control; OFA, opioid-free anesthesia; MAP, mean arterial pressure; HR, heart rate.
Table 3.
Summary of the Intra- and Postoperative Parameters in the Two Groups
| CON | OFA | P | |
|---|---|---|---|
| Parameters | (n=61) | (n=60) | Value |
| Intraoperative | |||
| Adverse events | |||
| Hypertension, n (%) | 0 (0) | 5 (8.3) | 0.065 |
| Hypotension, n (%) | 58 (95.1) | 37 (61.7) | <0.001 |
| Bradycardia, n (%) | 20 (32.8) | 9 (15.0) | 0.022 |
| Propofol, mg | 230[214.5–255.5] | 252[225.5–277.5] | 0.067 |
| Vasoactive agents | |||
| Phenylephrine, μg | 816[374.6–1404.8] | 203.8[0–556.7] | <0.001 |
| Ephedrine, mg | 0[0–6] | 0[0–0] | <0.001 |
| Urapidil, mg | 0[0–0] | 0[0–0] | 0.022 |
| Postoperative | |||
| Time to extubation, min | 11[6–24.5] | 16.5[10.3–25] | 0.007 |
| RASS scores | |||
| Immediate in AICU | −1[−2–0] | −2[−2–1] | <0.001 |
| 15 min in AICU | 0[−1–0] | −1[−2–0] | <0.001 |
| 30 min in AICU | 0[0–0] | 0[−1–0] | 0.047 |
| Adverse events | |||
| Hypertension, n (%) | 1 (1.7) | 4 (6.6) | 0.365 |
| Hypotension, n (%) | 4 (6.6) | 8 (13.3) | 0.212 |
| Bradycardia, n (%) | 11(18.3) | 5 (8.2) | 0.100 |
| Rescue analgesia, n (%) | 1 (1.6) | 3 (5.0) | 0.30 |
| Tramadol consumption, mg | 348[318–408] | 348[288–408] | 0.281 |
| Sleep quality | 30[20–35] | 35[25.3–40] | 0.020 |
| Length of hospital stay after surgery, d | 5[4–7] | 5[4–7] | 0.866 |
| Unplanned ICU admission, n (%) | 0 (0) | 1 (1.7) | 0.496 |
Notes: Data are expressed as the median [interquartile range] or number (%).
Abbreviations: CON, control; OFA, opioid-free anesthesia; RASS, Richmond Agitation Sedation Scale; AICU, anesthesia intensive care unit; ICU, intensive care unit.
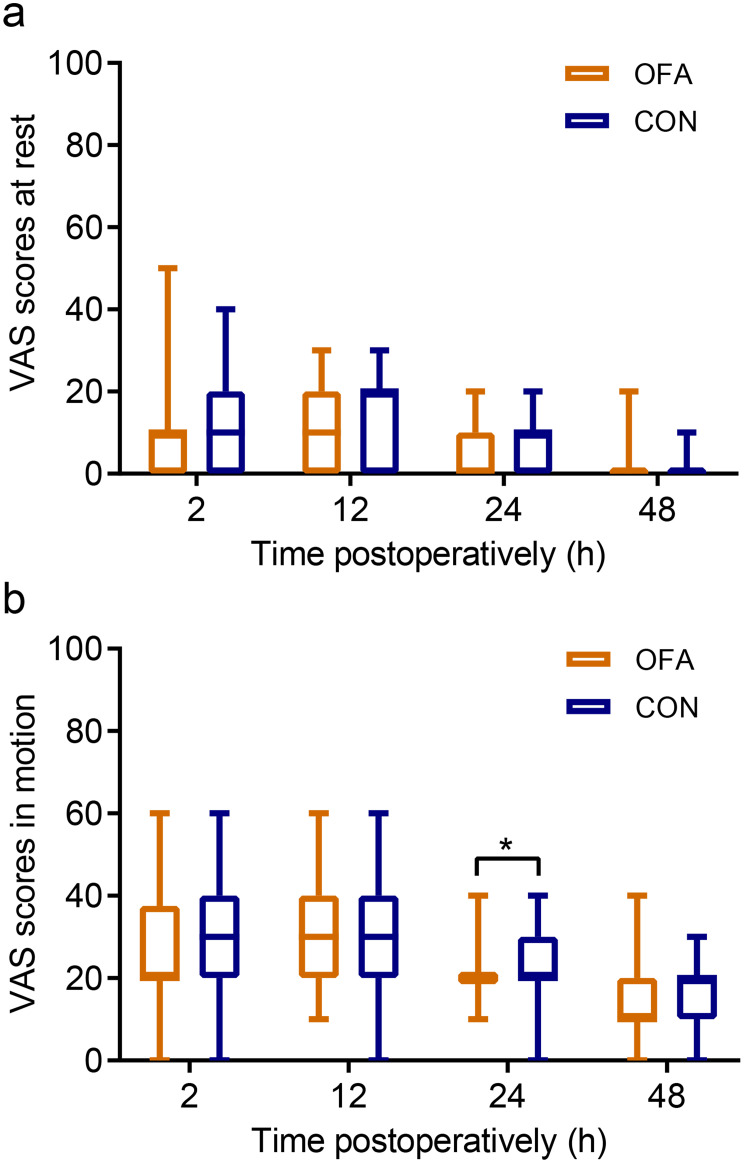
Postoperatively, patients in the OFA group had a longer time to extubation (P = 0.007) and lower RASS scores during the first 30 min in the AICU (P <0.05). No significant differences in the incidences of hypertension, hypotension, bradycardia or the use of rescue analgesia of the fascia iliaca compartment block were observed (Table 3). Patients receiving opioid-anesthesia experienced significantly less pain in motion than those in the CON group at 24 postoperatively (P = 0.010) (Figure 4), and had a higher sleep quality during the surgical night (P = 0.020). No severe cardiovascular events were observed. Also, no significant differences were found in terms of the consumption of tramadol, length of hospitalization or incidence of unplanned ICU admission after surgery (Table 3).
Figure 4.
The VAS scores at rest (a) and during motion (b) within 48 hours after surgery.
Notes: *P <0.05 indicates statistically significant differences between the groups. Data are expressed as the median (horizontal bar), interquartile range (box) and the maximum and minimum values (whiskers).
Abbreviations: CON, control; OFA, opioid-free anesthesia; VAS, visual analogue scale.
Totally, 11 patients were lost during the 30-day follow-up. No significant differences in the pain scores and quality of life between the two groups were found during the postoperative 30 d period. Two patients in the CON group were readmitted due to pneumonia and nephrolithiasis, and three patients in the OFA group were readmitted due to pneumonia and hip dislocation. One patient in the CON group died (Table 4).
Table 4.
Summary of the Follow-Up Outcomes at 30 Days After Surgery
| CON | OFA | P Value | |
|---|---|---|---|
| Outcome | (n=56) | (n=54) | |
| Death, n (%) | 1(1.8) | 0(0) | 1.0 |
| Total patients, n | 55 | 54 | |
| VAS scores at rest | 0[0–0] | 0[0–0] | 0.475 |
| VAS scores in motion | 10[0–20] | 10[7.5–20] | 0.417 |
| Quality of life | 70[70–70] | 70[60–70] | 0.612 |
| Readmission, n (%) | 2(3.6) | 3(5.6) | 0.679 |
Notes: Data are expressed as the median [interquartile range] or number (%).
Abbreviations: CON, control; OFA, opioid-free anesthesia; VAS, visual analogue scale.
Discussion
In this randomized controlled trial, opioid-free anesthesia with dexmedetomidine and esketamine resulted in a lower incidence of postoperative anesthetic-related adverse events compared with balanced anesthesia with opioids in geriatric patients undergoing hip surgery. Notably, patients receiving opioid-free anesthesia had lower incidences of PONV and hypoxemia after surgery, as well as lower incidences of intraoperative hypotension and bradycardia. However, patients in the OFA group exhibited a prolonged time to awakening.
The routine perioperative use of opioids has recently been questioned due to opioid-related side effects that can significantly impede an otherwise uneventful postoperative course.19 For enhanced recovery after surgery, the use of perioperative opioids should be kept to a minimum or not used, particularly in patients with specific risk factors.20 Aging is associated with normal progressive functional decline and reduced ability to respond to intrinsic or extrinsic stimuli.21 It is also an independent risk factor for adverse drug reactions.22 In this context, our randomized controlled trial helped to develop the evidence base for perioperative pain management in the elderly.
Alpha2-agonists are drugs with considerable analgesic potency with the potential to almost replace opioids in the perioperative period. Beloeil et al have reported that opioid-free anesthesia with dexmedetomidine significantly reduced postoperative morphine consumption and the incidence of PONV compared with balanced anesthesia with remifentanil.23 Consistently, we found a significantly reduced risk of PONV in the OFA group. Apart from the effect of reducing opioid-induced PONV, we speculate that dexmedetomidine acts through a sympatholytic effect as it has been demonstrated that sympatho-excitation and catecholamine release increase PONV.24 However, with a decline in cardiovascular reserves, geriatric patients are vulnerable to reduction in sympathetic drive by prolonged infusion of alpha2-agonists, resulting decreased blood pressure and heart rate values during general anesthesia.25 For this reason, we administered dexmedetomidine only over 10 min with a loading dose, which resulted less bradycardia happened in our study.
Multimodal drug combinations ideally have higher analgesic efficacy with reduced side effects. Esketamine, acting as a potent NMDA receptor antagonists, has been demonstrated to improve pain relief and reduce rescue analgesic requirement after surgery.26 Specifically, esketamine increases vascular tone by inducing the release of catecholamines via sympathetic activation.27 Combination of dexmedetomidine and esketamine may be complementary to provide adequate pain control and maintain hemodynamic stability in the context of opioid-free anesthesia. As expected, patients in the OFA group exhibited an improved hemodynamic stability.
Opioids can produce life-threatening respiratory depression whereas dexmedetomidine induces a sleep-like sedation with strong analgesia yet patients remain fully arousable without the risk of respiratory depression.28 Recent studies also suggest that ketamine is a respiratory stimulant and consequently may offset opioid-induced respiratory depression.29 Thus, opioid-free anesthesia with dexmedetomidine and esketamine had favorable efficacy on respiratory management after hip surgery compared with the CON group.
Patients in the OFA group reported lower pain scores in motion 24 h after surgery and experienced higher sleep quality during the surgical night. Ketamine has been shown to prolong the average duration of deep sleep and modulate circadian rhythms by regulating clock genes whilst dexmedetomidine is an integral part of the neuronal pathways of natural sleep.30,31 In recent years, the OFA strategy integrating dexmedetomidine and esketamine has been demonstrated to be safely applicable in thoracoscopic and laparoscopic surgeries. This approach can minimize postoperative complications,32 enhance postoperative sleep quality,33 and expedite recovery.7 A case report on a centenarian patient undergoing colorectal cancer surgery demonstrated the safe and effective application of the OFA strategy, which combines nerve blockade with dexmedetomidine and esketamine, in frail elderly patients with multiple comorbidities.34 These findings further suggest that opioid-free anesthesia with dexmedetomidine and esketamine may be a favorable anesthetic regimen due to its strong analgesic and dormant effects.
It is worth noting that patients in the OFA group showed a prolonged time to extubation and lower RASS scores after surgery. However, opioid-free anesthesia has been reported to reduce extubation delay.35 These conflicting data may be related to different subjects and surgical procedures, since the previous study focused on young bariatric patients undergoing gastric bypass surgery, whilst our subjects were older than 60 and were undergoing hip surgery. Elderly people may be more sensitive to combinations of multiple sedatives.
We did not find any significant differences in the risks of postoperative ileus, urinary retention, delirium and further, quality of life during 30 days of follow-up. We hypothesize that these data may result from the multimodal analgesia with fascia iliaca block that provided effective analgesia and reduced the consumption of systemic anesthetics and associated side effects.
Despite the compelling evidence presented, our study has several limitations. Firstly, nerve blocks were used as part of a multimodal analgesia regimen that could potentially affect the outcomes. However, we considered this desirable as it was close to real-world scenarios in clinical practice. Secondly, the definition of opioid-free anesthesia is not definitive and other opioid-free anesthesia approaches could be explored. Thirdly, there is a lack of reliable tools to assess intraoperative nociceptive stimuli to objectively evaluate analgesic efficacy and demonstrate the advantages of opioid-free anesthesia. Finally, we did not evaluate the role of opioid-free anesthesia in the treatment of chronic pain.
Conclusion
In summary, opioid-free anesthesia with dexmedetomidine and esketamine reduced postoperative anesthetic-related complications and provided hemodynamic stability in geriatric patients undergoing hip surgery. Optimal dosing strategies for other high-risk surgeries in future research are needed to provide the best balance between perioperative analgesia, patient safety and quick recovery.
Acknowledgments
We thank our colleagues and nursing teams of the Second Affiliated Hospital of Anhui Medical University for their support in this study. This paper has been uploaded to ResearchSquare as a preprint: https://www.researchsquare.com/article/rs-2324065/v1.
Funding Statement
This work was supported by the Clinical Trial Cultivation Program of the Second Affiliated Hospital of Anhui Medical University (Grant number: 2021LCZD17 to YW).
Abbreviations
AICU, anesthesia intensive care unit; ASA, American Society of Anesthesiologists; CI, confidence interval; CON, control group with on intervention; CONSORT, Consolidated Standards of Reporting Trials; EQ-5D-5L, EuroQol 5 Dimension 5 Level; HR, heart rate; ICU, intensive care unit; IQR, interquartile range; MAP, mean arterial pressure; NMDA, N-methyl-D-aspartate; OFA, opioid free anesthesia; PCIA, patient–controlled intravenous analgesia; PONV, postoperative nausea and vomiting; RASS, Richmond Agitation Sedation Scale; RCT, randomized controlled trial; SD, standard deviation; VAS, visual analogue scale.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
Written informed consent was obtained from all participants. The study was conducted in accordance with the Consolidated Standards of Reporting Trials criteria and in compliance with the Helsinki Declaration. The Ethics Committee of the Second Hospital of Anhui Medical University approved the experimental protocols of this study (YX2021-124).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All authors have no conflicts of interest in this work.
References
- 1.Cooper C, Campion G, Melton LJ, et al. Hip fractures in the elderly: a world-wide projec-tion. Osteoporos Int. 1992;2(6):285–289. [DOI] [PubMed] [Google Scholar]
- 2.Murthy S, Hepner DL, Cooper Z, et al. Controversies in anaesthesia for noncardiac surgery in older adults. Br J Anaesth. 2015;115(Suppl 2):ii15–25. doi: 10.1093/bja/aev396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desai V, Chan PH, Prentice HA, et al. Is Anesthesia Technique Associated With a Higher Risk of Mortality or Complications Within 90 Days of Surgery for Geriatric Patients With Hip Fractures? Clin Orthop Relat Res. 2018;476(6):1178–1188. doi: 10.1007/s11999.0000000000000147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shafi S, Collinsworth AW, Copeland LA, et al. Association of Opioid-Related Adverse Drug Events With Clinical and Cost Outcomes Among Surgical Patients in a Large Integrated Health Care Delivery System. JAMA Surg. 2018;153(8):757–763. doi: 10.1001/jamasurg.2018.1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hwang W, Lee J, Park J, et al. Dexmedetomidine versus remifentanil in postoperative pain control after spinal surgery: a randomized controlled study. BMC Anesthesiol. 2015;15(1):21. doi: 10.1186/s12871-015-0004-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan H, Chen W, Chen Y, et al. Opioid-Free Versus Opioid-Based Anesthesia on Postoperative Pain After Thoracoscopic Surgery: the Use of Intravenous and Epidural Esketamine. Anesth Analg. 2023;137(2):399–408. doi: 10.1213/ANE.0000000000006547 [DOI] [PubMed] [Google Scholar]
- 7.Léger M, Perrault T, Pessiot-Royer S, et al. Opioid-free Anesthesia Protocol on the Early Quality of Recovery after Major Surgery (SOFA Trial): a Randomized Clinical Trial. Anesthesiology. 2024;140(4):679–689. doi: 10.1097/ALN.0000000000004840 [DOI] [PubMed] [Google Scholar]
- 8.Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Trials. 2010;11(1):32. doi: 10.1186/1745-6215-11-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–1344. doi: 10.1164/rccm.2107138 [DOI] [PubMed] [Google Scholar]
- 10.Abbott TEF, Fowler AJ, Pelosi P, et al. A systematic review and consensus definitions for standardised end-points in perioperative medicine: pulmonary complications. Br J Anaesth. 2018;120(5):1066–1079. doi: 10.1016/j.bja.2018.02.007 [DOI] [PubMed] [Google Scholar]
- 11.Chanques G, Ely EW, Garnier O, et al. The 2014 updated version of the Confusion Assessment Method for the Intensive Care Unit compared to the 5th version of the Diagnostic and Statistical Manual of Mental Disorders and other current methods used by intensivists. Ann Intensive Care. 2018;8(1):33. doi: 10.1186/s13613-018-0377-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ritmala-Castren M, Axelin A, Richards KC, et al. Investigating the construct and concurrent validity of the Richards-Campbell Sleep Questionnaire with intensive care unit patients and home sleepers. Aust Crit Care. 2022;35(2):130–135. doi: 10.1016/j.aucc.2021.04.001 [DOI] [PubMed] [Google Scholar]
- 13.Wang P, Luo N, Tai ES, et al. The EQ-5D-5L is More Discriminative Than the EQ-5D-3L in Patients with Diabetes in Singapore. Value Health Reg Issues. 2016;9:57–62. doi: 10.1016/j.vhri.2015.11.003 [DOI] [PubMed] [Google Scholar]
- 14.Duan XZ, Zhang X, Tong DK, et al. Risk factors for and predictive nomogram of postoperative hypoxaemia in elderly patients with femoral neck fractures. J Int Med Res. 2020;48(10):300060520945132. doi: 10.1177/0300060520945132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Yang Q, Lin J, et al. Risk factors of postoperative nausea and vomiting after total Hip arthroplasty or total knee arthroplasty: a retrospective study. Ann Transl Med. 2020;8(17):1088. doi: 10.21037/atm-20-5486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krenk L, Kehlet H, Bæk Hansen T, et al. Cognitive dysfunction after fast-track Hip and knee replacement. Anesth Analg. 2014;118(5):1034–1040. doi: 10.1213/ANE.0000000000000194 [DOI] [PubMed] [Google Scholar]
- 17.Horowitz JA, Jain A, Puvanesarajah V, et al. Risk Factors, Addi-tional Length of Stay, and Cost Associated with Postoperative Ileus Following Anterior Lumbar Interbody Fusion in Elderly Patients. World Neurosurg. 2018;115:e185–e9. doi: 10.1016/j.wneu.2018.04.006 [DOI] [PubMed] [Google Scholar]
- 18.Baldini G, Bagry H, Aprikian A, et al. Postoperative urinary retention: anesthetic and perioperative considerations. Anesthesiology. 2009;110(5):1139–1157. doi: 10.1097/ALN.0b013e31819f7aea [DOI] [PubMed] [Google Scholar]
- 19.De Boer HD, Detriche O, Forget P. Opioid-related side effects: postoperative ileus, urinary retention, nausea and vomiting, and shivering. A review of the literature. Best Pract Res Clin Anaesthesiol. 2017;31(4):499–504. doi: 10.1016/j.bpa.2017.07.002 [DOI] [PubMed] [Google Scholar]
- 20.Sultana A, Torres D, Schumann R. Special indications for Opioid Free Anaesthesia and Analgesia, patient and procedure related: including obesity, sleep apnoea, chronic obstructive pulmonary disease, complex regional pain syndromes, opioid addiction and cancer surgery. Best Pract Res Clin Anaesthesiol. 2017;31(4):547–560. doi: 10.1016/j.bpa.2017.11.002 [DOI] [PubMed] [Google Scholar]
- 21.Schmucker DL. Age-related changes in liver structure and function: implications for disease? Exp Gerontol. 2005;40(8–9):650–659. doi: 10.1016/j.exger.2005.06.009 [DOI] [PubMed] [Google Scholar]
- 22.Priebe HJ. The aged cardiovascular risk patient. Br J Anaesth. 2000;85(5):763–778. doi: 10.1093/bja/85.5.763 [DOI] [PubMed] [Google Scholar]
- 23.Beloeil H, Garot M, Lebuffe G, et al. Balanced Opioid-free Anesthesia with Dexmedetomidine versus Balanced Anesthesia with Remifentanil for Major or Intermediate Noncardiac Surgery. Anesthesiology. 2021;134(4):541–551. doi: 10.1097/ALN.0000000000003725 [DOI] [PubMed] [Google Scholar]
- 24.Jeffs SA, Hall JE, Morris S. Comparison of morphine alone with morphine plus clonidine for postoperative patient-controlled analgesia. Br J Anaesth. 2002;89(3):424–427. doi: 10.1093/bja/89.3.424 [DOI] [PubMed] [Google Scholar]
- 25.Blaudszun G, Lysakowski C, Elia N, et al. Effect of perioperative systemic α2 agonists on postoperative morphine consumption and pain intensity: systematic review and meta-analysis of randomized controlled trials. Anesthesiology. 2012;116(6):1312–1322. doi: 10.1097/ALN.0b013e31825681cb [DOI] [PubMed] [Google Scholar]
- 26.Argiriadou H, Himmelseher S, Papagiannopoulou P, et al. Improvement of pain treatment after major abdominal surgery by intravenous S+-ketamine. Anesth Analg. 2004;98(5):1413–1418. doi: 10.1213/01.ANE.0000111204.31815.2D [DOI] [PubMed] [Google Scholar]
- 27.Peltoniemi MA, Hagelberg NM, Olkkola KT, et al. Ketamine: a Review of Clinical Pharmacokinetics and Pharmacodynamics in Anesthesia and Pain Therapy. Clin Pharmacokinet. 2016;55(9):1059–1077. doi: 10.1007/s40262-016-0383-6 [DOI] [PubMed] [Google Scholar]
- 28.Aho M, Lehtinen AM, Erkola O, et al. The effect of intravenously administered dexmedetomidine on perioperative hemodynamics and isoflurane requirements in patients undergoing abdominal hysterectomy. Anesthesiology. 1991;74(6):997–1002. doi: 10.1097/00000542-199106000-00005 [DOI] [PubMed] [Google Scholar]
- 29.Jonkman K, van Rijnsoever E, Olofsen E, et al. Esketamine counters opioid-induced respiratory depression. Br J Anaesth. 2018;120(5):1117–1127. doi: 10.1016/j.bja.2018.02.021 [DOI] [PubMed] [Google Scholar]
- 30.Ahnaou A, Huysmans H, Biermans R, et al. Ketamine: differential neurophysiological dynamics in functional networks in the rat brain. Transl Psychiatry. 2017;7(9):e1237. doi: 10.1038/tp.2017.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson LE, Lu J, Guo T, et al. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98(2):428–436. doi: 10.1097/00000542-200302000-00024 [DOI] [PubMed] [Google Scholar]
- 32.Feng CD, Xu Y, Chen S, et al. Opioid-free anaesthesia reduces postoperative nausea and vomiting after thoracoscopic lung resection: a randomised controlled trial. Br J Anaesth. 2024;132(2):267–276. doi: 10.1016/j.bja.2023.11.008 [DOI] [PubMed] [Google Scholar]
- 33.Chen L, He W, Liu X, et al. Application of opioid-free general anesthesia for gynecological laparoscopic surgery under ERAS protocol: a non-inferiority randomized controlled trial. BMC Anesthesiol. 2023;23(1):34. doi: 10.1186/s12871-023-01994-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dai J, Yang M, Li S. Application of an OFA strategy to ERAS in a 102-year-old patient undergoing colon cancer surgery: a case report. Medicine. 2023;102(29):e34431. doi: 10.1097/MD.0000000000034431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feld JM, Hoffman WE, Stechert MM, et al. Fentanyl or dexmedetomidine combined with desflurane for bariatric surgery. J Clin Anesth. 2006;18(1):24–28. doi: 10.1016/j.jclinane.2005.05.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.