Abstract
Purpose
Despite significant advancements in the treatment of acute ischemic stroke (AIS) with endovascular thrombectomy (EVT), post-EVT mortality remains a considerable concern. However, there is a lack of real-world epidemiological data delineating the characteristics of mortality for EVT, particularly in recent years following the widespread promotion of EVT treatment for stroke patients.
Methods
This multicenter, retrospective study collected data from 721 AIS patients who died following EVT across 33 hospitals in Sichuan Province, China, from January 2019 to September 2022. The analysis sought to identify the primary causes of death within 30 days post-EVT and explore their related clinical features.
Results
The leading causes of death were malignant cerebral edema (MCE) in 365 patients (50.6%), pneumonia in 180 patients (25%), and symptomatic intracranial hemorrhage (sICH) in 94 patients (13%). MCE was the predominant cause of death in anterior circulation strokes, while pneumonia prevailed in posterior circulation strokes. MCE was also the primary cause of death within one week post-EVT, but pneumonia became increasingly dominant over time. Large vessel occlusion and lower reperfusion success rate were significantly correlated with MCE. Advanced age increases the risk of death from pneumonia. Tandem occlusion and procedural complications tend to correlate with mortality from sICH.
Conclusion
This study revealed that the principal causes of death after EVT included MCE, sICH, and pneumonia. MCE was found to be correlated with unsuccessful reperfusion. sICH was associated with procedural complications and the operators’ experience. Pneumonia was linked to post-EVT management, particularly for those who survived for one week.
Keywords: acute ischemic stroke, endovascular thrombectomy, mortality, cerebral edema, intracranial hemorrhage, pneumonia
Introduction
Stroke is a leading cause of death worldwide and presents significant challenges to public health systems, especially in low- and middle-income countries.1,2 In China, the mortality rate of stroke reaches 343.4 per 100,000 person-years.3 Acute ischemic stroke (AIS) constitutes approximately 87% of all strokes.4 Endovascular thrombectomy (EVT) is recognized as a standard treatment for resolving large-vessel occlusions (LVO) leading to AIS.
Although existing studies have shown that EVT significantly reduces mortality rates and enhances functional outcomes, a significant number of patients still die after EVT treatment.5–7 Meta-analyses of randomized trials indicate that the mortality rate at three months for patients treated with EVT within six hours is approximately 15%.6 Further studies, such as the DEFUSE-3 and DAWN trials, have demonstrated that extending the EVT time window could result in 14–19% three-month mortality rates.8,9 However, the optimal approach for further reducing the mortality rate in AIS patients after reperfusion therapy remains uncertain. Thus, addressing the characteristics of mortality is crucial for continuously improving the prognosis of stroke patients undergoing EVT.
Most research on mortality following EVT has focused on predictive factors for mortality in cohort studies.10–14 These studies often reveal inconsistencies in mortality predictors due to variations in sample sizes, study designs (prospective or retrospective), and demographic and ethnic differences. Common predictors include age, initial National Institutes of Health Stroke Scale (NIHSS) score, occlusion site, recanalization status, and symptomatic intracranial hemorrhage (sICH). In addition, a study based on the MR CLEAN registry revealed that common causes of death post-EVT include pneumonia (26.2%), intracranial hemorrhage (17.3%), and cerebral edema (12.3%).15 However, these data primarily pertain to patients treated within six hours and may not reflect true real-world scenarios due to selective patient inclusion. Moreover, there is a lack of epidemiological data describing the characteristics of mortality for stroke patients undergoing EVT, as preoperative assessments and EVT treatment techniques have been broadly promoted and refined in recent years.
Therefore, we collected a large sample of epidemiological data of patients who died within 30 days post-EVT treatment from January 2019 to September 2022 from multicenter disease surveillance system data in Sichuan Province, China. We aim to describe the causes of death and related clinical characteristics in EVT patients. This study may provide a scientific basis and practical guidelines for reducing postoperative deaths in future interventions.
Methods
Population and Design
The data were sourced from the Sichuan Provincial Neurology Quality Control Center’s stroke quality control data platform in China. This study selected data from patients who underwent EVT and subsequently died at 33 high-capacity stroke centers. These centers are characterized by more advanced facilities, broader patient coverage, and a more significant number of trained professionals. At each center, specially trained doctors and nurses were responsible for verifying the accuracy of the collected data on patient deaths. The provincial quality control center also arranged for two senior neurologists to independently review 10% of the case records in the electronic health records system to ensure data consistency and accuracy.
This study screened AIS patients who underwent EVT and died within 30 days post-treatment from January 2019 to September 2022. Mortality was ascertained either during hospitalization or through reports of death within a 1-month follow-up period post-discharge. The integrity of the mortality records and the precise timing of death were corroborated by cross-referencing with data from the Civil Registry Office. The cause of death during hospitalization was determined based on hospital records and death certificates. Patients who died within one month after discharge, if there were records from local hospitals and diagnoses by specialists at the time of follow-up, and if the cause of death could be determined, were included in this study. Those who died from accidents or unknown causes and for whom the actual cause of death could not be determined were not included in this study. This study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Ethics
The ethics committee of West China Hospital, Sichuan University, approved the study (2020(69)) and waived the need to obtain patient informed consent since all the data were retrospectively collected and individual information was not disclosed.
Data Collection
Demographic information included age and gender. Clinical data included smoking history, pre-mRS, vascular risk factors [including hypertension, diabetes, atrial fibrillation (AF), previous stroke, dyslipidemia], perioperative laboratory values (neutrophils, lymphocyte count), serum creatinine, serum low-density lipoprotein, and serum glucose levels, as well as systolic/diastolic blood pressure measurements at admission and initial NIHSS scores. Stroke neurologists calculated NIHSS pre-treatment scores at each center.
Imaging Data: Imaging assessments before thrombectomy included the Alberta Stroke Program Early CT Score (ASPECTS) using non-contrast brain computed tomography (CT) and the presence and location of large vessel occlusion and tandem arterial occlusion determined by CT angiography (CTA). The imaging assessment was conducted by independently trained neuro-interventional physicians blinded to the clinical outcomes.
Treatment Data: Treatment-related data collected included the use of intravenous tPA, the application of stent retrievers, aspiration techniques, and their frequency. Additionally, time intervals from onset to door, door to puncture, and puncture to reperfusion, the interventionalist’s experience, and operative time (including daytime or nighttime) were recorded. Patients treated during working hours (08:00–17:59) are referred to as daytime, whereas those receiving treatment during night shift hours (18:00–07:59) are categorized as nighttime. Anesthesia methods used during the procedure and complications (such as arterial perforation, arterial dissection, new arterial occlusions in different regions, etc.) were also documented. Tandem lesions refer to the presence of severe stenosis in the vessel proximal to the site of occlusion. The pre-EVT DSA collateral status was assessed using the ASITN/SIR grading system to evaluate collateral compensation by neuro-interventional physicians blinded to the clinical outcomes. A good collateral status is classified as ASITN/SIR16 grades 3–4, while grades 0–2 indicate poorer collateral status. Reperfusion outcomes were evaluated using the modified Thrombolysis in Cerebral Infarction (mTICI) score,16 with no or minimal reperfusion: grade 0/1; partial arterial filling <50%: grade 2a; partial arterial filling >50%: grade 2b; and near-complete or complete reperfusion: grade 2c or 3. Successful reperfusion was defined as a post-EVT mTICI score of 2b, 2c, or 3. ICH refers to high-density shadows indicated on a follow-up CT scan during hospitalization 24 hours after EVT. sICH was defined as neurological deterioration post-EVT (increase in NIHSS score ≥4 points) accompanied by ICH.17 Procedural-related complications include intrasurgical events such as vascular dissection, new vascular embolism, thrombosis within stents, and vascular perforation, among other complications that occur during the procedure itself.
Definitions of Causes of Death
The study established clear diagnostic criteria for causes of death to ensure the accuracy and consistency of the data. Predetermined causes of death were categorized into pneumonia, symptomatic intracranial hemorrhage(sICH), malignant cerebral edema(MCE), cardiogenic death, withdrawal of life-sustaining treatment, gastrointestinal bleeding, sepsis, pulmonary embolism, recurrent ischemic stroke, and tumor. Each cause of death met specific diagnostic criteria and was independently reviewed by two senior neurologists to ensure accuracy.
The predetermined causes of death were defined as follows:
1. Pneumonia: The presence of infection signs with evident respiratory symptoms or chest CT anomalies, which the treating physician diagnoses as the cause of death.
2. Symptomatic intracranial hemorrhage (sICH): Imaging evidence of hemorrhage (according to the Heidelberg classification18) with neurological deterioration (an increase of ≥4 on the NIHSS) diagnosed by the treating physician as the ultimate cause of death.
3. Malignant cerebral edema (MCE): Neurological deterioration, along with clinical and imaging evidence of space-occupying cerebral edema, was the cause of death, without progression to PH2 on follow-up CT.
4. Cardiogenic death: Death caused by heart failure, cardiogenic asthma, myocardial infarction, or cardiac arrest.
5. Withdrawal of life-sustaining treatment: Refusal of life-sustaining therapy due to lack of improvement in initial stroke symptoms and no other apparent cause of death.
6. Sepsis: A systemic inflammatory response syndrome caused by infection.
7. Pulmonary embolism: Circulatory disturbance caused by pulmonary artery blockage confirmed by pulmonary artery CTA.
8. Recurrent ischemic stroke: A second ischemic stroke occurring in the same or a different location, leading to neurological deterioration and cessation of life-sustaining treatment.
9. Tumor: Cachexia and multi-organ failure caused by a tumor.
Statistical Analysis
Continuous variables were characterized by their mean and standard deviation (SD). Parametric Student’s t-tests were employed to compare normally distributed continuous variables, while the non-parametric Mann–Whitney U-tests were utilized for variables with a non-normal distribution. Categorical variables were depicted as counts and corresponding percentages and subjected to comparison using Chi-square tests or Fisher’s exact tests.
To explore the associations between potential predictor variables (p<0.05 in the group difference analysis) and the three most prevalent causes of death following EVT (MCE, Pneumonia, sICH), both univariable and multivariable logistic regression analyses were conducted. Odds ratios (ORs) and their respective 95% confidence intervals (CIs) were computed to assess this relationship. The multivariable logistic regression analysis was adjusted for age, gender, and variables, demonstrating a p-value less than 0.1 in group difference analysis. Statistical significance was established at p-values less than 0.05 for all analyses. IBM’s Statistical Package for the Social Sciences (SPSS) version 26.0 was employed for the data analysis.
Results
A total of 7985 patients with AIS underwent EVT across 33 hospitals from January 2019 to September 2022. Among these, 721 patients who died within 30 days were incorporated into the study. The average age of the participants was 72.36 years. The mean NIHSS score upon admission was 19.27, and the average ASPECTS was 7.1 (Supplementary Table S1). The average time from symptom onset to hospital admission was 260.36 minutes, admission to puncture was 126.08 minutes, puncture to recanalization was 100.05 minutes, and 76.7% of patients achieved successful recanalization (Supplementary Table S1).
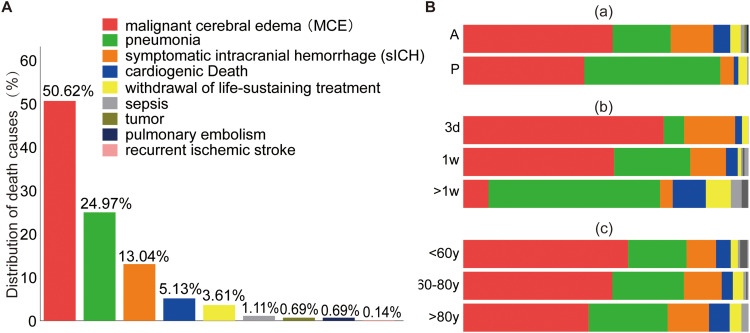
As shown in Figure 1, the primary causes of death were MCE (365/721, 50.6%), sICH (94/721, 13%), and pneumonia (180/721, 25%). A more significant proportion of deaths due to pneumonia was observed in patients with posterior circulation strokes than in patients with anterior circulation strokes (posterior vs anterior, 47.2% vs 20.2%) (Figure 1 and Supplementary Table S2). Over half of these patients (368/721, 51%) died within the first three days, primarily due to MCE (258/368, 70%) and sICH (65/368, 17.7%). After one week, pneumonia (109/183, 59.6%) became the predominant cause of death post-EVT (Figure 1 and Supplementary Table S3). sICH was more common in patients with anterior circulation large vessel occlusion (anterior vs posterior, 14.8% vs 4.7%). The causes of death were similar across the different age groups (Figure 1 and Supplementary Table S4).
Figure 1.
Distribution of the proportion of causes of death. (A) Proportion of various causes of death among AIS patients who died within 30 days post-EVT treatment. (B) Distribution of cause of death in panel A categorized by anterior circulation or posterior circulation (a), onset to death time within 3 days, 3 days-1 week, and over 1 week (b), and age group of <60 years, 60–80 years, and older than 80 years (c).
Further analysis of the clinical characteristics associated with each cause of death indicated that patients who died from MCE were younger (non-MCE vs MCE, 71.22 vs 73.53, p=0.008), had lower pre-treatment ASPECT scores (non-MCE vs MCE, 6.95 vs 7.25, p=0.04), experienced longer puncture to reperfusion(PTR) times (non-MCE vs MCE, 109.9 vs 89.94 minutes, p<0.001), had more large vessel involvement (non-MCE vs MCE, 95.5% vs 80.2%, p<0.001), and had lower successful reperfusion rates (non-MCE vs MCE, 68.5% vs 85.1%, p<0.001). Further univariate logistic regression analysis indicated that younger age (OR=0.98 [0.971–0.996]), longer PTR time (OR=1.005 [1.003–1.008]), large vessel occlusion (OR=5.233 [2.842–9.638]), and lower reperfusion success rate (OR=0.38 [0.264–0.548]) were associated with higher potential risk of death caused by MCE (Table 1). Adjusted multivariable regression analysis revealed that large vessel occlusion and a lower reperfusion success rate significantly associated with death caused by MCE (Table 1).
Table 1.
Demographics, Clinical Data, and Treatment Characteristics Between Patients Deceased Due to Malignant Cerebral Edema (MCE) and Non-MCE Causes, Along with Logistic Regression Analysis of Their Associations
| Variables | Non-MCE | MCE | pValue | OR (95% CI) | OR adj (95% CI) | |
|---|---|---|---|---|---|---|
| Age, mean years (SD) | 73.53(11.84) | 71.22(11.49) | 0.008 | 0.98 (0.971–0.996) | - | |
| Gender, Male | 181(50.8%) | 172(47.1%) | 0.318 | - | - | |
| pre-mRS≥2 | 16(4.5%) | 13(3.6%) | 0.524 | - | - | |
| Admission NIHSS score, mean (SD) | 19.18(7.72) | 19.36(7.30) | 0.741 | - | - | |
| Admission ASPECTS, mean (SD) | 7.25(1.89) | 6.95(2.02) | 0.04 | 1.003(0.984–1.023) | - | |
| SBP, mean mmHg (SD) | 147.19 (27.41) | 146.23(27.30) | 0.636 | - | - | |
| DBP, mean mmHg (SD) | 84.23 (17.49) | 85.56 (17.15) | 0.303 | - | - | |
| FPG, mean mmol/L (SD) | 9.38(3.98) | 9.66 (4.58) | 0.372 | - | - | |
| Neutrophils, mean 109/L(SD) | 11.21(15.17) | 9.83(10.98) | 0.17 | - | - | |
| Lymphocyte, mean 109/L (SD) | 1.43(1.55) | 1.40(1.20) | 0.782 | - | - | |
| sCr, mean μmol/L (SD) | 91.76 (47.99) | 90.50 (86.30) | 0.812 | - | - | |
| LDL, mean mmol/L (SD) | 2.46(0.92) | 3.13(9.09) | 0.217 | - | - | |
| Smoking, n (%) | 119(33.4%) | 111(30.4%) | 0.385 | - | - | |
| Alcohol, n (%) | 96(27%) | 98(26.8%) | 0.972 | - | - | |
| Hypertension, n (%) | 196(55.1%) | 213(58.4%) | 0.371 | - | - | |
| DM, n (%) | 97(27.2%) | 89(24.4%) | 0.38 | - | - | |
| Previous ischemic stroke, n (%) | 59(16.6%) | 43(11.8%) | 0.065 | - | - | |
| Previous hemorrhagic stroke | 7(2%) | 8(2.2%) | 0.832 | - | - | |
| Atrial fibrillation, n (%) | 212(59.6%) | 226(61.9%) | 0.515 | - | - | |
| TOAST classification, n (%) | LAA | 138(38.8%) | 133(36.4%) | 0.17 | - | - |
| Cardioembolism | 207(58.1%) | 224(61.4%) | - | - | ||
| Other etiology | 10(2.8%) | 4(1.1%) | - | - | ||
| UE | 1(0.3%) | 4(1.1%) | - | - | ||
| Wake-up stroke | 58(16.5%) | 63(17.5%) | 0.729 | - | - | |
| Onset to admission, min (SD) | 259.27(464.24) | 261.42(525.92) | 0.954 | - | - | |
| Admission to puncture, min (SD) | 124.13(195.38) | 127.99(161.12) | 0.772 | - | - | |
| Puncture to reperfusion, min (SD) | 89.94(55.67) | 109.90(67.23) | <0.001 | 1.005(1.003–1.008) | - | |
| IVT | 94(26.4%) | 111(30.4%) | 0.233 | - | - | |
| Occlusion site, n (%) | MeVO | 56(19.8%) | 14(4.5%) | <0.001 | 5.233(2.842–9.638) | 4.303(1.966–9.415) |
| LVO | 227(80.2%) | 297(95.5%) | - | - | ||
| Tandem occlusion | 49(13.8%) | 48(13.2%) | 0.809 | - | - | |
| pre-ASITN/SIR | Good | 10(2.8%) | 10(2.7%) | 0.96 | - | - |
| Poor | 346(97.2%) | 354(97.3%) | - | - | ||
| Time from onset to puncture | <6h | 254(71.3%) | 257(70.4%) | 0.782 | - | - |
| ≥6h | 102(28.7%) | 108(29.6%) | - | - | ||
| General anesthesia, n (%) | 180(50.6%) | 186(51%) | 0.915 | - | - | |
| *Stenting, n (%) | 48(13.5%) | 38(10.4%) | 0.203 | - | - | |
| Operative time | Daytime | 171(48%) | 166(45.5%) | 0.492 | - | - |
| Nighttime | 185(52%) | 199(54.5%) | - | - | ||
| Successful revascularization, n (%) | 303(85.1%) | 250(68.5%) | <0.001 | 0.38(0.264–0.548) | 0.518(0.302–0.889) | |
| Procedural complications, n (%) | 24(6.7%) | 28(7.7%) | 0.622 | - | - | |
| Interventionalist’s experience, mean years (SD) | 4.08 (1.89) | 4.23 (2.15) | 0.32 | - | - | |
| Aspiration, n (SD) | 1.33(1.27) | 1.47(1.37) | 0.174 | - | - | |
| Stent retrievers, n (SD) | 1.36(1.29) | 1.54(1.27) | 0.059 | - | - | |
| ICH, n (%) | 138(38.8%) | 116(31.8%) | 0.05 | - | - | |
| sICH, n (%) | 120(33.7%) | 100(27.4%) | 0.066 | - | - | |
| Brain herniation | 153(43%) | 357(97.8%) | <0.001 | 59.21(28.49–123.04) | 123.13(46.57–325.61) |
Notes: *= extracranial stenting and/or intracranial stenting; . adj, Adjusted for age, gender, admission ASPECTS, interval of Puncture to reperfusion, occlusion site, successful revascularization, number of stent retrievers, ICH, sICH, brain herniation.
Abbreviations: SD, Standard deviation; NIHSS, National Institute of Health Stroke Scale; ASPECTS, Alberta Stroke Program Early CT Score; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; sCr, serum creatinine; LDL, low density lipoprotein; DM, diabetes mellitus; IVT, intravenous thrombolysis; TOAST, Trial of Org 10172 in acute stroke treatment; LAA, Large artery atherosclerosis. UE, Undetermined etiology; LVO, large-vessel occlusions; MeVO, medium-vessel occlusions; ASITN/SIR, American Society of Interventional and Therapeutic Neuroradiology/ Society of Interventional Radiology; ICH, intracranial hemorrhage; sICH, symptomatic intracranial hemorrhage.
A more significant proportion of patients who died from sICH had tandem lesions (sICH vs non-sICH, 22.3% vs 12.1%, p=0.007), more procedural-related complications (sICH vs non-sICH,16% vs 5.9%, p<0.001). It was treated by less experienced surgeons (sICH vs non-sICH, mean experience 3.53 vs 4.25 years, p<0.001). Further univariate logistic regression analysis indicated that tandem lesions (OR=2.086 [1.214–3.584]), procedural complications (OR=3.023 [1.587–5.757]), and less experienced surgeons (OR=0.817 [0.722–0.925]) increased the potential risk of death caused by sICH. Adjusted multivariable regression analysis showed that tandem lesions and procedural complications remained associated with an increased risk of death due to sICH (Table 2).
Table 2.
Demographics, Clinical Data, and Treatment Characteristics Between Patients Deceased Due to Symptomatic Intracranial Hemorrhage (sICH) and Non-sICH Causes, Along with Logistic Regression Analysis of Their Associations
| Variables | Non-sICH | sICH | pValue | OR (95% CI) | ORadj(95% CI) | |
|---|---|---|---|---|---|---|
| Age, mean years (SD) | 72.32(11.59) | 72.65(12.54) | 0.798 | - | - | |
| Gender, Male | 304(48.5%) | 49(52.1%) | 0.51 | - | - | |
| pre-mRS≥2 | 26(4.1%) | 3(3.2%) | 0.66 | - | - | |
| Admission NIHSS score, mean (SD) | 19.45(7.523) | 18.06(7.28) | 0.094 | - | - | |
| Admission ASPECTS, mean (SD) | 7.08(1.98) | 7.26(1.83) | 0.413 | - | - | |
| SBP, mean mmHg (SD) | 146.27 (27.49) | 149.56 (26.27) | 0.277 | - | - | |
| DBP, mean mmHg (SD) | 84.97 (17.51196) | 84.46(16.02) | 0.788 | - | - | |
| FPG, mean mmol/L (SD) | 9.60 (4.31) | 8.98(4.10) | 0.194 | - | - | |
| Neutrophils, mean 109/L (SD) | 10.17 (12.35) | 12.8544(18.10) | 0.179 | - | - | |
| Lymphocyte, mean 109/L (SD) | 1.37(1.27) | 1.76 (1.99) | 0.071 | - | - | |
| sCr, mean μmol/L (SD) | 92.24(73.55) | 83.60(35.32) | 0.274 | - | - | |
| LDL, mean mmol/L (SD) | 2.87 (6.99) | 2.31(0.92) | 0.463 | - | - | |
| Smoking, n (%) | 203(32.4%) | 27(28.7%) | 0.479 | - | - | |
| Alcohol, n (%) | 175(27.9%) | 19(20.2%) | 0.117 | - | - | |
| Hypertension, n (%) | 366(58.4%) | 43(45.7%) | 0.021 | 0.601(0.389–0.93) | - | |
| DM, n (%) | 166(26.5%) | 20(21.3%) | 0.283 | - | - | |
| Previous ischemic stroke, n (%) | 86(13.7%) | 16(17%) | 0.391 | - | - | |
| Previous hemorrhagic stroke | 14(2.2%) | 1(1.1%) | 0.707 | - | - | |
| Atrial fibrillation, n (%) | 379(60.4%) | 59(62.8%) | 0.668 | - | - | |
| TOAST classification, n (%) | LAA | 236(37.6%) | 35(37.2%) | 0.288 | - | - |
| Cardioembolism | 376(60%) | 55(58.5%) | - | - | ||
| Other etiology | 10(1.6%) | 4(4.3%) | - | - | ||
| UE | 5(0.8%) | 0(0%) | - | - | ||
| Wake-up stroke | 108(17.4%) | 13(14.1%) | 0.437 | - | - | |
| Onset to admission, min (SD) | 264.32(480.74) | 233.96(590.59) | 0.58 | - | - | |
| Admission to puncture, min (SD) | 128.99 (189.70) | 106.66(68.49) | 0.259 | - | - | |
| Puncture to reperfusion, min (SD) | 100.41 (64.27) | 97.63(49.83) | 0.629 | - | - | |
| IVT | 174(27.8%) | 31(33%) | 0.295 | - | - | |
| Occlusion site, n (%) | MeVO | 57(11.3%) | 13(14.8%) | 0.346 | - | - |
| LVO | 449(88.7%) | 75(85.2%) | - | - | ||
| Tandem occlusion | 76(12.1%) | 21(22.3%) | 0.007 | 2.086(1.214–3.584) | 2.554(1.122–5.816) | |
| pre-ASITN/SIR | Good | 17(2.7%) | 3(3.2%) | 0.794 | - | - |
| Poor | 609(97.3%) | 91(96.8%) | - | - | ||
| Time from onset to puncture | <6h | 437(69.7%) | 74(78.7%) | 0.072 | - | - |
| ≥6h | 190(30.3%) | 20(21.3%) | - | - | ||
| General anesthesia, n (%) | 324(51.7%) | 42(44.7%) | 0.206 | - | - | |
| *Stenting, n (%) | 70(11.2%) | 16(17%) | 0.102 | - | - | |
| Operative time | Daytime | 293(46.7%) | 44(46.8%) | 0.989 | - | - |
| Nighttime | 334(53.3%) | 50(53.2%) | - | - | ||
| Successful revascularization, n (%) | 474(75.6%) | 79(84%) | 0.071 | - | - | |
| Procedural complications, n (%) | 37(5.9%) | 15(16%) | <0.001 | 3.023(1.587–5.757) | 3.205(1.322–7.77) | |
| Interventionalist’s experience, mean years (SD) | 4.25 (2.05) | 3.53 (1.71) | 0.001 | 0.817(0.722–0.925) | - | |
| Aspiration, n (SD) | 1.40 (1.31) | 1.41 (1.42) | 0.904 | - | - | |
| Stent retrievers, n (SD) | 1.43 (1.25) | 1.62(1.45) | 0.178 | - | - | |
| ICH, n (%) | 165(26.3%) | 89(94.7%) | <0.001 | 49.84(19.89–124.84) | 9.82(2.38–40.51) | |
| sICH, n (%) | 133(21.2%) | 87(92.6%) | <0.001 | 46.16 (20.88–102.07) | 7.73(2.32–25.75) | |
| Brain herniation | 419(66.8%) | 91(96.8%) | <0.001 | 15.06(4.71–48.13) | 7.96(2.19–28.98) |
Notes: *= extracranial stenting and/or intracranial stenting; adj: Adjusted for age, gender, admission NIHSS score, lymphocyte, hypertension, tandem occlusion, time from onset to puncture, successful revascularization, procedural complications, interventionalist’s experience, ICH, sICH, brain herniation;
Abbreviations: SD, Standard deviation; NIHSS, National Institute of Health Stroke Scale; ASPECTS, Alberta Stroke Program Early CT Score; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; sCr, serum creatinine; LDL, low density lipoprotein; DM, diabetes mellitus; IVT, intravenous thrombolysis; TOAST, Trial of Org 10172 in acute stroke treatment; LAA, Large artery atherosclerosis; UE, Undetermined etiology; LVO, large-vessel occlusions; MeVO, medium-vessel occlusions; ASITN/SIR, American Society of Interventional and Therapeutic Neuroradiology/ Society of Interventional Radiology; ICH, intracranial hemorrhage; sICH, symptomatic intracranial hemorrhage.
Patients who died from pneumonia were older (pneumonia vs non-pneumonia, mean age 74.35 vs 71.7, p<0.001), had shorter PTR (pneumonia vs non-pneumonia, mean 84.31 vs 105.28 minutes, p<0.001), had lower rates of intravenous thrombolysis (pneumonia vs non-pneumonia, 21.7% vs 30.7%, p = 0.02), had a lower proportion of large vessel occlusion (pneumonia vs non-pneumonia, 75.8% vs 91.4%, p<0.001), and had a higher successful reperfusion rate (pneumonia vs non-pneumonia, 85.6% vs 73.8%, p=0.001). Further univariate logistic regression analysis indicated that older age (OR=1.021 [1.005–1.036]), shorter PTR time (OR=0.994 [0.991–0.997]), intravenous thrombolysis (OR=1.6 [1.074–2.385]), large vessel occlusion (OR=0.297 [0.175–0.503]), higher reperfusion success rate (OR=2.108 [1.334–3.331]), and a lower incidence of procedural complications (OR=0.37 [0.155–0.882]) increased the potential risk of death caused by pneumonia. Adjusted multivariable regression analysis showed that older age and poorer preoperative collateral circulation status remained associated with an increased risk of death due to pneumonia (Table 3).
Table 3.
Demographics, Clinical Data, and Treatment Characteristics Between Patients Deceased Due to Pneumonia and Non-Pneumonia Causes, Along with Logistic Regression Analysis of Their Associations
| Variables | Non-pneumonia | Pneumonia | pValue | OR (95% CI) | ORadj(95% CI)a | |
|---|---|---|---|---|---|---|
| Age, mean years (SD) | 71.7(11.86) | 74.35(11.03) | <0.001 | 1.021(1.005–1.036) | 1.04(1.012–1.069) | |
| Gender, Male | 258(47.7%) | 95(52.8%) | 0.237 | - | - | |
| pre-mRS≥2 | 22(4.1%) | 7(3.9%) | 0.916 | - | - | |
| Admission NIHSS score, mean (SD) | 18.99(7.31) | 20.12(8.01) | 0.079 | - | - | |
| Admission ASPECTS, mean (SD) | 7.08(2.00) | 7.16(1.85) | 0.64 | - | - | |
| SBP, mean mmHg (SD) | 146.60(27.16) | 147.01(27.96) | 0.864 | - | - | |
| DBP, mean mmHg (SD) | 85.06 (16.92) | 84.43(18.49) | 0.669 | - | - | |
| FPG, mean mmol/L (SD) | 9.41 (4.39) | 9.86 (3.97) | 0.221 | - | - | |
| Neutrophils, mean 109/L (SD) | 10.21(12.39) | 11.42(15.46) | 0.292 | - | - | |
| Lymphocyte, mean 109/L (SD) | 1.44(1.34) | 1.37(1.52) | 0.568 | - | - | |
| sCr, mean μmol/L (SD) | 89.57(74.09) | 95.71(55.52) | 0.311 | - | - | |
| LDL, mean mmol/L (SD) | 2.89(7.46) | 2.50 (0.92) | 0.535 | - | - | |
| Smoking, n (%) | 166(30.7%) | 64(35.6%) | 0.224 | - | - | |
| Alcohol, n (%) | 141(26.1%) | 53(29.4%) | 0.376 | - | - | |
| Hypertension, n (%) | 304(56.2%) | 105(58.3%) | 0.616 | - | - | |
| DM, n (%) | 132(24.4%) | 54(30%) | 0.137 | - | - | |
| Previous ischemic stroke, n (%) | 73(13.5%) | 29(16.1%) | 0.383 | - | - | |
| Previous hemorrhagic stroke | 13(2.4%) | 2(1.1%) | 0.379 | - | - | |
| Atrial fibrillation, n (%) | 333(61.6%) | 105(58.3%) | 0.444 | - | - | |
| TOAST classification, n (%) | LAA | 197(36.4%) | 74(41.1%) | 0.785 | - | - |
| Cardioembolism | 328(60.6%) | 103(57.2%) | - | - | ||
| Other etiology | 12(2.2%) | 2(1.1%) | - | - | ||
| UE | 4(0.7%) | 1(0.6%) | - | - | ||
| Wake-up stroke | 89(16.6%) | 32(18%) | 0.679 | - | - | |
| Onset to admission, min (SD) | 262.29 (555.46) | 254.55(243.06) | 0.856 | - | - | |
| Admission to puncture, min (SD) | 120.55(137.23) | 142.72 (267.07) | 0.149 | - | - | |
| Puncture to reperfusion, min (SD) | 105.28 (64.03) | 84.31 (55.11) | <0.001 | 0.994(0.991–0.997) | - | |
| IVT | 166(30.7%) | 39(21.7%) | 0.02 | 1.6(1.074–2.385) | - | |
| Occlusion site, n (%) | MeVO | 41(8.6%) | 29(24.2%) | <0.001 | 0.297(0.175–0.503) | - |
| LVO | 433(91.4%) | 91(75.8%) | - | - | ||
| Tandem occlusion | 74(13.7%) | 23(12.8%) | 0.759 | - | - | |
| pre-ASITN/SIR | good | 19(3.5%) | 1(0.6%) | 0.036 | 6.528(0.868–49.112) | 10.63(1.119–100.977) |
| poor | 521(96.5%) | 179(99.4%) | - | - | ||
| Time from onset to puncture | <6h | 389(71.9%) | 122(67.8%) | 0.291 | - | - |
| ≥6h | 152(28.1%) | 58(32.2%) | - | - | ||
| General anesthesia, n (%) | 273(50.5%) | 93(51.7%) | 0.779 | - | - | |
| *Stenting, n (%) | 62(11.5%) | 24(13.3%) | 0.502 | - | - | |
| Operative time | daytime | 245(45.3%) | 92(51.1%) | 0.175 | - | - |
| nighttime | 296(54.7%) | 88(48.9%) | - | - | ||
| Successful revascularization, n (%) | 399(73.8%) | 154(85.6%) | 0.001 | 2.108(1.334–3.331) | - | |
| Procedural complications, n (%) | 46(8.5%) | 6(3.3%) | 0.02 | 0.37(0.155–0.882) | - | |
| Interventionalist’s experience, mean years (SD) | 4.12 (2.04) | 4.27(1.99) | 0.404 | - | - | |
| Aspiration, n (SD) | 1.44 (1.37) | 1.27(1.19) | 0.097 | - | - | |
| Stent retrievers, n (SD) | 1.54 (1.30) | 1.17 (1.18) | <0.001 | 0.778(0.671–0.902) | - | |
| ICH, n (%) | 227(42%) | 27(15%) | <0.001 | 0.244(0.157–0.38) | - | |
| sICH, n (%) | 204(37.7%) | 16(8.9%) | <0.001 | 0.161(0.094–0.277) | 0.213(0.069–0.661) | |
| Brain herniation | 475(87.8%) | 35(19.4%) | <0.001 | 0.034(0.021–0.053) | 0.047(0.026–0.084) |
Notes: *= extracranial stenting and/or intracranial stenting; adj: Adjusted for age, gender, admission NIHSS score, lymphocyte, hypertension, tandem occlusion, time from onset to puncture, successful revascularization, procedural complications, interventionalist’s experience, ICH, sICH, brain herniation.
Abbreviations: SD, Standard deviation; NIHSS, National Institute of Health Stroke Scale; ASPECTS, Alberta Stroke Program Early CT Score; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; sCr, serum creatinine; LDL, low density lipoprotein; DM, diabetes mellitus; IVT, intravenous thrombolysis; TOAST, Trial of Org 10172 in acute stroke treatment; LAA, Large artery atherosclerosis; UE, Undetermined etiology; LVO, large-vessel occlusions; MeVO, medium-vessel occlusions; ASITN/SIR, American Society of Interventional and Therapeutic Neuroradiology/ Society of Interventional Radiology; ICH, intracranial hemorrhage; sICH, symptomatic intracranial hemorrhage.
Discussion
Despite a meta-analysis demonstrating that EVT can significantly reduce mortality rates in AIS patients,6 15% of patients still succumb within three months post-EVT.5–7 A better understanding of the characteristics of post-EVT mortality could guide future treatment approaches, further improving patient survival rates.
Using extensive sample data, we revealed the most common causes of death among AIS patients who underwent EVT, including MCE, sICH, and pneumonia. We observed that the causes of death after ischemic stroke vary over time. Within the first week, MCE is a leading cause of death. Consistent with domestic and international studies, cerebrovascular causes are the main reasons for short-term post-stroke mortality.15,19–21 Large cerebral infarction is primarily due to low recanalization success rates and prolonged PTR. Although we did not assess technical issues, longer PTR times might reflect our study’s challenges in achieving rapid reperfusion.
Furthermore, sICH occurs relatively frequently in patients with tandem lesions and those who undergo stent placement, possibly related to the postoperative use of potent antiplatelet medications, though the lack of detailed data on the timing and regimen of these medications limits our ability to fully assess their role in the bleeding risk. Additionally, our study highlights that surgical complications and the limited experience of surgeons are closely associated with sICH. Previous research indicates that outcomes of endovascular procedures, such as carotid artery stenting, intracranial aneurysm coiling, and percutaneous coronary interventions, are poorer in smaller medical centers than in larger centers.22–24 Studies have found that hospitals with a higher volume of stroke cases have lower mortality and adverse outcome rates.25–27 As the volume of cases increases, improvements in the EVT workflow also contribute to better treatment outcomes. Critical factors for successful treatment include rapid symptom recognition by emergency personnel, swift imaging acquisition and review, well-trained neurological assessments, timely interventions by skilled neurointerventionalists, advanced neurointensive care, and proactive rehabilitation measures.28 Therefore, it is reasonable to infer that hospitals with lower volumes and less specialization experience higher complication and mortality rates. However, intraoperative technical complications and the resultant sICH are directly linked to the proficiency level of the EVT surgeons. A study involving 955 EVT patients treated consecutively at 17 Korean stroke centers demonstrated that the cumulative number of cases at a center positively correlates with revascularization and favorable outcomes and negatively correlates with sICH and mortality.29 Additionally, another retrospective cohort study showed that an increase in the number of surgical cases reduced mortality and improved prognoses.30
When EVT fails to reduce neurological deficits significantly, strategies to prevent post-EVT complications such as pneumonia may enhance survival rates, as these are often the primary causes of death. Pneumonia, as the leading cause of death one week after surgery, highlights the need for enhanced respiratory system monitoring and early intervention. Stroke often occurs with accompanying consciousness disorders or difficulty swallowing.20,31 As a result, pneumonia is more common in posterior circulation strokes. Pneumonia is considered the most common cause of death within one year following AIS.32 In our study, older age may be associated with pneumonia-induced deaths. Moreover, pneumonia deaths were more common in patients with medium-vessel occlusions. Although not statistically significant, these deaths occurred more frequently in patients who experienced disease onset for more than 6 hours, who had lower thrombolysis rates, and who had poorer collateral status. This finding suggested that patients with non-extensive infarctions, despite lacking significant cerebral edema, exhibited substantial neurological deficits. Prolonged bed rest, advanced age, or poor nutritional intake increase the risk of pulmonary infection. Thus, enhanced postoperative infection prevention and management are crucial for these patients. Early rehabilitation after stroke may reduce the risk of pneumonia.33,34
Predictive variables for mortality in patients post-EVT have been extensively studied in the previous literature.10–15 Common predictive factors include age, prior stroke, admission NIHSS, fasting blood glucose, occlusion site, reperfusion status, sICH, and discharge modified Rankin Scale, etc. Our study investigated the characteristics of real-world deceased patients, which is essential for improving future treatment strategies and reducing mortality rates. For example, for patients with extensive cerebral infarction, early attention to alleviating cerebral edema is crucial. In patients with relatively more minor infarct areas, emphasis should be placed on preventing pneumonia complications, especially in elderly patients. During EVT procedures, it is essential to minimize surgical complications and reduce deaths related to hemorrhagic transformation. High-risk patient groups (such as older patients or those with severe disease) may require more personalized, precise treatment approaches and closer monitoring. Enhancing EVT techniques and preventing and treating these complications could also improve survival rates for AIS patients.
Our study is subject to several limitations. First, the absence of non-deceased patients for comparison restricts our ability to ascertain the exclusive impact of various factors on AIS management improvements aimed at reducing post-EVT mortality. Second, the retrospective data collection across multiple hospitals may have led to inconsistencies in assessing baseline characteristics such as the NIHSS and mTICI scores. Nevertheless, the multicenter design of the study enhances the applicability of the findings to real-world conditions. Third, our study cohort comprised patients of Asian descent from the western region of China, raising questions about the generalizability of our findings to other populations and whether these patients exhibit a heightened risk of post-EVT mortality. Fourth, we included patients who died within 30 days, focusing on the assessment of early mortality characteristics following EVT. Due to the lack of longer follow-up, detailed data on mid- to long-term mortality post-EVT are unavailable. Fifth, several factors contributed to the observed slightly lower mortality rate, including missing or incomplete follow-up data, potential overestimation of EVT patient numbers due to the inclusion of those receiving emergency angiography or thrombolysis, and underreporting of deaths across centers due to discrepancies in mortality reporting through the provincial quality control platform.
Conclusion
This study provides important insights into the 30-day mortality rates and associated factors for AIS patients following EVT treatment. The primary causes of death were MCE, pneumonia, and sICH. Strategies aimed at reducing MCE involve achieving successful reperfusion. Efforts to lower sICH rates include minimizing procedural complications and requiring the use of skilled operators. Decreasing pneumonia rates are associated with effective postoperative care, especially for those who survive one week post-stroke. Future research should explore comprehensive management strategies for patients post-EVT to elucidate further ways to enhance patient survival and functional recovery.
Funding Statement
This study was funded by the National Natural Science Foundation of China (grant no. 82271360), the Sichuan Science and Technology Program (Funding number: 2022YFS0139), and China’s Postdoctoral Science Foundation (No.2022M722265).
Institutional Review Board Statement
This study was conducted according to the ethical principles of the 1964 Declaration of Helsinki and approved by the Ethics Committee of West China Hospital [No. 2020(69)].
Data Sharing Statement
The data supporting this study’s findings are available from the corresponding author on reasonable request. Supplemental Material for this article is available online.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no potential conflicts of interest concerning the research, authorship, and publication of this article.
References
- 1.Collaborators GBDCo D. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392(10159):1736–1788. doi: 10.1016/S0140-6736(18)32203-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2019;394(10204):1145–1158. doi: 10.1016/S0140-6736(19)30427-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tu WJ, Zhao Z, Yin P, et al. Estimated burden of stroke in China in 2020. JAMA Network Open. 2023;6(3):e231455. doi: 10.1001/jamanetworkopen.2023.1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Writing Group M, Mozaffarian D, Benjamin EJ, et al. Heart disease and stroke statistics-2016 update: a report from the American heart association. Circulation. 2016;133(4):e38–360. doi: 10.1161/CIR.0000000000000350 [DOI] [PubMed] [Google Scholar]
- 5.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723–1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 6.Katsanos AH, Malhotra K, Goyal N, et al. Mortality risk in acute ischemic stroke patients with large vessel occlusion treated with mechanical thrombectomy. J Am Heart Assoc. 2019;8(21):e014425. doi: 10.1161/JAHA.119.014425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yaeger KA, Martini ML, Hardigan T, et al. Mortality reduction after thrombectomy for acute intracranial large vessel occlusion: meta-analysis of randomized trials. J Neurointerv Surg. 2020;12(6):568–573. doi: 10.1136/neurintsurg-2019-015383 [DOI] [PubMed] [Google Scholar]
- 8.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708–718. doi: 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11–21. doi: 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 10.Li H, Ye SS, Wu YL, et al. Predicting mortality in acute ischaemic stroke treated with mechanical thrombectomy: analysis of a multicentre prospective registry. BMJ Open. 2021;11(4):e043415. doi: 10.1136/bmjopen-2020-043415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karamchandani RR, Rhoten JB, Strong D, Chang B, Asimos AW. Mortality after large artery occlusion acute ischemic stroke. Sci Rep. 2021;11(1):10033. doi: 10.1038/s41598-021-89638-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Zhou S, Yang S, et al. Developing and predicting of early mortality after endovascular thrombectomy in patients with acute ischemic stroke. Front Neurosci. 2022;16:1034472. doi: 10.3389/fnins.2022.1034472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gattringer T, Posekany A, Niederkorn K, et al. Predicting early mortality of acute ischemic stroke. Stroke. 2019;50(2):349–356. doi: 10.1161/STROKEAHA.118.022863 [DOI] [PubMed] [Google Scholar]
- 14.Oliveira ADP, Andrade-Valenca LPA, Valenca MM. Factors associated with in-hospital mortality in very elderly patients with ischemic stroke: a cohort study. J Stroke Cerebrovasc Dis. 2019;28(10):104281. doi: 10.1016/j.jstrokecerebrovasdis.2019.06.039 [DOI] [PubMed] [Google Scholar]
- 15.Sluis WM, Hinsenveld WH, Goldhoorn RB, et al. Timing and causes of death after endovascular thrombectomy in patients with acute ischemic stroke. Eur Stroke J. 2023;8(1):215–223. doi: 10.1177/23969873221143210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaidat OO, Yoo AJ, Khatri P, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44(9):2650–2663. doi: 10.1161/STROKEAHA.113.001972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rao NM, Levine SR, Gornbein JA, Saver JL. Defining clinically relevant cerebral hemorrhage after thrombolytic therapy for stroke: analysis of the national institute of neurological disorders and stroke tissue-type plasminogen activator trials. Stroke. 2014;45(9):2728–2733. doi: 10.1161/STROKEAHA.114.005135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Kummer R, Broderick JP, Campbell BC, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46(10):2981–2986. doi: 10.1161/STROKEAHA.115.010049 [DOI] [PubMed] [Google Scholar]
- 19.Nagel H, Pinho J, Hasan D, et al. Causes of death in endovascularly treated patients with acute stroke. AJNR Am J Neuroradiol. 2022;43(9):1299–1303. doi: 10.3174/ajnr.A7599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vernino S, Brown RD Jr, Sejvar JJ, Sicks JD, Petty GW, O’Fallon WM. Cause-specific mortality after first cerebral infarction: a population-based study. Stroke. 2003;34(8):1828–1832. doi: 10.1161/01.STR.0000080534.98416.A0 [DOI] [PubMed] [Google Scholar]
- 21.Aked J, Delavaran H, Lindgren AG. Survival, causes of death and recurrence up to 3 years after stroke: a population-based study. Eur J Neurol. 2021;28(12):4060–4068. doi: 10.1111/ene.15041 [DOI] [PubMed] [Google Scholar]
- 22.Brinjikji W, Rabinstein AA, Lanzino G, Kallmes DF, Cloft HJ. Patient outcomes are better for unruptured cerebral aneurysms treated at centers that preferentially treat with endovascular coiling: study of the national inpatient sample 2001-2007. AJNR Am J Neuroradiol. 2011;32(6):1065–1070. doi: 10.3174/ajnr.A2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pandey AS, Gemmete JJ, Wilson TJ, et al. High subarachnoid hemorrhage patient volume associated with lower mortality and better outcomes. Neurosurgery. 2015;77(3):462–470. doi: 10.1227/NEU.0000000000000850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jalbert JJ, Gerhard-Herman MD, Nguyen LL, et al. Relationship between physician and hospital procedure volume and mortality after carotid artery stenting among medicare beneficiaries. Circ Cardiovasc Qual Outcomes. 2015;8(6 Suppl 3):S81–9. doi: 10.1161/CIRCOUTCOMES.114.001668 [DOI] [PubMed] [Google Scholar]
- 25.Rinaldo L, Brinjikji W, Rabinstein AA. Transfer to high-volume centers associated with reduced mortality after endovascular treatment of acute stroke. Stroke. 2017;48(5):1316–1321. doi: 10.1161/STROKEAHA.116.016360 [DOI] [PubMed] [Google Scholar]
- 26.Gupta R, Horev A, Nguyen T, et al. Higher volume endovascular stroke centers have faster times to treatment, higher reperfusion rates and higher rates of good clinical outcomes. J Neurointerv Surg. 2013;5(4):294–297. doi: 10.1136/neurintsurg-2011-010245 [DOI] [PubMed] [Google Scholar]
- 27.Adamczyk P, Attenello F, Wen G, et al. Mechanical thrombectomy in acute stroke: utilization variances and impact of procedural volume on inpatient mortality. J Stroke Cerebrovasc Dis. 2013;22(8):1263–1269. doi: 10.1016/j.jstrokecerebrovasdis.2012.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mocco J, Fargen KM, Goyal M, et al. Neurothrombectomy trial results: stroke systems, not just devices, make the difference. Int J Stroke. 2015;10(7):990–993. doi: 10.1111/ijs.12614 [DOI] [PubMed] [Google Scholar]
- 29.Kim BM, Baek JH, Heo JH, Kim DJ, Nam HS, Kim YD. Effect of cumulative case volume on procedural and clinical outcomes in endovascular thrombectomy. Stroke. 2019;50(5):1178–1183. doi: 10.1161/STROKEAHA.119.024986 [DOI] [PubMed] [Google Scholar]
- 30.Stein LK, Mocco J, Fifi J, Jette N, Tuhrim S, Dhamoon MS. Correlations between physician and hospital stroke thrombectomy volumes and outcomes: a nationwide analysis. Stroke. 2021;52(9):2858–2865. doi: 10.1161/STROKEAHA.120.033312 [DOI] [PubMed] [Google Scholar]
- 31.Bosel J. Use and timing of tracheostomy after severe stroke. Stroke. 2017;48(9):2638–2643. doi: 10.1161/STROKEAHA.117.017794 [DOI] [PubMed] [Google Scholar]
- 32.Ingeman A, Andersen G, Hundborg HH, Svendsen ML, Johnsen SP. In-hospital medical complications, length of stay, and mortality among stroke unit patients. Stroke. 2011;42(11):3214–3218. doi: 10.1161/STROKEAHA.110.610881 [DOI] [PubMed] [Google Scholar]
- 33.Li S, Fang S, Zhang D, Lu Y, Wang L, Peng B. Association between rehabilitation after reperfusion treatment and in-hospital mortality: results from a national registry study. Front Neurol. 2022;13:949669. doi: 10.3389/fneur.2022.949669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coleman ER, Moudgal R, Lang K, et al. Early rehabilitation after stroke: a narrative review. Curr Atheroscler Rep. 2017;19(12):59. doi: 10.1007/s11883-017-0686-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting this study’s findings are available from the corresponding author on reasonable request. Supplemental Material for this article is available online.