Abstract
Background
Cholelithiasis areis a common digestive system disorder, with cholesterol gallstones being the most prevalent type. Gallstones lead to many severe complications, posing a significant burden on global healthcare systems. Many studies have shown associations between biliary microbiota, gallbladder immune microenvironment, and gallstone formation. However, the specific immune mechanisms underlying the cholesterol gallstone formation have not been fully elucidated.
Methods
In this study, gallbladderand bile samples from 8 asymptomatic patients with cholelithiasis undergoing cholecystectomy and 11 healthy liver transplant donors were collected for tissue transcriptome sequencing and differential analysis. Male C57BL/6J mice were fed a normal or lithogenic diet for 6 weeks. Starting from the third week, lipopolysaccharide (LPS) or specific regulators were injected intraperitoneally once a week for a total of 3 times. Enzyme-linked immunosorbent assay, quantitative polymerase chain reaction, Western blot, immunohistochemistry, and immunofluorescence were employed for quantitative, qualitative or localization analysis of LPS, neutrophil extracellular traps (NETs), inflammatory factors, proteins, and mRNAs using samples collected from mice.
Results
In patients with cholelithiasis, the gallbladder mechanical barrier is impaired, resulting in an immune-activated state. LPS induces damage to the gallbladder mucosal mechanical barrier through the Toll-like receptor 4 (TLR4)/myeloid differentiation factor 88 (MyD88)/nuclear factor kappa-B (NF-κB) signaling pathway. Furthermore, it stimulates the continuous production of NETs through the TLR4/Phosphoinositide 3-kinase (PI3K)/Protein kinase B (Akt) signaling pathway, aggravating the formation of gallstones.
Conclusion
With the biliary dysbiosis, excessive LPS can invade the submucosa of the gallbladder, leading to chronic inflammation that recruits neutrophils to form NETs, which are ultimately expelled into bile, thereby promoting the formation of gallstones.
Keywords: gallstone, lipopolysaccharide, immune barrier, neutrophil extracellular traps
Graphical Abstract
Introduction
Cholelithiasis is the most common disease of the biliary, which can cause a variety of complications, such as acute cholecystitis, pancreatitis and cholangitis often leading to the high mortality rates in Europe and the United States every year.1 In Western countries, approximately 5%–25% of adults suffer from cholelithiasis, of which 75%–80% of cases are cholesterol gallstones, with an annual management and treatment costs of approximately 6.5 billion US dollars.2,3 The specific mechanism underlying cholesterol gallstone formation has not yet been fully elucidated. Previous studies have indicated that the excessive secretion of hepatic cholesterol leads to supersaturation of bile; rapid phase transition of cholesterol in bile, resulting in the precipitation of solid cholesterol crystals; excessive secretion and accumulation of mucin gel in the gallbladder lumen, leading to immune-mediated cholecystitis; as well as intestinal factors involving cholesterol absorption, slow intestinal movement, and changes in intestinal microbiota, which are the main factors for cholesterol gallstone formation.4
Bile secreted by the liver is concentrated in the gallbladder, and its main components include water, bile salts, lipids, and lecithin.5,6 It was once believed that a healthy biliary tract system was sterile, but current research has shown that there is complex microbiota in the gallbladder, with Proteobacteria, Firmicutes, and Bacteroidetes distributed in the gallbladder ecosystem.7 Studies have also indicated that Helicobacter pylori in the gallbladder contributes to the gallstone formation.8,9
With the in-depth exploration of the gallbladder microbiota in cholelithiasis, its interaction with the immune microenvironment of gallbladder has gradually emerged. Recent studies have shown that gallbladder neutrophils can also promote the gallstone formation. In healthy individuals, neutrophils play a role in the immune surveillance of the gallbladder. Activated neutrophils can promote the formation and growth of gallstones by forming neutrophil extracellular traps (NETs) that aggregate calcium and cholesterol crystals. The extrachromosomal DNA (ecDNA) covering the surface of gallstones can further induce neutrophil recruitment and NETs formation.10,11 In addition, neutrophils can produce inflammatory mediators, recruit inflammatory cells, and damage gallbladder interstitial Cajal-like cells, leading to damage to gallbladder wall endothelial cells, manifesting as impaired smooth muscle contraction function of the gallbladder wall, and subsequently promoting gallstone formation.12
It can be seen that there are many correlations between gallbladder microbiota, gallbladder immune microenvironment, and gallstone formation in cholelithiasis. Therefore, it is crucial to evaluate the changes in the gallbladder and bile in cholelithiasis patients and clarify their roles in the occurrence and development of cholelithiasis. Hence, this study aims to investigate gallbladder and bile samples of asymptomatic cholelithiasis patients and healthy controls, and the content of bile lipopolysaccharide (LPS) and changes in the gallbladder immune microenvironment as well as their potential mechanisms in gallstone formation.
Materials and Methods
Clinical Samples Collection
This study collected a total of 8 gallbladder and bile samples from patients with asymptomatic cholelithiasis undergoing cholecystectomy (GS group). The inclusion criteria were as follows: no history of antibiotic or probiotic treatment within three months before sample collection, no history of other digestive system-related diseases, no history of allergic or autoimmune diseases or ongoing immunomodulatory therapy, no history of neurological or neurodevelopmental disorders or chronic pain syndrome, non-obesity based on body mass index (BMI) ≤ 30 kg/m2, no metabolic syndrome or severe malnutrition, no history of malignancy or ongoing cancer treatment, non-vegetarian diet, and no history of fecal microbiota transplantation. For comparison, 11 gallbladders and bile samples from healthy liver transplant donors were included as the negative control group (NC group) using the same inclusion criteria. Approximately 5 mL of bile was collected intraoperatively using a 24-gauge needle from the gallbladder or bile duct. After removing the gallbladder, tissue samples encompassing all layers of the gallbladder were promptly obtained using tissue forceps containing five soybean-sized fragments. Four of these fragments were frozen in liquid nitrogen, and one was preserved in 4% formalin at room temperature. Additionally, a brief flowchart of this study is provided in the supplementary materials (Figure S1).
Experimental Animal Model Establishment
A gallbladder LPS microinjection-induced gallstone model was established using 6-8 week-old, specific pathogen-free C57BL/6J male mice (Liaoning Changsheng Biotechnology Co., Ltd, Shenyang, China). The mice were randomly divided into each group (n=25): normal diet with an intragallbladder injection of saline (NC group), normal diet with an intragallbladder injection of LPS (NC + LPS group), lithogenic diet with an intragallbladder injection of saline (GS group), and lithogenic diet with an intragallbladder injection of LPS (GS + LPS group). The mice were fed for 6 weeks, and starting from the third week, they received an intragallbladder microinjection of 20 μL of LPS (30 ng/mL) once a week for a total of three times. The NC groups was administered an equal volume of saline solution. After 6 weeks, the mice were euthanized, and samples including gallstones, gallbladders, and bile, were collected for subsequent experiments. Owing to the small size of the mouse gallbladders, the gallbladder from a single mouse is insufficient to provide adequate RNA, protein, and bile for detection. Therefore, a pooled sample extraction method for mice within the same group was adopted for mice in the same group. Each group consisted of 25 mice, from which four gallbladders were randomly selected and fixed with 4% paraformaldehyde. Additionally, five randomly selected gallbladders were selected for pooled protein and bile extraction, with three extractions performed. Furthermore, two gallbladders were randomly selected for pooled RNA extraction, also with a total of three extractions conducted.
Drug Administration Method for Model Construction
Toll-like receptor 4 (TLR4) inhibitor TAK-242, nuclear factor kappa-B (NF-κB) inhibitor PDTC, phosphoinositide 3-kinase (PI3K) inhibitor LY294002 dissolved in DMSO, and PI3K activator Recilisib was administered via intraperitoneal injection at a dose of 3, 100, 50, and 10 mg/kg once daily, respectively. Similarly, the NF-κB inhibitor (PDTC) was injected intraperitoneally at a dose of 100 mg/kg once daily. The injection schedules for all four regulators were synchronized with the LPS injection period.
RNA Sequencing
DESeq2 was used to analyze the differential expression genes (DEGs) between the cholelithiasis and control group, the P value was corrected using the Benjamini and Hochberg method. The corrected P-value and |log2foldchange| were used as thresholds for significantly different expressions.
Differential Gene Enrichment Analysis
The enrichment analysis was performed using the hypergeometric test. For the Kyoto encyclopedia of genes and genomes (KEGG) analysis, the hypergeometric distribution test was performed with the unit of the pathway, whereas for gene ontology (GO), it was performed based on the GO term.
Weighted Gene Co-Expression Analysis (WGCNA)
WGCNA was performed under the subgroup‐specific signatures to identify potential functional modules that could characterize the biological function of each subgroup. Co-expression networks were created utilizing the “WGCNA” package in R software. The gallbladder samples were clustered to determine the presence of outliers. Subsequently, the soft threshold was computed using the PickSoftThreshold function. The soft threshold for a scale-free network was determined based on the maximal R2 (power = 26). The topological overlap matrix similarity was used to evaluate the distance between each gene pair. Moreover, hierarchical clustering analysis with average and dynamic method was used to build the cluster tree and classify the genes into modules. Based on these two evaluation criteria, the gene set related to gallstone disease was classified into 27 modules. The protein-protein interaction network was constructed using Genemania (http://genemania.org/).
Detection of LPS and NETs using the enzyme-linked immunosorbent assay (ELISA).
The bile NETs and LPS were detected using the Human NETs ELISA Kit (JL47452-96T, Jianglai Biotech, China), and the LPS ELISA Kit (JL12996-96T, Jianglai Biotech, China), respectively. Before the detection, the bile samples were diluted with PBS at a ratio of 1:100, and the detection was performed using a Thermo Scientific Varioskan Flash (Thermo Fisher Scientific, Waltham, MA, USA).
Histopathology and Immunohistochemical (IHC) Quantification Analysis
Tissues were fixed and embedded, and 4μm sections were stained with hematoxylin and eosin (H&E). Two pathologists blindly evaluated gallbladder mucosal mechanical barrier injury. In brief, gallbladder histopathological assessment included four categories: edema, inflammatory cell infiltration, necrosis, and vacuolization. IHC testing was performed for mucin 5 Subtype B (MUC5B,1:500, Cat.ab77995, Abcam, UK), occludin (OCLN, 1:1000, Cat.27260-1-AP, Proteintech, USA), and tight junction protein 1 (TJP1, 1:500, Cat.66452-1-Ig, Proteintech, USA). Quantification was performed using Image J software.
Quantitative Polymerase Chain Reaction (qPCR)
Total RNA was extracted from cells and tissues using the AxyPrep Multisource Total RNA Miniprep Kit (Axygen Scientific, Inc., CA, USA) according to the manufacturer’s instructions. Following RNA quantification, the RNA was reverse transcribed into cDNA using the qPCR RT Kit (TOYOBO, Shanghai, China). Real-time PCR was performed using the THUNDERBIRD SYBR qPCR Mix (TOYOBO, Shanghai, China) on an ABIPRISM 7500HT instrument (Applied Biosystems, CA, USA). The expression levels of target mRNA were normalized to the expression level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and determined using the 2-ΔΔCt method. Table S1 in the supplementary materials list all primers used.
Western Blot (WB) Analysis
Whole-cell and cellular extracts from cells and tissues were prepared using radioimmunoprecipitation assay (RIPA) buffer containing proteinase and phosphatase inhibitors. Approximately 60 μg of protein from whole-cell lysates was separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and subjected to protein immunoblotting. Antibodies against OCLN (1:5000, Cat.27260-1-AP, Proteintech, USA), TJP1 (1:1000, Cat.66452-1-Ig, Proteintech, USA), TLR4 (1:1000, Cat.19811-1-AP, Proteintech, USA), Toll-like receptor 2 (TLR2, 1:1000, Cat.17236-1-AP, Proteintech, USA), protein arginine deiminase type 4 (PADI4, 1:1000, Cat.17373-1-AP, Proteintech, USA), myeloperoxidase (MPO, 1:1000, Cat.66177-1-IG, Proteintech, USA), MyD88 (1:5000, 67969-1-Ig, Proteintech, USA), NF-κB (1:3000, 10745-1-AP, Proteintech, USA), p-NFκB (1:5000, 82335-1-RR, Proteintech, USA), inhibitor of NF-κB (IκB, 1:20,000, 10268-1-AP Proteintech, USA), p-IκB (1:1000, 2859L, CST, USA), PI3K (1:2000, 67071-1-Ig, Proteintech, USA), p-PI3K (1:400, ab182651, Abcam, UK), Akt (1:20,000, 60203-2-Ig, Proteintech, USA), and p-Akt (1:2000, 28731-1-AP, Proteintech, USA) were used for protein detection. After appropriate secondary antibody incubation, proteins were visualized and analyzed using the Odyssey® Imaging System (LI-COR, AZ, USA).
Immunofluorescence (IF) Quantification Analysis
IF was performed using paraffin-embedded tissue sections. Gallbladder sections were incubated with primary antibodies against tumor necrosis factor α (TNF-α, 1:5000, Cat.ab183218, Abcam, UK), interleukin-1 beta (IL-1β, 1:50, Cat.ab254360, Abcam, UK), interleukin-6 (IL-6, 1:200, Cat.YT5348, Immunoway, USA), Citrullinated Histone H3 (1:2000, Cat.ab281584, Abcam, UK), and MPO (1:1000, Cat.66177-1-IG, Proteintech, USA). Subsequently, the sections were incubated with the corresponding fluorescent labelled secondary antibodies (1:100, Immunoway, USA) for further staining.
Statistical Analysis
The sample size for the experiment was determined based on previous experience studies rather than statistical methods. All error bars represent the standard error of the mean (SEM). The samples distribution type was determined using the Shapiro–Wilk test. For two groups of data with normal distribution and equal variances, a two-sided Student’s t-test was used to evaluate the significance of the statistical differences. Welch’s correction t-test was used to analyze data with unequal variance. All statistical analyses were performed using SPSS (version 25.0, IBM Corporation, NY, USA), and graphs were generated using GraphPad Prism 8.0 (GraphPad Software, CA, USA). P ≤ 0.05 was considered statistically significant. Outliers were excluded using the method of mean ± 3 standard deviations.
Results
In Cholelithiasis Patients, the Secretion of Gallbladder Mucin Is Reduced, and the Gallbladder’s Mechanical Barrier Is Impaired
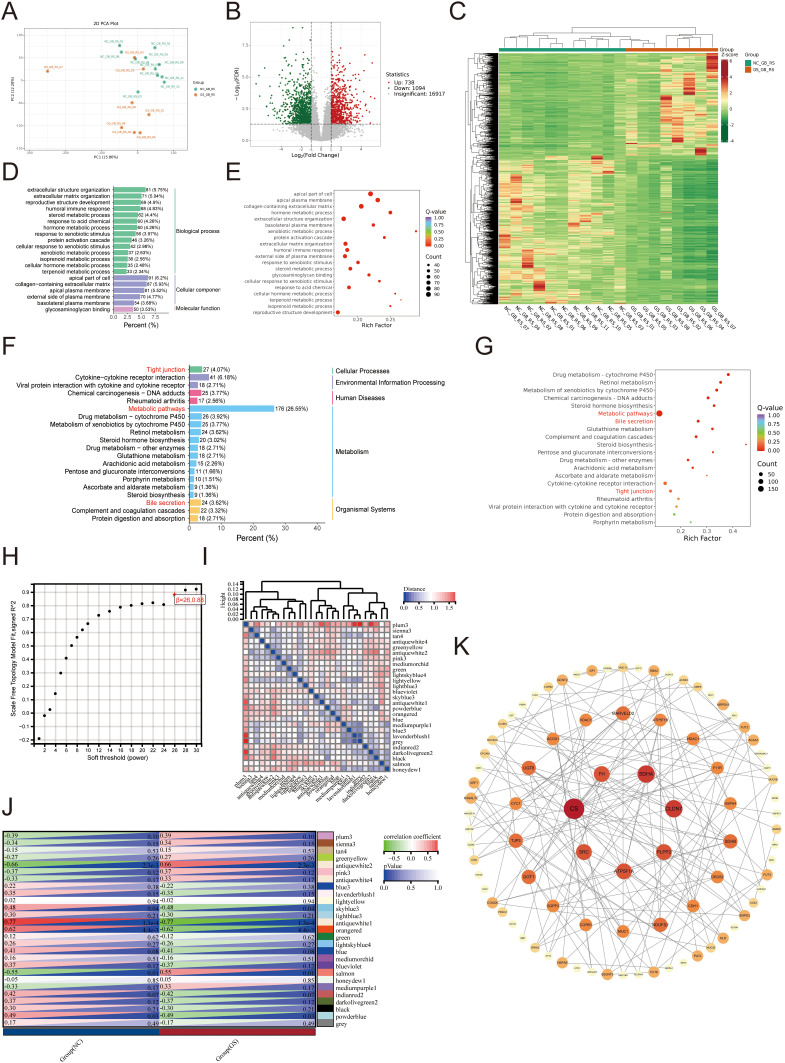
We performed transcriptome sequencing on collected clinical samples of gallbladder tissue. Principal component analysis (PCA) revealed significant differences between the GS and NC group (Figure 1A), with 738 upregulated and 1094 downregulated genes (Figure 1B and C). GO enrichment analysis revealed significant enrichment in the apical part of the cell, apical plasma membrane, and collagen-containing extracellular matrix (Figure 1D and E). Furthermore, KEGG enrichment analysis indicated enrichment in metabolic pathways, bile secretion, and tight junction pathways (Figure 1F and G). Among all the differentially expressed genes, metabolic pathways accounted for the highest proportion. Notably, the enrichment ratio of tight junction-related genes was 4.07%, which ranked third. Similar results were obtained from the WGCNA analysis (Figure 1H and I). The antiquewhite1 module was significantly reduced in the GS group (Figure 1J). Network analysis on the hub genes revealed that mucin and mechanical connection-related genes were significantly downregulated in the GS group (Figure 1K).
Figure 1.
Transcriptome analysis of patients with cholelithiasis. (A-C) DEGs between the GS and the NC group. (A: PCA; B: Volcano plot; C: Heatmap) (D-G) Classification and enrichment results of differentially expressed metabolites between the GS and the NC group. (D and E: GO; F and G: KEGG) (H) The nature of the network topology constructed using unique Power values. (I and J) Correlation between different modules and the proportion of normal and cholecystolithiasis samples. (K) Venn plot of DEGs.
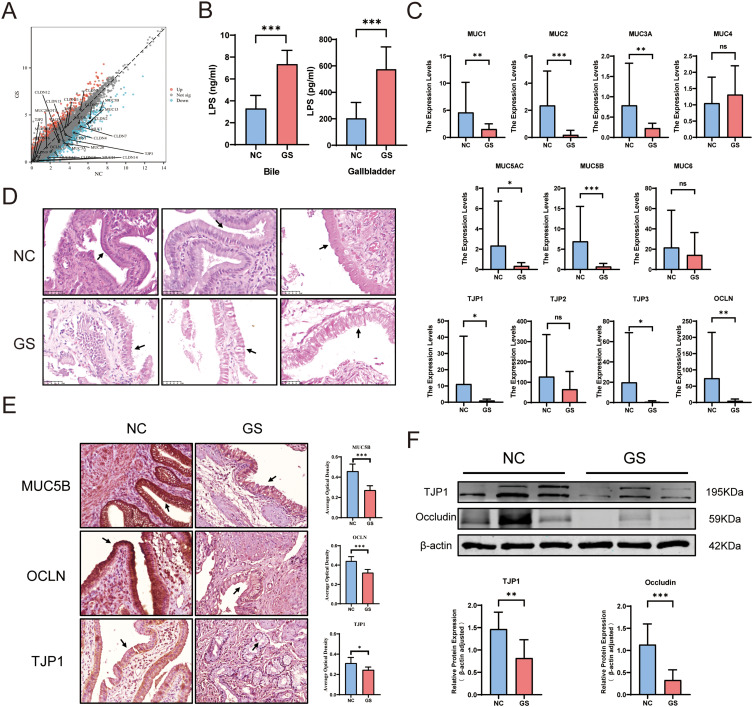
No statistically significant differences were observed in detectable gallbladder mucosal barrier-related indicators, except for MUC6 and MUC21. The expression of other mucin-related genes including MUC1, MUC3A, MUC4, MUC5AC, MUC5B, MUC13, and MUC20 decreased (P <0.05), indicating a reduction in mucin secretion in the gallbladder of the GS group. Except for TJP1, TJP2, CLDN5, CLDN9, CLDN11, CLDN15, CLDN16, and CLDN23, which showed no statistically significant difference, the expression of OCLN, CLDN1, CLDN2, CLDN3, CLDN4, CLDN7, CLDN10, CLDN12, CLDN14, CLDN18, and TJP3 related to the intercellular tight junctions detected was decreased (Figure 2A; P <0.05). This suggests the mechanical barrier of the gallbladder mucosa in the GS group was damaged. Subsequently, ELISA was used to detect LPS levels in the gallbladder and bile. The results showed that significantly higher LPS levels in the gallbladder tissue and bile of the GS group (Figure 2B, p <0.001). qPCR was performed to verify the accuracy of the transcriptome sequencing results. The results showed that the expression of mucin-related genes MUC1, MUC2, MUC3A, MUC5AC, and MUC5B in the GS group were significantly decreased (P < 0.05) and the expression of tight junction-related genes OCLN, TJP1, and TJP3 was also significantly decreased (P < 0.05). These results were consistent with the transcriptome sequencing data (Figure 2C). The H&E staining results showed that the gallbladder mucosal layer in the control group was smooth, even with closely arranged gallbladder epithelial cells. However, in the GS group, the integrity of the gallbladder mucosa was poor, and the gallbladder epithelial cells were loosely arranged. This suggested the presence of mucosal barrier damage in the gallbladders of the GS group (Figure 2D). Furthermore, IHC and WB analysis of mucin and tight junction-related proteins were performed. The results showed that the expression levels of MUC5B, OCLN, and TJP1 in the GS group was significantly lower than that in the control group (Figure 2E and F; P<0.05).
Figure 2.
Impairment of gallbladder mucosal barrier in patients with cholelithiasis. (A) Volcano plot of gene expression related to the mucosal barrier from RNA sequencing results. (B) LPS levels in gallbladder tissues and bile detected by ELISA. (C) qPCR analysis of the expression levels of mucosal barrier-related genes (GS vs NC, outliers were excluded based on the mean ± 3*standard deviation criterion). (D) H&E staining of the gallbladder mucosal barrier (GS vs NC). (E) IHC detection of MUC5B, OCLN, and TJP1 expression (GS vs NC), the gallbladder mucosal epithelial cells have been indicated by arrows. (F) Protein expression levels of TJP1 and OCLN (GS vs NC). (mean ± SD, *p <0.05, **p <0.01, ***p <0.001).
LPS Induces Mechanical Barrier Injury and Activates Immune Response in Mouse Gallbladder
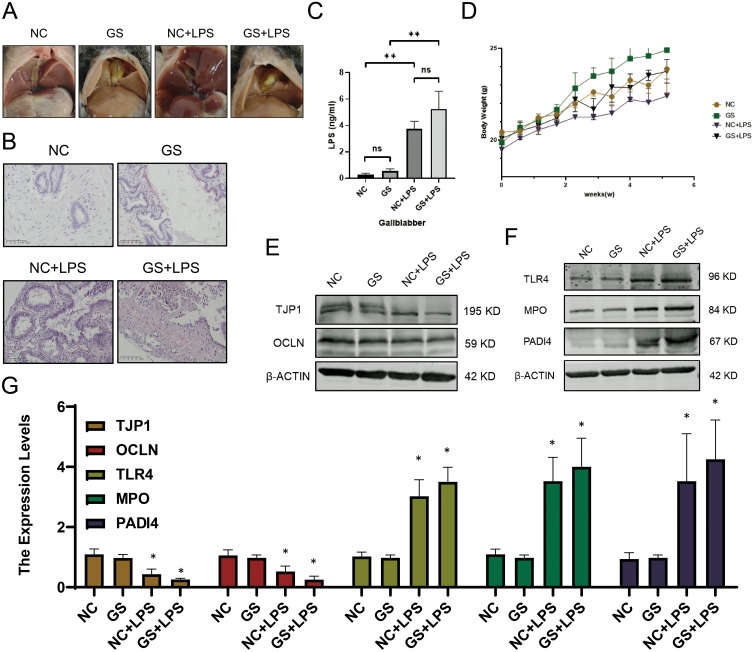
Gallstones in the common gallstone model mice were sand-like, whereas gallstones in gallstone-induced mice after LPS injection were clumpy. No significant differences were observed among the control groups (Figure 3A). Additionally, changes in body weight and gallbladder LPS content of mice in each group was monitored to ensure the accuracy of the model (Figure 3C and D). Subsequently, HE staining was performed on the mouse gallbladders, revealing poor integrity of the gallbladder mucosa with obvious necrosis and shedding after LPS injection, similar to the phenomenon observed in gallbladders of patients with gallstones (Figure 3B).
Figure 3.
LPS as a potential key factor in gallbladder mechanical barrier impairment and NETs formation promotion. (A) Gallstone formation in mice. (B) H&E staining of the gallbladder in mice. (C) LPS concentration in bile from mice measured by ELISA. (D) Changes in body weight of mice over time. (E) Expression profiles of genes related to the gallbladder mucosal barrier. (F) Expression profiles of genes associated with NETs formation. (G) Transcriptional levels of relevant genes in model mice. (mean ± SD, *p <0.05, **p<0.01).
Previous studies have reported that NETs are important inducing factors for gallstones, and LPS is one of the main causes of NETs formation. Therefore, while detecting the genes related to the mechanical barrier of gallbladder mucosal, the key genes involved in NETs formation was also examined. The results of qPCR and WB showed that after LPS treatment, the expression of TJP1 and OCLN decreased significantly, whereas that of TLR4, MPO, and PADI4 increased significantly (Figure 3E-G). Based on these findings, it was hypothesized that increased LPS in the gallbladder can disrupt the gallbladder mucosal mechanical barrier and induce the NETs formation by gallbladder neutrophils, thereby promoting gallstone formation.
LPS Induces Damage to the Gallbladder Mechanical Barrier by Activating the NF-κB Pathway
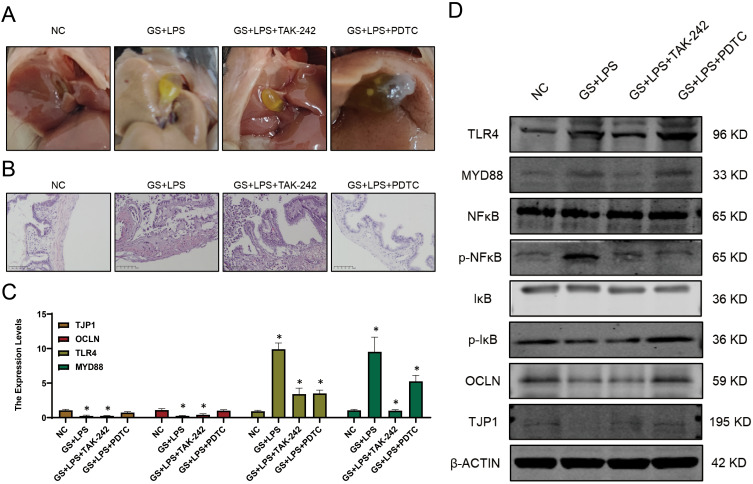
Specific mechanism underlying LPS-induced gallbladder mucosal barrier damage (Figure 4A) was further investigated. Drawing parallels from previous studies on LPS-induced intestinal mucosal barrier damage, it was hypothesized that a similar damage mechanism exists in the gallbladder, specifically, that LPS damages the gallbladder mucosal barrier through the NF-κB pathway. H&E staining on the mouse gallbladders indicated that after treatment with TLR4 inhibitor and NF-κB inhibitor, necrotic cells in the gallbladder mucosa was decreased compared to that of the LPS-treated group, and the gallbladder mucosa was intact (Figure 4B). qPCR and WB results showed that the TLR4-MyD88-NF-κB signaling axis was significantly activated after LPS treatment, and the expression of OCLN and TJP1 was significantly downregulated. However, inhibition of the TLR4 and NF-κB pathways significantly alleviated the degree of gallbladder mucosal mechanical barrier damage (Figure 4C and D) separately.
Figure 4.
LPS induces gallbladder mucosal mechanical barrier impairment via the TLR4/MyD88/NF-κB signaling pathway. (A) Gallstone formation in mice from different groups. (B) H&E staining of the gallbladder in mice from different groups. (C) Transcriptional levels of TLR4, OCLN, and MyD88 in each group. (D) Expression profiles of genes related to the TLR4/MyD88/NF-κB signaling pathway in different groups (mean ± SD, *p <0.05).
The Gallbladder of Cholelithiasis Patients Is in an Immune-activated State
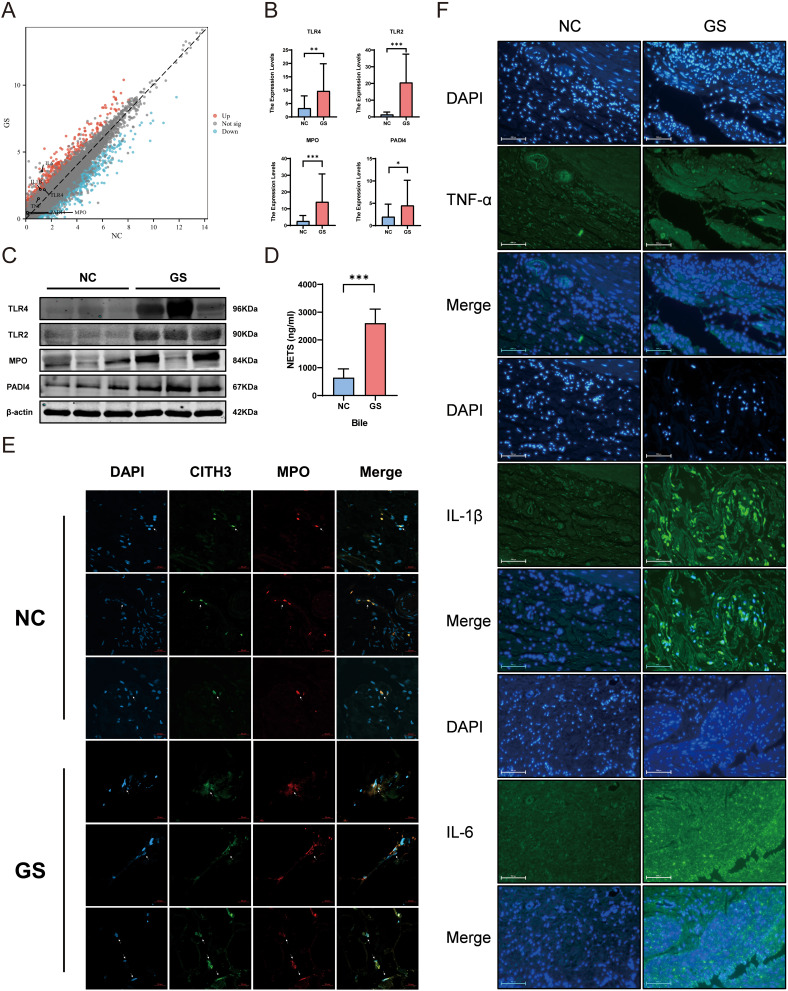
The results of transcriptome sequencing suggested an upward trend in the expression of inflammatory factors such as TNF-α, IL-1β, and IL-6. Meanwhile, immunofluorescence analysis revealed increased expression of inflammatory factors IL-1β and IL-6 in the GS group, while there was no significant difference in TNF-α expression, consistent with the sequencing results (Figure 5F). The expression of key genes involved in NETs formation, including TLR4, MPO, and PADI4, also showed an increasing trend (Figure 5A). Further verification by qPCR and WB indicated a significant upregulation of NETs-related genes in the GS group (Figure 5B and C). ELISA of bile samples revealed an increase in NETs content in the GS group (Figure 5D). IF analysis of gallbladder tissues showed the presence of a large number of NETs in the gallbladders of patients with cholelithiasis (Figure 5E), indicating immune activation in the gallbladder of patients with cholelithiasis.
Figure 5.
Increased formation of NETs in the gallbladder and bile of patients with cholelithiasis, indicating chronic inflammation in the gallbladder. (A) Volcano plot of gene expression related to NETs formation in gallbladder tissues of patients with cholelithiasis based on RNA sequencing. (B) Expression levels of key genes involved in NETs formation in GS and NC group determined by qPCR. (C) Protein expression levels of key genes involved in NETs formation. (GS vs NC). (D) Quantification of NETs content in bile samples from GS and NC groups using ELISA. (E) Formation of NETs in gallbladder tissues of GS and NC groups. (F) Immunofluorescence results for inflammatory cytokines TNF-α, IL-1β, and IL-6 in gallbladder tissues of GS and NC groups. (mean ± SD, *p <0.05, **p <0.01, ***p <0.001).
LPS Induces the Sustained Production of NETs in the Gallbladder
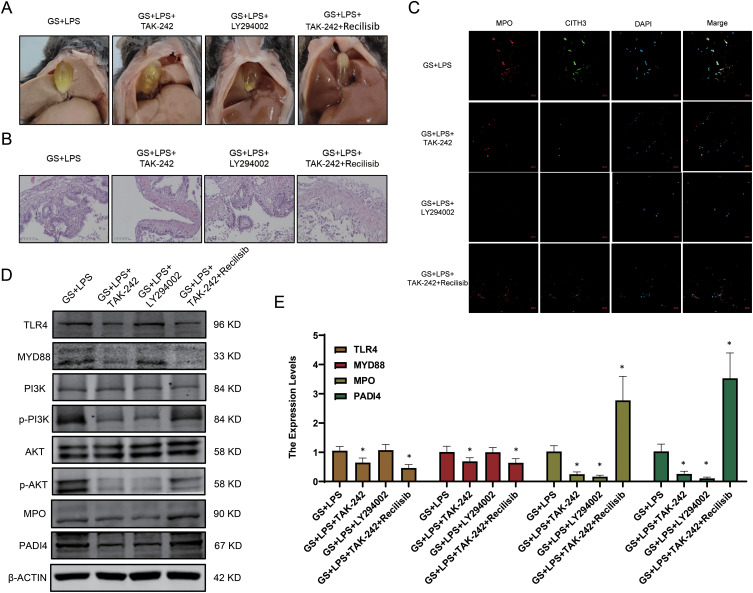
By searching the KEGG database, it is found that LPS often induces NETs formation through the PI3K/Akt signaling pathway. To verify whether the PI3K/Akt signaling pathway is the key pathway for LPS-induced NETs formation, TAK-242, LY294002, and recilisib was administered via intraperitoneal injection into a mouse model of gallstones treated with LPS. The results showed that LPS-treated gallstone mice that received the LY294002 exhibited significantly reduced gallstone formation, whereas those injected with TAK-242 and recilisib showed increased gallstone content (Figure 6A). Subsequently, H&E staining was performed on the gallbladders of the mice and it was determined that the gallbladder mucosa of mice injected with LY294002 remained relatively intact, whereas those injected with TAK-242 and Recilisib exhibited aggravated damage. This suggests that PI3K activation can further exacerbate damage to the gallbladder mucosal barrier (Figure 6B). The IF results suggested that inhibiting the activation of PI3K could reduce the NETs formation, which is consistent with our hypothesis (Figure 6C). Furthermore, the PCR and WB results indicated that LPS-induced NETs formation occurred via the TLR4/PI3K/Akt signaling pathway (Figure 6D and E).
Figure 6.
LPS promotes NETs formation in the gallbladder via the TLR4/PI3K/Akt signaling pathway. (A) Formation of gallstones in mice. (B) H&E staining of gallbladder tissues from mice. (C) NETs formation in different groups. (D) Protein expression levels of genes related to the PI3K/Akt signaling pathway. (E) Transcription levels of NETs-related genes (mean ± SD, *p <0.05).
Discussion
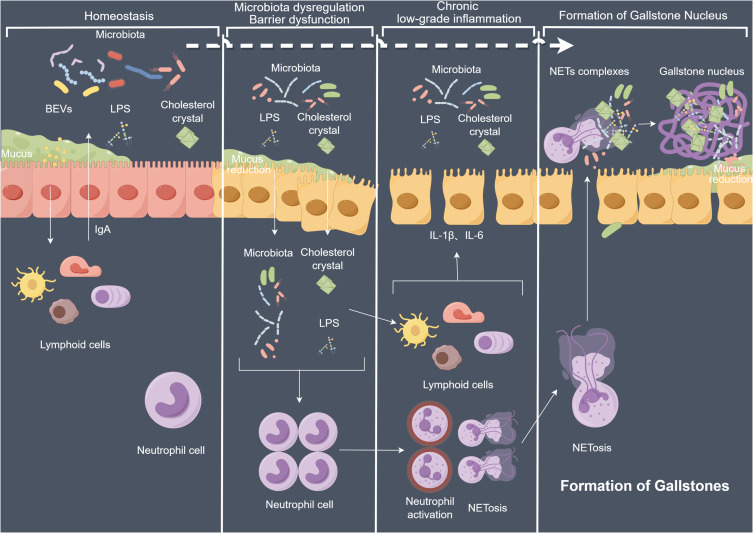
Cholelithiasis is a major global public health concern. Currently, the accepted understanding of the pathogenesis of cholelithiasis mainly involves the interaction of five factors including genetic factors, gallbladder cholesterol supersaturation, rapid phase transition caused by the growth of gallbladder cholesterol crystals, impaired gallbladder motility, and intestinal factors (excessive delivery of cholesterol from the small intestine to the liver, changes in the intestinal microbiota, and damage to the intestinal mucosal barrier).13 In recent years, numerous studies have been conducted on intestinal mucosal barriers. However, there are relatively few reports on gallbladder mucosal barriers. This study analyzed the intestinal mucosal barrier and explored the roles of the gallbladder microbial barrier (bile microbiota), chemical barrier (gallbladder mucins), mechanical barrier (tight junction-related proteins), and immune barrier (chronic low-grade inflammation and NETs) in the gallstones formation.14
Complex microbiota exists in the healthy biliary tract. Biological culture results of gallstones and bile suggest that multiple microorganisms are present in gallstones.15–17 Bacterial cell wall decomposition products such as LPS and lipoteichoic acid have been detected in human bile.18 Previous studies have suggested that the gallbladder microbiota may be a core factor in initiating gallstone formation.19,20 In this study, it was determined that bile samples from patients with gallstone disease contained higher concentrations of LPS, which explains why bile leakage during gallbladder surgery often leads to severe infectious complications.21
The chemical barrier of the intestinal mucosa is a protective layer located on the surface of the intestinal mucosa and is primarily composed of viscous substances such as mucin, water, and electrolytes.22 The gallbladder also possesses a comparable chemical barrier in which gallbladder mucin is primarily present on the microvilli of gallbladder mucosal epithelial cells, forming a mucus layer that covers the surface of the gallbladder mucosa. This mucus layer protects the gallbladder mucosa from erosion by harmful substances and cholesterol in bile, thereby reducing damage to the gallbladder mucosa.23 Mucins are classified into two categories based on their structure and function: membrane-bound mucins such as MUC1, MUC3, MUC4, MUC12, MUC17, and mucin-like protocadherin (MUPCDH), which are primarily located on the cell surface; and gel-forming mucins such as MUC2, MUC5AC, MUC5B, and MUC6, which are secreted by epithelial cells to provide a protective layer for the underlying mucosa.24 Known mucins in gallbladder tissue include MUC1, MUC2, MUC3A, MUC3B, MUC4, MUC5AC, MUC5, MUC6, and MUPCDH.25 Among them, MUC5B was the major expressed mucin.26 Previous in vivo studies have demonstrated increased mucin secretion in gallstone disease.27–29 Interestingly, decreased expression of MUC1, MUC2, MUC3A, MUC5AC, and MUC5B was observed. The mechanical barrier of the intestinal mucosa primarily comprises the epithelial cell layers and adhesive junction proteins. The intestinal mucosal epithelial cells are connected by tight junction proteins that reduce the penetration of harmful substances. The intestinal adhesive junction proteins mainly include cadherins and integrins, which strengthen the mechanical barrier stability. Analogously, the expressions of the key genes OCLN and TJPs of the mechanical barrier was decreased in this study, suggesting impairment of the mechanical barrier of the gallbladder.
Regarding the activation of the gallbladder immune barrier, it is speculated that the fundamental reason lies in the impairment of the gallbladder mucosal mechanical barrier, and that long-term and chronic low-grade inflammatory stimulus is the primary cause of gallstone formation. Through retrospective studies on multiple patients, it was determined that in acute cholecystitis, the neutrophil index can be used to predict the severity of the disease and the risk of mortality early in patients.30–32 Immunologically mediated neutrophils, which form NETs after death, have been proven to be the core of the process of gallstone formation.33 This study utilized transcriptome sequencing data to construct an immune microenvironment map and found increased expression of indicators of chronic low-grade inflammation in the gallbladder of the GS group, accompanied by increased neutrophil infiltration.
Most neutrophils remain quiescent and stable. However, upon exposure to infectious or inflammatory mediators, neutrophils are eliminated through NETs formation. We observed an increased expressions of NETs and their key pathway genes (TLR2, TLR4, MPO, and PADI4) in both the gallbladder and bile in the GS group, suggesting that in gallstone disease, there is an increase in neutrophils undergoing NETs formation and their migration into the bile through the damaged gallbladder mucosal barrier, initiating gallstone formation.
Based on the above experiments and analyses, we hypothesize that an increase in LPS with the biliary dysbiosis leads to damage of the gallbladder mucosal barrier. This allows the LPS and cholesterol crystals to invade the submucosal layer of the gallbladder, resulting in a chronic low-grade inflammatory state that persists for an extended period. The inflammation recruits neutrophils, which are stimulated by bacteria, LPS, and cholesterol crystals to form NETs that are expelled into the bile, thereby promoting the formation of gallstones.
Conclusion
Compared to intestinal flora alterations, the relation between biliary dysbiosis and gallstone formation has not garnered sufficient attention from clinical researchers. Here, it is determined that excessive LPS in bile with the biliary dysbiosis promoted the NETs-induced gallstones formation by activating the gallbladder immune barrier. Inhibiting biliary dysbiosis through fecal microbiota transplantation (FMT) and dietary therapy may contribute to the prevention of gallstones and the treatment of chronic cholecystitis.
Acknowledgments
We thank all the relevant institutions and individuals who provided experimental reagents and RNA sequencing analyses. These data will be made available upon reasonable request. Jingjing Yu and Ziang Meng are the first co-authors of this study.
Funding Statement
This study was supported by the National Natural Science Foundation of China (No. 82100675) and the Excellent Youth Foundation of the First Affiliated Hospital of Harbin Medical University (NO. 2021Y12).
Abbreviations
NETs, Neutrophil extracellular traps; LPS, lipopolysaccharide; TLR, Toll-like receptor; NF-κB, nuclear factor kappa-B; PI3K, phosphoinositide 3-kinase; DEGs, differential expression genes; KEGG, the Kyoto encyclopedia of genes and genomes; GO, gene ontology; WGCNA, Weighted Gene Co-expression Analysis; ELISA, enzyme-linked immunosorbent assay; IHC, immunohistochemical; H&E, hematoxylin and eosin; MUC, Mucin; OCLN, occludin; TJP, tight junction protein; qPCR, quantitative polymerase chain reaction; WB, Western blot; PADI4, protein arginine deiminase type 4; MPO, myeloperoxidase; MyD88, myeloid differentiation factor 88; Akt, protein kinase B; IκB, inhibitor of NF-κB; IF, immunofluorescence; TNF-α, tumor necrosis factor α; IL-1β, interleukin-1 beta; IL-6, interleukin-6; PCA, principal component analysis; MUPCDH, mucin-like protocadherin.
Ethics Statement
Written informed consent, including permission to collect the gallbladder samples, was obtained from all patients before enrollment in this study. The study protocol was approved by the Ethics Committee of The First Affiliated Hospital of Harbin Medical University (No.202322), in strict accordance with the Declaration of Helsinki. This study was conducted in strict accordance with the Guide for the Care and Use of Laboratory Animals formulated by the Ministry of Science and Technology of China in 2006, and all procedures involving animal experiments were conducted according to the regulations of the Institutional Animal Care and Use Committee of The First Affiliated Hospital of Harbin Medical University (No. 2022083).
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Shaffer EA. Gallstone disease: epidemiology of gallbladder stone disease. Best Pract Res Clin Gastroenterol. 2006;20(6):981–996. doi: 10.1016/j.bpg.2006.05.004 [DOI] [PubMed] [Google Scholar]
- 2.Stinton LM, Shaffer EA. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver. 2012;6(2):172–187. doi: 10.5009/gnl.2012.6.2.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States Part III: liver, biliary tract, and pancreas. Gastroenterology. 2009;136(4):1134–1144. doi: 10.1053/j.gastro.2009.02.038 [DOI] [PubMed] [Google Scholar]
- 4.Di Ciaula A, Wang DQ, Portincasa P. An update on the pathogenesis of cholesterol gallstone disease. Curr Opin Gastroenterol. 2018;34(2):71–80. doi: 10.1097/MOG.0000000000000423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyer JL. Bile formation and secretion. Compr Physiol. 2013;3(3):1035–1078. doi: 10.1002/cphy.c120027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Ciaula A, Baj J, Garruti G, et al. Liver steatosis, gut-liver axis, microbiome and environmental factors. a never-ending bidirectional cross-talk. J Clin Med. 2020;9(8):2648. doi: 10.3390/jcm9082648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molinero N, Ruiz L, Milani C, et al. The human gallbladder microbiome is related to the physiological state and the biliary metabolic profile. Microbiome. 2019;7(1):100. doi: 10.1186/s40168-019-0712-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abayli B, Colakoglu S, Serin M, et al. Helicobacter pylori in the etiology of cholesterol gallstones. J Clin Gastroenterol. 2005;39(2):134–137. PMID:15681909. [PubMed] [Google Scholar]
- 9.Zhang FM, Yu CH, Chen HT, et al. Helicobacter pylori infection is associated with gallstones: epidemiological survey in China. World J Gastroenterol. 2015;21(29):8912–8919. doi: 10.3748/wjg.v21.i29.8912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niyonzima N, Halvorsen B, Sporsheim B, et al. Complement activation by cholesterol crystals triggers a subsequent cytokine response. Mol Immunol. 2017;84:43–50. doi: 10.1016/j.molimm.2016.09.019 [DOI] [PubMed] [Google Scholar]
- 11.García-Prieto J, Villena-Gutiérrez R, Gómez M, et al. Neutrophil stunning by metoprolol reduces infarct size. Nat Commun. 2017;8:14780. doi: 10.1038/ncomms14780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang L, Yang B, Xiao Y, Zhang B, Yu B, Kuang Y. Neutrophil depletion reduces interstitial cajal-like cell injury and alleviates inflammation-induced motor dysfunction in Guinea-pig gallbladder during acute cholecystitis. Iran J Basic Med Sci. 2022;25(4):435–441. doi: 10.22038/IJBMS.2022.59415.13195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang HH, Portincasa P, Afdhal NH, Wang DQ. Lith genes and genetic analysis of cholesterol gallstone formation. Gastroenterol Clin North Am. 2010;39(2):185–207,vii–viii. doi: 10.1016/j.gtc.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 14.De Santis S, Cavalcanti E, Mastronardi M, Jirillo E, Chieppa M. Nutritional keys for intestinal barrier modulation. Front Immunol. 2015;6:612. doi: 10.3389/fimmu.2015.00612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart L, Griffiss JM, Jarvis GA, Way LW. Gallstones containing bacteria are biofilms: bacterial slime production and ability to form pigment solids determines infection severity and bacteremia. J Gastrointest Surg. 2007;11(8):977–983. doi: 10.1007/s11605-007-0168-1 discussion 983-984. [DOI] [PubMed] [Google Scholar]
- 16.Tajeddin E, Sherafat SJ, Majidi MR, Alebouyeh M, Alizadeh AH, Zali MR. Association of diverse bacterial communities in human bile samples with biliary tract disorders: a survey using culture and polymerase chain reaction-denaturing gradient gel electrophoresis methods. Eur J Clin Microbiol Infect Dis. 2016;35(8):1331–1339. doi: 10.1007/s10096-016-2669-x [DOI] [PubMed] [Google Scholar]
- 17.Monstein HJ, Jonsson Y, Zdolsek J, Svanvik J. Identification of Helicobacter pylori DNA in human cholesterol gallstones. Scand J Gastroenterol. 2002;37(1):112–119. doi: 10.1080/003655202753387455 [DOI] [PubMed] [Google Scholar]
- 18.Kawata K, Kobayashi Y, Gershwin ME, Bowlus CL. The immunophysiology and apoptosis of biliary epithelial cells: primary biliary cirrhosis and primary sclerosing cholangitis. Clin Rev Allergy Immunol. 2012;43(3):230–241. doi: 10.1007/s12016-012-8324-0 [DOI] [PubMed] [Google Scholar]
- 19.Kaufman HS, Magnuson TH, Lillemoe KD, Frasca P, Pitt HA. The role of bacteria in gallbladder and common duct stone formation. Ann Surg. 1989;209(5):584–591. doi: 10.1097/00000658-198905000-00011 discussion 591-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maurer KJ, Ihrig MM, Rogers AB, et al. Identification of cholelithogenic enterohepatic helicobacter species and their role in murine cholesterol gallstone formation. Gastroenterology. 2005;128(4):1023–1033. doi: 10.1053/j.gastro.2005.01.008 [DOI] [PubMed] [Google Scholar]
- 21.Lévay B, Szabó G, Szijártó A, Gamal EM. The frequency of bacteria in human gallstones. Magy Seb. 2013;66(6):353–356. doi: 10.1556/MaSeb.66.2013.6.8 [DOI] [PubMed] [Google Scholar]
- 22.Wang ZE, Peng J, Wu D, Zheng JJ, Peng X. Effects of intestinal trefoil factor on intestinal mucus barrier in burned mice. Am J Transl Res. 2020;12(11):7187–7198. PMID:33312359. [PMC free article] [PubMed] [Google Scholar]
- 23.Jüngst D, Niemeyer A, Müller I, et al. Mucin and phospholipids determine viscosity of gallbladder bile in patients with gallstones. World J Gastroenterol. 2001;7(2):203–207. doi: 10.3748/wjg.v7.i2.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sasaki M, Ikeda H, Nakanuma Y. Expression profiles of MUC mucins and trefoil factor family (TFF) peptides in the intrahepatic biliary system: physiological distribution and pathological significance. Prog Histochem Cytochem. 2007;42(2):61–110. doi: 10.1016/j.proghi.2007.02.001 [DOI] [PubMed] [Google Scholar]
- 25.Pigny P, Guyonnet-Duperat V, Hill AS, et al. Human mucin genes assigned to 11p15.5: identification and organization of a cluster of genes. Genomics. 1996;38(3):340–352. doi: 10.1006/geno.1996.0637 [DOI] [PubMed] [Google Scholar]
- 26.Chuang SC, Hsi E, Lee KT. Mucin genes in gallstone disease. Clin Chim Acta. 2012;413(19–20):1466–1471. doi: 10.1016/j.cca.2012.06.015 [DOI] [PubMed] [Google Scholar]
- 27.Womack NA. The development of gallstones. Surg Gynecol Obstet. 1971;133(6):937–945. [PubMed] [Google Scholar]
- 28.E E Jr, Harman CG, Freston JW, Straight RC, Wales EE Jr. Studies on the pathogenesis of diet-induced dog gallstones. Am J Dig Dis. 1977;22(4):305–314. doi: 10.1007/BF01072187 [DOI] [PubMed] [Google Scholar]
- 29.Freston JW, Bouchier IA, Newman J. Biliary mucous substances in dihydrocholesterol-induced cholelithiasis. Gastroenterology. 1969;57(6):670–678. doi: 10.1016/S0016-5085(19)33822-3 [DOI] [PubMed] [Google Scholar]
- 30.Ünsal A, Öztürk D, Buluş H, Turhan VB. Predictive value of immature granulocyte and delta neutrophil index in the diagnosis of complicated acute cholecystitis. Eur Rev Med Pharmacol Sci. 2022;26(18):6505–6511. doi: 10.26355/eurrev_202209_29749 [DOI] [PubMed] [Google Scholar]
- 31.Lee SJ, Park EJ, Lee KJ, Cha YS. The delta neutrophil index is an early predictive marker of severe acute cholecystitis. Dig Liver Dis. 2019;51(11):1593–1598. doi: 10.1016/j.dld.2019.03.026 [DOI] [PubMed] [Google Scholar]
- 32.Romano L, Giuliani A, Pessia B, et al. The early prediction of mortality in acute cholecystitis: temperature, Neutrophils and Multiple organ failure (TNM) score. Eur Rev Med Pharmacol Sci. 2021;25(20):6339–6348. doi: 10.26355/eurrev_202110_27006 [DOI] [PubMed] [Google Scholar]
- 33.Muñoz LE, Boeltz S, Bilyy R, et al. Neutrophil extracellular traps initiate gallstone formation. Immunity. 2019;51(3):443–450.e4. doi: 10.1016/j.immuni.2019.07.002 [DOI] [PubMed] [Google Scholar]