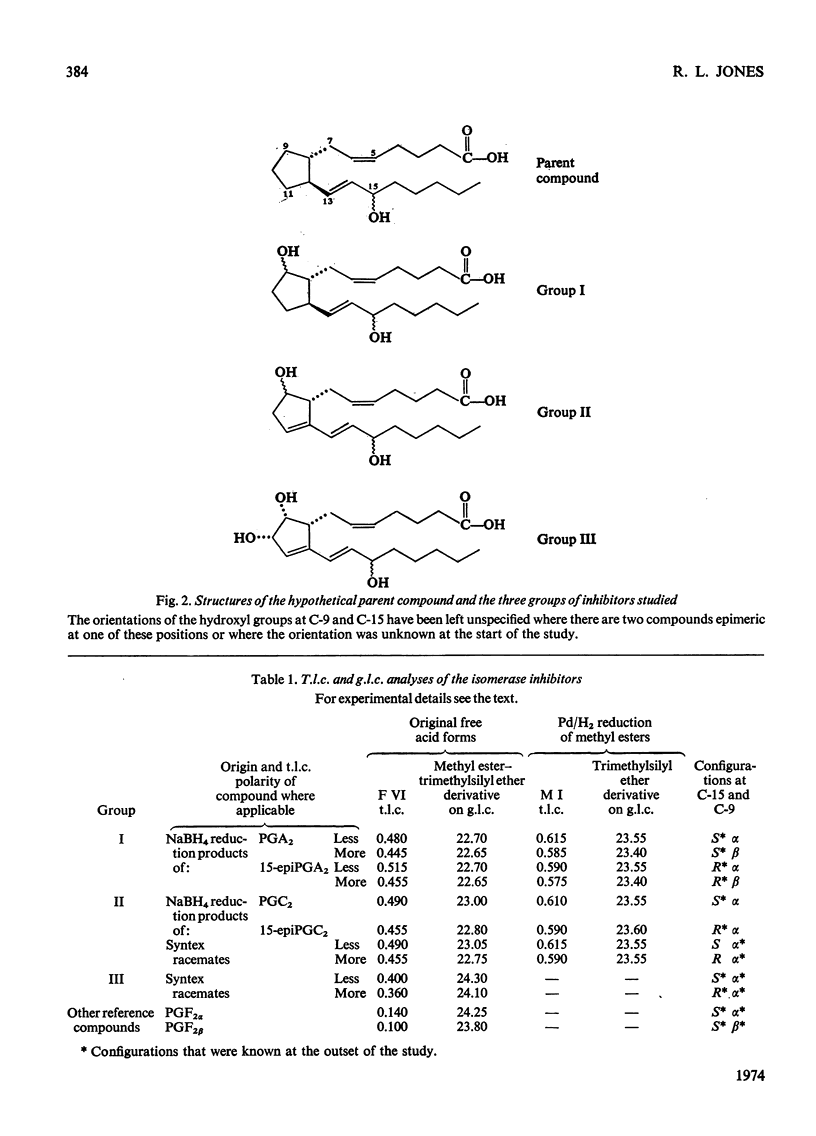
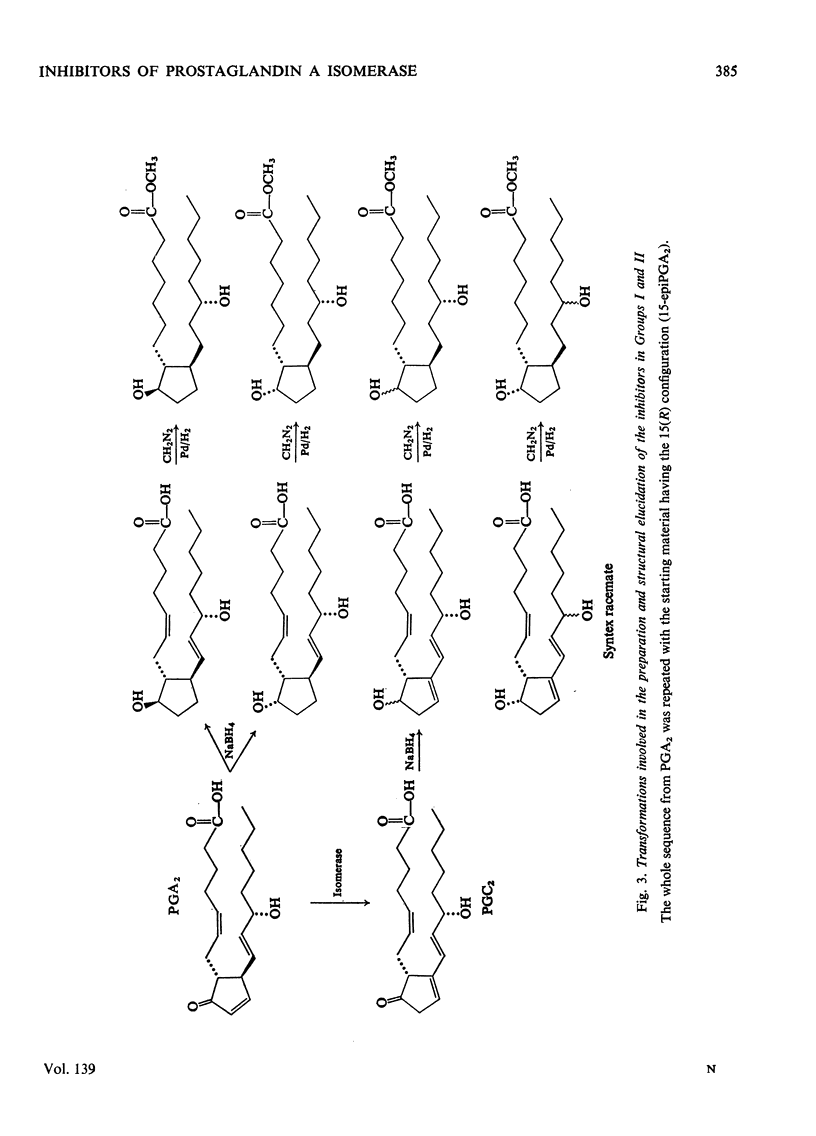
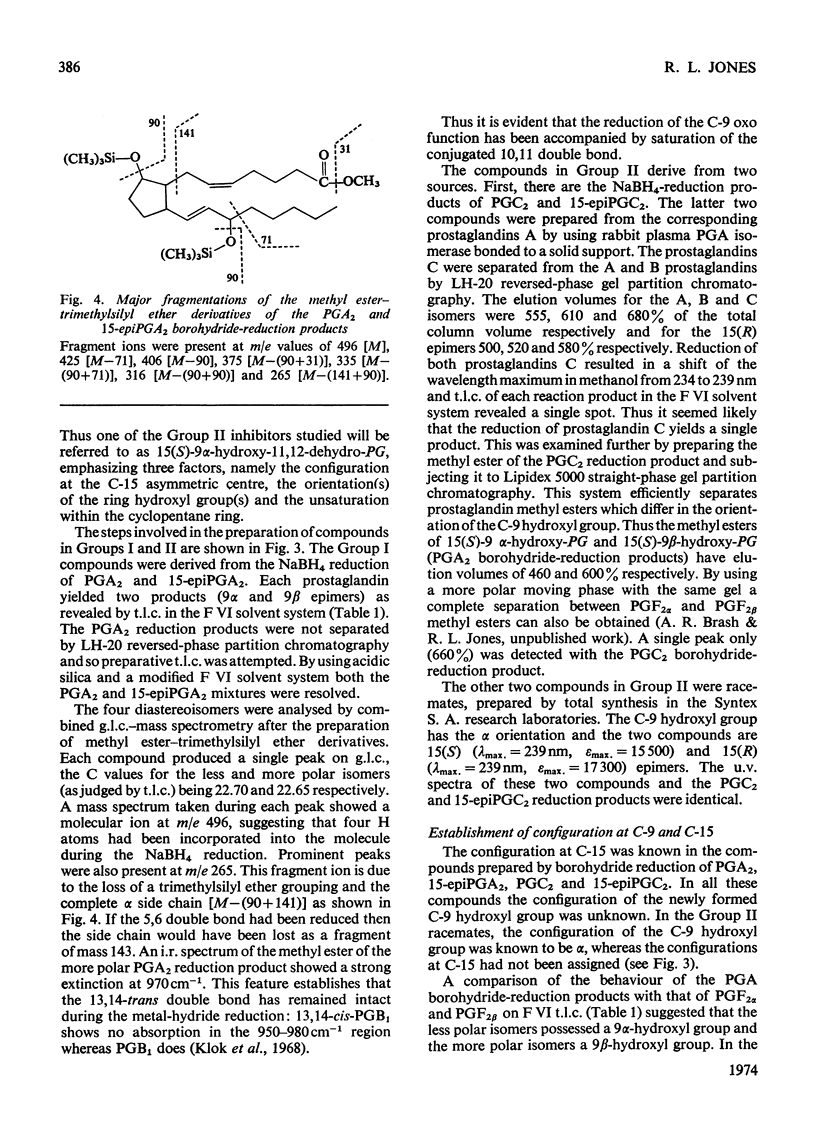
Abstract
1. The potent inhibitory activities of three groups of prostaglandin analogues on the prostaglandin A isomerase of rabbit plasma were demonstrated. 2. Six of the compounds were prepared by NaBH4 reduction of the C-9 oxo groups of prostaglandin A2 and prostaglandin C2 and their C-15 epimers. The remaining four were racemates and were synthesized in another laboratory. Unknown configurations at C-9 and at C-15 were assigned. 3. All the compounds were found to be competitive inhibitors of the isomerase in vitro. Km/Ki ratios were determined and it was found that both the 15(S) and 15(R) epimers have potent inhibitory activity. 4. One of the inhibitors was used to study the reversibility of the isomerase. 5. It is suggested that these compounds may be useful for determining the biological significance of prostaglandin A isomerase. In view of its weak biological activity and possibly extended half-life in vivo, the reduction product of 15-epiprostaglandin C2 may be the most suitable agent for this purpose.
Full text
PDF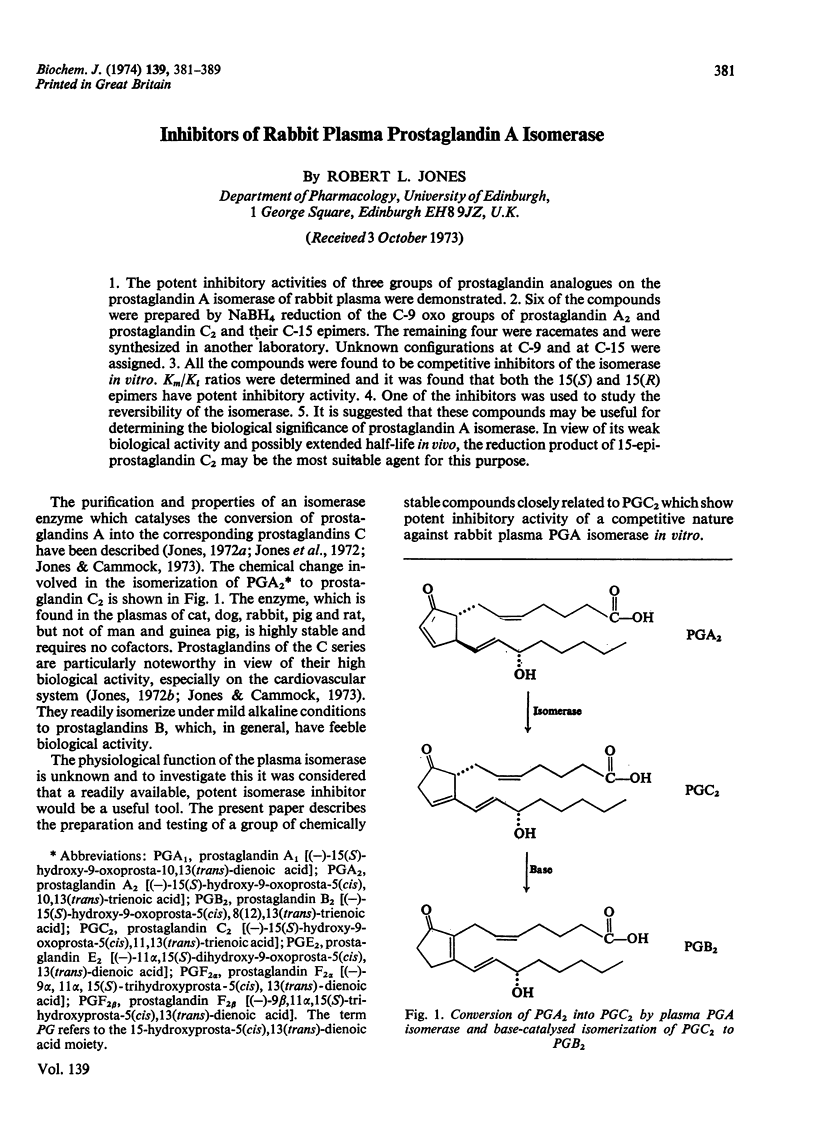




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders N. H. Preparative thin-layer and column chromatography of prostaglandins. J Lipid Res. 1969 May;10(3):316–319. [PubMed] [Google Scholar]
- Daniels E. G., Krueger W. C., Kupiecki F. P., Pike J. E., Schneider W. P. Isolation and characterization of a new prostaglandin isomer. J Am Chem Soc. 1968 Oct 9;90(21):5894–5895. doi: 10.1021/ja01023a041. [DOI] [PubMed] [Google Scholar]
- GREEN K., SAMUELSSON B. PROSTAGLANDINS AND RELATED FACTORS: XIX. THIN-LAYER CHROMATOGRAPHY OF PROSTAGLANDINS. J Lipid Res. 1964 Jan;5:117–120. [PubMed] [Google Scholar]
- Jones R. L. 15-hydroxy-9-oxoprosta-11, 13-dienoic acid as the product of a prostaglandin isomerase. J Lipid Res. 1972 Jul;13(4):511–518. [PubMed] [Google Scholar]
- Jones R. L., Cammock S., Horton E. W. Partial purification and properties of cat plasma prostaglandin A isomerase. Biochim Biophys Acta. 1972 Dec 8;280(4):588–601. doi: 10.1016/0005-2760(72)90139-7. [DOI] [PubMed] [Google Scholar]
- Lincoln F. H., Schneider W. P., Pike J. E. Prostanoic acid chemistry. II. Hydrogenation studies and preparation of 11-deoxyprostaglandins. J Org Chem. 1973 Mar 9;38(5):951–956. doi: 10.1021/jo00945a024. [DOI] [PubMed] [Google Scholar]
- Nakano J., Anggård E., Samuelsson B. 15-Hydroxy-prostanoate dehydrogenase. Prostaglandins as substrates and inhibitors. Eur J Biochem. 1969 Dec;11(2):386–389. doi: 10.1111/j.1432-1033.1969.tb00784.x. [DOI] [PubMed] [Google Scholar]
- Nakano J. Cardiovascular effect of a prostaglandin isolated from a gorgonian Plexaura homomalla. J Pharm Pharmacol. 1969 Nov;21(11):782–783. doi: 10.1111/j.2042-7158.1969.tb08172.x. [DOI] [PubMed] [Google Scholar]
- Nakano J., Kessinger J. M. Effects of 8-isoprostaglandin E1 on the systemic and pulmonary circulations in dogs. Proc Soc Exp Biol Med. 1970 Apr;133(4):1314–1317. doi: 10.3181/00379727-133-34679. [DOI] [PubMed] [Google Scholar]
- Shio H., Ramwell P. W., Andersen N. H., Corey E. J. Stereospecificity of the prostaglandin 15-dehydrogenase from swine lung. Experientia. 1970 Apr 15;26(4):355–357. doi: 10.1007/BF01896883. [DOI] [PubMed] [Google Scholar]
- THORN M. B. Inhibition by malonate of succinic dehydrogenase in heart-muscle preparations. Biochem J. 1953 Jul;54(4):540–547. doi: 10.1042/bj0540540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinheimer A. J., Spraggins R. L. The occurrence of two new prostaglandin derivatives (15-epi-PGA2 and its acetate, methyl ester) in the gorgonian Plexaura homomalla chemistry of coelenterates. XV. Tetrahedron Lett. 1969 Dec;(59):5185–5188. doi: 10.1016/s0040-4039(01)88918-8. [DOI] [PubMed] [Google Scholar]


