Abstract
Objective
To investigate the relationship between the expression levels of serum forkhead box protein M1(FOXM1) and insulin-like growth factor 2 (IGF2) mRNA in patients with acute respiratory distress syndrome (ARDS) condition and prognosis.
Methods
Ninety patients with ARDS admitted to our hospital were regarded as the ARDS group, according to the prognosis, they were grouped into death group (n = 64) and survival group (n = 126); the control group consisted of 190 healthy individuals.
Results
Compared with the control group, the level of serum FOXM1 mRNA in ARDS group was obviously lower, and the level of IGF2 mRNA was higher. The serum IGF2 mRNA, serum creatinine, inhaled oxygen concentration (FiO2), and mechanical ventilation time in the death group were higher than those in the control group, and the arterial oxygen partial pressure (PaO2), FOXM1 mRNA, and oxygenation index (PaO2/FiO2) were lower than those in control group. Logistic regression analysis indicated that FOXM1, IGF2, and PaO2/FiO2 were significant factors influencing the prognosis and mortality in ARDS patients. Correlation analysis showed that there was a negative correlation between serum FOXM1 and IGF2 mRNA levels in patients with ARDS.
Conclusion
Serum FOXM1 and IGF2 mRNA in patients with ARDS are correlated with the severity and prognosis of ARDS.
Keywords: acute respiratory distress syndrome, forkhead box protein M1, insulin-like growth factor 2, oxygenation index, prognosis
1. Introduction
In acute respiratory distress syndrome (ARDS), multiple pathogenic factors including trauma, pneumonia, sepsis incur lung injury, leading to acute respiratory failure and persistent hypoxemia [1]. With complicated etiology, ARDS has a high fatality rate despite the improvement of clinical respiratory support technology and the continuous update in diagnosis and pulmonary re-expansion technique [2]. Hence, efficient and unique prognostic indexes will help start early symptomatic treatment and promote the outcome of the disease. Previous studies verify that the pathological mechanism of ARDS closely correlates with inflammatory response. ARDS is mainly caused by various inflammatory mediators that activate the inflammatory cascade and induce diffuse lung parenchymal injury [3]. Fork head box M1 (FOXM1), a member of the FOX transcription factor family, acts as an important regulator of cell cycle. By direct or indirect activation of target gene transcription expression, it takes part in multiple pathophysiological processes such as DNA damage repair and angiogenesis, which is indispensable in normal lung development [4]. Recent scholars found that the overexpression of FOXM1 enhances the therapeutic effects of bone marrow mesenchymal stem cells in treating ARDS [5]. Insulin-like growth factor 2 (IGF2) is a multifunctional growth hormone that regulates cell activity. Abnormally high expression of IGF2 has been proved to facilitate lipopolysaccharide (LPS)-induced inflammatory damage and aggravate acute pneumonia [6]. It is noteworthy that IGF2 knockout can inhibit the secretion of inflammatory cytokines to reduce LPS-induced cell damage in acute pneumonia [7]. It is thus speculated that FOXM1 and IGF2 are involved in the progression of ARDS, but their correlation in ARDS remains unclear. This study aims to investigate the serum levels of FOXM1 and IGF2 mRNA in ARDS patients, analyze its relationship with the disease, and assess its prognostic prediction value.
2. Data and methods
2.1. General data
A total of 190 ARDS patients (ARDS group) admitted to Ganzhou People’s Hospital from January 2020 to December 2022 were retrospectively selected, including 105 males and 85 females aged 30–70 (55.75 ± 6.84) years, with body mass index (BMI) in the range of 18–26 (22.73 ± 2.06) kg/m2. Inclusion criteria were (1) meet the diagnostic criteria for ARDS [8], (2) onset time <5 days, and (3) complete clinical data. Exclusion criteria were (1) with pulmonary diseases such as pulmonary tuberculosis, cardiogenic edema, lung tumors, and chronic obstructive pulmonary disease; (2) with contraindications of mechanical ventilation; (3) administration of hormone drugs 3 months before admission; (4) with respiratory failure due to fluid overload or heart failure; and (5) related to COVID-19. This study was approved by the hospital ethics committee. The 190 healthy volunteers in physical examination of our hospital during the same period were the control group, including 96 males and 94 females aged 31–69 (54.39 ± 7.54) years, with BMI in the range of 18–26 (22.08 ± 2.27) kg/m2. The above data have no significant difference between the control group and ARDS group (P > 0.05).
According to clinical outcome within 28 days of hospitalization (defined as ICU or in-hospital death), the ARDS patients were divided into death group (n = 64) and survival group (n = 126). According to the oxygenation index (PaO2/FiO2) within 24 h after admission, the 190 ARDS were divided into three groups in light of the disease severity, with >200 mmHg for mild group (n = 59), 100–200 mmHg for moderate group (n = 76), and ≤100 mmHg for severe group (n = 55) (Figure 1).
Figure 1.
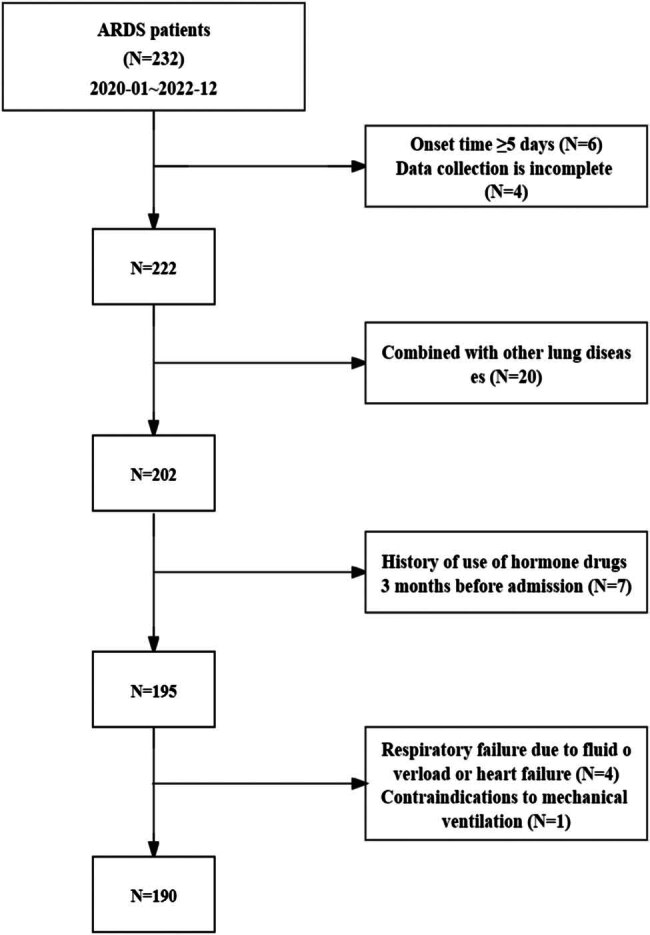
Study flow chart.
2.2. Methods
2.2.1. Collection of clinical baseline data
Basic data of ARDS patients were collected, including general demographic data (age, etc.), heart rate, diabetes history, respiratory rate, hypertension history, biochemical indexes, ARDS etiology (pulmonary embolism, sepsis, trauma, etc.), blood gas indexes, mechanical ventilation duration, etc. The biochemical indexes of serum creatinine (Scr), albumin (Alb), and urate nitrogen (BUN) were detected by automatic biochemical analyzer (Beckmann AU680). Arterial blood gas analyzer (Danish ray ABL80) was used for blood gas analysis. The indexes included arterial partial pressure of carbon dioxide (PaCO2), arterial partial pressure of oxygen (PaO2), inhaled oxygen concentration (FiO2), and calculated PaO2/FiO2.
2.2.2. Detection of serum FOXM1 and IGF2 levels
Fasting peripheral blood was collected from ARDS group at admission and control group during physical examination. The serum was collected by centrifugation, and the serum expressions of FOXM1 and IGF2 mRNA were detected by real-time quantitative PCR (qRT-PCR). RNA extraction kit (article No: 9112) was used to extract total RNA from serum samples, and RNA concentration and purity were determined by spectrophotometer (NanoDrop ND-1000, Kailebo (Beijing) Technology Development Co., LTD). After obtaining qualified RNA samples, cDNA was extracted using a reverse transcription kit (article No. 639504) and qRT-PCR expression analysis was performed according to the instructions of the TB Green®Premix Ex Taq™II kit (article No. RR820A), with sequence shown in Table 1. β-actin was used as the internal parameter of FOXM1 and IGF2. The relative expression levels of FOXM1 and IGF2 mRNA were analyzed by 2−ΔΔCt. All reagents and primers were provided by TaKaRa Biomedical Technology (Beijing) Co., Ltd.
Table 1.
Primer sequence
| Gene | Sequence |
|---|---|
| FOXM1 | Forward: 5′-ATACGTGGATTGAGGACCACT-3′ |
| Reverse: 5′-TCCAATGTCAAGTAGCGGTTG-3′ | |
| IGF2 | Forward: 5′-CTTGGACTTTGAGTCAAATTGG-3′ |
| Reverse: 5′-GGTCGTGCCAATTACATTTCA-3ʹ | |
| β-actin | Forward: 5ʹ-TGGAATCCTGTGGCATCCATGAAAC-3″ |
| Reverse: 5ʹ-ACGCAGCTCAGTAACAGTCCG-3″ |
2.3. Statistical analysis
SPSS 25.0 software was used. Statistical data including gender, etiology, and smoking were represented as n (%) and tested by χ 2. Measurement data such as FOXM1 and IGF2 mRNA were expressed as (x̅ ± s). t test was used if two groups were involved, one-way analysis of variance was used when three groups were involved, and SNK-q test was used for pairwise group comparison. Pearson coefficient was used to analyze the correlation between FOXM1 and IGF2 mRNA levels. Logistic regression was used to identify prognostic factors of ARDS patients. The receiver operating characteristic (ROC) curve was taken to evaluate the efficacy of serum FOXM1 and IGF2 mRNA in predicting prognosis of ARDS patients, and the area under the curve (AUC) difference was compared by Z-test. P < 0.05 indicates statistically significant difference.
Informed consent: Informed consent was obtained from the patient or their guardian, with signatures on the consent form.
Ethical approval: This study involving human participants was in accordance with the ethical standards of the Medical Ethics Committee of the Ganzhou People's Hospital and with the 1964 Helsinki Declaration.
3. Results
3.1. Comparison of serum FOXM1 and IGF2 mRNA levels between ARDS group and control group
Compared with the control group, ARDS group had significantly decreased serum FOXM1 mRNA level and increased serum IGF2 mRNA level (P < 0.05), as shown in Table 2.
Table 2.
Comparison of serum FOXM1 and IGF2 mRNA levels between ARDS group and control group (x̅ ± s)
| ARDS group (n = 190) | Control group (n = 190) | t | P | |
|---|---|---|---|---|
| FOXM1 mRNA | 0.66 ± 0.20 | 1.12 ± 0.21 | 21.864 | 0.000 |
| IGF2 mRNA | 2.29 ± 0.62 | 1.04 ± 0.16 | 26.909 | 0.000 |
3.2. Comparison of serum FOXM1 and IGF2 mRNA levels in patients with different disease severity
Serum FOXM1 mRNA level was increased in turn in severe group, moderate group, and mild group, with serum IGF2 mRNA level decreased successively in the three groups, showing statistically significant difference (P < 0.05), as shown in Table 3.
Table 3.
Comparison of serum FOXM1 and IGF2 mRNA levels in patients with different disease levels (x̅ ± s)
| Mild group (n = 59) | Moderate group (n = 76) | Severe group (n = 55) | F | P | |
|---|---|---|---|---|---|
| FOXM1 mRNA | 0.85 ± 0.24 | 0.64 ± 0.21a | 0.48 ± 0.14ab | 47.826 | 0.000 |
| IGF2 mRNA | 1.30 ± 0.41 | 1.78 ± 0.44a | 2.57 ± 0.68ab | 88.705 | 0.000 |
Note: Compared with mild group, a P < 0.05; compared with moderate group, b P < 0.05.
3.3. Comparison of clinical data of patients with different prognosis
PaO2/FiO2 and PaO2 were lower in the death group than in the control group, while Scr, FiO2, and mechanical ventilation duration were higher in the former than in the latter (P < 0.05), as shown in Table 4.
Table 4.
Comparison of clinical data of patients with different prognosis [(x̅ ± s)/n (%)]
| Clinical data | Death group (n = 64) | Survival group (n = 126) | t/χ 2 | P |
|---|---|---|---|---|
| Gender (male/female) | 37/27 | 68/58 | 0.254 | 0.614 |
| Age (years) | 56.18 ± 7.04 | 55.53 ± 6.73 | 0.620 | 0.536 |
| BMI (kg/m2) | 22.45 ± 2.27 | 22.87 ± 1.95 | 1.326 | 0.186 |
| Smoking history | 24 (37.50) | 48 (38.10) | 0.006 | 0.936 |
| Drinking history | 15 (23.44) | 22 (17.46) | 0.967 | 0.325 |
| Diabetes mellitus | 7 (10.94) | 16 (12.70) | 0.124 | 0.725 |
| Hypertension | 9 (14.06) | 25 (19.84) | 0.965 | 0.326 |
| Pathogenesis | ||||
| Wound | 17 (26.56) | 31 (24.60) | 2.480 | 0.479 |
| Pneumonia | 26 (40.63) | 65 (51.59) | ||
| SEPSIS | 14 (21.88) | 21 (16.67) | ||
| Pulmonary embolism | 7 (10.94) | 9 (7.14) | ||
| Mechanical ventilation time (d) | 9.04 ± 1.88 | 6.75 ± 1.46 | 8.523 | 0.000 |
| Respiratory rate (time/min) | 25.39 ± 4.85 | 24.47 ± 4.66 | 1.227 | 0.221 |
| Heart rate (time/min) | 105.09 ± 15.49 | 103.62 ± 16.63 | 0.589 | 0.557 |
| PaO2 (mmHg) | 65.73 ± 7.02 | 72.65 ± 3.77 | 8.847 | 0.000 |
| FiO2 (%) | 49.05 ± 5.56 | 40.03 ± 3.10 | 14.359 | 0.000 |
| PaCO2 (mmHg) | 49.91 ± 6.24 | 48.51 ± 5.49 | 1.586 | 0.115 |
| PaO2/FiO2 (mmHg) | 134.01 ± 38.17 | 181.49 ± 54.28 | 6.253 | 0.000 |
| BUN (mmol/L) | 15.22 ± 3.08 | 14.56 ± 2.94 | 1.439 | 0.152 |
| Scr (μmol/L) | 186.39 ± 14.92 | 161.04 ± 11.73 | 12.815 | 0.000 |
| Alb (g/L) | 36.84 ± 3.47 | 35.92 ± 3.01 | 1.890 | 0.060 |
3.4. Comparison of serum FOXM1 and IGF2 mRNA levels in patients with different prognosis
Compared with the survival group, the death group had decreased serum FOXM1 mRNA level and increased serum IGF2 mRNA level (P < 0.05), as shown in Table 5.
Table 5.
Comparison of serum FOXM1 and IGF2 mRNA levels in patients with different prognosis (x̅ ± s)
| Death group (n = 64) | Survival group (n = 126) | t | P | |
|---|---|---|---|---|
| FOXM1 mRNA | 0.50 ± 0.16 | 0.74 ± 0.22 | 7.745 | 0.000 |
| IGF2 mRNA | 2.83 ± 0.71 | 2.02 ± 0.57 | 8.505 | 0.000 |
3.5. Correlation between serum FOXM1 and IGF2 mRNA levels in ARDS patients
The correlation analysis showed that serum FOXM1 was negatively correlated with IGF2 mRNA levels in ARDS patients (r = −0.358, P = 0.000), as shown in Figure 2.
Figure 2.
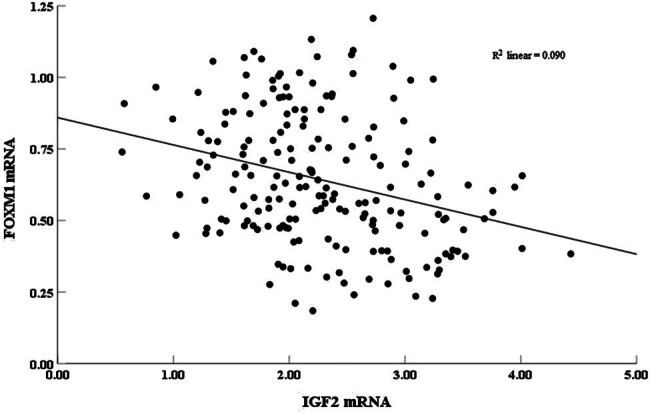
Correlation of serum FOXM1 and IGF2 mRNA levels in ARDS patients.
3.6. Logistic regression analysis of multiple factors influencing prognosis in ARDS patients
The prognosis of ARDS patients was taken as the dependent variable (death = 1, survival = 0), and the significant difference factors in univariate analysis were used as independent variables, including mechanical ventilation duration (measured value), FiO2 (measured value), PaO2 (measured value), PaO2/FiO2 (measured value), Scr (measured value), serum FOXM1 (measured value), and IGF2 (measured value). The multivariate logistic regression model was established. The results showed that FOXM1, IGF2, and PaO2/FiO2 were the factors influencing prognostic death of ARDS patients (P < 0.05), as shown in Table 6.
Table 6.
Logistic regression analysis of multiple factors influencing prognosis of ARDS patients
| Factors | B | SE | Wald | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| Mechanical ventilation time | 0.160 | 0.159 | 1.007 | 0.316 | 1.173 | 0.859–1.602 |
| FiO2 | 0.230 | 0.166 | 1.921 | 0.165 | 1.259 | 0.909–1.743 |
| PaO2 | −0.028 | 0.095 | 0.089 | 0.765 | 0.972 | 0.807–1.170 |
| PaO2/FiO2 | −0.180 | 0.071 | 6.450 | 0.011 | 0.835 | 0.726–0.956 |
| Scr | 0.268 | 0.184 | 2.117 | 0.146 | 1.307 | 0.911–1.875 |
| FOXM1 mRNA | −0.179 | 0.075 | 5.704 | 0.017 | 0.836 | 0.722–0.968 |
| IGF2 mRNA | 0.583 | 0.158 | 13.631 | 0.000 | 1.792 | 1.314–2.442 |
3.7. Predictive efficacy of serum FOXM1 and IGF2 mRNA on ARDS patients’ prognosis
The cut-off values in prognostic death prediction of ARDS patients by serum FOXM1 and IGF2 mRNA were 0.61 and 2.39, respectively, and the AUC in prognostic death prediction by serum FOXM1 mRNA combined with IGF2 mRNA was 0.902, which was superior to 0.789 and 0.812 (Z/P = 3.161/0.002, 3.278/0.001) in single detection, as shown in Table 7 and Figure 3.
Table 7.
Predictive efficacy of serum FOXM1 and IGF2 mRNA on prognosis of ARDS patients
| AUC | 95% CI | Critical value | Sensitivity (%) | Specificity (%) | Youden index | |
|---|---|---|---|---|---|---|
| FOXM1 mRNA | 0.789 | 0.715–0.864 | 0.61 | 71.90 | 81.70 | 0.536 |
| IGF2 mRNA | 0.812 | 0.745–0.878 | 2.39 | 75.00 | 80.20 | 0.552 |
| FOXM1+IGF2 | 0.902 | 0.854–0.950 | — | 87.50 | 81.00 | 0.685 |
Figure 3.
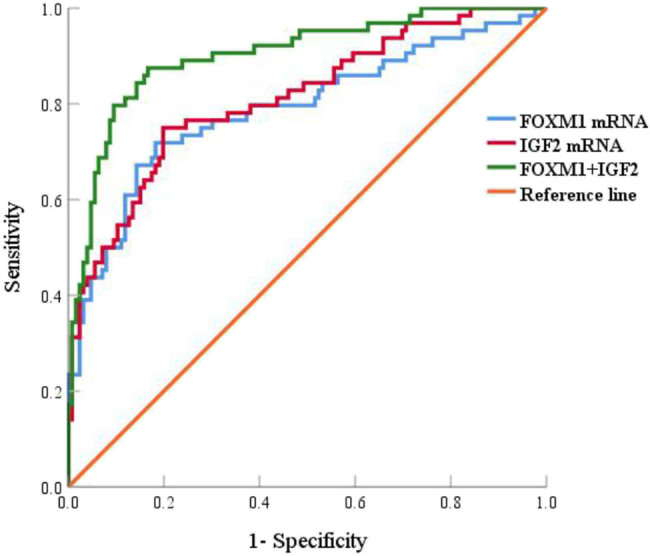
ROC curve of serum FOXM1 and IGF2 mRNA predicting prognosis of ARDS patients.
4. Discussion
4.1. Background introduction of ARDS
ARDS as a common severe respiratory system disease in clinical practice has complex pathogenic factors and high morbidity and mortality [9,10]. This study retrospectively analyzed 190 ARDS patients, finding that the prognostic death rate was as high as 33.68%, which suggested a high death rate of ARDS in our center, so attention was needed. Previous scholars found that lung injury severity score, APACHE II score, etc., had certain value in judging prognosis, but the results were subjective [11]. Therefore, it is necessary to find prognostic indexes for ARDS patients to provide early intervention and increase the survival rate.
4.2. Correlation between FOXM1 and ARDS
Studies have shown that the pathogenesis of ARDS is inseparable from inflammatory response. Inflammatory factors secreted in large amounts break the original anti-inflammatory and pro-inflammatory system of the body, activate inflammatory mediators, damage alveolar epithelium, increase capillary permeability, aggravate lung injury, and accelerate ARDS progression [9].
Located in human chromosome 12p13.3, FOXM1 is a key factor in regulating cell cycle. Several previous studies have verified that abnormal expression of FOXM1 correlates with lung diseases such as acute lung injury, asthma, pulmonary arterial hypertension [4]. Wang et al. [12] found that FOXM1 had abnormal expression in the serum of pneumonia patients, and miR-370-3p alleviated LPS-induced lung injury by targeting FOXM1. In this study, FOXM1 mRNA had decreased expression level in the serum of ARDS patients, suggesting the important role of FOXM1 in the pathogenesis of ARDS. Previous studies have shown that, by activating Wnt/β-catenin signaling pathway, transplantation of BMSC with FOXM1 overexpression can inhibit inflammation (by reducing white blood cell count, total protein concentration, inflammatory cytokines, etc.) and apoptosis, partially restore vascular integrity, thus preventing LPS-induced ARDS [13]. Relevant studies also confirmed that endothelial cell FOXM1 was a key endogenous mediator in BMPC-induced anti-inflammatory lung injury, which can restore vascular integrity, speed up the regression of inflammation, thus contributing to the recovery of inflammatory lung injury [14]. This study showed that FOXM1 mRNA level decreased with the disease aggravation, and the FOXM1 mRNA level was lower in the death group than in the survival group, suggesting that FOXM1 was closely related to the progression and prognosis of ARDS. It was speculated that decreased FOXM1 expression would lead to reduced inhibition of inflammatory response, massive secretion of inflammatory factors and mediators, which then promote acute lung inflammation and exacerbate lung damage. It is worth noting that the abnormal expression of FOXM1 in several malignant tumors is associated with poor prognosis, and targeting FOXM1 is considered as a potential therapeutic target for improving prognosis [15]. The regression analysis in this study showed that FOXM1 was a factor influencing prognosis of ARDS, confirming that FOXM1 participates in the progression of ARDS, so monitoring its level changes can provide clinical early warning.
4.3. Correlation between IGF2 and ARDS
IGF2, an imprinted gene located on chromosome 7, is an acidic peptide formed by 67 amino acids expressed in tissues like liver, which can regulate cell proliferation and differentiation. Previous reports on IGF2 mostly focused on tumors, and it was found to promote disease progression as an oncogene [16]. Recently, scholars have found that IGF2 also participated in lung-related diseases. Zhang et al. [6] found that acute pneumonia patients had increased serum expression level of IGF2, while miR-370-3p inhibited LPS-induced apoptosis and inflammatory damage by targeting IGF2. LPS-treated WI-38 cells had increased expression level of IGF2, and overexpression of IGF2 could eliminate the inhibitory effect of miR-24-3p on inflammatory damage [17]. This study found that ARDS group had increased serum IGF2 mRNA level, indicating that IGF2 may be involved in the occurrence and development of ARDS. IGF2 mRNA level increased as ARDS patients’ condition aggravated, indicating that IGF2 correlated with disease severity. A higher IGF2 level correlates with a more severe patient condition. It is speculated that the increased IGF2 level lead to an exacerbated inflammatory response, thus aggravating the inflammatory injury of the lung. Other scholars have confirmed that overexpression of IGF2 inhibits the protective effect of miR-218-5p on injury induced by oxidative modification of low-density lipoprotein (ox-LDL) in human umbilical vein endothelial cells (HUVECs), thus promoting the progression of AS, while ox-LDL stimulates HUVEC injury by promoting inflammation and inhibiting cell proliferation [18]. Further analysis of the relationship between IGF2 and prognosis revealed that patients with ARDS prognostic death had significantly higher serum IGF2 mRNA level than survivors. Moreover, the regression analysis found that IGF2 was a factor influencing prognostic death, suggesting that progression of ARDS lesions may correlate with abnormally high levels of IGF2. It is inferred that high levels of IGF2 would regulate the expression of key factors in inflammatory signaling pathway, exacerbate inflammatory response, thereby playing a role in the prognosis of ARDS. It is noteworthy that IGF2 sends signals through IGF1R, one of the key receptor tyrosine kinases involved in tumor development. IGF2 and IGF1R are considered as ideal therapeutic targets for tumors [19]. The results of this study suggested that the detection of serum FOXM1 and IGF2 mRNA levels was of great significance in predicting prognostic death of ARDS patients. Hence, in this study, the serum FOXM1 and IGF2 mRNA levels were used for prognosis evaluation. It was found that AUC prediction by combining the two was superior to FOXM1 and IGF2 mRNA alone. In addition, the combined detection could enhance the sensitivity and improve the evaluation efficiency without reducing specificity, which would help early prediction of prognostic death events in ARDS patients to guide clinical practice. In addition, Xing et al. [20] found that miR-4521 inhibited the AKT-GSK3β/Snail1 pathway by targeting IGF2 and FOXM1, thus inhibiting the epithelial mesenchymal transition process. According to the correlation analysis results in this study, FOXM1 was negatively correlated with IGF2 mRNA, suggesting that FOXM1 and IGF2 might jointly participate in the progression of ARDS through some inflammatory signaling pathway, but the specific mechanism remains unclear.
4.4. Conclusion
To conclude, ARDS patients had decreased serum FOXM1 mRNA level and increased IGF2 mRNA level. The two presented negative correlation, showing relevance with ARDS disease severity and 28-day prognosis. The combination of the two can be used as a reference index for evaluating the prognosis.
4.5. Study limitation
As for limitations of this study, the specific regulation mechanism for FOXM1 and IGF2 mRNA to participate in ARDS was not discussed, and the cases were sourced from a single center, which may lead to biased results. Sample size can be expanded to verify the conclusions. Meanwhile, the mechanism of action can be discussed from molecular pathways based on basic experiments to provide a basis for clinical diagnosis and treatment.
Acknowledgements
Not applicable.
Footnotes
Funding information: No funding was provided for this research.
Author contributions: Chao Liu: project development, data collection, and manuscript writing; Shengrui Zhang: data collection and data analysis; Weiwei Zhang: data collection; Jinfeng Huang: project development, data collection, data analysis, and manuscript editing.
Conflict of interest: Authors declare that they have no conflict of interest.
Data availability statement: The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
References
- [1].Kotas ME, Thompson BT. Toward optimal acute respiratory distress syndrome outcomes: recognizing the syndrome and identifying its causes. Crit Care Clin. 2021;37:733–48. [DOI] [PMC free article] [PubMed]
- [2].Battaglini D, Fazzini B, Silva PL, Cruz FF, Ball L, Robba C, et al. Challenges in ARDS definition, management, and identification of effective personalized therapies. J Clin Med. 2023;12(4):1–25. [DOI] [PMC free article] [PubMed]
- [3].Sinha P, Bos LD. Pathophysiology of the acute respiratory distress syndrome: insights from clinical studies. Crit Care Clin. 2021 statement;37:795–815. [DOI] [PMC free article] [PubMed]
- [4].Li Y, Wu F, Tan Q, Guo M, Ma P, Wang X, et al. The multifaceted roles of FOXM1 in pulmonary disease. Cell Commun Signal. 2019;17:35. [DOI] [PMC free article] [PubMed]
- [5].Luo Y, Ge S, Chen Q, Lin S, He W, Zeng M. Overexpression of FoxM1 optimizes the therapeutic effect of bone marrow mesenchymal stem cells on acute respiratory distress syndrome. Stem Cell Res Ther. 2023;14:27. [DOI] [PMC free article] [PubMed]
- [6].Zhang J, Mao F, Zhao G, Wang H, Yan X, Zhang Q. Long non-coding RNA SNHG16 promotes lipopolysaccharides-induced acute pneumonia in A549 cells via targeting miR-370-3p/IGF2 axis. Int Immunopharmacol. 2020;78:106065. [DOI] [PubMed]
- [7].Fei S, Cao L, Pan L. microrna‑3941 targets IGF2 to control LPS‑induced acute pneumonia in A549 cells. Mol Med Rep. 2018;17:4019–26. [DOI] [PubMed]
- [8].Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–33. [DOI] [PubMed]
- [9].Liu Z, Liu D, Wang Z, Zou Y, Wang H, Li X, et al. Association between inflammatory biomarkers and acute respiratory distress syndrome or acute lung injury risk: a systematic review and meta-analysis. Wien Klin Wochenschr. 2022;134:24–38. [DOI] [PMC free article] [PubMed]
- [10].Jabaudon M, Blondonnet R, Ware LB. Biomarkers in acute respiratory distress syndrome. Curr Opin Crit Care. 2021;27:46–54. [DOI] [PMC free article] [PubMed]
- [11].Zhang C, Huang Q, He F. Correlation of small nucleolar RNA host gene 16 with acute respiratory distress syndrome occurrence and prognosis in sepsis patients. J Clin Lab Anal. 2022;36:e24516. [DOI] [PMC free article] [PubMed]
- [12].Wang P, Zhang H, Zhao W, Dai N. Silencing of long non-coding RNA KCNQ1OT1 alleviates LPS-induced lung injury by regulating the miR-370-3p/FOXM1 axis in childhood pneumonia. BMC Pulm Med. 2021;21:247. [DOI] [PMC free article] [PubMed]
- [13].Luo Y, Lin S, Mao X, Yang Y, He W, Guo M, et al. Overexpression of FoxM1 enhanced the protective effect of bone marrow-derived mesenchymal stem cells on lipopolysaccharide-induced acute lung injury through the activation of wnt/β-catenin signaling. Oxid Med Cell Longev. 2023;2023:8324504. [DOI] [PMC free article] [PubMed]
- [14].Zhao YD, Huang X, Yi F, Dai Z, Qian Z, Tiruppathi C, et al. Endothelial FoxM1 mediates bone marrow progenitor cell-induced vascular repair and resolution of inflammation following inflammatory lung injury. Stem Cells. 2014;32:1855–64. [DOI] [PMC free article] [PubMed]
- [15].Wei G, Yang X, Lu H, Zhang L, Wei Y, Li H, et al. Prognostic value and immunological role of FOXM1 in human solid tumors. Aging (Albany NY). 2022;14:9128–48. [DOI] [PMC free article] [PubMed]
- [16].Kasprzak A, Adamek A. Insulin-like growth factor 2 (IGF2) signaling in colorectal cancer-from basic research to potential clinical applications. Int J Mol Sci. 2019;20(19):1–28. [DOI] [PMC free article] [PubMed]
- [17].Yu Y, Yang T, Ding Z, Cao Y. Circ_0026579 alleviates LPS-induced WI-38 cells inflammation injury in infantile pneumonia. Innate Immun. 2022;28:37–48. [DOI] [PMC free article] [PubMed]
- [18].Mao P, Liu X, Wen Y, Tang L, Tang Y. LncRNA SNHG12 regulates ox-LDL-induced endothelial cell injury by the miR-218-5p/IGF2 axis in atherosclerosis. Cell Cycle. 2021;20:1561–77. [DOI] [PMC free article] [PubMed]
- [19].Sélénou C, Brioude F, Giabicani E, Sobrier M-L, Netchine I. IGF2: development, genetic and epigenetic abnormalities. Cells. 2022;11(12):1–18. [DOI] [PMC free article] [PubMed]
- [20].Xing S, Tian Z, Zheng W, Yang W, Du N, Gu Y, et al. Hypoxia downregulated miR-4521 suppresses gastric carcinoma progression through regulation of IGF2 and FOXM1. Mol Cancer. 2021;20:9. [DOI] [PMC free article] [PubMed]


