Abstract
Feed quality is among the most determinative criteria for aquaculture success. Along with feed ingredient quality and its production process, feed storage conditions would also affect feed quality, especially in terms of adventitious toxins. Mycotoxins are frequent food and feed contaminants and are considered important health threats to both human and animal health. In this context, the effects of mycotoxins on aquatic animals were reviewed with an emphasis on aflatoxin B1 (AFB1), which is obviously reported in aquafeed. Severe tissue damage, increased susceptibility to infectious diseases, compromised immune system function, and increasing unknown death risks are among the most frequent symptoms of aflatoxicosis in aquatic animals. The lowest observable effect level for AFB1 has also been documented for different fish species. Considering the importance of such fungal toxins on the economic viability of aquaculture enterprises, it is recommended that further knowledge be obtained concerning the safe levels of AFB1 in terms of fish health and final product safety to human consumers.
Keywords: aflatoxicosis, aquaculture, aquafeed, feed contaminants, fungal toxins, mycotoxins
1. Introduction
World aquaculture production is projected to increase by 62% (35 million tons) from 2010 to 2030, with over 90% of such growth occurring in lower middle-income countries [1]. Aquaculture has recently transformed into a more sustainable and productive industry model, mainly due to cost feed formulation strategy and search for fishmeal alternatives in the last decade [2, 3]. Fish feed is an essential part of the aquaculture industry and significantly contributes to fish production costs and quality [4]. It plays a pivotal role in determining the success and profitability of fish farming. Ensuring high-quality feed is vital for optimizing fish health and growth. However, it has been reported that the feed is prone to being contaminated by fungal toxins [5, 6]. The risk of toxin occurrence in feeds increases at temperatures above 27°C, humidity above 62%, and feed moisture content above 14%. Inappropriate feed storage is a common predisposing factor for fungal and mold growth [7] and poses severe health issues in terms of human health and animal production costs and health [8, 9]. Contamination with mycotoxins might result in decreased nutritional values of ingredients and finished feed [10]. Mycotoxins might be responsible for hepatocellular and neurologic injuries, hypoimmunity, cancer, and even an increased mortality rate [11]. The toxic effects of mycotoxins depend not only on their dietary contents but also on the duration of toxin exposure and fish species, gender, and ontogenic stage [12]. Although there have been some reports regarding decreasing toxin bioavailability using dietary additives, including yeast cell wall, clay minerals, and pro/post-biotics, mycotoxins are still among the main risks of reduced fish growth performance and immune competence [5, 13–17]. Moreover, the long-term ingestion of feeds with low levels of mycotoxins or acute exposure to high dietary contents might be a reason for the unexplained mortalities occasionally observed in fish farms [18–20]. While the effects of mycotoxins are relatively well-known in most terrestrial farm animals, the outcomes of dietary mycotoxin contamination on aquaculture species have yet to be extensively studied. Therefore, the present review focused on the effect of mycotoxins, especially aflatoxin B1 (AFB1), on aquatic animals in terms of fish growth performance, digestive tract physiology, immune system functionality, and intestinal barrier integrity. Such understanding plays a crucial role in managing the adverse effects caused by mycotoxins and increasing social awareness regarding the presence of such toxins in aquafeed.
2. Mycotoxins
Mycotoxins are low molecular weight secondary fungal metabolites (MW∼700 Da) produced by Aspergillus, Penicillium, Fusarium, and Alternaria species [21–23]. Fungi frequently contaminate agricultural commodities throughout the world [24]. Humans and animals are exposed to mycotoxins mainly through the alimentary tract; however, inhalation and skin contact might also be possible [25]. Environmental factors, especially warm climates and irregular precipitation due to climate change, naturally promote fungal growth and increase the risk of mycotoxin occurrence in agricultural products [26, 27].
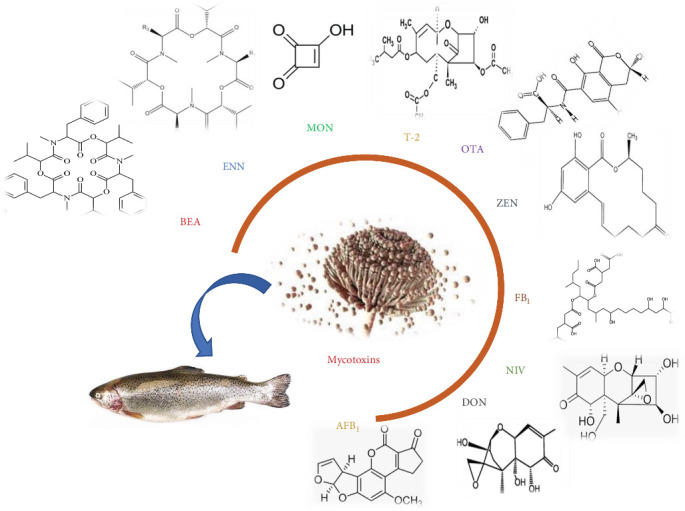
Mycotoxins come in various structural forms (Figure 1), from four simple-carbon compounds to complex substances [28]. More than 500 different mycotoxins have been isolated and chemically characterized according to previous data [29]. The 10 most common and hazardous feed mycotoxins include AFB1, deoxynivalenol (DON), nivalenol (NIV), zearalenone (ZEN), ochratoxin A (OTA), T-2 toxin (T-2), fumonisin B1 (FB1), moniliformin (MON), enniatins (ENN), and beauvericin (BEA) [30]. Among them, aflatoxins (AFs) are mostly studied as fungal toxins in aquaculture species and seem to affect industry development worldwide [31]. The effects of mycotoxins on fish are shown in Figure 2.
Figure 1.
Chemical structure of the most common mycotoxins. AFB1, aflatoxin B1; BEA, beauvericin; DON, deoxynivalenol; ENN, enniatins; FB1, fumonisin B1; MON, moniliformin; NIV, nivalenol; OTA, ochratoxin A; T-2, T-2 toxin; ZEN, zearalenone.
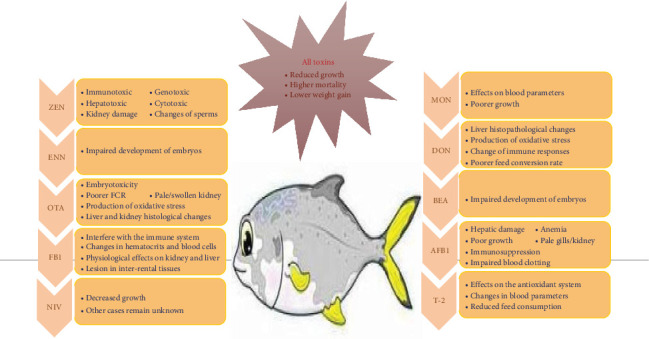
Figure 2.
The effect of mycotoxins on fish. AFB1, aflatoxin B1; BEA, beauvericin; DON, deoxynivalenol; ENN, enniatins; FB1, fumonisin B1; MON, moniliformin; NIV, nivalenol; OTA, ochratoxin A; T-2, T-2 toxin; ZEN, zearalenone.
2.1. AFs
AFs are the first mycotoxins discovered after a case of what was later found to be acute aflatoxicosis, turkey “X” disease, that resulted in the death of around 100,000 turkeys in the 1960s [32]. They are the most studied and well-characterized mycotoxins. AFs are highly toxic, carcinogenic, teratogenic, and mutagenic secondary metabolites primarily produced by the conidial fungi of the genus Aspergillus, mainly A. flavus and A. parasiticus [9, 33, 34]. The potential teratogenic characteristics of AFs play a vital role in human and animal malignancies. It is worth mentioning that about 4.5 billion people worldwide are at risk of AF exposure, mainly in poor and undeveloped countries [11].
Feed AF contamination has been reported to result in decreased fish growth performance, anemia, hemorrhage, liver function impairment, higher vulnerability to infectious diseases, and increased mortality [7]. Clinical signs associated with aflatoxicosis in fish include pale gill coloration and pathological tissue changes, altered blood indices, and lower growth rates with subsequently decreased weight gain (WG), reduced survival rate, darkening/yellowing of the body, and abnormal behavior [35–37]. In addition, dietary AFs might affect the nutritional value of fish muscle tissues [38, 39].
According to their natural blue or green fluorescence, AFs have several main types, including AFB1, AFG1, AFM1, AFB2, AFG2, and AFM2 [30, 40], which are illustrated in Figure 3. They are common in feed ingredients, finished feeds, and aquatic environments [42]. Among different AFs, AFB1 is considered the most common natural carcinogenic compound by the United States Food and Drug Administration. Its hepatotoxic effects, mutagenicity, carcinogenicity, teratogenicity, and immune system suppression have been confirmed in fish species [14, 31]. Meanwhile, AFM1 does not seem to be an important threat to fish health [30].
Figure 3.
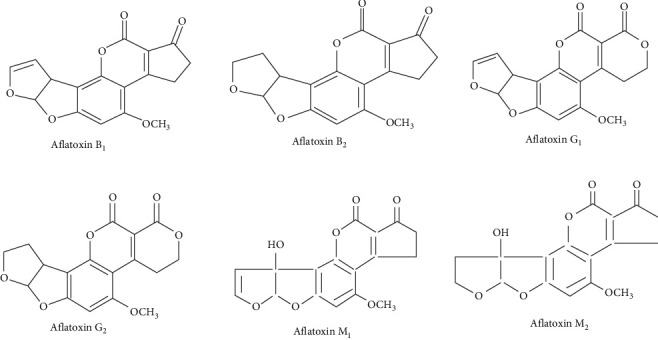
Chemical structure of the most important aflatoxins [41], with some modifications.
2.1.1. AFB1
The toxin is the major mycotoxin that globally contaminates aquafeeds, especially in tropical regions. It is involved in disease and the mortality of aquaculture species [43]. AFB1 exposure in fish might result in changes in hematological indices and serum biochemistry of fish [44]. According to previous evidence (e.g., [7, 14, 45–50]), elevated toxin levels may lead to reduced growth performance, histopathological changes in the liver and kidneys, and alterations in hematological and biochemical serum parameters in common carp (cyprinos carpio), rainbow trout (Oncorhynchus mykiss), rohu (Labeo rohita), and silver catfish (Rhamdia quelen). It has been shown that rainbow trout is a more susceptible fish species to AFB1; susceptibility to infectious diseases and mortality might increase depending on the dietary concentration of the toxin and the duration of exposure [51]. In aquaculture production, considerable research has been performed on the toxicity of AFB1 on fish species, including rainbow trout [52], sea bass (Dicentrarchus labrax) [53], sea bream (Sparus murata) [54], and beluga (Huso huso) [55]. The other studied species are juvenile hybrid sturgeon (A. ruthenus × A. Baeri) [18], Nile tilapia [56–59], rohu (L. rohita) [49, 60], and red drum (Sciaenops ocellatus) [61]. The remaining species included gibel carp (Carassius gibelio) [62, 63], channel catfish (Ictalurus punctatus) [64], and juvenile hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂) [65]. The toxic effects of AFB1 on various fish species are summarized in Table 1.
Table 1.
Effects of dietary AFB1 contamination on different fish species.
| Species | Dose | Effect | Reference |
|---|---|---|---|
| Nile tilapia (Oreochromis niloticus) |
100 ppb 200 ppb |
Growth impaired Mortality |
El-Banna et al. [56] |
| 2000 or 4000 ppb | Reduced weight gain and decreased body lipid content | Hussain, Manteen, and Gatlin [5] | |
| 200 ppb | Decreased total erythrocyte and leucocyte count, serum liver enzymes leakage, hemoglobin count decreased; reduced weight gain | Selim, El-hofy, and Khalil [44] | |
| 375, 752, 940, 1500, 1880, 3000 ppb | Decreased FI and growth rate, liver atrophy | Chávez-Sánchez, Martinez-Palacios, and Osorio-Moreno [57] | |
| 100,000 ppb | Lipofuscin and irregularly hepatocellular nuclei, weight loss, severe hepatic necrosis, and mortality | Tuan et al. [58] | |
| 100 ppb | Increased liver enzymes, reduced growth rate, and weight gain | Mahfouz and Sherif [66] | |
| 100 ppb | Weight loss, changes in blood parameters, and liver necrosis | Abdelhamid et al. [67] | |
| 3000 ppb | Lower SGR | Shehata, El-Melegy, and Ebrahim [68] | |
| 200, 250 ppb | Mortality | Naiel, Ismael, and Shehata [69] | |
| 100 ppb | Severe liver tissue vacuolation and lipid accumulation | Kenawy et al. [70] | |
| 100 ppb | Decreased growth | Encarnacao et al. [71] | |
| 150 ppb | Serious toxic impacts and negative effects on health performance | Mehrim and Salem [72]; Zychowski et al. [73] | |
|
| |||
| Tetrahybrid red tilapia | <5 ppb | Pale gills, liver damage, poor growth rates, and immune suppression | Conroy [74] |
|
| |||
| Rohu (L. rohita) | 1250 ppb | Reduction in nonspecific immunity | Sahoo and Mukherjee [75] |
| 7500, 1125 ppb 1250, 2500 ppb 12,000, 13,300 ppb |
Acute toxicity Subchronic toxicity Necrosis of gill lamellae, primary lamellar hyperplasia |
Sahoo and Mukerjee [76] | |
| 10, 20 ppb 40 ppb |
Liver tissue mild edema Swollen hepatocytes, kidney mild hemorrhages, decrease total erythrocyte, and hemoglobin count |
Mohapatra et al. [77] | |
| 25, 50 ppb 100 ppb |
Reduced growth performance, growth depression Reduced growth indices and survival rate |
Bhatt et al. [49] | |
| 100 ppb | Cytoplasmic vacuolization in hepatocytes and hepatic tissue showing loss of membrane integrity, along with diffused hepatocytes and hyperplasia | Bhatt et al. [78] | |
| 1250 ppb | Increased serum lysozyme activity, enhanced phagocytic ratio, and immunostimulatory effects | Sahoo and Mukherjee [76] | |
|
| |||
| Seabass (D. labrax) | 180 ppb 18 ppb 4.25 ± 0.85 ppb |
Abnormal behavioral Increased ALT, AST, and ALP enzymes, decrease in plasma proteins Serious health problems in exposed fish and a high risk to fish consumers |
El-Sayed and Khalil [79] |
|
| |||
| Rainbow trout (O. mykiss) | 500 ppb | Acute toxicities | Lovell [80] |
| 810 ppb | Acute toxicities | Bauer, Li, and Sinnuhuber [81] | |
| 25 ppb≥ 50 ppb≤ |
Persisting inflammatory response without mortality Decreased LYZ, TP, and ALB and increased inflammatory cytokines Villi destruction and necrosis, hyperplasia, and edema of gill lamellae |
Ghafarifarsani et al. [50] | |
| 25 ppb≥ 50 ppb≤ 50 ppb |
Infiltration of inflammatory cells into the underlying layers, necrosis, hyperplasia, atrophy, and severe destruction of gills Liver tissue damage and hepatocyte changes |
Imani et al. [82] | |
| 50 ppb | Destruction of intestinal villi | Mahmoudi et al. [83] | |
|
| |||
| Juvenile turbot (Scophthalmus maximus L.) |
100 ppb | Negatively affected liver catalase activity and intestinal microbiota | Zhang et al. [84] |
|
| |||
| Silver catfish, Jundia (R. quelen) |
204 ppb 350 ppb |
Lower weight and length gain Alterations in the liver and tissues |
Lopes et al. [85] |
|
| |||
| Channel catfish (I. punctatus) |
10,000 ppb | Decreased growth performance, anemia, and liver and gastric necrosis | Jantrarotai and Lovell [64] |
| 12,000 ppb | Regurgitating stomach contents, pale organs of moribund fish, istological lesions, and mortality | Jantrarotai, Lovell, and Grizzle [86] | |
|
| |||
| Yellow catfish (Pelteobagrus fulvidraco) |
200 ppb< | Growth performance (WG, SGR), lower survival rate, and increased FCR | Wang et al. [87] |
|
| |||
| Tra catfish (Pangasius hypophthalmus) |
500, 1000 ppb 50, 100, 250 ppb |
Increased HIS AST, ALT, and liver damage |
Gonçalves et al. [88] |
|
| |||
| White surgeon (H. huso) | 75, 100 ppb | Altered feed conversion and weight gain and decreases in growth | Sepahdari et al. [55] |
|
| |||
| Juvenile hybrid sturgeon (A. ruthenus ♂ × A. baeri ♀) |
40 ppb 80 ppb |
Mortality High mortality, decreased hematocrit value, nuclear hypertrophy, and hyperchromasia |
Raghavan et al. [18] |
|
| |||
| Stellate sturgeon (Acipenser stellatus) |
1500 ppb 3500 ppb |
8% mortality 50% mortality |
Santacroce et al. [7] |
| 75, 100 ppb | Bleeding points in the gills and head, hyperplasia and destruction of the epithelial tissue of the gills lamellae, and necrosis of the liver cell | Motallebi Moghanjouei [89] | |
| 1500, 1850, 2300, 2850, 3500 ppb | Increased liver enzymes (AST, ALT, and ALP) and mortality rate | Jalilpour et al. [90] | |
|
| |||
| Grass carp (Ctenopharyngodon idella) |
147 ppb | Suppressed Nrf2 signaling—a decrease in growth and antioxidant enzymes—and disruption of the integrity and TJ protein | Zeng et al. [91] |
| 85.94 ppb< | Decreased expression of genes β-defensin-1, LEAP-2A, Mucin2, and LEAP-2B | He et al. [92] | |
|
| |||
| Gibel crap (C. gibelio) |
5 ppb | Reduced growth | Han et al. [45] |
| 2000 ppb< | Fecundity is reduced and tissue accumulation | Huang et al. [93] | |
|
| |||
| Common carp (C. carpio) | 100,000 ppb | Immunosuppression | Sahoo and Mukherjee [94] |
| 200, 400 ppb | No mortality | Al-Rubaiy et al. [95]; Tasa et al. [96] | |
| 500, 1000, 2000 ppb | Decreased weight gain, histopathological changes | Rhadi, Rudainy, and Attee [97] | |
|
| |||
| Salmon (Oncorhynchus kisutch) |
10,000 ppb | Acute toxicities | Schoental [98] |
|
| |||
| Mosquitofish (Gambusia affinis) | 4640 ppb | Acute toxicities and mortality | McKean et al. [99] |
|
| |||
| Tambaqui fingerlings (Colossoma macropomum) |
500 ppb< | Decreases in the WG, FI, and FE | Nunes et al. [100] |
|
| |||
| Lambari fish (Astyanax altiparanae) |
10 ppb | Accumulation in fish liver and muscle | Michelin et al. [101] |
Abbreviations: ALB, albumin; ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate aminotransferase; FCR, feed conversion ratio; FE, feed efficiency; FI, feed intake; HIS, hepatosomatic index; LYZ, lysozyme; Nrf2, NF-E2-related factor 2; SGR, specific growth rate; TJ, tight junction; TP, total protein; WG, weight gain.
2.1.1.1. Fish Growth Performance
From an economical point of view, feed AF contamination is one of the most crucial worries for aquaculture and feed industries [102] since it might affect the growth performance of fish [19, 103].
Salem et al. [104] found a significant reduction in the growth performance and survival rate of Nile tilapia following dietary exposure to AFB1. It has been reported that dietary AFB1 decreased the growth rate of the gibel carp by impairing liver function and metabolic disorders [105]. Likewise, Hasanpour et al. [106] concluded that dietary AFB1 or ZEN reduced growth indices and affected fish body composition. However, severe changes in fish growth have been noticed in the simultaneous contamination of the diet by both toxins. Barany et al. [107] reported that chronic exposure of sea bream to AFB1 impairs growth as well as metabolic and physiological responses of fish to environmental stress, including increased stocking density (i.e., crowding). Conversely, according to Baglodi et al. [108], Indian carp raised on diets containing AFB1 at 50, 100, and 150 ppb for 130 days demonstrated no differences in survival, WG, length, or feed conversion ratio (FCR). Meanwhile, Huang et al. [109] and Liu et al. [110] found that AFB1 could negatively affect the growth performance and antioxidative capacity of juvenile marbled eel (Anguilla marmorata) and growth indices, intestinal health, and muscle quality of hybrid grouper (E. fuscoguttatus ♀ × E. lanceolatus ♂).
Moreover, it might infiltrate through the blood–brain barrier and affect the brain development of zebrafish embryos by stimulating apoptosis in the brain and axons [34]. In addition, the cytotoxic effects of AFB1 in the endothelial cells of the blood–brain barrier were proposed to be connected to silver catfish's behavioral dysfunction [111]. Further, intake of AFB1-contaminated food is associated with neurological diseases such as neuropathy, neurological defects, cerebral edema, and even death [112]. Park et al. [113] also concluded that AFB1 affected human and zebrafish nervous systems via its antiproliferative and apoptotic properties.
It has been shown that the gastrointestinal tract would be affected by AFB1, which might lead to growth deterioration due to mal-nutrition/absorption or endogenous protein loss from increased digestive enzyme synthesis/release [96, 114]. In addition, AFB1 might affect intestinal physiology through changes in electrophysiological and morphological properties and mRNA expression of cell-to-cell adhesion proteins. The binding of AFB1 to tight junction (TJ) components might result in damage to the intestine and the integrity of the tissue, consequently leading to a leaky gut [114].
2.1.1.2. Digestive Enzyme Activity
Digestive enzymes are essential physiological components of fish growth and development [115]. Their activity also indicates the nutritional status of fish, dietary composition, and digestive tract health [116, 117]. It has been reported that dietary AFB1 contamination increases the activity of alkaline protease, lipase, and amylase in common carp [96]. Similar results have been found for various fish species, including rainbow trout [14, 48], tilapia [118], common carp [95], and Chinese sea bass [119] exposed to dietary AFB1. However, Fan et al. [120] concluded that feeding on a diet with 50 ppb AFB1 decreased the digestive enzyme activity of yellow river carp (Cyprinus carpio haematopterus).
2.1.1.3. Immune Indices
Pathogens can breach physical barriers and enter the host, leading to a decrease in immune function and disease resistance [121]. AFB1 could affect immune system function in aquatic organisms [122]. Recently, Nazdar et al. [123] reported that AFB1 could decrease the survival and functionality of the mouse macrophage RAW264.7 cell line in a dose-dependent manner. Various immune pathways might be inhibited or even stimulated, depending on the concentration of AF to which the animal is exposed or the extent of toxin metabolites produced in the course of toxin biotransformation [124].
Aflatoxicosis leads to the development of deformed cells, eosinophilic cytoplasm, lymphocyte leakage, and cell necrosis in rainbow trout [125]. Further, El-Enbaawy et al. [126] found a decrease in phagocytic activity and neutrophil count in rainbow trout fish [127], as well as a decline in serum immunoglobulin content in Oreochromis niloticus. Sepahdari et al. [55] also concluded that feeding beluga with diets containing 100 ppb AFB1 remarkably decreased the red blood cell count and blood hemoglobin content of the fish. Moreover, He et al. [92] demonstrated that dietary AFB1 decreased the content of antibacterial activity, immunoglobulins, and expression of antimicrobial peptides in the immune organs of grass carp (C. idella). Additionally, dietary AFB1 affected the expression of various cytokines, including interleukin (IL)-6, IL-8, IL-15, interferon-gamma 2, tumor necrosis factor-α, IL-17D, and IL-12. Therefore, this AFB1 could affect the immunological competence of the skin, the spleen, and the kidney of fish since the spleen and head kidney, along with the skin, are the main body immune organs [55, 92]. In addition, He et al. [92] reported that AFB1 decreased the activities of immunological parameters, including lysozyme (LYZ), complement C3 (C3), complement C4 (C4), and immunoglobulin M in grass carp. Similarly, Yang et al. [15] indicated that feeding a diet containing 20 ppb AFB1 reduced C3, C4, and immunoglobulin M in juvenile turbot (S. maximus).
2.1.1.4. Antioxidant Capacity
Any exposure to AFs might result in increased free radical production/liberation in cells, which could lead to increased tissue malondialdehyde (MDA) content, indicative of increased lipid peroxidation [128]. In other words, lipid peroxidation causes increased tissue MDA content, which might further induce oxidative stress [129, 130]. For instance, Peng et al. [119] found that AFB1 increased the MDA content of up to 1.0 ppm in Chinese sea bass. Xue et al. [131] inferred that AFB1 induced severe oxidative stress, including increased reactive oxygen species (ROS) and MDA content in gibel carp exposed to 50–100 ppb AFB1.
Superoxide dismutase (SOD) and catalase (CAT) are actively involved in decreasing cellular oxidative stress via scavenging ROS [17]. It has been reported that SOD could catalyze the dismutation of superoxide free radicals and thereby alleviate DNA damage. CAT also protects the cell from oxidative injury by catalyzing hydrogen peroxide radicals [132]. Peng et al. [119] concluded that dietary AFB1 up to 1.0 ppm resulted in reduced growth, enhanced antioxidant and immune response, decreased intestinal trypsin activity, and impaired intestinal morphology in spotted Leporinus (Leporinus maculatus). Further, AFB1 has been shown to undesirably affect thyroid gland function and decrease serum T3 and T4 titers in zebrafish larvae. The toxin would also affect the expression of genes involved in oxidative stress and apoptosis [133].
2.1.1.5. Expression of Immune and Inflammatory Genes
Dietary AFB1 contamination considerably affects inflammatory and immune responses in different fish species [92, 133, 134]. However, the immune toxicity of the toxin might vary in different fish since they might possess different AFB1 biotransformation capabilities [135]. Immune responses and growth performance of fish are interdependent so that any changes in the immune system functionality will finally affect animal growth and body protein accretion. The first immune organ, including the skin, mainly contributes to fish immune responses, where many lymphocytes are naturally present and secrete immunoglobulin and antibacterial compounds. Reduced body protein synthesis might decrease serum antibody content, interfering with proper/suitable lymphocyte functioning and immunological responses. According to the literature, AFB1 could adversely affect the structural integrity of highly important supporting organs (the spleen and head kidney) and restrict immunological response in fish [92]. Moreover, the activation of the target of rapamycin (TOR) and nuclear factor kappa B (NF-κB) pathways might be dose-dependently affected by AFB1 [136]. It has been reported that any inflammation following the activation of TOR and NF-κB pathways resulted in increased pro-inflammatory cytokine production/liberation and decreased synthesis of anti-inflammatory cytokines [137, 138]. According to Ottinger and Kaattari [139], lymphocytes, monocytes, and neutrophils are responsible for alterations in the expression of LZ, IL-4, and IL-8, so dietary exposure to AFB1 might affect their serum content/activity. It has been found that AFB1, on the one hand, drastically decreases arginine contents of the spleen and head kidney, which also influences the organ TOR mRNA expression. In addition, the toxin might affect the cell mRNA contents of antibacterial peptides, namely, LAEP-2A, LEAP-2B, hepcidin and β-defensin-1, and Mucin-2 immune organs in fish. AFB1 also influences the expression of IL-6, IL-8, IL-15, interferon-gamma 2, tumor necrosis factor-α, IL-17D, and IL-12p40 cytokines [92]. Recently, Ghafarifarsani, Kachuei, and Imani [48] have demonstrated that the expression of IL1-β, INF-γ, and TNF-α genes was increased in rainbow trout fed a diet containing 25 ppb AFB1.
2.1.1.6. The Liver Tissue Injury and Expression of Hepatic Antioxidant Enzymes
The liver is the main organ that is responsive to absorbed AFB1 [5, 7, 140], and hepatic enzymes are considered indicators of cellular damage and tissue function impairment [141, 142].
The liver is involved in metabolizing different xenobiotics, including toxins, and might be affected by aflatoxicosis [124, 143]. Through blood circulation, AFB1 is immediately transferred to the liver and metabolized by hepatocytes. Cytochrome P450 (CYP450) enzymes metabolize AFB1 to AFB1-exo-8,9-epoxide, a highly toxic and reactive AFB1 metabolite that can react with different biomolecules, including DNA, RNA, and protein. It could also finally inactivate the p53 gene. The event might eventually result in GC to TA mutagenesis [10]. AFB1-DNA conjugate was reported in the liver of AFB1-exposed rainbow trout and Atlantic salmon. Naturally, a higher half-life of AFB1-DNA in fish hepatocytes compared to mammals might imply that its enzymatic removal is insufficient in fish, indicating a higher probability of mutation in fish [144].
The living cells contain antioxidant enzymes (e.g., SOD, CAT, glutathione peroxidase, and glutathione reductase), for protection against oxidative stress due to xenobiotic metabolism and/or resultant ROS. The immune system of zebrafish (Danio rerio) was responsive to oxidative damage following excess ROS production via NF-E2-related factor 2 [145]. AFB1 damages the hepatocyte cell membrane and results in serum liver enzyme leakage. Those enzymes activity in serum samples were used as the biological markers of liver tissue damage in common carp and northern snakehead (Channa argus) [146, 147]. Recently, Di Paola et al. [133] investigated the effect of AFB1 on Zebrafish embryos and found that AFB1 increased oxidative stress indices, including activity of SOD, CAT, GST, and CYP450, along with tissue MDA and apoptotic protein contents. Disturbed cellular oxidation-reduction status and tissue damage following oxidative stress were reported in the liver of Chinese sea bass [119] and Stellate sturgeon (A. stellatus) fingerlings [90]. Oxidative stress increased hepatic lipid peroxidation and tissue ROS production in common carp. Lipid transportation was adversely affected following hepatic AFB1 bioaccumulation [148]. Indeed, increased liver lipid deposition was reported in red drum [61] and juvenile rainbow trout [46] exposed to AFB1. Impaired hepatic lipid metabolism, lipid peroxidation, or lipoprotein synthesis following AFB1 exposure was also reported in gibel carp [62]. Furthermore, hepatic cell damage by AFB1 exposure resulted in decreased whole-body protein and lipid contents in hybrid striped bass (Morone chrysops × M. saxatilis) [124]. It has also been shown that any liver damage caused by oxidative stress leads to increased tissue protein degradation and reduced protein synthesis in animals [46, 149].
2.1.1.7. Intestinal Tissue Structure and Barrier Proteins
The intestinal epithelial integrity by adhesion junctions, TJs, and desmosomes plays a vital role in intestinal permeability, nutrient uptake, toxins uptake, bacterial translocation, and immune response, recently known as the main gut health components. TJs, as the major functioning components of the intestinal barrier, seal the intercellular spaces of epithelial cells [150, 151]. It has been shown that intestinal integrity through clathrin-mediated endocytosis was affected by AFB1 [151].
Huang et al. [152] concluded that feeding an AFB1-contaminated diet imposed intestinal oxidative damage, TJ destruction, and epithelial cell apoptosis, which could adversely affect the integrity of the intestine in juvenile grass carp. In addition, claudin and occludin and their interaction with signaling molecules regulate the permeability of junctions in the gastrointestinal tract. In sea bream (S. aurata), AFB1 affected claudin proteins in intestinal TJs and resulted in cell necrosis with mononuclear cell penetration [114]. Feeding rainbow trout with an AFB1 contaminated diet caused infiltration of inflammatory cells into the underlying intestinal mucosal layer [82]. Moreover, the expression of caspase-3, a central effector of cell apoptosis, increased in goldfish (Carassius auratus) [63] and in common carp [95] following AFB1 exposure.
The digestive tract is the main route of feed-born toxins' entry to the body [153], so that any dietary exposure to AFB1 might affect fish susceptibility to secondary infectious microorganisms [154]. For instance, gastrointestinal microbiota was affected in turbot feed AFB1-contaminated diet [15]. Further, the structural disruption of intestinal epithelial cells leads to increased feed-born toxins or antigen uptake into blood circulation with subsequent susceptibility to pathogens [150, 155].
AFB1 increased the expression of IL-1β and TNF-α mRNA in rainbow trout, which resulted in intestinal inflammation, severe tissue damage, and reduced nutrient bioavailability [48]. Changes in intestinal villus morphology and damaged enterocytes were observed following dietary AFB1 exposure in juvenile gilthead seabream [114], Chinese sea bass [119], and rainbow trout [156]. Furthermore, Zhang et al. [84] reported considerable changes in the abundance of intestinal bacteria in turbots fed a diet containing AFB1 in comparison to the control group.
2.1.1.8. Gill Tissue Damage and Disturbed Lamellar Ventilation
Pathological changes in gill tissue might be indicative of exposure to toxins or xenobiotics [63, 157]. For instance, AFB1 adversely altered the structural barrier of gills and remarkably lowered TJ proteins and anti-inflammatory gene expression in grass carp [158]. The gill lamellae hypertrophy, increased secondary lamella thickness, and increased mucus secretion were found in rohu exposed to AFB1 [76]. It has been recently reported that any dietary exposure to AFB1 resulted in pathological changes in goldfish gills [63]. Similarly, lamellae edema and epithelial necrosis with physiological consequences were confirmed in Nile tilapia [159]. Cell necrosis and lamellae hemorrhage in major Indian carp, rohu, were detected following aflatoxicosis [160]. In addition, gill hyperplasia and epithelial disruption were observed in Stellate sturgeon (A. stellatus) [89], rainbow trout (O. mykiss) [82, 161], and rohu (L. rohita) [76].
The exfoliation of epithelial cells in lamellae could lead to an increased distance between oxygen-containing water flow and blood circulation and an insufficient supply of oxygen, and consequently, severe secondary lamellae necrosis, which was a principal limiting factor for metabolite excretion via gills [161, 162].
3. Worldwide AFB1 Occurrence in Aquafeed and Lowest Observable Effect Levels (LOELs)
In spite of national and international constant surveillance to limit/manage fungal toxins, it has been reported that approximately 475 million tons of feedstuffs and forages have been consumed only in the EU. While the mycotoxin contents of the feedstuffs are well below the accepted maximum levels for animals, their co-occurrence is now a worldwide feed supply chain concern [27, 163, 164]. Generally, AFB1 is more prevalent in tropical regions due to warm, humid conditions [165]. For instance, AFB1 content of fish feed in Asia and Africa typically ranges from 51.83 μg/kg (51.83 ppb) to 220.61 μg/kg (220.61 ppb), while in the EU region, it is 0.43 μg/kg (0.43 ppb) on average [88, 166, 167].
As discussed earlier in the present review, some aquatic species, including channel catfish, Coho salmon, and tilapia, are less susceptible to aflatoxicosis thanks to their higher metabolic capacity to biotransform AFs [168]. According to the International Agency for Research on Cancer, AFs are the primary cause of human carcinoma [169]. It is highly recommended that allowable ranges of feed/food (ingredients) toxins should be established as a safe standard rate with a safety margin. Generally, the acceptable value for Nile tilapia is <100 ppb. The legal limit of AFs in feed for all animal species is 50 ppb in Brazil. In the United States and EU, however, the safe level is 10 ppb for some agricultural products/commodities and livestock products, respectively. The acceptable daily intake of 5 ppb AFB1 is introduced by the Food and Drug Administration. However, the maximum authorized concentration of 20 ppb is also established by the United States Food and Drug Administration [170] for total AFs (AFB1 mixed with AFB2, AFG1, and AFG2). However, the EU has defined maximum limits of 5 and 12 ppb for AFs in feed and foods, respectively [14].
Raghavan et al. [18] found that juvenile hybrid sturgeons (A. ruthenus × A. baeri) are sensitive to dietary AFB1 contents of >10 μg/kg feed (10 ppb). AFB1 content of commercial fish feed was demonstrated to be less than 10 μg/kg (10 ppb) [171, 172]. However, higher dietary contents were also unavoidable [173]. Meanwhile, Pietsch [134] considered that 4.30 μg/kg feed (4.30 ppb) might be a safe AFB1 contamination threshold in commercial feeds. Similarly, Nácher-Mestre et al. [174] concluded that the overall level of mycotoxin in fish feed was below the maximum residue limit suggested by Commission Recommendation 2006/576/EC, and no mycotoxin transfer might occur from feeds to fish fillets.
Furthermore, the lowest and highest threshold concentrations are 1.69 and 8.70 ppb, respectively, and with an average concentration of 4.30 ppb, 5% of the fish population might be at risk of aflatoxicosis. Early signs of body composition changes and oxidative stress following dietary AFB1 exposure would be observable at 563 ± 252 and 1598 ± 1467 ppb, respectively [30]. LOEL is different for various fish species. For instance, exposure to 20–200 ppb AFB1 did not affect common carp [175, 176]. However, juvenile common carp showed decreased growth indices following dietary exposure to 100 ppb AFB1 [177]. Meanwhile, exposing common carp to 2 ppb AFB1 resulted in liver injury and histopathological alterations at 20–200 ppb AFB1. However, the lowest LOEL for genotoxicity and immunosuppression were 317 ± 136 and 1770 ± 630 ppb in different fish species, respectively [30]. He et al. [92] also discussed that LOEL for the normal functioning of the immune organs (the skin, spleen, and head kidney) would be 29.48 ppb AFB1 in grass carp. According to Alinezhad et al. [46], the early signs of reduced growth indices would be observed following dietary exposure to >5 ppb AFB1 in rainbow trout. As discussed above, there are considerable reports regarding harmful and lethal concentrations of AFB1 in aquafeed. However, acceptable thresholds of AFB1 in different fish species require further studies [48, 50, 135].
4. Conclusion
AFB1 is considered a potential threat to the aquaculture industry regarding aquatics and consumer health. Dietary AFB1 contamination influences the immune system and growth performance, resulting in economic worries and decreased farm profitability. Therefore, it is highly recommended that safety thresholds or standards be determined for feed and final products of AFB1 contents. To introduce such standards, various factors, including fish species, developmental stage, culture condition, and facilities, along with final product safety, should be taken into consideration. Studies on how to manage/control the occurrence of toxins in aquafeed must also be conducted as well. Considering that there is high variation in feed (stuff) mycotoxin content from one place to another, close collaboration between scientists and legislation authorities is required regarding sampling methods/frequencies, processing, and/or analyses. In addition, developing quick and handy methods of detecting (multi)-mycotoxin contamination is necessary.
Contributor Information
Ahmad Imani, Email: a.imani@urmia.ac.ir.
Hamed Ghafarifarsani, Email: hamed_ghafari@alumni.ut.ac.ir.
Data Availability Statement
Data sharing is not applicable to this article, as no new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Mina Ziarati was responsible for writing the early draft and preparing figures and tables. Ahmad Imani contributed to supervision, funding acquisition, resources, and writing–review and editing. Hamed Ghafarifarsani participated in the conceptualization and review of the draft. Deepa Bhatt took part in writing–review and editing.
Funding
Ahmad Imani is employed by Urmia University and his researches are financially supported by the research council of Urmia University.
References
- 1.World Bank. Agriculture and Environmental Services Discussion Paper . Vol. 3. World Bank; 2013. Fish to 2030: Prospects for Fisheries and Aquaculture. [Google Scholar]
- 2.Tacon A. G. J., Metian M. Global Overview on the use of Fish Meal and Fish Oil in Industrially Compounded Aquafeeds: Trends and Future Prospects. Aquaculture . 2008;285:1–4, 7, 146–158. [Google Scholar]
- 3.Nasopoulou C., Zabetakis I. Benefits of Fish Oil Replacement by Plant Originated Oils in Compounded Fish Feeds. A Review. LWT—Food Science and Technology . 2012;47(2):217–224. doi: 10.1016/j.lwt.2012.01.018. [DOI] [Google Scholar]
- 4.Kjørsvik E., Mangor-Jensen A., Holmefjord I. Egg Quality in Fishes. Advances in Marine Biology . 1990;26:71–113. doi: 10.1016/S0065-2881(08)60199-6. [DOI] [Google Scholar]
- 5.Hussain D., Mateen A., III, Gatlin D. M. Alleviation of Aflatoxin B1 (AFB1) Toxicity by Calcium Bentonite Clay: Effects on Growth Performance, Condition Indices and Bioaccumulation of AFB1 Residues in Nile Tilapia (Oreochromis niloticus) Aquaculture . 2017;475:8–15. [Google Scholar]
- 6.Magouz F. I., Salem M. S., Hashad M. A. Effect of Some Mycotoxin on Growth Performance and Feed Utilization of Nile Tilapia (Oreochromis niloticus) Iraqi Journal of Veterinary Sciences . 2018;32(1):99–108. doi: 10.33899/ijvs.2018.153830. [DOI] [Google Scholar]
- 7.Santacroce M. P., Conversano M. C., Casalino E., et al. Aflatoxins in Aquatic Species: Metabolism, Toxicity and Perspectives. Reviews in Fish Biology and Fisheries . 2008;18:99–130. [Google Scholar]
- 8.Belhassen H., Jiménez-Díaz I., Arrebola J., et al. Zearalenone and Its Metabolites in Urine and Breast Cancer Risk: A Casecontrol Study in Tunisia. Chemosphere . 2015;128:1–6. doi: 10.1016/j.chemosphere.2014.12.055. [DOI] [PubMed] [Google Scholar]
- 9.Dirican S. A Review of Effects of Aflatoxins in Aquaculture. Applied Research . 2015;1:1191–1196. [Google Scholar]
- 10.Bennett J. W., Klich M. Mycotoxins. Clinical Microbilogy Reviews . 2003:497–516. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Francis S., Kortei N. K., Sackey M., Richard S. A. Aflatoxin B1 Induces Infertility, Fetal Deformities, and Potential Therapies. Open Med . 2024;19 doi: 10.1515/med-2024-0907.20240907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pleadin J., Frece J., Lešic T., Zadravec M., Vahcić N., Markov K. Deoxynivalenol and Zearalenone in Unprocessed Cereals and Soybean From Different Cultivation Regions in Croatia. Food Additives & Contaminants: Part B . 2017;4:1–7. doi: 10.1080/19393210.2017.1345991. [DOI] [PubMed] [Google Scholar]
- 13.Ellis R. W., Clements M., Tibbetts A., Winfree R. Reduction of the Bioavailability of 20 μg/Kg Aflatoxin in Trout Feed Containing Clay. Aquaculture . 2000;183(1-2):179–188. doi: 10.1016/S0044-8486(99)00292-6. [DOI] [Google Scholar]
- 14.Imani A., Bani M. S., Noori F., Farzaneh M., Moghanlou K. S. The Effect of Bentonite and Yeast Cell Wall Along With Cinnamon Oil on Aflatoxicosis in Rainbow Trout (Oncorhynchus mykiss): Digestive Enzymes, Growth Indices, Nutritional Performance and Proximate Body Composition. Aquaculture . 2017;476:160–167. [Google Scholar]
- 15.Yang J., Wang T., Lin G., et al. The Assessment of Diet Contaminated With Aflatoxin b in Juvenile turbot (Scophthalmus maximus) and the Evaluation of the Efficacy of Mitigation of a Yeast Cell Wall Extract. Toxins . 2020;12(9) doi: 10.3390/toxins12090597.597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nogueira W. V., Moyano F. J., Aznar-Garcia M. J., Tesser M. B., Garda-Buffon J. Preliminary Assessment of Bioaccessibility of Aflatoxin B1 in Fish. Aquaculture International . 2022;30(3):1315–1325. doi: 10.1007/s10499-022-00860-4. [DOI] [Google Scholar]
- 17.Peng K., Lv X., Zhao H., Chen B., Chen X., Huang W. Antioxidant and Intestinal Recovery Function of Condensed Tannins in Lateolabrax maculatus Responded to In Vivo and In Vitro Oxidative Stress. Aquaculture . 2022;547737399 [Google Scholar]
- 18.Raghavan P. R., Zhu X., Lei W., Han D., Yang Y., Xie S. Low Levels of Aflatoxin B1 Could Cause Mortalities in Juvenile Hybrid Sturgeon, Acipenser ruthenus Male Chi A. baeri Female. Aquaculture Nutrition . 2011;17(2):e39–e47. doi: 10.1111/j.1365-2095.2009.00725.x. [DOI] [Google Scholar]
- 19.Michelin E. C., Bedoya-Serna C. M., Carrion L. C. S., et al. Long-Term Exposure of Pacu (Piaractus mesopotamicus) Fish to Dietary Aflatoxin B1: Residues in Tissues and Performance. World Mycotoxin Journal . 2021;14(3):411–420. doi: 10.3920/WMJ2020.2659. [DOI] [Google Scholar]
- 20.Li J. L., Guo J. R., Wang P., et al. Assessment of the Hormesis Effect and Toxic Damage of Short-Term Low-Dose Aflatoxin B1 in Grass Carp (Ctenopharyngodon Idellus) Frontiers in Marine Science . 2024;11 doi: 10.3389/fmars.2024.1451204. [DOI] [Google Scholar]
- 21.Kabak B., Dobson A. D., Var I. I. Strategies to Prevent Mycotoxin Contamination of Food and Animal Feed: A Review. Critical Reviews in Food Science and Nutrition . 2006;46(8):593–619. doi: 10.1080/10408390500436185. [DOI] [PubMed] [Google Scholar]
- 22.Gonçalves R. A., Naehrer K., Santos G. A. Occurrence of Mycotoxins in Commercial Aquafeeds in Asia and Europe: A Real Risk to Aquaculture? Reviews in Aquaculture . 2016;10:263–280. [Google Scholar]
- 23.Koletsi P., Schrama J. W., Graat E. A. M., Wiegertjes G. F., Lyons P., Pietsch C. The Occurrence of Mycotoxins in Raw Materials and Fish Feeds in Europe and the Potential Effects of Deoxynivalenol (DON) on the Health and Growth of Farmed Fish Species—A Review. Toxins . 2021;13(6) doi: 10.3390/toxins13060403.403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu F., Groopman J. D., Pestka J. J. Public Health Impacts of Foodborne Mycotoxins. Annual Review of Food Science and Technology . 2014;5(1):351–372. doi: 10.1146/annurev-food-030713-092431. [DOI] [PubMed] [Google Scholar]
- 25.Mwihia E. W., Lyche J. L., Mbuthia P. G., et al. Co-Occurrence and Levels of Mycotoxins in Fish Feeds in Kenya. Toxins . 2020;12(10) doi: 10.3390/toxins12100627.627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohd-Redzwan S., Jamaluddin R., Abd-Mutalib M. S., Ahmad Z. A Mini Review on Aflatoxin Exposure in Malaysia: Past, Present and Future. Frontiers in Microbiology . 2013;4 doi: 10.3389/fmicb.2013.00334.334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinotti L., Ottoboni M., Giromini C., Dell’Orto V., Cheli F. Mycotoxin Contamination in the EU Feed Supply Chain: A Focus on Cereal Byproducts. Toxins . 2016;8(2) doi: 10.3390/toxins8020045.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zain M. E. Impact of Mycotoxins on Humans and Animals. Journal of Saudi Chemical Society . 2011;15(2):129–144. doi: 10.1016/j.jscs.2010.06.006. [DOI] [Google Scholar]
- 29.Stein R. A., Bulboacӑ A. E. Foodborne Diseases . 2017. Mycotoxins; pp. 407–446. [DOI] [Google Scholar]
- 30.Pietsch C. Risk Assessment for Mycotoxin Contamination in Fish Feeds in Europe. Mycotoxin Research . 2020;36(1):41–62. doi: 10.1007/s12550-019-00368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matejova I., Svobodova Z., Vakula J., Mares J., Modra H. Impact of Mycotoxins on Aquaculture Fish Species: A Review. Journal of the World Aquaculture Society . 2017;48(2):186–200. doi: 10.1111/jwas.12371. [DOI] [Google Scholar]
- 32.Richard J. L. Discovery of Aflatoxins and Significant Historical Features. Toxin Reviews . 2008;27(3-4):171–201. doi: 10.1080/15569540802462040. [DOI] [Google Scholar]
- 33.Alshannaq A. F., Gibbons J. G., Lee M.-K., Han K.-H., Hong S.-B., Yu J.-H. Controlling Aflatoxin Contamination and Propagation of Aspergillus flavus by a Soy-Fermenting Aspergillus oryzae Strain. Scientific Reports . 2018;8(1) doi: 10.1038/s41598-018-35246-1.16871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richard S. A., Manaphraim N. Y. B., Kortei N. K. The Novel Neurotoxic and Neuroimmunotoxic Capabilities of Aflatoxin B1 on the Nervous System: A Review. ABCmed . 2020;8(3):1–8. [Google Scholar]
- 35.Farabi S. M. V., Yousefia M., Hajimoradloo A. Aflatoxicosis in Juvenile Huso huso Fed a Contamined Diet. Journal of Applied Ichthyology . 2006;22:234–237. [Google Scholar]
- 36.Marijani E., Kigadye E., Okoth S. Occurrence of Fungi and Mycotoxins in Fish Feeds and Their Impact on Fish Health. International Journal of Microbiology . 2019;2019:17. doi: 10.1155/2019/6743065.6743065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oliveira M., Vasconcelos V. Occurrence of Mycotoxins in Fish Feed and Its Effects: A Review. Toxins . 2020;12(3) doi: 10.3390/toxins12030160.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Z. Y., Jiang Z. Y., Lv H. B., et al. Dietary Aflatoxin Impairs Flesh Quality Through Reducing Nutritional Value and Changing Myofiber Characteristics in Yellow Catfish (Pelteobagrus fulvidraco) Animal Feed Science and Technology . 2021;274 doi: 10.1016/j.anifeedsci.2020.114764.114764 [DOI] [Google Scholar]
- 39.He X. N., Zeng Z. Z., Jiang W. D., et al. Aflatoxin B1 Decreased Flesh Flavor and Inhibited Muscle Development in Grass Carp (Ctenopharyngodon idella) Animal Nutrition . 2024;18:27–38. doi: 10.1016/j.aninu.2024.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshida S., Zhang H., Takahashi R., Yoshida S., Abiko Y., Toriba A. Identification and Removal of Aflatoxin Coprecipitates Derived From Plant Samples on Immunoaffinity Chromatographic Purification. Journal of Chromatography A . 2022;1678 doi: 10.1016/j.chroma.2022.463382.463382 [DOI] [PubMed] [Google Scholar]
- 41.Dharumadurai D., Shanmugapriya S., Thajuddin N., Annamalai P. Aflatoxins —Biochemistry and Molecular Biology . InTechOpen; 2011. Aflatoxins and Aflatoxicosis in Human and Animals. [DOI] [Google Scholar]
- 42.Gell R. M., Carbone I. HPLC Quantitation of Aflatoxin B 1 From Fungal Mycelium Culture. Journal of Microbiological Methods . 2019;158:14–17. doi: 10.1016/j.mimet.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 43.Wu T.-S., Cheng Y.-C., Chen P.-J., Huang Y.-T., Yu F.-Y., Liu B.-H. Exposure to Aflatoxin B1 Interferes With Locomotion and Neural Development in Zebra Fish Embryos and Larvae. Chemosphere . 2019;217:905–913. doi: 10.1016/j.chemosphere.2018.11.058. [DOI] [PubMed] [Google Scholar]
- 44.Selim K. M., El-hofy H., Khalil R. H. The Efficacy of Three Mycotoxin Adsorbents to Alleviate Aflatoxin B 1-Induced Toxicity in Oreochromis niloticus. Aquaculture International . 2014;22(2):523–540. [Google Scholar]
- 45.Han D., Xie S., Zhu X., Yang Y., Guo Z. Growth and Hepatopancreas Performances of Gibel Carp Fed Diets Containing Low Levels of Aflatoxin B1. Aquaculture Nutrition . 2010;16(4):335–342. [Google Scholar]
- 46.Alinezhad S., Faridi M., Falahatkar B., Nabizadeh R., Davoodi D. Effects of Nanostructured Zeolite and Aflatoxin B1 in Growth Performance, Immune Parameters and Pathological Conditions of Rainbow Trout Oncorhynchus mykiss. Fish & Shellfish Immunology . 2017;70:648–655. doi: 10.1016/j.fsi.2017.08.021. [DOI] [PubMed] [Google Scholar]
- 47.Anater A., Araújo C. M. T. D., Rocha D. C. C., et al. Evaluation of Growth Performance, Hematological, Biochemical and Histopathological Parameters of Rhamdia quelen Fed With a Feed Artificially Contaminated With Aflatoxin B1. Aquaculture Reports . 2020;17 doi: 10.1016/j.aqrep.2020.100326.100326 [DOI] [Google Scholar]
- 48.Ghafarifarsani H., Kachuei R., Imani A. Dietary Supplementation of Garden Thyme Essential Oil Ameliorated the Deteriorative Effects of Aflatoxin B1 on Growth Performance and Intestinal Inflammatory Status of Rainbow Trout (Oncorhynchus mykiss) Aquaculture . 2021;531735928 [Google Scholar]
- 49.Bhatt D., Holeyappa S. A., Pandey A., Bansal N., Hundal J. S., Khairnar S. O. Growth Performance, Physiological Response, and Tissue Microarchitecture of the Carp Labeo rohita Challenged With AFB1 Are Improved by Supplementing With Turmeric. Spanish Journal of Agricultural Research . 2024;22(2)e0501 [Google Scholar]
- 50.Ghafarifarsani H., Imani A., Niewold A. A., Pietsch-Schmied T. C., Moghanlou K. S. Synergistic Toxicity of Dietary Aflatoxin B1 (AFB1) and Zearalenone (ZEN) in Rainbow Trout (Oncorhynchus mykiss) Is Attenuated by Anabolic Effects. Aquaculture . 2021;541736793 [Google Scholar]
- 51.Greco M., Pardo A., Pose G. Mycotoxigenic Fungi and Natural Co-Occurrence of Mycotoxins in Rainbow Trout (Oncorhynchus mykiss) Feeds. Toxins . 2015;7(11):4595–4609. doi: 10.3390/toxins7114595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hendricks J. D. Carcinogenicity of Aflatoxins in Nonmammalian Organisms. In: Eaton D. L., Groopman J. D., editors. Toxicology of Aflatoxins: Human Health, Veterinary, and Agricultural Significance . San Diego: Academic Press; 1994. [Google Scholar]
- 53.El-Sayed Y. S., Khalil R. H., Saad T. T. Acute Toxicity of Ochratoxin-A in Marine Water-Reared Sea Bass (Dicentrarchus labrax L.) Chemosphere . 2009;75(7):878–882. doi: 10.1016/j.chemosphere.2009.01.049. [DOI] [PubMed] [Google Scholar]
- 54.Centoducati G., Santacroce M. P., Lestingi A., Casalino E., Crescenzo G. Characterization of the Cellular Damage Induced by Aflatoxin B1 in Sea Bream (Sparus aurata Linnaeus, 1758) Hepatocytes. Proceedings of the 18th ASPA Congress, Italian Journal of Animal Science, Palmero . 2010 [Google Scholar]
- 55.Sepahdari A., Ebrahimzadeh-Mosavi H. A., Sharifpour I., et al. Effects of Different Dietary Levels of AFB1 on Survival Rate and Growth Factors of Beluga (Huso huso) Iranian Journal of Fisheries Sciences . 2010;9(1):14–150. [Google Scholar]
- 56.El-Banna R., Teleb H. M., Hadi M. M., Fakhry F. M. Performance and Tissue Residue of Tilapias Fed Dietary Aflatoxin. Journal of Veterinary Medicine . 1992;40:17–23. [Google Scholar]
- 57.Chávez-Sánchez C., Martinez-Palacios C. A., Osorio-Moreno I. Pathological Effects of Feeding Young Oreochromis niloticus Diets Supplemented With Different Levels of Aflatoxin B1. Aquaculture . 1994;127(1):49–60. doi: 10.1016/0044-8486(94)90191-0. [DOI] [Google Scholar]
- 58.Tuan N. A., Grizzle J. M., Lovell R. T., Manning B. B., Rottinghaus G. E. Growth and Hepatic Lesions of Nile Tilapia (Oreochromis niloticus) Fed Diets Containing Aflatoxin B1. Aquaculture . 2002;212:311–319. [Google Scholar]
- 59.Oliveira S. T. L., Veneroni-Gouveia G., Santurio J. M., Costa M. M. Aeromonas hydrophila in Tilapia (Oreochromis niloticus) After the Intake of Aflatoxins. Arquivos do Instituto Biológico . 2013;80(4):400–406. doi: 10.1590/S1808-16572013000400005. [DOI] [Google Scholar]
- 60.Madhusudhanan N., KavithaLakshmi S. N., Radha Shanmugasundaram K., Shanmugasundaram E. R. B. Oxidative Damage to Lipids and Proteins Induced by Aflatoxin B1 in Fish (Labeo rohita) Protective Role of Amrita Bindu. Environmental toxicology and pharmacology . 2004;17(2):73–77. doi: 10.1016/j.etap.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 61.Zychowski K. E., Hoffmann A. R., Ly H. J., et al. The Effect of Aflatoxin-B1 on Red Drum (Sciaenops ocellatus) and Assessment of Dietary Supplementation of NovaSil for the Prevention of Aflatoxicosis. Toxins . 2013;5(9):1555–1573. doi: 10.3390/toxins5091555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang Y., Han D., Zhu X., et al. Response and Recovery of Gibel Carp From Subchronic Oral Administration of Aflatoxin B1. Aquaculture . 2011;319(2):89–97. [Google Scholar]
- 63.Hoseyni S. Z., Imani A., Vazirzadeh A., Sarvi Moghanlou K., Farhadi A., Razi M. Dietary Aflatoxin B1 and Zearalenone Contamination Affected Growth Performance, Intestinal and Hepatopancreas Gene Expression Profiles and Histology of the Intestine and Gill in Goldfish, Carassius auratus. Aquaculture Reports . 2024;34 doi: 10.1016/j.aqrep.2023.101887.101887 [DOI] [Google Scholar]
- 64.Jantrarotai W., Lovell B. T. Subchronic Toxicity of Aflatoxin B1 to Channel Catfish. Journal of Aquatic Animal Health . 1990;2:248–275. [Google Scholar]
- 65.Liu P., Xie R., Huang W., et al. Negative Effects of Aflatoxin B1 (AFB1) in the Diet on Growth Performance, Protein and Lipid Metabolism, and Liver Health of Juvenile Hybrid Grouper (Epinephelus fuscoguttatus ♀×Epinephelus lanceolatus ♂) Aquaculture Reports . 2023;33101779 [Google Scholar]
- 66.Mahfouz M. E., Sherif A. H. A. Multiparameter Investigation Into Adverse Effects of Aflatoxin on Oreochromis niloticus Health Status. The Journal of Basic & Applied Zoology . 2015;71:48–59. doi: 10.1016/j.jobaz.2015.04.008. [DOI] [Google Scholar]
- 67.Abdelhamid A. M., Salem M. F. I., Mehrim M. A. M., EL-Sharavi M. Nutritions Attempts to Detoxify Aflatoxic Diets of Tilapia Fish. Proceedings of the First Annual Scientific Conference on Animal and Fish Production; 2007; Egypt. Mansura University; pp. 207–230. [Google Scholar]
- 68.Shehata S. A., El-Melegy KhM., Ebrahim M. S. Toxicity Reduction of Aflatoxin B1 by Vitamin C in Fish. Journal of the World Aquaculture Society . 2009;4(2):73–85. [Google Scholar]
- 69.Naiel M. A. E., Ismael N. E. M., Shehata S. A. Ameliorative Effect of Diets Supplemented With Rosemary (Rosmarinus officinalis) on Aflatoxin B1 Toxicity in Terms of the Performance, Liver Histopathology, Immunity and Antioxidant Activity of Nile Tilapia (Oreochromis niloticus) Aquaculture . 2019;511 doi: 10.1016/j.aquaculture.2019.734264.734264 [DOI] [Google Scholar]
- 70.Kenawy A. M., El-Genaidy H. M., Authman M. M. N., Abdel-Wahab M. A. Pathological Studies on Effects of Aflatoxin on Oreochromis niloticus With Application of Different Trials of Control. Egyptain Journal of Comparative Pathology and Clinical Pathology . 2009;22(1):175–193. [Google Scholar]
- 71.Encarnacao P., Srikhum B., Rodrigues I., Hofstetter U. Book of Abstracts, World Aquaculture 2009 . Veracruz, Mexico: 2009. Growth Performance of Red Tilapia (O. niloticus × O. mossambicus) Fed Diets Contaminated With Aflatoxin B1 and the use of a Commercial Product to Suppress Negative Effects. [Google Scholar]
- 72.Mehrim A. I., Salem M. F. Medicinal Herbs Against Aflatoxicosis in Nile Tilapia (Oreochromis niloticus): Clinical Signs, Postmortem Lesions and Liver Histopathological Changes. Egyptian Journal of Aquatic Research . 2013;3:13–25. [Google Scholar]
- 73.Zychowski K. E., Pohlenz C., Mays T., et al. The Effect of NovaSil Dietary Supplementation on the Growth and Health Performance of Nile Tilapia (Oreochromis niloticus) Fed Aflatoxin-B1 Contaminated Feed. Aquaculture . 2013;376-379:117–123. doi: 10.1016/j.aquaculture.2012.11.020. [DOI] [Google Scholar]
- 74.Conroy G. Associacion Americana De Soya . Boletin Informativo, Caracas-Venezuela; 2000. Alteraciones Asociadas Condos Alimentos Comerciales en Tetrahibridos De Tilapia Roja Cultivados en Venezuela; p. p. 33. [Google Scholar]
- 75.Sahoo P. K., Mukherjee S. C. Effect of Dietary β-1,3 Glucan on Immune Responses and Disease Resistance of Healthy and Aflatoxin B1-Induced Immunocompromised Rohu (Labeo rohita Hamilton) Fish & Shellfish Immunology . 2001;11(8):683–695. doi: 10.1006/fsim.2001.0345. [DOI] [PubMed] [Google Scholar]
- 76.Sahoo P. K., Mukherjee S. C. Immunomodulation by Dietary Vitamin c in Healthy and Aflatoxin B1-Induced Immunocompromised Rohu (Labeo rohita) Comparative Immunology, Microbiology and Infectious Diseases . 2003;26(1):65–76. doi: 10.1016/S0147-9571(01)00038-8. [DOI] [PubMed] [Google Scholar]
- 77.Mohapatra S., Sahu N. P., Pal A. K., Prusty A. K., Kumar V., Kumar S. Haematoimmunology and Histo-Architectural Changes in Labeo rohita Fingerlings: Effect of Dietary Aflatoxin and Mould Inhibitor. Fish Physiology and Biochemistry . 2011;37(1):177–186. doi: 10.1007/s10695-010-9428-1. [DOI] [PubMed] [Google Scholar]
- 78.Bhatt D., Pandey A., Holeyappa S. A., Bansal N., Khairnar S. O. Histomorphological Alterations in the Liver of Labeo rohita Exposed to Aflatoxin B1 and Quercetin-Enriched Diets, -Enriched Diets. National Academy Science Letters . 2023:1–8. [Google Scholar]
- 79.El-Sayed Y. S., Khalil R. H. Toxicity, Biochemical Effects and Residue of Aflatoxin B1 in Marine Water-Reared Sea Bass (Dicentrarchus labrax L.) Food and Chemical Toxicology . 2009;47(7):1606–1609. doi: 10.1016/j.fct.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 80.Lovell R. T. Nutrition and Feeding of Fish . New York: Van Nostrand Reinhold; 1989. [DOI] [Google Scholar]
- 81.Bauer D. H., Lee D. J., Sinnhuber R. O. Acute Toxicity of Aflatoxins B1 and G1 in the Rainbow Trout (Salmo gairdneri) Toxicology and Applied Pharmacology . 1969;15(2):415–419. doi: 10.1016/0041-008x(69)90039-8. [DOI] [PubMed] [Google Scholar]
- 82.Imani A., Sarvi Moghanlo K., Mahmoudi S. S., Ghafarifarsani H., Noori F., Farzaneh F. Histopathological Effect of Aflatoxin B1 on Some Internal Tissues of Rainbow Trout (Oncorhynchus mykiss) Journal of Fisheries . 2020;73(2):149–161. [Google Scholar]
- 83.Mahmoudi Kia Z., Imani A., Sarvi Moghanloo K., Razi M. Effect of Simultaneous Rearing Densities, Aflatoxin B1 Toxin and Herb Powder Mixture on Digestive Physiology of Rainbow Trout. Iranian Journal of Fisheries Sciences . 2019;28(1):107–117. [Google Scholar]
- 84.Zhang Z., Zhou Y., Yang J., Zhang W., Mai K., Zhang Y. Evaluation of the Effects of Dietary Mycotoxin-Degrading Adsorbent on Juvenile Turbot (Scophthalmus maximus L.) Fed Aflatoxin B1-Contaminated Diets. Aquaculture Reports . 2023;30 doi: 10.1016/j.aqrep.2023.101539.101539 [DOI] [Google Scholar]
- 85.Lopes P. R. S., Neto J. R., Mallmann C. A., Lazzari R., Pedron F. A., Veiverberg C. A. Crescimento e AlteracËões No fõÂgado e na CarcacËa De Alevinos De Jundia Alimentados Com Dietas Com Aflatoxinas. Pesquisa Agropecuaria Brasileira . 2005;40:1029–1034. [Google Scholar]
- 86.Jantrarotai W., Lovell R. T., Grizzle J. M. Acute Toxicity of Aflatoxin B1 to Channel Catfish. Journal of Aquatic Animal Health . 1990;2:237–247. [Google Scholar]
- 87.Wang X., Wang Y., Li Y., et al. Response of Yellow Catfish (Pelteobagrus fulvidraco) to Different Dietary Concentrations of Aflatoxin B1 and Evaluation of an Aflatoxin Binder in Offsetting Its Negative Effects. Ciencias Marinas . 2016;42(1):15–29. doi: 10.7773/cm.v42i1.2595. [DOI] [Google Scholar]
- 88.Gonçalves R. A., Cam T. D., Tri N. N., Santos G. A., Encarnação P., Hung LTh. Aflatoxin B1 (AFB1) Reduces Growth Performance, Physiological Response, and Disease Resistance in Tra Catfish (Pangasius hypophthalmus) Aquaculture International . 2018 [Google Scholar]
- 89.Motallebi Moghanjouei M. Research Project of the Fisheries Sciences Research Institute of Iran . 2016. Study and Evaluation of Economical and Wellbeing Effects of Aflatoxins in Some Iranian Aquaculture; p. p. 298 P. [Google Scholar]
- 90.Jalilpour J., Sepahdari A., Kakoolaki S., et al. Effects of Aflatoxin B1-Contaminated Feeds on Growth Performance, Blood Parameters and Liver Enzymes of Farmed Acipenser stellatus Fingerlings. Iranian Journal of Aquatic Animal Health . 2018;4(1):95–108. [Google Scholar]
- 91.Zeng Z. Z., Jiang W. D., Wu P., et al. Dietary Aflatoxin B1 Decreases Growth Performance and Damages the Structural Integrity of Immune Organs in Juvenile Grass Carp (Ctenopharyngodon idella) Aquaculture . 2019;500:1–17. doi: 10.1016/j.aquaculture.2018.09.064. [DOI] [Google Scholar]
- 92.He X.-N., Zeng Z.-Z., Wu P., et al. Dietary Aflatoxin B1 Attenuates Immune Function of Immune Organs in Grass Carp (Ctenopharyngodon idella) by Modulating NF-kB and the TOR Signaling Pathway. Frontiers in Immunology . 2022;13 doi: 10.3389/fimmu.2022.1027064.1027064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang Y., Han D., Xiao X., et al. Effect of Dietary Aflatoxin B1 on Growth, Fecundity and Tissue Accumulation in Gibel Carp During the Stage of Gonad Development. Aquaculture . 2014;428(429):236–242. [Google Scholar]
- 94.Sahoo P. K., Mukherjee S. C. Immunosuppressive Effects of Aflatoxin B1 in Indian Major Carp (Labeo rohita) Comparative Immunology, Microbiology and Infectious Diseases . 2001;24(3):143–149. doi: 10.1016/S0147-9571(00)00017-5. [DOI] [PubMed] [Google Scholar]
- 95.Al-Rubaiy A. G., Abdulhassan I. A., Al-Rudainy A. J., Falih I. B. Toxicity Effects of Aflatoxin B1 on Growth Indices and Histopathological Alteration in Cyprinus carpio. Iraqi Journal of Biotechnology . 2018;17(3):17–31. [Google Scholar]
- 96.Tasa H., Imani A., Sarvi Moghanlou K., Nazdar N., Moradi-Ozarlou M. Aflatoxicosis in Fingerling Common Carp (Cyprinus carpio) and Protective Effect of Rosemary and Thyme Powder: Growth Performance and Digestive Status. Aquaculture . 2020;527735437 [Google Scholar]
- 97.Rhadi A. G., Al-Rudainy A. J., Attee R. S. Impact of Aflatoxin B1 on Growth and Histopathological Changes in, Cyprinus carpio. Iraqi Journal of Agricultural Sciences . 2024;55(4):1346–1352. doi: 10.36103/4vnewz86. [DOI] [Google Scholar]
- 98.Schoental R. Aflatoxins. Annual Review of Pharmacology and Toxicology . 1967;7:343–356. doi: 10.1146/annurev.pa.07.040167.002015. [DOI] [PubMed] [Google Scholar]
- 99.McKean C., Tang L., Billam M., et al. Comparative Acute and Combinative Toxicity of Aflatoxin B1 and T-2 Toxin in Animals and Immortalized Human Cell Lines. Journal of Applied Toxicology . 2006;26(2):139–147. doi: 10.1002/jat.1117. [DOI] [PubMed] [Google Scholar]
- 100.Nunes E. M. C. G., Pereira M. M. G., Costa A. P. R., et al. Effects of Aflatoxin B1 on Performance and Health of Tambaqui Fingerlings (Colossoma macropomum) International Aquatic Research . 2019;11(1):73–83. doi: 10.1007/s40071-019-0220-2. [DOI] [Google Scholar]
- 101.Michelin E. C., Massocco M. M., Godoy S. H. S., et al. Carryover of Aflatoxins From Feed to Lambari Fish (Astyanax altiparanae) Tissues. Food Additives & Contaminants: Part A . 2017;34:256–272. doi: 10.1080/19440049.2016.1266097. [DOI] [PubMed] [Google Scholar]
- 102.Souza C. F., Baldissera M. D., Baldisserotto B., et al. Dietary Vegetable Choline Improves Hepatic Health of Nile Tilapia (Oreochromis niloticus) Fed Aflatoxin-Contaminated Diet. Comparative Biochemistry and Physiology Part C . 2020;227 doi: 10.1016/j.cbpc.2019.108614.108614 [DOI] [PubMed] [Google Scholar]
- 103.Goncalves R. A., Schatzmayr D., Albalat A., Mackenzie S. Mycotoxins in Aquaculture: Feed and Food. Reviews in Aquaculture . 2020;12:145–175. [Google Scholar]
- 104.Salem M. F. I., El-Raou E. M. A., Eweedah N. M., Mohamed B. S. Influence of Some Medicinal Plants as Antimycotoxins in Nile Tilapia (Oreochromis niloticus) Diets. Proceedings of the 2nd Global Fisheries and Aquaculture Research Conference, Cairo International Convention Center, Massive Conferences and Trade Fairs; 2009; CABI Digital Library; pp. 227–242. [Google Scholar]
- 105.Huang Y., Zhu X. M., Han D., Yang Y. X., Xie S. Q. Growth and Aflatoxin B1 Accumulation of Gibel Carp Adult Fed With Diets of Different Levels of Alfatoxin B1. Acta Hydrobiologica Sinica . 2012;36:818–825. [Google Scholar]
- 106.Hassanpour S., Moghanlou K. M. S., Razi M., Imani A. Alterations in Growth Indices and Body Composition of Goldfish (Carassius auratus) Fed on Diets Contaminated With Different Levels of Aflatoxin B1 and Zearalenone Toxins. J. Fish . 2021;74(1):153–163. doi: 10.22059/jfisheries.2021.318417.1229. [DOI] [Google Scholar]
- 107.Barany A., Guilloto M., Cosano J., et al. Dietary Aflatoxin B1 (AFB1) Reduces Growth Performance, Impacting Growth Axis, Metabolism, and Tissue Integrity in Juvenile Gilthead Sea Bream (Sparus aurata) Aquaculture. 2020;533736189 [Google Scholar]
- 108.Baglodi V., Jayaraj E. G., Nesara K. M., Abhiman P. B. Effect of Dietary Incorporated Aflatoxin (AFB1) on the Survival and Growth Performance of Labeo rohita. Journal of Experimental Zoology-India . 2015;18:603–607. [Google Scholar]
- 109.Huang Y., Han J. G., Zhu X. M., et al. Effects of Dietary Aflatoxin b1 on Growth, Antioxidant Capacity and Tissue Accumulation of Juvenile Marbled Eel (Anguilla marmorata) Acta Hydrobiologica Sinica . 2021;45(3):566–572. [Google Scholar]
- 110.Liu H., Xie R., Huang W., et al. Effects of Dietary Aflatoxin B1 on Hybrid Grouper (Epinephelus fuscoguttatus × Epinephelus lanceolatus) Growth, Intestinal Health, and Muscle Quality. Aquaculture Nutrition . 2024;120:43–49. doi: 10.1155/2024/3920254. 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Baldissera M. D., Souza C. F., Zeppenfeld C. C., et al. Aflatoxin B1-Contaminated Diet Disrupts the Blood–Brain Barrier and Affects Fish Behavior: Involvement of Neurotransmitters in Brain Synaptosomes. Environmental Toxicology and Pharmacology . 2018;60:45–51. doi: 10.1016/j.etap.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 112.Seidu A. R., Manaphraim N. Y. B., Kortei N. K. The Novel Neurotoxic and Neuro-Immunotoxic Capabilities of Aflatoxin B1 on the Nervous System: A Review. ABC Medical . 2020;8(3):1–8. [Google Scholar]
- 113.Park S., Lee J.-Y., You S., Song G., Lim W. Neurotoxic Effects of Aflatoxin B1 on Human Astrocytes in Vitro and on Glial Cell Development in Zebrafish in Vivo. Journal of Hazardous Materials . 2020;386 doi: 10.1016/j.jhazmat.2019.121639.121639 [DOI] [PubMed] [Google Scholar]
- 114.Barany A., Oliva M., Gregório S. F., Martínez-Rodríguez G., Mancera J. M., Fuentes J. Dysregulation of Intestinal Physiology by Aflatoxicosis in the Gilthead Seabream (Sparus aurata) Frontiers in Physiology . 2021;12 doi: 10.3389/fphys.2021.741192.741192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ghasemi N., Imani A., Noori F., Shahrooz R. Ontogeny of Digestive Tract of Stellate Sturgeon (Acipenser stellatus) From Hatching to Juvenile Stage: Digestive Enzymes Activity, Stomach and Proximal Intestine. Aquaculture . 2020;519734751 [Google Scholar]
- 116.Wei L., Xiu-Mei Z., Li-Bo W. Digestive Enzyme and Alkaline Phosphatase Activities During the Early Stages of Silurus soldatovi Development. Zooological Research . 2010;31(6):627–632. doi: 10.3724/SP.J.1141.2010.06627. [DOI] [PubMed] [Google Scholar]
- 117.Nazdar N., Imani A., Noori F., Sarvi Moghanlou K. Effect of Silymarin Supplementation on Nickel Oxide Nanoparticle Toxicity to Rainbow Trout (Oncorhynchus mykiss) Fingerlings: Pancreas Tissue Histopathology and Alkaline Protease Activity. Iranian Journal of Science and Technology, Transactions A: Science . 2018;42:353–361. [Google Scholar]
- 118.Deng S. X., Tian L. X., Liu F. J., et al. Toxic Effects and Residue of Aflatoxin B1 in Tilapia (O. niloticus × O. aureus) During Long-Term Dietary Exposure. Aquaculture . 2010;307:233–240. [Google Scholar]
- 119.Peng K., Chen B., Zhao H., Huang W. Toxic Effects of Aflatoxin B1 in Chinese Sea Bass (Lateolabrax maculatus) Toxins . 2021;13(12) doi: 10.3390/toxins13120844.844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fan Y., Liu L., Zhao L., et al. Influence of Bacillus subtilis ANSB060 on Growth, Digestive Enzyme and Aflatoxin Residue in Yellow River Carp Fed Diets Contaminated With Aflatoxin B1. Food and Chemical Toxicology . 2018;113:108–114. doi: 10.1016/j.fct.2018.01.033. [DOI] [PubMed] [Google Scholar]
- 121.Wang B., Thompson K. D., Wangkahart E., et al. Strategies to Enhance Tilapia Immunity to Improve Their Health in Aquaculture. Review in Aquaculture . 2022 [Google Scholar]
- 122.Rodriguez-Cervantes C. H., Giron-Perez M. I., Robledo-Marenco M. L., et al. Aflatoxin B1 and Its Toxic Effects on Immune Response of Teleost Fishes: A Review. World Mycotoxin Journal . 2010;3(2):193–199. [Google Scholar]
- 123.Nazdar N., Imani A., Froushani S. M. A., Farzaneh M., Moghanlou K. S. Antioxidative Properties, Phenolic Compounds, and in Vitro Protective Efficacy of Multi-Herbal Hydro-Alcoholic Extracts of Ginger, Turmeric, and Thyme Against the Toxicity of Aflatoxin B1 on Mouse Macrophage RAW264.7 Cell Line. Food Science and Nutrition . :1–17. doi: 10.1002/fsn3.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang Y., Rossi W., Jr., Yamamoto F. Y., Velasquez A. M., Wang A., Gatlin D. M., III Effects of Dietary Aflatoxin B1 on Hybrid Striped Bass (Morone chrysops × M. saxatilis) and Assessment of Supplemental Arginine as a Potential Aflatoxicosis Alleviator. Aquaculture Nutrition . 2022;2022:11. doi: 10.1155/2022/5161222.5161222 [DOI] [Google Scholar]
- 125.Ngethe S., Horsberg T. E., Ingebrigtsen K. The Disposition of H-Aflatoxin B1 in the Rainbow Trout (Oncorhynchus mykiss) After Oral and Intravenous Administration. Aquaculture . 1992;108(3-4):323–332. doi: 10.1016/0044-8486(92)90116-3. [DOI] [Google Scholar]
- 126.El-Enbaawy M., Adel M., Marzouk M., AAS The Effect of Acute and Chronic Aflatoxicosis on the Immune Functions of Oreochromis niloticus in Egypt. VMJ-Giza . 1994;42:47–52. [Google Scholar]
- 127.Arkoosh M. R., Kaattari S. L. Effect of Early Aflatoxin B1 Exposure on in Vivo and in Vitro Antibody Responses in Rainbow Trout, Salmo gairdneri. Journal of Fish Biology . 1987;31(sA):19–22. doi: 10.1111/j.1095-8649.1987.tb05287.x. [DOI] [Google Scholar]
- 128.Marin D. E., Taranu I. Overview on Aflatoxins and Oxidative Stress. Toxin Reviews . 2012;31(3-4):32–43. doi: 10.3109/15569543.2012.730092. [DOI] [Google Scholar]
- 129.Karaman M., Basmacioglu H., Ortatatli M., Oguz H. Evaluation of the Detoxifying Effect of Yeast Glucomannan on Aflatoxicosis in Broiler as Assessed by Gross Examination and Histopathology. British Poultry Science . 2005;46(3):394–400. doi: 10.1080/00071660500124487. [DOI] [PubMed] [Google Scholar]
- 130.Ding Z., Zhang Y., Ye J., Du Z., Kong Y. An Evaluation of Replacing Fish Meal With Fermented Soybean Meal in the Diet of Macrobrachium nipponense: Growth, Nonspecific Immunity, and Resistance to Aeromonas, A Review of Effects of Aflatoxins in Aquaculture. Fish and Shellfish Immunology . 2015;44:295–301. doi: 10.1016/j.fsi.2015.02.024. 2015. [DOI] [PubMed] [Google Scholar]
- 131.Xue M., Fu M., Zhang M., et al. Aflatoxin B1 Induced Oxidative Stress and Gut Microbiota Disorder to Increase the Infection of Cyprinid Herpesvirus 2 in Gibel Carp (Carassius auratus gibelio) Antioxidants . 2023;12(2) doi: 10.3390/antiox12020306.306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Peng K., Shirley D. C., Xu Z., et al. Effect of Purple Prairie Clover (Dalea purpurea Vent.) Hay and Its Condensed Tannins on Growth Performance, Wool Growth, Nutrient Digestibility, Blood Metabolites and Ruminal Fermentation in Lambs Fed Total Mixed Rations. Animal Feed Science and Technology . 2016;222:100–110. doi: 10.1016/j.anifeedsci.2016.10.012. [DOI] [Google Scholar]
- 133.Di Paola D., Iaria G., Capparucci F., et al. Impact of Mycotoxin Contaminations on Aquatic Organisms: Toxic Effect of Aflatoxin B1 and Fumonisin B1 Mixture. Toxins . 2022;14(8) doi: 10.3390/toxins14080518.518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pietsch C. Food Safety: The Risk of Mycotoxin Contamination in Fish. 2019. [DOI]
- 135.Bedoya-Serna C. M., Michelin E. C., Massocco M. M., et al. Effects of Dietary Aflatoxin B1 on Accumulation and Performance in Matrinxã Fish (Brycon cephalus) PloS One . 2018;13(8) doi: 10.1371/journal.pone.0201812.e0201812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cordaro M., Trovato Salinaro A., Siracusa R., et al. Hidrox Roles in Neuroprotection: Biochemical Links Between Traumatic Brain Injury and Alzheimer’s Disease. Antioxidants . 2021;10(5) doi: 10.3390/antiox10050818.818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Weichhart T., Costantino G., Poglitsch M., et al. The TSC-mTOR Signaling Pathway Regulates the Innate Inflammatory Response. Immunity . 2008;29(4):565–577. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 138.Chen S., Zhu J., Chen G., et al. 1,25-Dihydroxyvitamin D3 Preserves Intestinal Epithelial Barrier Function From Tnf-A Induced Injury Via Suppression of Nf-Kb P65 Mediated Mlck-P-Mlc Signaling Pathway. Biochemical and Biophysical Research Communications . 2015;460(3):873–878. doi: 10.1016/j.bbrc.2015.03.125. [DOI] [PubMed] [Google Scholar]
- 139.Ottinger C., Kaattari S. Sensitivity of Rainbow Trout Leucocytes to Aflatoxin B1. Fish & Shellfish Immunology . 1998;8(7):515–530. doi: 10.1006/fsim.1998.0154. [DOI] [Google Scholar]
- 140.Mishra H. N., Das C. A Review on Biological Control and Metabolism of Aflatoxin. Critical Reviews in Food Science and Nutrition . 2003;43(3):245–264. doi: 10.1080/10408690390826518. [DOI] [PubMed] [Google Scholar]
- 141.Ronyai A., Peteri A., Radics F. Cross Breeding of Starlet and Lena River Sturgeon. Aquaculture Hungrica Szarwas . 1990;6:13–18. [Google Scholar]
- 142.Hassaan M. S., Wafa M. A., Soltan M. A., Goda A. S., Mogheth N. M. A. Effect of Dietary Organic Salts on Growth, Nutrient Digestibility, Mineral Absorption and Some Biochemical Indices of Nile Tilapia; Oreochromis niloticus L Fingerlings. World Applied Sciences Journal . 2014;29(1):47–55. [Google Scholar]
- 143.Tung H.-T., Cook F. W., Wyatt R. D., Hamilton P. B. The Anemia Caused by Aflatoxin. Poultry Science . 1975;54(6):1962–1969. doi: 10.3382/ps.0541962. [DOI] [PubMed] [Google Scholar]
- 144.Bailey G. S., Williams D. E., Wilcox J. S., Loveland P. M., Coulombe R. A., Hendricks J. D. Aflatoxin B1 Carcinogenesis and Its Relation to DNA Adduction Formation and Adduct Per-Sistence in Sensitive and Resistant Salmonid Fish. Carcinogenesis . 1988;9(11):1919–1926. doi: 10.1093/carcin/9.11.1919. [DOI] [PubMed] [Google Scholar]
- 145.Nakajima H., Nakajimatakagi Y., Tsujita T., et al. Correction: Tissue-Restricted Expression of Nrf2 and Its Target Genes in Zebrafish With Gene-Specific Variations in the Induction Profiles. Plos One . 2012;7(9) doi: 10.1371/annotation/50ee3aff-3010-4c42-a130-70509c88a67e.e26884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.He C. H., Fan Y. H., Wang Y., Huang C. Y., Wang X. C., Zhang H. B. The Individual and Combined Effects of Deoxynivalenol and Aflatoxin B1 on Primary Hepatocytes of Cyprinus carpio. International Journal of Molecular Sciences . 2010;11(10):3760–3768. doi: 10.3390/ijms11103760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li M., Kong Y., Guo W., et al. Dietary Aflatoxin B1 Caused the Growth Inhibition, and Activated Oxidative Stress and Endoplasmic Reticulum Stress Pathway, Inducing Apoptosis and Inflammation in the Liver of Northern Snakehead (Channa argus) Science of the Total Environment . 2022;850 doi: 10.1016/j.scitotenv.2022.157997.157997 [DOI] [PubMed] [Google Scholar]
- 148.Shankar Murti A., Panda P. C., Sreenivasa Murthy V., Murti I. A. Growth Retardation and Change in Organ Weights due to Aflatoxicosis in Rats and Guineapigs. Indian Veterinary Journal . 1979;56:279–283. [PubMed] [Google Scholar]
- 149.Stoskopf M. K. In: Fish Medicine . Phelps T. H., Bauer B. A., editors. Philadelphia: Saunders Company; 1993. p. p. 882. [Google Scholar]
- 150.Liew W. P. P., Mohd-Redzwan S., Lim H. A., Ng W. K., Lim S. L., Ibrahim C. O. Mycotoxin: Its Impact on Gut Health and Microbiota. Frontiers in Cellular and Infection Microbiology . 2018;8 doi: 10.3389/fcimb.2018.00060.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gao Y., Bao X., Meng L., Liu H., Wang J., Zheng N. Aflatoxin B1 and Aflatoxin M1 Induce Compromised Intestinal Integrity Through Clathrin-Mediated Endocytosis. Toxins . 2021;13(3) doi: 10.3390/toxins13030184.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Huang C., Wu P., Jiang W. D., et al. Deoxynivalenol Decreased the Growth Performance and Impaired Intestinal Physical Barrier in Juvenile Grass Carp (Ctenopharyngodon idella) Fish & Shellfish Immunology . 2018;80:376–391. doi: 10.1016/j.fsi.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 153.Banerjee S., Bhattacharya S. Histopathological Changes Induced by Chronic Nonlethal Levels of Elsan, Mercury, and Ammonia in the Small Intestine of Channa Punctatus. Ecotoxicology and Environmental Safety . 1995;31:62–68. doi: 10.1006/eesa.1995.1044. [DOI] [PubMed] [Google Scholar]
- 154.Ayyat M. S., Ayyat A. M. N., Al-Sagheer A. A., El-Hais A. E. A. M. Effect of Some Safe Feed Additives on Growth Performance, Blood Biochemistry, and Bioaccumulation of Aflatoxin Residues of Nile Tilapia Fed Aflatoxin-B1 Contaminated Diet. Aquaculture . 2018;495:27–34. doi: 10.1016/j.aquaculture.2018.05.030. [DOI] [Google Scholar]
- 155.Brown R., Priest E., Naglik J. R., Richardson J. P. Fungal Toxins and Host Immune Responses. Frontiers in Microbiology . 2021;12 doi: 10.3389/fmicb.2021.643639.643639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Verdile N., Pasquariello R., Scolari M., Scirè G., Brevini T. A. L., Gandolfi F. A Detailed Study of Rainbow Trout (Onchorhynchus mykiss) Intestine Revealed That Digestive and Absorptive Functions Are Not Linearly Distributed Along Its Length. Animals . 2020;10(4) doi: 10.3390/ani10040745.745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yancheva V., Velcheva I., Stoyanova S., Georgieva E. Histological Biomarkers in Fish as a Tool in Ecological Risk Assessment and Monitoring Programs: A Review. Applied Ecology and Environmental Research . 2016;14(1):47–75. doi: 10.15666/aeer/1401_047075. [DOI] [Google Scholar]
- 158.He X. N., Zeng Z. Z., Feng L., et al. 2023. Aflatoxin B1 Damaged Structural Barrier Through Keap1a/Nrf2/ MLCK Signaling Pathways and Immune Barrier Through NF-κB/ TOR Signaling Pathways in Gill of Grass Carp (Ctenopharyngodon idella) Aquatic Toxicology . 2023;257 doi: 10.1016/j.aquatox.2023.106424.106424 [DOI] [PubMed] [Google Scholar]
- 159.Mehrim A. I., Abdelhamid A. M., Abo Shosha A. A. M., Salem M. F. I., El-Sharawy M. A. M. M. Nutrtious Attempts to Detoxify Aflatoxic Diets of Tilapia Fish 2-Clinical, Biochemical and Histological Parameters. Journal of the World Aquaculture Society . 2006;1(2):69–90. [Google Scholar]
- 160.Sahoo P. K., Mukherjee S. C., Nayak S. K., Dey S. Acute and Subchronic Toxicity of Aflatoxine B1 to Rohum Labeo rohita (Hamilton) Indian Journal of eXperimental Biology . 2001;39(5):453–458. [PubMed] [Google Scholar]
- 161.Ashley L. M. Histopathology of Rainbow Trout Aflatoxicosis. In: Halver J. E., Mitchell) I. A., editors. Trout Hepatoma Research Conference Papers . Washington: Bureau of Sport Fisheries and Wildlife; 1967. pp. 103–120. [Google Scholar]
- 162.Tuurala H. Relationships Between Secondary Lamellar Structure and Dorsal Aortic Oxygen Tension in Salmo gairdneri With Gills Damaged by Zinc. Annals De Zoologica Fennici . 1983;20:235–238. [Google Scholar]
- 163.Fumagalli F., Ottoboni M., Pinotti L., Cheli F. Integrated Mycotoxin Management System in the Feed Supply Chain. Innovative Approaches . 2021;13(8) doi: 10.3390/toxins13080572.572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nogueira W. V., Tesser M. B., Buffon J. G. Assessment of Mycotoxins Found in Farmed Fish Feed. Aquaculture International . 2024;32(7):9795–9851. doi: 10.1007/s10499-024-01638-6. [DOI] [Google Scholar]
- 165.Barbosa T. S., Pereyra C. M., Soleiro C. A., et al. Mycobiota and Mycotoxins Present in Finished Fish Feeds From Farms in the Rio De Janeiro State, Brazil. International Aquatic Research . 2013;5:p. 3. [Google Scholar]
- 166.Fallah A. A., Pirali-Kheirabadi E., Rahnama M., Saei-Dehkordi S. S., Pirali-Kheirabadi K. Mycoflora, Aflatoxigenic Strains of Aspergillus Section Flavi and Aflatoxins in Fish Feed. Quality Assurance and Safety of Crops & Foods . 2014;6(4):419–424. doi: 10.3920/QAS2012.0186. [DOI] [Google Scholar]
- 167.Anater A., Manyes L., Meca G., et al. Mycotoxins and Their Consequences in Aquaculture: A Review. Aquaculture . 2016;451:1–10. doi: 10.1016/j.aquaculture.2015.08.022. [DOI] [Google Scholar]
- 168.Mwihia M., Eriksen P., Gathumbi G. Occurrence and Levels of Aflatoxins in Fish Feeds and Their Potential Effects on Fish in Nyeri, Kenya. Toxins . 2018;10(12) doi: 10.3390/toxins10120543.543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.World Health Organization (WHO) International Agency for Research on Cancer (IARC). Monographs on the Evaluation of Carcinogenic Risks to Humans . Lyon: IARC; 2002. p. p. 82. [Google Scholar]
- 170.Food and Drug Administration (FDA) CPG Section 683.100 Action Levels for Aflatoxins in Animal Feeds. 2015. https://www.fda.gov/iceci/compliancemanuals/compliancepolicy,guidancemanual/ucm074703.htm,guidancemanual/ucm074703.htm .
- 171.María E., Gonçalves-Nunes C., Gomes-Pereira M. M., et al. Screening of Aflatoxin B1 and Mycobiota Related to Raw Materials and Finished Feed Destined for Fish. Latin American Journal of Aquatic Research . 2015;43(3):595–600. [Google Scholar]
- 172.Samuel T. O., Odunigba O. Aflatoxins Associated With Storage Fungi in Fish Feeds. IFE Journal of Science . 2015;17(2):519–523. [Google Scholar]
- 173.Marijani E., Wainaina J. M., Charo-Karisa H., et al. Mycoflora and Mycotoxins in Finished Fish Feed and Feed Ingredients From Smallholder Farms in East Africa. Egyptian Journal of Aquatic Research . 2017. pp. 169–176.
- 174.Nácher-Mestre J., Serrano R., Beltrán E., et al. Occurrence and Potential Transfer of Mycotoxins in Gilthead Sea Bream and Atlantic Salmon by use of Novel Alternative Feed Ingredients. Chemosphere . 2015;128:314–320. doi: 10.1016/j.chemosphere.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 175.Svobodova Z., Piskac A. Effect of Feeds With a Low Content of Aflatoxin B1 on the Health of Carp Cyprinus carpio. Zivocisna Vyroba-UVTIZ . 1980;25(11):809–814. [Google Scholar]
- 176.Svobodova Z., Piskac A., Havlikova J., Groch L. Influence of Feed With Different Contents of B Sub (1) Aflatoxin on the Carp Health Condition. Zivocisna Vyroba . 1982;27(11):811–820. [Google Scholar]
- 177.Akhter A., Rahman M., Hasan M. Effects of Aflatoxin B1 on Growth and Bioaccumulation in Common Carp Fingerling in Bangladesh. Asia-Pacific Journal of Rural Development . 2010;20(2):1–14. doi: 10.1177/1018529120100201. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article, as no new data were created or analyzed in this study.


