Abstract
Background:
Per- and polyfluoroalkyl substances (PFAS) are fluorinated chemicals linked to adverse pregnancy and birth outcomes. However, the underlying mechanisms, specifically their effects on maternal inflammatory processes, are not well characterized.
Objective:
We examined associations between prenatal PFAS exposure and repeated measures of inflammatory biomarkers, including C-reactive protein (CRP) and four cytokines [Interleukin-10 (IL-10), IL-1β, IL-6, and tumor necrosis factor-α (TNF-α)].
Methods:
We analyzed data from 469 pregnant women in a nested case-control study of preterm birth at Brigham and Women’s Hospital in Boston, Massachusetts (2006–2008). We measured nine PFAS in early pregnancy plasma samples (median gestation: 10 weeks), with inflammatory biomarkers measured at median gestations of 10, 18, 26, and 35 weeks. We used linear mixed models for repeated measures and multivariable regression for visit-specific analysis to examine associations between each PFAS and inflammation biomarker, adjusting for maternal demographics, pre-pregnancy BMI, and parity. We examined the effects of PFAS mixture using sum of all PFAS (∑PFAS) and quantile-based g-computation approaches.
Results:
We observed consistent inverse associations between most PFAS and cytokines, specifically IL-10, IL-6, and TNF-α, in both single pollutant and mixture analyses. For example, an interquartile range increase in perfluorooctanesulfonic acid was associated with −10.87 (95% CI: −19.75, −0.99), −13.91 (95% CI: −24.11, −2.34), and −8.63 (95% CI: −14.51, −2.35) percent change in IL-10, IL-6, and TNF-α levels, respectively. Fetal sex, maternal race, and visit-specific analyses showed associations between most PFAS and cytokines were generally stronger in mid-pregnancy and among women who delivered males or identified as African American.
Conclusions:
The observed suppression of both regulatory (IL-10) and pro-inflammatory (TNF-α) cytokines suggests that PFAS may alter maternal inflammatory processes or immune functions during pregnancy. Further research is needed to understand the effects of both legacy and newer PFAS on inflammatory pathways and their broader clinical implications.
Keywords: PFAS, Cytokines, C-reactive protein, Inflammation, Pregnancy, LIFECODES
1. Introduction
Per- and polyfluoroalkyl substances (PFAS) are a class of synthetic fluorinated chemicals that have been extensively used in consumer products and industrial applications for over six decades (Buck et al., 2011). These compounds, characterized by their water- and oil-repelling properties, are valuable additives in a wide range of products, including non-stick cookware, water-repellent clothing, stain-resistant fabrics, firefighting foams, and various cleaning products (Sunderland et al., 2019). Certain long-chain PFAS (≥ 6 carbons) tend to be persistent, bioaccumulative, and toxic at low levels (Sunderland et al., 2019; Fenton et al., 2021), and have been detected in the bodies of more than 90 % of the US adult population (Calafat et al., 2019), raising concerns about their impact on human health and the environment. Recently, the United States Environmental Protection Agency (EPA) established new drinking water regulations for PFAS, setting individual maximum contaminant levels of 4 parts per trillion (ppt) for perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS), and 10 ppt for perfluorohexane sulfonic acid (PFHxS), perfluorononanoic acid (PFNA), and hexafluoropropylene oxide dimer acid (HFPO-DA, also known as GenX chemicals). Additionally, it implemented a hazard index approach for a mixture of PFHxS, PFNA, HFPO-DA, and perfluorobutane sulfonic acid (PFBS) to account for their additive health effects (USEPA, 2023). The ubiquity and potential toxicity of PFAS has raised important health concerns for vulnerable populations, particularly pregnant women and developing fetuses. Notably, PFAS can cross the placental barrier, exposing fetuses during crucial developmental periods (VanNoy et al., 2018; Gützkow et al., 2012). Increasing evidence has linked prenatal PFAS exposure to adverse pregnancy outcomes, including preeclampsia, altered birthweight, and preterm birth, with potential long-term implications for postnatal development (Gao et al., 2021).
The mechanisms underlying the adverse health effects of PFAS exposure during pregnancy are not fully understood. However, disruptions in inflammatory processes, oxidative stress, and endocrine functions may play a role (Welch et al., 2022; Rivera-Núñez et al., 2023). Cytokines, signaling molecules that direct and regulate immune responses and other biological processes, are central to inflammatory processes, which are intricately linked to the maternal immune response and play crucial roles across different phases of pregnancy. In the early stage, a pro-inflammatory state, characterized by an increase in Type 1 helper (Th1) cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), is dominant to ensure successful endometrial remodeling, embryo implantation, and placentation (Mor et al., 2011; Dekel et al., 2014). As pregnancy advances into the mid-phase, the inflammatory response mostly shifts toward an anti-inflammatory state. This shift, marked by an increase in Type 2 helper (Th2) or regulatory cytokines such as IL-10, ensures rapid fetal growth and development while maintaining effective placental function (Mor et al., 2011). The last phase of pregnancy, usually occurring after the fetus has fully developed with functional organs and leading to parturition, is marked by a reactivation of the pro-inflammatory state. This shift towards a Th1 environment promotes uterine contractions, which aid in expelling the baby and placenta (Mor et al., 2011). Any perturbation of these tightly regulated inflammatory processes could have significant implications for pregnancy outcomes (Al-Gubory, 2016; Orsi and Tribe, 2008). Indeed, dysregulation of cytokine networks has been implicated in the onset of multiple pregnancy complications (Orsi and Tribe, 2008; Spence et al., 2021; Ferguson et al., 2014a; Eick et al., 2022; Pandey et al., 2017; Prairie et al., 2021). For instance, changes in maternal IL-10 and IL-6 levels have been linked with preterm birth (Ferguson et al., 2014a; Wei et al., 2010), while changes in several cytokine levels have been associated with preeclampsia (Spence et al., 2021; Aggarwal et al., 2019), or intrauterine growth restriction (Bartha et al., 2003; Kirici et al., 2023).
Several factors, including maternal infections (Goldenberg et al., 2000; Urakubo et al., 2001), chronic conditions (Steptoe et al., 2007; Blackmore et al., 2011; Coussons-Read et al., 2007; Pendeloski et al., 2017), genetic or epigenetic factors (Varner and Esplin, 2005; Munro et al., 2021), and environmental factors (Ferguson et al., 2014b; Kelley et al., 2019; Thompson González et al., 2022) could lead to the modulation of cytokine networks or other inflammatory signaling pathways during pregnancy. While numerous studies have examined the effects of PFAS exposure on various aspects of immune function in both experimental and epidemiological studies (Ehrlich et al., 2023a; Phelps et al., 2024; Zhang et al., 2023; Grandjean et al., 2017), information specifically on PFAS and maternal cytokine levels during pregnancy remains limited and inconsistent. A handful of studies have reported positive associations between prenatal PFAS and circulating levels of cytokines across pregnancy (Tan et al., 2023; Zota et al., 2018; Palaniyandi et al., 2023). One study involving African American pregnant women in Atlanta found positive associations between several PFAS (and their mixture) and levels of IL-6, IL-10, TNF-α, and IFN-γ in early and mid-gestation (Tan et al., 2023). Another study involving obese and overweight pregnant women in California reported a positive association between PFAS and IL-6 (Zota et al., 2018), with similar findings between a few PFAS and pro-inflammatory index consisting of several cytokines in a group of pregnant women in Canada (Palaniyandi et al., 2023). Conversely, a cross-sectional study in Illinois observed a negative association between PFAS mixture and TNF-α in pregnant women (Cinzori et al., 2024). Studies in non-pregnant women have shown varied patterns, with both positive and negative associations between certain PFAS and cytokines (Nian et al., 2022; Cauble et al., 2024; Matta et al., 2022; Bassler et al., 2019). Most of these studies, while informative, have several limitations, including their focus on the four most common PFAS, limited number of cytokine measurements during pregnancy, small sample size in some cases, and the use of non-generalizable population subgroups. Additionally, the sex-specific effects of PFAS on inflammatory biomarkers have not been well explored, which is a crucial data gap considering the sexually dimorphic effects of PFAS reported in earlier studies (Everson et al., 2024; Svoboda et al., 2022; Roth et al., 2021).
Therefore, the objective of this study was to further evaluate associations between prenatal PFAS exposure and inflammatory biomarkers in a group of pregnant women in the LIFECODES cohort. We analyzed repeated measures (up to four observations per participant) of different circulating cytokines and a protein biomarker of inflammation. We examined the effects of individual PFAS as well as their mixture, hypothesizing that elevated PFAS levels would lead to the dysregulation of these biomarkers. Additionally, we tested for effect modification by fetal sex and maternal race to examine whether the effects of PFAS on these biomarkers vary across different categories.
2. Materials and methods
2.1. Study population
The current study is based on a nested case-control design within the LIFECODES birth cohort, which enrolled 1,648 pregnant women at Brigham and Women’s Hospital (BWH) in Boston, Massachusetts, between 2006 and 2008 (Ferguson et al., 2014c; Cantonwine et al., 2015). Eligibility criteria for the LIFECODES cohort included seeking prenatal care before 15 weeks of gestation, being over 18 years old, and planning to deliver at BWH. Women with higher-order multiple pregnancies (triplets or higher) were excluded. Written informed consent was obtained from all participants, and the cohort study protocols were approved by the Institutional Review Board (IRB) at BWH.
In 2011, a nested case-control study was established within the LIFECODES cohort to investigate the effects of prenatal environmental exposures on preterm birth. This study included 130 singleton preterm birth cases (delivery before 37 weeks gestation) and 352 randomly selected controls (delivery at or after 37 weeks gestation). The current secondary analysis included 469 participants (127 preterm cases; 342 controls) after excluding 13 participants due to insufficient data. Information on sociodemographic characteristics and personal medical history were collected using detailed questionnaires at the first study visit [median gestational age (GA) = 10 weeks], while birth outcomes data were collected at delivery. Urine and plasma samples were also collected at the first visit and three follow-up visits (median GA = 18, 26, and 35 weeks, respectively). The IRBs at both BWH and the University of Michigan approved the study protocols for the nested case-control study.
2.2. PFAS measurements
We used maternal plasma samples collected during the first study visit (91.1% of participants) or, if unavailable, during the second visit (8.9% of participants) to measure concentrations of nine PFAS compounds, namely N-methylperfluoro-1-octanesulfonamidoacetic acid (MPAH), perfluorodecanoic acid (PFDA), perfluoroheptanoic acid (PFHP), PFHxS, PFNA, PFOA, PFOS, perfluorooctane sulfonamide (PFOSA), and perfluoroundecanoic acid (PFUA). These PFAS were selected because they are among the most commonly detected in the U. S. population (Calafat et al., 2019; NCHS, 2020) and could be reliably measured using the available analytical methods and standards at the time of analysis. The samples were analyzed by NSF International (Ann Arbor) using a validated method based on the Centers for Disease Control and Prevention’s Polyfluoroalkyl Chemicals laboratory method No. 6304.1.
In brief, all plasma samples, stored at −80 °C, were thawed and vortex-mixed to ensure homogeneity; and standards and reagents were allowed to reach room temperature before use. All samples, method blanks, plasma blanks, calibration standards, and quality control (QC) samples were manually prepared in 2 mL autosampler vials. Optima water, control human plasma, and plasma samples were added to appropriate vials, while isotopically labeled internal standards were added to all vials. Methanol was added to plasma samples, method blanks, and plasma blanks, while standard solutions were added to calibration standards and QC samples. Ninety percent formic acid was then added to all samples, and the vials were sealed and briefly vortexed. The samples then underwent pre-concentration using on-line solid phase extraction (SPE) with a Thermo Scientific Transcend TXII system and Cyclone-P extraction column. Analytes were separated using a Dionex UltiMate 3000 UHPLC system with a Waters XBridge C18 analytical column and analyzed using liquid chromatography-tandem mass spectrometry (LC-MS/MS) with heated electrospray ionization (HESI) on a Thermo Scientific Quantiva mass spectrometer, operating in negative mode. The method was validated with an analyte calibration curve correlation coefficient (R2) of 0.998 or greater. The method accuracy, determined through six replicate analyses of plasma samples spiked at three different concentrations across three days, ranged from 90–108% for all analytes. The precision, indicated by the relative standard deviation (RSD), was between 1.6–8%. Calibration checks using internal standards were conducted every 10 samples to ensure system performance, and any samples exceeding the calibration range were appropriately diluted. The standards and internal standards had purity greater than 98%, and other reagents exceeded 99% purity.
The limit of detection (LOD) was 0.5 ng/mL for PFOA and 0.1 ng/mL for all other compounds. All PFAS were detected in over 75% of samples, except for PFHP and PFOSA. PFHP was detected in 43% of samples and treated as a dichotomous variable (above/below the LOD) in the statistical analysis (Siwakoti et al., 2024). PFOSA was detected in only 1% of samples and was excluded from further analysis. For samples with PFAS concentrations below LOD, machine-read values (MRVs) were used when available. When MRVs were not available, the levels were imputed by dividing the LOD by the square root of 2 (LOD/√2). The concentrations of all PFAS except PFHP were natural log-transformed to minimize the influence of outliers in statistical analysis.
2.3. Inflammation biomarkers measurements
We analyzed maternal plasma samples collected during each study visit (one to four samples per participant) to measure concentrations of inflammation biomarkers at the University of Michigan Cancer Center Immunological Monitoring Core (Ann Arbor, MI). Detailed methods for measuring these biomarkers are described elsewhere (Ferguson et al., 2014a). Briefly, the concentration of C-reactive protein (CRP) was determined using a DuoSet enzyme-linked immunosorbent assay (ELISA) with a sensitivity of 10 pg/mL. Only one sample was detected below the LOD, and its value was replaced with LOD/√2. The concentrations of IL-1β, IL-6, IL-10, and TNF-α were measured using a Milliplex MAP High Sensitivity Human Cytokine Magnetic Bead Panel, which allowed for simultaneous measurement of multiple cytokines in small sample volumes. Most samples were measured in duplicate with high correlation (Spearman ρ ranging from 0.96 to 0.99), while a subset (N = 176) was measured once. Detection rates for IL-6, IL-10, and TNF-α were greater than 98%, while IL-1β was detected in 78% of samples. For samples with measured concentrations below the LOD (0.128 pg/mL), MRVs were used when available. For samples below the LOD, but without MRVs, values were imputed using LOD/√2. After these substitutions, the arithmetic average of duplicate measures was calculated for statistical analysis. The distributions of all inflammation biomarkers were right-skewed and were natural log-transformed to approximate a normal distribution for statistical analysis.
2.4. Covariates assessments
We collected data on sociodemographic and behavioral characteristics, as well as personal medical history, through questionnaires during the initial visit. Covariates collected at the baseline visit included self-reported maternal age (measured continuously in years), maternal race (Caucasian, African American, Hispanic, Asian, Mixed, or Other), insurance status (private/HMO or Medicaid/uninsured), educational attainment (high school or less, some college, undergraduate degree or higher), nulliparity (yes/no), smoking status during pregnancy (yes/no), alcohol consumption during pregnancy (yes/no), and personal history of renal disease (yes/no). The pre-pregnancy body mass index (BMI) (kg/m2) was deduced from self-reported maternal weight and height during the first visit. Gestational age at the time of sample collection was estimated using the last menstrual period and ultrasound measurements, following guidelines established by the American College of Obstetrics and Gynecologists (ACOG, 2017). The biological sex of the newborn was recorded post-delivery.
For primary statistical analysis, we combined participants identifying as Asian (N = 37), Hispanic (N = 66), Mixed (N = 10), and Other (N = 14) into a single category due to smaller sample sizes within these groups. However, we explored more granular racial categories in the sensitivity analysis. It is important to note that we do not anticipate maternal race to directly impact the inflammatory processes at the cellular level in pregnant women. Instead, we regard race as a possible proxy for various unmeasured cultural or societal factors, including structural or environmental racism and health disparities, which may not be entirely captured by other socioeconomic indicators included in our study (Williams et al., 2016).
2.5. Descriptive statistics of study population, exposures, and outcomes
We performed univariate analyses to summarize the demographic and socioeconomic characteristics of our study participants, calculating both unweighted and weighted proportions for categorical variables, and medians with first (Q1) and third (Q3) quartiles for continuous variables. The weighted statistics (Lumley et al., 2010) were calculated to account for the case-control design of the study, and details on the weighting methodology are provided in the statistical analysis section. Additionally, we computed the distributions [median (Q1, Q3)] of PFAS and inflammatory biomarker concentrations across different categories. We assessed correlations between PFAS compounds and inflammatory biomarkers using Spearman’s rank correlation (Hauke and Kossowski, 2011). The stability of inflammatory biomarker measurements across pregnancy visits was evaluated using intraclass correlation coefficients (ICCs), with higher values indicating greater consistency or lower variability (Rosner BA: Fundamentals of biostatistics, 2006). The ICCs for inflammatory biomarkers in this cohort have also been published elsewhere (Ferguson et al., 2014a). All hypotheses were tested at α = 0.05 unless otherwise noted. In line with recommendations from various statistical and scientific organizations, we interpreted our findings by considering the magnitude, direction, and precision of the effect estimates, rather than relying solely on p-values (Savitz et al., 2024). All statistical analyses were performed using RStudio version 2024.04.2 (Posit, 2024).
2.6. Adjusted pairwise associations between PFAS and inflammatory biomarkers
In single pollutant analysis, we examined pairwise associations between individual PFAS and inflammatory biomarkers separately. For repeated measures analysis, linear mixed-effects regression models with random intercepts (implemented using the ‘lme4′ package in R) (Bates et al., 2014) were used to estimate covariate-adjusted differences (β) and 95% confidence intervals (CIs) in log-transformed biomarker concentrations associated with a 1-unit increase in log-transformed PFAS concentrations. The β coefficients and CIs were expressed as percent changes in biomarker levels per interquartile range (IQR) increase in PFAS concentrations, derived using the equation: [exp(β × IQR) − 1] × 100. We adjusted all models for maternal age, pre-pregnancy BMI, maternal education, maternal race, insurance status, and parity, which were selected a priori based on published literature and a directed acyclic graph (Fig. S1). We applied inverse probability weights (IPW) to account for the nested case-control study design and to generalize our findings to the broader LIFECODES population (Jiang et al., 2006). The weights applied were 1.1 for participants with preterm deliveries and 2.95 for those with term deliveries, reflecting selection probabilities of 90.1% and 33.9%, respectively.
We assessed potential non-linear associations between each PFAS and repeated measures of an inflammatory biomarker using generalized additive mixed models (GAMMs), as implemented in the ‘mgcv’ package in R (Wood, 2001). To examine whether the associations between early pregnancy PFAS concentrations and inflammatory biomarkers varied throughout pregnancy, we conducted visit-specific analyses using multivariable linear regression models, adjusting for the same covariates as those used in the repeated measures analysis.
We investigated potential effect modifications by fetal sex and maternal race on the effects of PFAS by including a product interaction term between each PFAS compound and fetal sex (male vs. female) or maternal race [White, African American, Other] in the mixed-effects regression models. Additionally, we ran separate models stratified by fetal sex and maternal race to obtain effect estimates for each stratum. We conducted all primary analyses using complete case data (N = 1485 observations from 448 participants, including 121 preterm cases and 327 controls), which included participants with available data on exposures, outcomes, and primary covariates.
2.7. Associations between PFAS mixture and inflammatory biomarkers
We examined the combined effects of PFAS compounds detected in more than 70% of samples on inflammatory biomarkers using two approaches. First, following the guidance from the National Academy of Science, Engineering, and Medicine (NASEM) on PFAS exposure, testing, and clinical follow-up among vulnerable populations in the US (NASEM, 2022), we calculated cumulative PFAS exposure (∑PFAS) for each participant by summing the concentrations of seven PFAS modeled as continuous exposures in the single pollutant analysis. The effect of ∑PFAS on each biomarker was then assessed using linear mixed-effects models and linear regression models, similar to the pairwise analysis described above.
We also used quantile-based g-computation (qgcomp), implemented using the ‘qgcomp’ package in R, to estimate the joint effect of the PFAS mixture on inflammation biomarkers. Qgcomp extends traditional g-computation approach within a generalized linear model framework to handle exposure mixtures (Keil et al., 2020; Keil et al., 2021). This method uses a marginal structural model to determine the expected change in an outcome per quartile increase in all mixture components. Since our data included repeated measures of inflammation biomarkers from the same participants across multiple visits, we employed the cluster bootstrapping feature in ‘qgcomp’, specifying participant identifier using the ‘id’ parameter in the ‘qgcomp.glm.boot’ function. This approach resamples data at the participant level, preserving the clustering structure within each bootstrap sample, and providing robust estimates of standard errors (Keil et al., 2021). For our analysis, we generated 2500 bootstrapped samples to estimate the expected differences and 95% CIs. For both mixture analyses, we adjusted for the same covariates as in the single pollutant analysis, accounted for case-control design using IPW, and natural log-transformed both exposures and outcomes. To ensure consistency, we expressed all estimates and 95% CIs from the mixture analyses as percent changes in biomarker levels per IQR increase in ∑PFAS (for the cumulative approach) or simultaneous quartile increase in all PFAS compounds (for qgcomp approach).
2.8. Sensitivity analysis
We conducted several sensitivity analyses to test the robustness of our findings. First, we reran our primary analysis with more detailed categories of maternal race (White, African American, Asian, Hispanic, Mixed, and Other) beyond the three categories used in the main analysis. This allowed us to assess potential residual confounding and the impact of broad racial recategorizations on the effect estimates. Second, to address concerns related to hemodynamic changes and plasma volume expansion affecting PFAS concentrations throughout pregnancy (Sagiv et al., 2017), we adjusted for GA at the time of sample collection and restricted our analysis to participants with Visit 1 PFAS only. Third, we restricted our analysis to term births or controls only to examine the PFAS-inflammatory biomarker associations in participants with more homogenous pregnancy-related physiology. Fourth, we used multiple imputation by chained equations (MICE) implemented via ‘mice’ package in R to create 10 imputed datasets, ensuring our findings were not affected by the exclusion of participants with missing covariate data (Zhang, 2016). The imputation model included all covariates, exposure variables, and outcome variables used in the primary analysis, assuming the missing data were missing at random. Results from each imputed dataset were combined using Rubin’s rules for final estimates and standard errors (Rubin, 1976). Fifth, given the low proportion of participants with a personal medical history of renal disease or smoking during pregnancy, we reran our analysis excluding these participants. This allowed us to assess whether the inclusion of participants with potentially altered PFAS levels due to renal dysfunction (Han et al., 2012) or baseline inflammation due to smoking (Goncalves et al., 2011) influenced our overall findings. Finally, we assessed the effects of using MRVs, when applicable, in our primary models by replacing all values below the LOD (for both PFAS and intermediary biomarkers) with respective LOD/√2.
3. Results
3.1. Descriptive statistics of study population, PFAS, and inflammatory biomarkers
Table 1 presents maternal and pregnancy characteristics for all participants considered in the current study. The median maternal age at enrollment was 32.7 years and the median pre-pregnancy BMI was 24.0 kg/m2. The study participants were diverse, with a majority being White (58.2%), followed by African American (16.4%), Hispanic (12.8%), Asian (7.7%), Mixed race (2.1%), and Other races (2.8%). Most participants were college educated (67.2%), had private health insurance (78.5%), and were parous (55.0%). An overwhelming majority of participants reported not smoking (94.2%) or consuming alcohol (94.0%) during pregnancy. Only 1.1% of participants had a personal history of renal conditions. The demographic characteristics of participants with complete case data were similar to those without (Table S1).
Table 1.
Characteristics of study participants and post-delivery outcomes (N = 469).
| Unweighted Statistics | Weighted Statistics1 | |
|---|---|---|
| Maternal age (y) | ||
| Median [Q1, Q3] | 32.7 [28.9, 35.8] | 32.7 [28.7, 35.7] |
| Maternal prepregnancy BMI (kg/m2) | ||
| Median [Q1, Q3] | 24.0 [21.5, 28.2] | 24.0 [21.5, 28.0] |
| Missing | 10 (2.1%) | 10 (2.1%) |
| Gestational age at sampling for PFAS (wk) | ||
| Median [Q1, Q3] | 9.9 [8.4, 12.4] | 9.9 [8.4, 12.3] |
| Missing | 2 (0.4%) | 2 (0.5%) |
| Maternal race/ethnicity | ||
| White | 273 (58.2%) | 273 (58.7%) |
| African American | 77 (16.4%) | 77 (15.9%) |
| Asian | 36 (7.7%) | 36 (7.0%) |
| Hispanic | 60 (12.8%) | 60 (13.2%) |
| Mixed | 10 (2.1%) | 10 (2.4%) |
| Other | 13 (2.8%) | 13 (2.9%) |
| Maternal education | ||
| High school or less | 67 (14.3%) | 67 (14.0%) |
| Some college/technical school | 76 (16.2%) | 76 (15.7%) |
| College or greater | 315 (67.2%) | 315 (67.7%) |
| Missing | 11 (2.3%) | 11 (2.7%) |
| Health insurance | ||
| Private insurance/HMO | 368 (78.5%) | 368 (77.6%) |
| Self-pay or Medicaid/Mass Health | 89 (19.0%) | 89 (19.6%) |
| Missing | 12 (2.6%) | 12 (2.8%) |
| Parity | ||
| Parous | 258 (55.0%) | 258 (54.7%) |
| Nulliparous | 211 (45.0%) | 211 (45.3%) |
| Smoking during pregnancy | ||
| No | 442 (94.2%) | 442 (94.5%) |
| Yes | 27 (5.8%) | 27 (5.5%) |
| Alcohol consumption during pregnancy | ||
| No | 441 (94.0%) | 441 (93.4%) |
| Yes | 18 (3.8%) | 18 (4.5%) |
| Missing | 10 (2.1%) | 10 (2.1%) |
| Personal history of renal disease | ||
| No | 464 (98.9%) | 464 (99.0%) |
| Yes | 5 (1.1%) | 5 (1.0%) |
| Fetal sex | ||
| Female | 209 (44.6%) | 209 (44.3%) |
| Male | 260 (55.4%) | 260 (55.7%) |
| Preterm birth | ||
| No | 342 (72.9%) | 342 (87.8%) |
| Yes | 127 (27.1%) | 127 (12.1%) |
Notes.
N values represent unweighted counts of participants in each category. Weighted quantiles and proportions were obtained using the ‘survey’ package in R, with inverse probability weights of 1.1 for preterm cases and 2.95 for controls. Abbreviations: Q1, 25th percentile; Q3, 75th percentile.
Table 2 presents the distributions of plasma PFAS concentrations and visit-specific inflammatory biomarkers among the study participants. The PFAS levels in this cohort have also been detailed in a previous study (Siwakoti et al., 2023). The median ∑PFAS among participants was 12.7 ng/mL, with the cumulative exposure levels for most participants (85.7%) falling in the 2–20 ng/mL range, which was identified by the NASEM as having potential for adverse effects in sensitive populations such as pregnant women (NASEM, 2022). Approximately 13.6% of participants had ∑PFAS concentrations above 20.0 ng/mL, a range that could lead to an increased risk of adverse health effects, while less than 1% of participants had summed exposure levels below 2.0 ng/mL, where adverse effects related to PFAS exposure are not expected.
Table 2.
Distributions [Median (Q1, Q3)] of inflammatory biomarkers and PFAS among study participants in LIFECODES.
| Visit 1 | Visit 2 | Visit 3 | Visit 4 | ICC2 | |
|---|---|---|---|---|---|
| Inflammatory biomarkers1 | N = 410 | N = 397 | N = 377 | N = 370 | |
| CRP (μg/mL) | 4.15 (2.19, 9.75) | 5.88 (3.18, 14.61) | 6.04 (3.24, 13.18) | 5.23 (2.95, 9.16) | 0.66 |
| Cytokines (pg/mL) | |||||
| IL-10 | 12.94 (8.59, 20.58) | 13.54 (9.25, 19.83) | 13.33 (9.36, 19.41) | 13.55 (8.91, 19.44) | 0.81 |
| IL-1β | 0.28 (0.16, 0.50) | 0.27 (0.14, 0.50) | 0.23 (0.13, 0.51) | 0.23 (0.12, 0.49) | 0.81 |
| IL-6 | 1.36 (0.85, 2.58) | 1.26 (0.75, 2.30) | 1.28 (0.81, 2.36) | 1.54 (0.96, 2.56) | 0.80 |
| TNF-α | 2.92 (2.13, 4.09) | 3.04 (2.25, 4.42) | 2.98 (2.19, 4.03) | 3.30 (2.19, 4.74) | 0.80 |
| PFAS (ng/mL) | N = 4693 | ||||
| PFOA | 2.49 (1.74, 3.24) | ||||
| PFOS | 7.04 (4.67, 9.83) | ||||
| PFNA | 0.86 (0.65, 1.12) | ||||
| PFHxS | 0.88 (0.54, 1.45) | ||||
| PFDA | 0.29 (0.21, 0.40) | ||||
| PFUA | 0.23 (0.15, 0.38) | ||||
| MPAH | 0.16 (0.10, 0.26) | ||||
| ∑PFAS4 | 12.69 (8.83, 16.47) |
Notes: Distributions of inflammatory biomarkers and PFAS were computed only for participants with both PFAS and biomarkers data for at least one visit. Abbreviations: ICC, intraclass correlation; CRP, C-reactive protein; IL, interleukin; TNF, tumor necrosis factor.
The sample sizes vary across visits due to missing biomarker data for some participants at certain visits.
The ICCs were calculated using the ‘performance’ package in R.
Most samples (~90%) for PFAS measurement were collected during Visit 1.
∑PFAS represents the sum of PFOA, PFOS, PFNA, PFHxS, PFDA, PFUA, and MPAH concentrations.
We observed no consistent visit-specific trend in the distribution of plasma biomarkers. However, they showed high consistency across visits, with an ICC of 0.66 for CRP and ≥ 0.80 for cytokines. Fig. S2a and b show correlations between different PFAS compounds and inflammatory biomarkers. The correlations ranged from 0.06 (between MPAH and PFOA) to 0.73 (between PFDA and PFNA) for PFAS, and from 0.08 (between CRP and IL-10) to 0.44 (between IL-6 and and IL-10) for biomarkers. Tables S2 and S3 present the distributions of PFAS and biomarker concentrations across different levels of covariates considered in our study. Briefly, PFAS concentrations were generally higher among participants with lower BMI, those identifying as Asian and White, those with private insurance or college education, and those who were nulliparous (Table S2). On the other hand, CRP and cytokine levels tended to be elevated among participants with higher BMI, African Americans, those with less than college education, those with public/self-pay insurance, and those who reported smoking (Table S3).
3.2. Pairwise associations between PFAS and inflammatory biomarkers
Covariate-adjusted associations between each PFAS and inflammatory biomarkers are shown in Fig. 1 and Table S4, with results for PFHP, modeled as a dichotomous variable, presented in Table S7. We observed inverse associations between most PFAS and cytokines measured in our study. For instance, all PFAS except MPAH were significantly associated with IL-10, with percent changes in IL-10 levels per IQR increase in each PFAS ranging from −9.98% (95% CI: −17.18, −2.15) for PFNA to −15.17% (95% CI: −24.04, −5.26) for PFUA. Four PFAS, namely PFOS, PFNA, PFDA, and PFUA, were also associated with IL-6 and TNF-α. The percent change in IL-6 concentrations ranged from −10.42% (95% CI: −18.97, −0.96) for PFNA to −13.91% (95% CI: −24.11, −2.34) for PFOS, while the percent change in TNF-α ranged from −8.49% (95% CI: −13.92, −2.71) for PFDA to −12.74% (95% CI: −18.61, −6.45) for PFUA. We also observed a suggestive inverse association between PFNA and IL-1β, with all other PFAS showing weaker negative associations. We did not observe a robust non-linear dose–response curve for any PFAS and inflammatory biomarkers (data not shown).
Fig. 1.
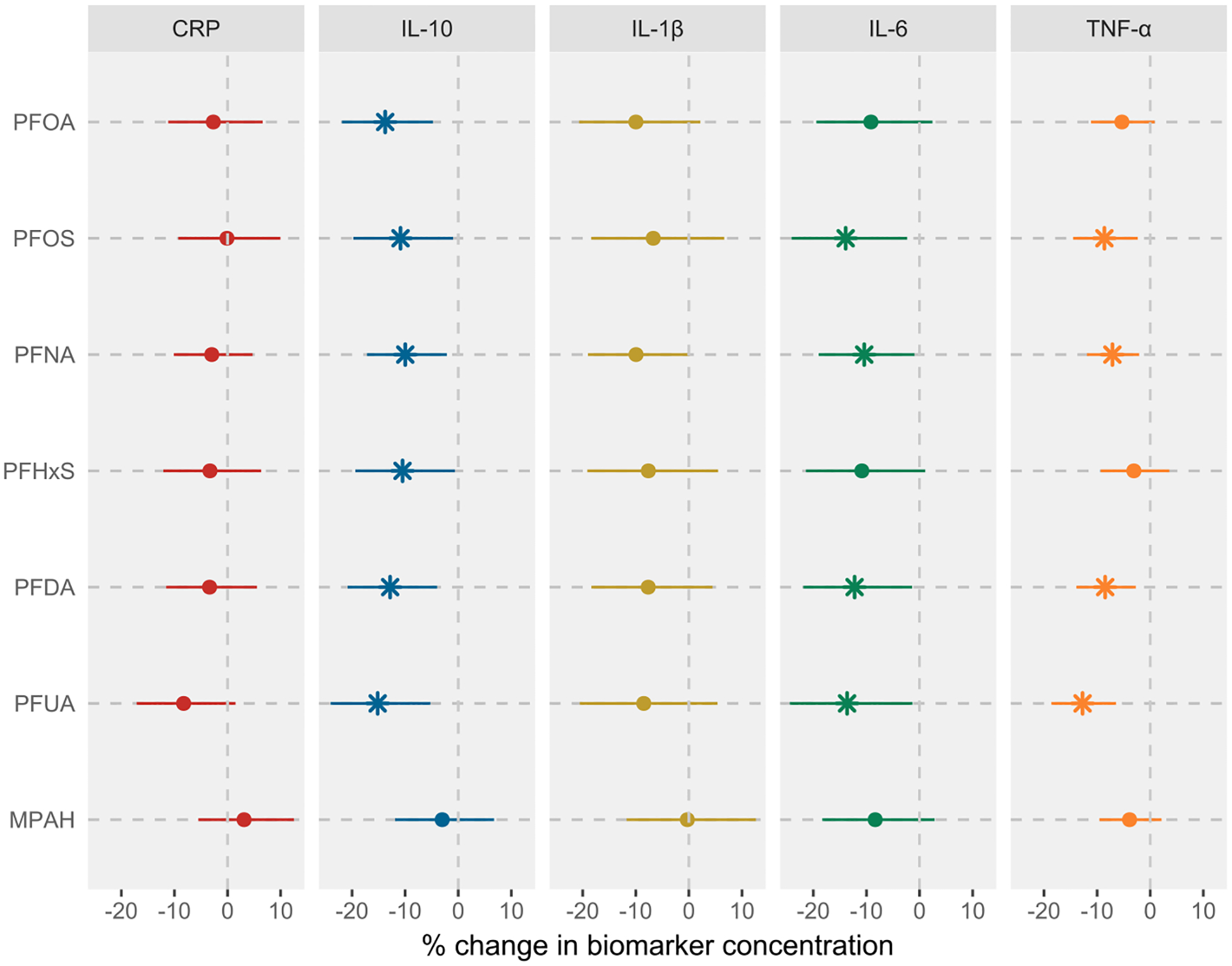
Associations between prenatal PFAS exposure and inflammatory biomarkers in LIFECODES. Analysis was based on the complete case data (N = 1485 observations from 448 participants). Linear mixed-effects models with random intercept were used to account for the correlation between repeated measures outcomes. Both PFAS and inflammatory biomarker concentrations were log-transformed prior to modeling. All models were adjusted for maternal age, prepregnancy BMI, race, education level, insurance status, and parity. Effect estimates were obtained as % change in biomarker concentrations per IQR increase in PFAS concentrations. All models were weighted using inverse probability weights (1.1 for preterm cases and 2.95 for controls) to account for the unequal selection of preterm cases in the case-control study. The vertical lines represent the null effect. * represents associations with 95% CIs that do not include null. Note: CRP, C-reactive protein; IL, interleukin; TNF, tumor necrosis factor; IQR, interquartile range.
The associations between most PFAS and inflammatory biomarkers were not significantly modified by fetal sex (Table S5a), although the inverse trends were broadly stronger in women who delivered males, including a notable negative association between PFUA and CRP (Fig. 2 and Table S5b). We observed some evidence of effect modification by maternal race on the associations between PFAS and inflammatory biomarkers (Table S6a). Specifically, the inverse associations of PFOS with IL-10, IL-6, and TNF-α were stronger in African American participants compared to White participants (Fig. S3 and Table S6b). Similarly, significant interaction effects were observed for the associations of MPAH with IL-6, and for PFNA and PFHxS with TNF-α, all showing stronger inverse effects in African American participants. While the interaction effects were not significant for some PFAS-biomarker pairs, we still observed stronger effects among African American participants, and in some instances (such as PFOA, PFOS, or PFNA, and IL-1β associations) in participants from the Other racial category.
Fig. 2.
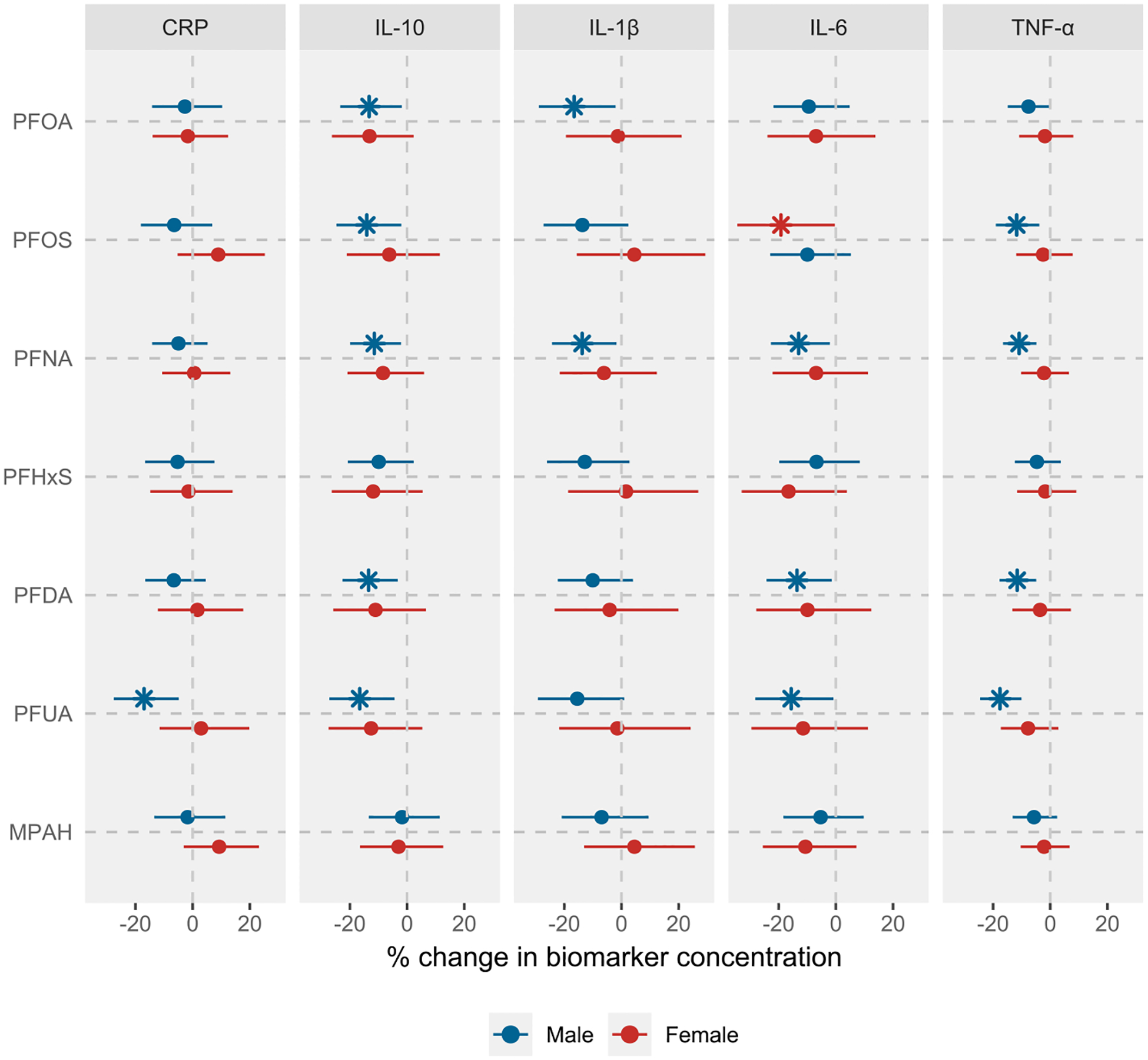
Associations between prenatal PFAS exposure and inflammatory biomarkers in LIFECODES, stratified by fetal sex. Analysis was based on the complete case data (N = 829 observations from 245 participants for males and 656 observations from 203 participants for females). Linear mixed-effects models with random intercept were used to account for the correlation between repeated measures outcomes. Both PFAS and inflammatory biomarker concentrations were log-transformed prior to modeling. All models were adjusted for maternal age, prepregnancy BMI, race, education level, insurance status, and parity. Effect estimates were obtained as % change in biomarker concentrations per IQR increase in PFAS concentrations. All models were weighted using inverse probability weights (1.1 for preterm cases and 2.95 for controls) to account for the unequal selection of preterm cases in the case-control study. The vertical lines represent the null effect. * represents associations with 95% CIs that do not include null. Note: CRP, C-reactive protein; IL, interleukin; TNF, tumor necrosis factor; IQR, interquartile range.
Fig. 3 and Table S8a show the results from the visit-specific analysis in which we examined the associations between each PFAS, measured once in early pregnancy, and inflammatory biomarkers measured at each visit separately. We observed some subtle visit-specific trends. For example, the inverse associations of most PFAS with IL-10 were strongest in mid-pregnancy, specifically visits 2 and 3. Likewise, the associations of most PFAS with IL-6 and TNF-α were typically more pronounced in visit 2, with effect estimates related to TNF-α becoming weaker in later visits. As detailed in Table S8b, c and Table S9a – c, the visit-specific analysis stratified by fetal sex and maternal race showed no consistent patterns among women who delivered males or females, nor across racial categories, except a notable trend of generally stronger associations with IL-10, IL-6, and TNF-α in visits 2 and 3.
Fig. 3.
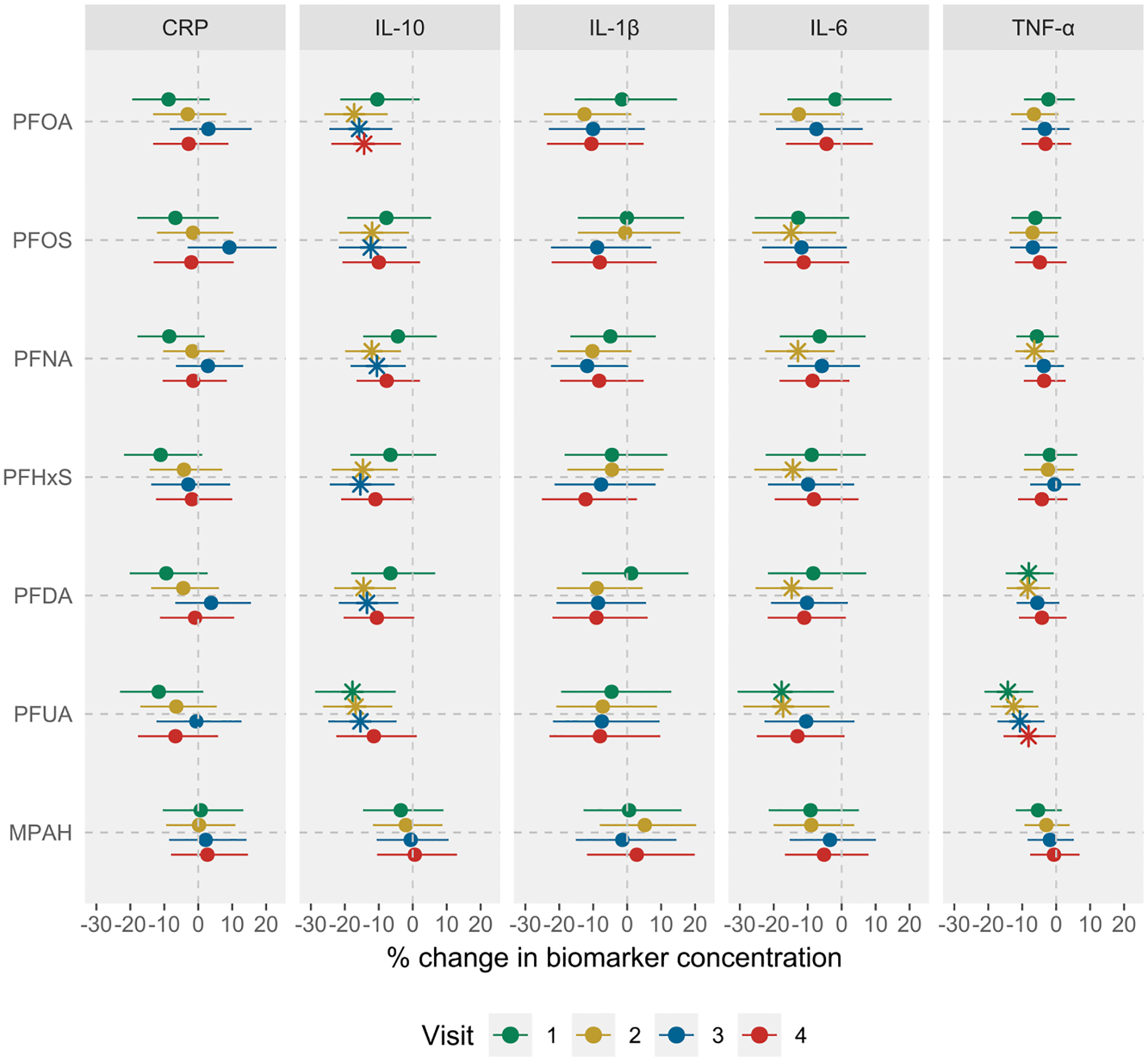
Cross sectional associations between prenatal PFAS exposure and visit-specific inflammatory biomarker concentrations among all participants in LIFECODES. Analysis was based on the complete case data (N = 390, 382, 360, and 353 participants for visits 1–4, respectively). Multivariable linear regression models were used to assess relationships between early pregnancy PFAS levels and visit-specific plasma biomarker concentrations. All models were adjusted for maternal age, race, prepregnancy BMI, education level, insurance status, and parity. Effect estimates were obtained as % change in biomarker concentrations per IQR increase in PFAS concentrations. All models were weighted using inverse probability weights (1.1 for preterm cases and 2.95 for controls) to account for the unequal selection of preterm cases in the case-control study. The vertical lines represent the null effect. * represents an association with 95% CIs that does not include null. Note: CRP, C-reactive protein; IL, interleukin; TNF, tumor necrosis factor; IQR, interquartile range.
3.3. Sensitivity analysis for pairwise associations between PFAS and inflammatory biomarkers
In the sensitivity analysis, adjusting for finer categories of maternal race did not alter the findings from the primary analysis (Table S10). Additionally, adjusting for GA at sample collection for PFAS measurement showed trends consistent with the primary findings (Table S11a), although attenuation in effect estimates was observed when the analysis was restricted to participants with visit 1 PFAS only (Table S11b). Restricting the analysis to term births (Table S12) and using multiply imputed datasets to account for missing covariate data (Table S13) did not substantially alter the primary findings, with results remaining largely consistent despite some minor variations in effect estimates. Finally, restricting the analysis to non-smokers (Table S14), those without a history of renal disease (Table S15), or replacing MRVs with LOD/√2 (Table S16) also did not meaningfully change the findings from the primary analysis.
3.4. Associations between PFAS mixture and inflammatory biomarkers
Fig. 4 and Table S17 show the results from the mixture analysis using the summed PFAS approach. The associations between cumulative PFAS exposure and inflammatory biomarkers were largely consistent with the inverse associations observed in the single pollutant analysis. For instance, an IQR increase in ∑PFAS was associated with −12.26% (95% CI: −20.84, −2.76), −14.09% (95% CI: −24.08, −2.79), and −7.82% (95% CI: −13.65, −1.61) change in IL-10, IL-6, and TNF-α levels, respectively. The fetal sex-specific results were also consistent, showing stronger inverse associations in women who delivered males (Table S17). Similarly, the visit-specific analysis generally showed stronger associations between ∑PFAS and inflammatory biomarkers measured in mid-pregnancy. The findings from the qgcomp analysis also showed similar trends, with inverse associations between the PFAS mixture and IL-10 and IL-6 (Fig. 5 and Table S18). For example, a simultaneous increase in all PFAS concentrations by their quartiles was associated with −14.50% (95% CI: −25.64, −1.70) change in IL-10 levels and −14.44% (95% CI: −28.53, 2.43) change in IL-6 levels, with consistently stronger effects in early and mid-gestation, and among women who delivered males. Interestingly, qgcomp analysis also showed an inverse association between PFAS mixture and CRP in visit 1.
Fig. 4.
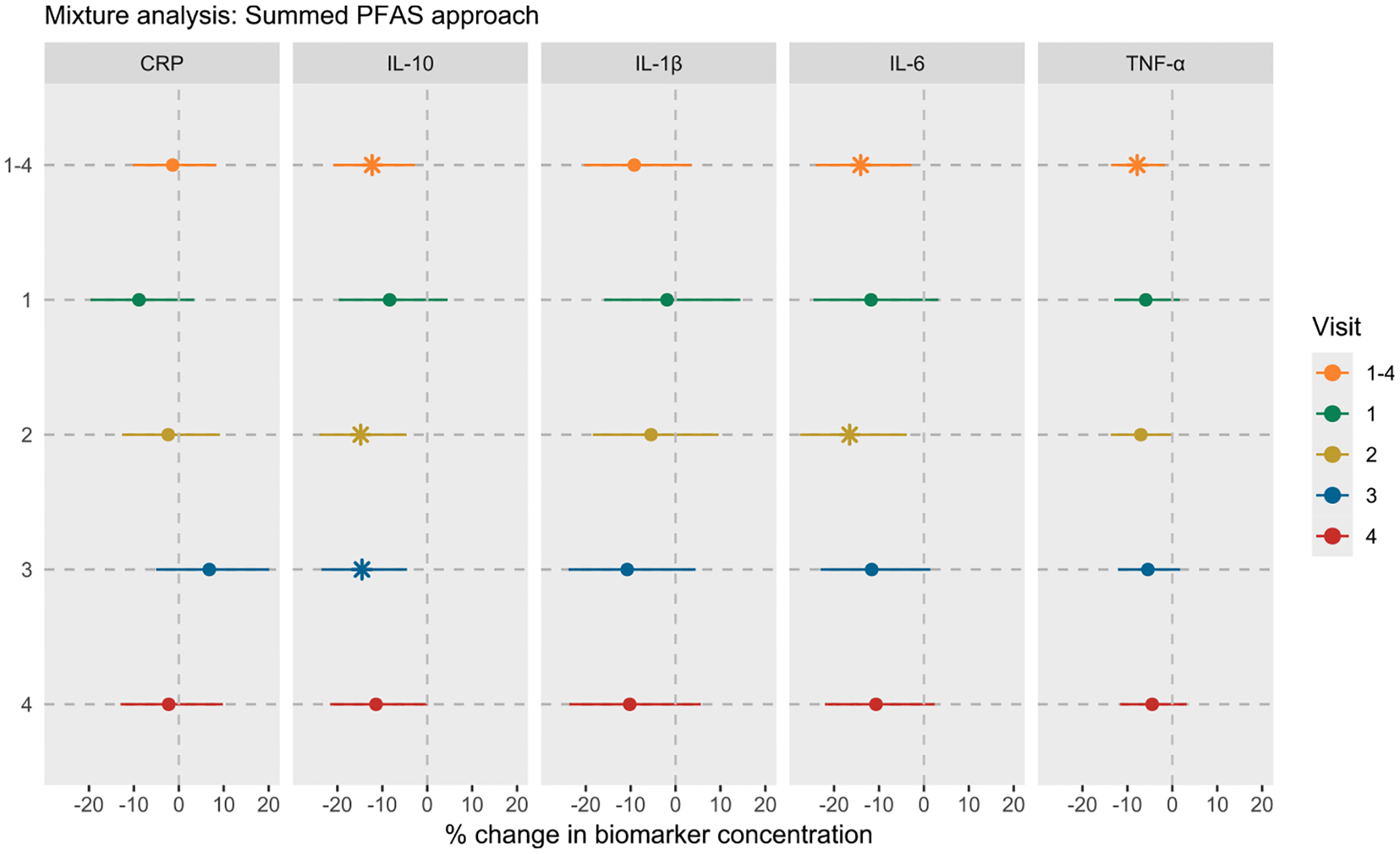
Associations between PFAS mixture and inflammatory biomarkers in LIFECODES using the summed PFAS approach. Visit 1–4 indicates the repeated measures analysis of biomarkers, while the individual visits indicate the visit-specific analysis. Summed PFAS was obtained by adding concentrations of seven PFAS with a detection rate >70% (PFOA, PFOS, PFNA, PFHxS, PFDA, PFUA, and MPAH). Linear mixed-effects models with random intercept were used for repeated measures analysis, while multivariable linear regression models were used for visit-specific analysis. All models were adjusted for maternal age, race, prepregnancy BMI, education level, insurance status, and parity. Effect estimates were obtained as % change in biomarker concentrations per IQR increase in sum of all PFAS concentrations. All models were weighted using inverse probability weights (1.1 for preterm cases and 2.95 for controls) to account for the unequal selection of preterm cases in the case-control study. The vertical lines represent the null effect. * represents associations with 95% CIs that do not include null. Note: CRP, C-reactive protein; IL, interleukin; TNF, tumor necrosis factor; IQR, interquartile range.
Fig. 5.
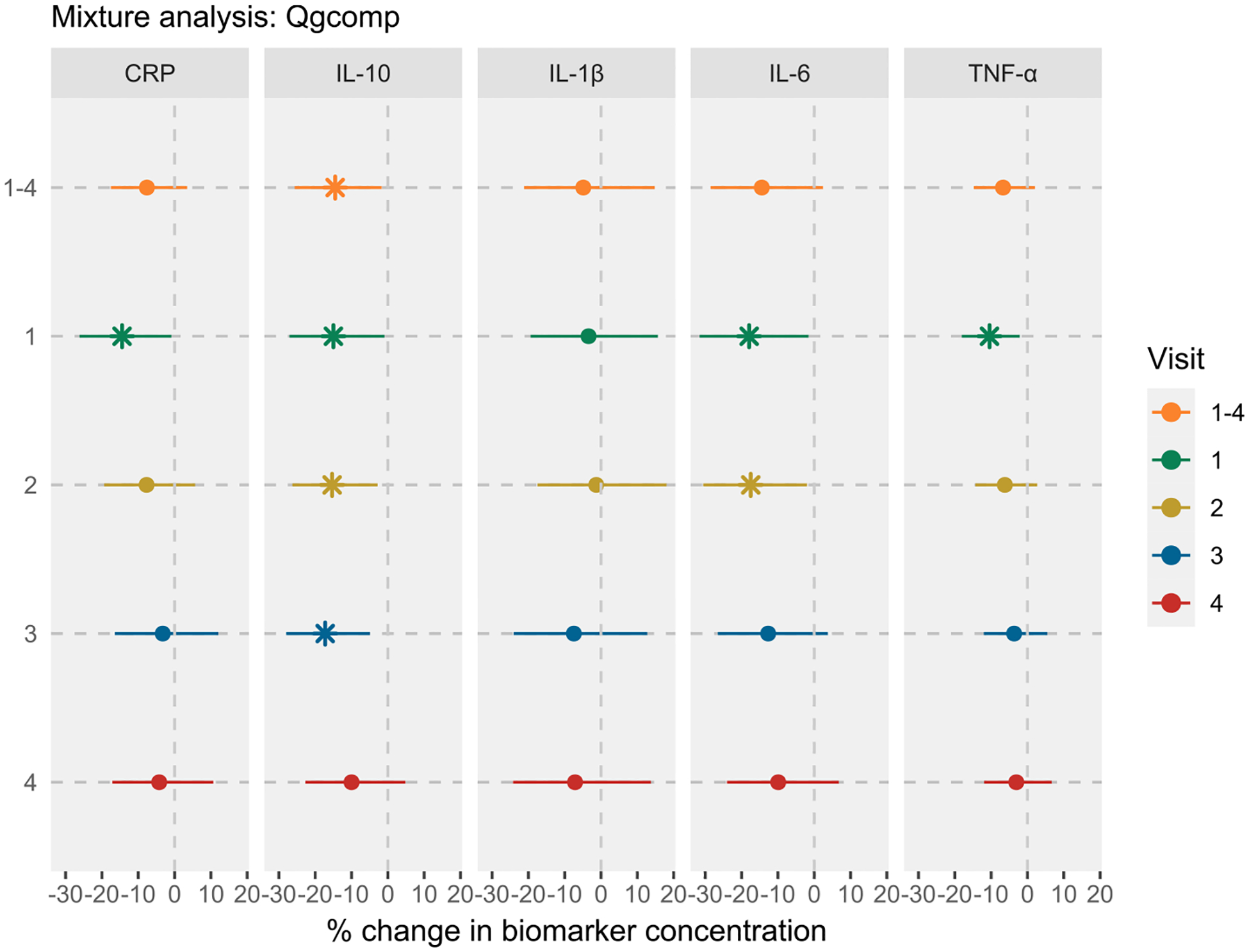
Associations between PFAS mixture and inflammatory biomarkers in LIFECODES using quantile-based g-computation (qgcomp) approach. Visit 1–4 indicates the repeated measures analysis of biomarkers, while the individual visits indicate the visit-specific analysis. For Visit 1–4, the bootstrapped implementation of qgcomp (2500 Monte Carlo simulations and 2500 bootstrapped samples) with linearity assumption was used to obtain robust standard errors. For visit-specific analysis, the non-bootstrapped implementation of qgcomp with linearity assumption was implemented. All models were adjusted for maternal age, race, prepregnancy BMI, education level, insurance status, and parity. Effect estimates were obtained as % change in biomarker concentrations per quartile increase in all PFAS levels. All models were weighted using inverse probability weights (1.1 for preterm cases and 2.95 for controls) to account for the unequal selection of preterm cases in the case-control study. The vertical lines represent the null effect. * represents associations with 95% CIs that do not include null. Note: CRP, C-reactive protein; IL, interleukin; TNF, tumor necrosis factor.
4. Discussion
In this analysis of pregnant women from the LIFECODES cohort, we found consistent inverse associations between early pregnancy plasma PFAS concentrations, both individually and as a mixture, and repeated measures of circulatory cytokines. Specifically, we observed decreased levels of IL-10, IL-6, and TNF-α with increasing concentrations of PFOS, PFNA, PFDA, and PFUA. PFOA and PFHxS were similarly inversely associated with IL-10. These associations were more likely to be pronounced in women who delivered males and those who identified as African Americans, although the estimates from the stratified analysis had larger variability due to smaller sample sizes. Similarly, the associations between most PFAS and specific cytokines (IL-10, IL-6, and TNF-α) tended to be slightly stronger in mid-pregnancy.
The findings in our study are in line with a few epidemiological studies that have examined the effects of PFAS on cytokines or CRP in pregnant or non-pregnant women. For instance, our observed inverse associations between PFAS and cytokines align with the results from Cinzori et al. (2024), which reported both a negative association of PFAS mixture with TNF-α (samples collected at median GA of 17 weeks) and a null association with CRP in a group of 452 pregnant women enrolled in the Illinois Kids Development Study (Cinzori et al., 2024). They also align with findings from two other studies conducted among non-pregnant women: Nian et al. (2022) reported negative associations between PFOA, PFOS, and PFAS mixture and IL-10 in women of reproductive age in China (N = 198), while Cauble et al. (2024) found a negative association between PFOA and IL-6 in the California Teachers Study (N = 722) (Nian et al., 2022; Cauble et al., 2024). However, our results differ from a few other studies focusing on pregnant women. Tan et al. (2023) found positive associations of a PFAS mixture with IL-10 and TNF-α (samples collected up to two times at mean GAs of 11 and 27 weeks) in African American women (N = 425), with the strongest effects observed at 24–30 weeks of gestation (Tan et al., 2023). Similarly, Zota et al. (2018) reported a positive association between a PFAS mixture and IL-6 (samples collected at median GA of 16 weeks) in a study of 103 obese and overweight pregnant women in California (Zota et al., 2018). In addition, Palaniyandi et al. (2023) reported positive associations of PFOA and PFHxS with a pro-inflammatory index (including TNF-α and IL-6 measured in samples collected at mean GA of 33 weeks) in the MIREC study (N = 1411), with stronger effects in women carrying male fetuses (Palaniyandi et al., 2023). Finally, in a comprehensive review of the effects of PFAS on innate immune system, Phelps et al. (2024) reported that several epidemiological studies with non-pregnant population or human cell lines, found both up- or down regulation of IL-6 and TNF-α in relation to elevated PFAS levels, although IL-10 levels were consistently downregulated (Phelps et al., 2024; Bassler et al., 2019). These discrepancies highlight the complexities of interpreting the effects of PFAS on inflammation in observational research.
Some factors that might contribute to directional inconsistencies across studies include variations in PFAS exposure profiles, demographic and lifestyle characteristics, genetics, co-exposures to other chemical or non-chemical stressors, timing of exposure and outcome assessments, and study selection criteria. For example, the distributions of PFOA, PFOS, and PFNA in the Atlanta cohort, measured in samples collected between 2016–2020, were much lower than those measured among all or African American participants in our study (Tan et al., 2023). While the covariates considered for modeling were similar across both cohorts, the participants in the Atlanta cohort were younger (24 vs. 32 years in the current study), and inflammatory biomarkers were measured only twice (versus four times in the current study). The cytokine levels between studies could not be directly compared due to differences in matrix or the unit of measurement. As for the study by Zota et al. (2018), both PFAS and cytokines (IL-10, IL-6, and TNF-α) were measured once during the second trimester, with levels lower compared to our study, and cytokines were additionally measured twice postpartum, which is beyond the scope of the current study (Zota et al., 2018). Notably, that study focused on overweight or obese pregnant women who might have a different risk profile than the general pregnant population. Restricting our analysis to match Zota et al.’s population characteristics – either to overweight participants (pre-pregnancy BMI ≥25 kg/m2; N = 198) for the PFAS mixture-IL-6 association, or to obese participants (BMI ≥30 kg/m2; N = 80) for the PFDA-IL-6 association did not yield similar results to their study (data not shown), suggesting that risk factors beyond just BMI may be contributing to the observed discrepancies. Palaniyandi et al. (2023), on the other hand, only measured three PFAS during the first trimester and multiple inflammatory biomarkers during the third trimester of pregnancy (Palaniyandi et al., 2023). That study, including largely white participants, reported lower PFOA and PFOS levels, but higher inflammatory biomarker (IL-10, IL-6, TNF-α, and CRP) levels compared to our study. Notably, the associations between individual PFAS and each of these biomarkers were null, indicating that the observed positive associations of PFAS with proinflammatory index may have been driven by a few chemokines such as MCP-1 and MIP-1β that were not part of our analysis. Furthermore, the creation of an inflammatory index by summing specific biomarkers, which assumes homogeneity in direction of associations, may mask more nuanced relationships with health outcomes or environmental exposures, especially since some cytokines like IL-6 can have both anti- or pro-inflammatory functions depending on the dose, cell type, or cell signaling mechanisms (Fuster and Walsh, 2014). It is worth noting that the studies with contrasting findings generally reported lower PFAS levels than those observed in LIFECODES, raising the question of whether discrepancies could be due to non-linear dose–response relationships, as sometimes observed with endocrine-disrupting chemicals (Vandenberg et al., 2012). Although we did not observe strong non-linear associations in our analysis, some studies with findings similar to ours also reported lower overall PFAS levels. This leaves it unclear whether the observed effects across these studies are driven by true non-linear dose–response relationships or by other study-specific factors.
Experimental studies involving human cell-lines and animal models have also reported modulation of cytokine levels with elevated PFAS exposure, with a few studies showing downregulation of both regulatory (or anti-inflammatory) and pro-inflammatory cytokines. For example, increasing levels of PFOA, PFOS, or PFDA have been shown to suppress the production of various cytokines, including TNF-α, IL-6, and IL-10 in whole blood and THP-1 human monocytic cell-lines (Brieger et al., 2011; Corsini et al., 2011; Corsini et al., 2012). Animal models have shown that PFOA, PFOS, and PFNA could perturb IL-6, TNF-α, or other cytokine expression, although effects varied by exposure route, dose, and tissue examined (Rockwell et al., 2013; Guo et al., 2021; Han et al., 2018; Rockwell et al., 2017; Qazi et al., 2010; Qazi et al., 2009; De Guise and Levin, 2021). A study in mice showed that PFOA and PFOS exposure increased TNF-α and IL-6 levels in bone marrow and peritoneal tissues, while simultaneously attenuating hepatic levels of TNF-α (Qazi et al., 2010; Qazi et al., 2009). Similarly, zebrafish models have also shown mixed findings, with PFOA leading to both up- or downregulation of mRNA levels of various cytokines (Zhang et al., 2014). The molecular mechanisms behind these alterations in cytokine homeostasis remain an area of active research, although several studies have linked modulation of nuclear factor kappa B (NF-κB) and peroxisome proliferator-activated receptors (PPARs) to PFAS-induced changes in cytokine production (Corsini et al., 2012; Ehrlich et al., 2023b). For instance, Corsini and colleagues (2011) demonstrated that PFOA, PFOS, and PFDA inhibited LPS-induced TNF-α production in THP-1 cells and human peripheral blood leukocytes, primarily by interfering with the NF-κB signaling pathway (Corsini et al., 2012), while other studies have reported contradictory findings (Ehrlich et al., 2023). These inconsistencies, coupled with the limited evidence for other legacy or newer PFAS compounds, highlight a significant gap in our understanding of their effects on cytokine regulation and downstream functional consequences of altered inflammatory responses.
We observed stronger inverse associations between several PFAS and cytokines, particularly IL-1β and TNF-α, in women who delivered males. The distributions of covariates, as well as PFAS and inflammatory biomarkers, were not substantially different between women who delivered males versus females (data not shown), suggesting that the observed sex-specific associations are unlikely due to confounding by these factors alone. Furthermore, multiple previous studies have reported sexually dimorphic effects of PFAS (Svoboda et al., 2022; Roth et al., 2021; Lucas et al., 2024). Notably, Lucas et al. (2024) observed that developmental (in-utero and immediate postnatal) PFOS exposure in mice resulted in male-specific suppression of proinflammatory cytokines, including TNF-α, in lung tissue and bronchoalveolar lavage fluid, with corresponding downregulation of cytokine gene expression (e.g., Cxcl1, Tnf, and Ccl2) in males but not in females (Lucas et al., 2024). While the mechanisms underlying such differences are not fully elucidated, hypothesized explanations include differences in placental functions, epimutations, hormonal regulation, or metabolic processes. For instance, the placenta, despite being of fetal origin, can influence maternal physiology by affecting gene expression, hormonal balance, or immune response in a fetal sex-dependent manner (Baines and West, 2023; Braun et al., 2022; Rosenfeld, 2015). Similarly, differences in epigenetic modifications, specifically DNA methylation, in response to environmental exposures (Everson et al., 2024; Svoboda et al., 2022; McCabe et al., 2017) could persist and influence gene expression patterns, potentially affecting maternal inflammatory responses.
We observed generally stronger inverse associations between multiple PFAS and IL-10, IL-6, or TNF-α in women who identified as African Americans. While most PFAS levels were lower and cytokine levels higher among African American women compared to others in our cohort, the exact causes for the observed differences are not clear. However, differences in dietary or behavioral patterns, socioeconomic factors, or psychosocial characteristics across racial categories could play a role. Nevertheless, these findings emphasize a need to comprehensively examine the disparate effects of PFAS on health outcomes across different socioeconomic or racial subgroups.
The consistent inverse associations we observed between PFAS and maternal circulatory levels of cytokines, which are also present in the endometrium, placenta, and at the maternal-fetal interface throughout pregnancy, raise important questions about their clinical implications for maternal and fetal health. Given the role of IL-10 in maintaining immune tolerance at the maternal-fetal interface and regulating Th1/Th2 cytokine balance throughout gestation, the dysregulation of this cytokine could disrupt important physiological processes such as trophoblast invasion, spiral artery remodeling, and placental angio-genesis (Cheng and Sharma, 2015). Moreover, as IL-10 has been shown to attenuate endoplasmic reticulum stress and regulate autophagy in placental tissue (Hasnain et al., 2013; Shkoda et al., 2007), its suppression might exacerbate oxidative stress, compromise placental function, and increase the risk of adverse outcomes such as preeclampsia, intrauterine growth restriction, or preterm birth. Similarly, the downregulation of IL-6, which plays a dual role in both promoting inflammatory responses necessary for processes like trophoblast invasion, spiral artery remodeling, and parturition, and in mediating immune-endocrine interactions essential for placental function, could potentially disrupt these critical processes (Vilotic et al., 2022). The decrease in levels of TNF-α, which is generally considered a proinflammatory Th1 cytokine that promotes inflammatory processes along with other cytokines, is particularly noteworthy. However, its suppression could also be problematic given its influence in cell-mediated immune responses across pregnancy, ranging from implantation to parturition, as well as its role in modifying the secretion pattern of placental immunomodulatory factors (Kelly, 1996; Romanowska-Prochnicka et al., 2021). Hence, any disruption of the delicate balance between various inflammatory agents, both pro- and anti-inflammatory, could induce negative downstream effects on pregnancy outcomes and fetal development. While the differences were subtle, the somewhat stronger inverse associations observed during early and mid-pregnancy suggest that cytokine regulation during these periods may be more susceptible to the impact of elevated PFAS levels, potentially affecting processes involved in placental formation and immune system development.
Our study has several strengths. First, it included a fairly large and diverse sample size, with repeated measures of inflammatory biomarkers, which allowed for robust statistical analyses and stratification by factors such as fetal sex and maternal race. In fact, to the best of our knowledge, this was the first study to evaluate the effects of PFAS in relation to inflammatory biomarkers from four different time points during pregnancy. Another key strength is that our study used historical samples collected from 2006 to 2008, a timeframe during which PFAS levels were higher before much of the regulatory actions were taken to limit PFAS exposure in the US. This provided a wider exposure distribution and larger exposure contrast for our statistical analyses. Additionally, our use of both single pollutant and mixture analysis approaches is directly applicable to risk assessment and policy strategy in the US and elsewhere, where PFAS are primarily assessed on an individual compound basis, with recent movement towards addressing them as a class (USEPA, 2023; USEPA, 2024). Furthermore, we investigated eight different PFAS, seven of which are identified in the NASEM’s clinical guidance on PFAS, providing a more complete picture of PFAS exposure and its impact on pregnancy-related processes. Finally, we implemented extensive sensitivity analyses, which ensured the robustness and validity of our findings.
Our study also has several limitations that need to be considered while interpreting its findings. First, since our study participants were selected as part of a nested case-control design, there might be concerns related to selection bias and the generalizability of our findings to the entire LIFECODES cohort. However, the use of IPW to account for unequal selection probability is usually thought to mitigate this bias and improve the representativeness of our findings (Jiang et al., 2006). Additionally, a sensitivity analysis restricted to controls only (Nagelkerke et al., 1995) showed consistent results, supporting the robustness of our findings and reducing the concerns about selection bias. Second, our analysis did not include newer non-legacy PFAS compounds, potentially limiting its relevance to future exposure scenarios. Given that our samples were collected in 2006–2008, the included PFAS compounds likely represent the predominant exposures of that period, and our summed PFAS approach reasonably approximates cumulative effects, as these compounds still remain among the most detected in the US (Hampson et al., 2024). Third, our focus on only four cytokines and CRP may be narrow, given the multifaceted role of numerous cytokines in pregnancy processes. While the high ICCs observed in this study indicate stable relative ranking of participants’ cytokine levels across pregnancy, this may not fully capture the dynamic nature of immune changes that occur throughout gestation. Nonetheless, our examination of IL-10 and TNF-α, two cytokines that play important and contrasting roles in regulating inflammatory process during pregnancy, provides a strong foundation for future investigations with a larger panel of biomarkers that also play a role in the patho-genesis of preterm birth and other pregnancy outcomes. Fourth, despite adjusting for an extensive set of potential confounders in both primary and sensitivity analyses, we were not able to account for dietary patterns, physical activity, recent infection status, medication use, or co-exposures to other stressors that might influence the PFAS-cytokines associations. In an ad-hoc analysis excluding participants with urinary tract infections, our findings remained largely unchanged (data not shown), however residual confounding from other unmeasured factors still cannot be fully ruled out. Limited knowledge exists on whether including dietary or physical activity data would shift the observed associations in specific directions, as some foods with anti- or proinflammatory properties may also contain PFAS, and the relationship between physical activity and PFAS levels remains unclear (Frangione et al., 2024). Finally, given the observational nature of the study, our findings are not necessarily causal.
In conclusion, our study suggests that prenatal exposure to PFAS is associated with the suppression of key inflammatory cytokines, specifically IL-10, IL-6, and TNF-α, during pregnancy, potentially altering maternal immune processes. These findings highlight the need for further research to understand the effect of PFAS-induced cytokine suppression on functional immune outcomes, ideally using larger sample sizes, more varied PFAS compounds, and an expanded panel of inflammatory biomarkers.
Supplementary Material
Funding
Support for this research was provided by grants R01ES031591, P30ES017885, and T32ES007062 from the National Institute of Environmental Health Sciences, National Institutes of Health. KKF was supported by the Intramural Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health (ZIA ES103321). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CRediT authorship contribution statement
Ram C. Siwakoti: Writing – original draft, Software, Methodology, Formal analysis, Conceptualization. Sean M. Harris: Writing – review & editing. Kelly K. Ferguson: Writing – review & editing, Methodology. Wei Hao: Writing – review & editing, Software, Methodology. David E. Cantonwine: Writing – review & editing, Resources, Methodology. Bhramar Mukherjee: Writing – review & editing, Methodology. Thomas F. McElrath: Writing – review & editing, Resources, Methodology. John D. Meeker: Writing – review & editing, Supervision, Funding acquisition, Conceptualization.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2024.109145.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
The authors do not have permission to share data.
References
- National Academies of Sciences E, Medicine, Health, Medicine D, Division on E, Life S, Board on Population H, Public Health P, Board on Environmental S, Toxicology, Committee on the Guidance on PT, Health O: The National Academies Collection: Reports funded by National Institutes of Health. In: Guidance on PFAS Exposure, Testing, and Clinical Follow-Up. Washington (DC): National Academies Press (US); 2022. [PubMed] [Google Scholar]
- ACOG: Committee Opinion No 700: Methods for Estimating the Due Date. In: Obstetrics & Gynecology. 129. 2017. e150–e154. [DOI] [PubMed] [Google Scholar]
- Aggarwal R, Jain AK, Mittal P, Kohli M, Jawanjal P, Rath G, 2019. Association of pro-and anti-inflammatory cytokines in preeclampsia. J. Clin. Lab. Anal 33 (4), e22834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Gubory KH, 2016. Multiple exposures to environmental pollutants and oxidative stress: Is there a sex specific risk of developmental complications for fetuses? Birth Defects Res C Embryo Today 108 (4), 351–364. [DOI] [PubMed] [Google Scholar]
- Baines KJ, West RC, 2023. Sex differences in innate and adaptive immunity impact fetal, placental, and maternal healthdagger. Biol Reprod 109 (3), 256–270. [DOI] [PubMed] [Google Scholar]
- Bartha JL, Romero-Carmona R, Comino-Delgado R, 2003. Inflammatory cytokines in intrauterine growth retardation. Acta Obstet. Gynecol. Scand 82 (12), 1099–1102. [DOI] [PubMed] [Google Scholar]
- Bassler J, Ducatman A, Elliott M, Wen S, Wahlang B, Barnett J, Cave MC, 2019. Environmental perfluoroalkyl acid exposures are associated with liver disease characterized by apoptosis and altered serum adipocytokines. Environ. Pollut 247, 1055–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S: Fitting linear mixed-effects models using lme4. arXiv preprint arXiv:14065823 2014. [Google Scholar]
- Blackmore ER, Moynihan JA, Rubinow DR, Pressman EK, Gilchrist M, O’Connor TG, 2011. Psychiatric symptoms and proinflammatory cytokines in pregnancy. Psychosom. Med 73 (8), 656–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun AE, Mitchel OR, Gonzalez TL, Sun T, Flowers AE, Pisarska MD, Winn VD, 2022. Sex at the interface: the origin and impact of sex differences in the developing human placenta. Biol Sex Differ 13 (1), 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieger A, Bienefeld N, Hasan R, Goerlich R, Haase H, 2011. Impact of perfluorooctanesulfonate and perfluorooctanoic acid on human peripheral leukocytes. Toxicol in Vitro 25 (4), 960–968. [DOI] [PubMed] [Google Scholar]
- Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, Jensen AA, Kannan K, Mabury SA, van Leeuwen SP, 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag 7 (4), 513–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Kato K, Hubbard K, Jia T, Botelho JC, Wong LY, 2019. Legacy and alternative per- and polyfluoroalkyl substances in the U.S. general population: Paired serum-urine data from the 2013–2014 National Health and Nutrition Examination Survey. Environ. Int 131, 105048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantonwine DE, Ferguson KK, Mukherjee B, McElrath TF, Meeker JD: Urinary Bisphenol A Levels during Pregnancy and Risk of Preterm Birth. In., vol. 123: Environ Health Perspectives; 2015: 895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauble EL, Reynolds P, Epeldegui M, Andra SS, Narasimhan S, Pulivarthi D, Von Behren J, Goldberg D, Spielfogel ES, Lacey JV, Wang SS: Associations between per- and poly-fluoroalkyl substance (PFAS) exposure and immune responses among women in the California Teachers Study: a cross-sectional evaluation. 2024. [DOI] [PubMed]
- Cheng SB, Sharma S, 2015. Interleukin-10: a pleiotropic regulator in pregnancy. Am J Reprod Immunol 73 (6), 487–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinzori ME, Pacyga DC, Rosas L, Whalen J, Smith S, Park JS, Geiger SD, Gardiner JC, Braun JM, Schantz SL, Strakovsky RS, 2024. Associations of per- and polyfluoroalkyl substances with maternal metabolic and inflammatory biomarkers in early-to-mid-pregnancy. Environ. Res 250, 118434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsini E, Avogadro A, Galbiati V, dell’Agli M, Marinovich M, Galli CL, Germolec DR, 2011. In vitro evaluation of the immunotoxic potential of perfluorinated compounds (PFCs). Toxicol Appl Pharmacol 250 (2), 108–116. [DOI] [PubMed] [Google Scholar]
- Corsini E, Sangiovanni E, Avogadro A, Galbiati V, Viviani B, Marinovich M, Galli CL, Dell’Agli M, Germolec DR, 2012. In vitro characterization of the immunotoxic potential of several perfluorinated compounds (PFCs). Toxicol Appl Pharmacol 258 (2), 248–255. [DOI] [PubMed] [Google Scholar]
- Coussons-Read ME, Okun ML, Nettles CD, 2007. Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain Behav. Immun 21 (3), 343–350. [DOI] [PubMed] [Google Scholar]
- De Guise S, Levin M, 2021. Suppression of Th2 cytokines as a potential mechanism for reduced antibody response following PFOA exposure in female B6C3F1 mice. Toxicol. Lett 351, 155–162. [DOI] [PubMed] [Google Scholar]
- Dekel N, Gnainsky Y, Granot I, Racicot K, Mor G, 2014. The role of inflammation for a successful implantation. Am. J. Reprod. Immunol 72 (2), 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich V, Bil W, Vandebriel R, Granum B, Luijten M, Lindeman B, Grandjean P, Kaiser A-M, Hauzenberger I, Hartmann C, 2023a. Consideration of pathways for immunotoxicity of per-and polyfluoroalkyl substances (PFAS). Environ. Health 22 (1), 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich V, Bil W, Vandebriel R, Granum B, Luijten M, Lindeman B, Grandjean P, Kaiser A-M, Hauzenberger I, Hartmann C, Gundacker C, Uhl M, 2023b. Consideration of pathways for immunotoxicity of per- and polyfluoroalkyl substances (PFAS). Environ. Health 22 (1), 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eick SM, Geiger SD, Alshawabkeh A, Aung M, Barrett ES, Bush N, Carroll KN, Cordero JF, Goin DE, Ferguson KK, Kahn LG, Liang D, Meeker JD, Milne GL, Nguyen RHN, Padula AM, Sathyanarayana S, Taibl KR, Schantz SL, Woodruff TJ, Morello-Frosch R, 2022. Urinary oxidative stress biomarkers are associated with preterm birth: an Environmental Influences on Child Health Outcomes program study. Am. J. Obstet. Gynecol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everson TM, Sehgal N, Barr DB, Panuwet P, Yakimavets V, Perez C, Shankar K, Eick SM, Pearson KJ, Andres A: Placental PFAS concentrations are associated with perturbations of placental DNA methylation at loci with important roles on cardiometabolic health. medRxiv 2024. [Google Scholar]
- Fenton SE, Ducatman A, Boobis A, DeWitt JC, Lau C, Ng C, Smith JS, Roberts SM, 2021. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem 40 (3), 606–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Chen YH, Mukherjee B, Meeker JD, 2014a. Longitudinal profiling of inflammatory cytokines and C-reactive protein during uncomplicated and preterm pregnancy. Am J Reprod Immunol 72 (3), 326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, Cantonwine DE, Rivera-González LO, Loch-Caruso R, Mukherjee B, Anzalota Del Toro LV, Jiménez-Vélez B, Calafat AM, Ye X, Alshawabkeh AN, 2014b. Urinary phthalate metabolite associations with biomarkers of inflammation and oxidative stress across pregnancy in Puerto Rico. Environ. Sci. Tech 48 (12), 7018–7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Meeker JD, 2014c. Environmental phthalate exposure and preterm birth. JAMA Pediatr 168 (1), 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangione B, Birk S, Benzouak T, Rodriguez-Villamizar LA, Karim F, Dugandzic R, Villeneuve PJ, 2024. Exposure to perfluoroalkyl and polyfluoroalkyl substances and pediatric obesity: a systematic review and meta-analysis. Int. J. Obes. (lond) 48 (2), 131–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JJ, Walsh K, 2014. The Good, the Bad, and the Ugly of interleukin-6 signaling. EMBO J. 33 (13), 1425–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Ni W, Zhu S, Wu Y, Cui Y, Ma J, Liu Y, Qiao J, Ye Y, Yang P, 2021. Per-and polyfluoroalkyl substances exposure during pregnancy and adverse pregnancy and birth outcomes: A systematic review and meta-analysis. Environ. Res 201, 111632. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Hauth JC, Andrews WW, 2000. Intrauterine infection and preterm delivery. N. Engl. J. Med 342 (20), 1500–1507. [DOI] [PubMed] [Google Scholar]
- Goncalves RB, Coletta RD, Silverio KG, Benevides L, Casati MZ, da Silva JS, Nociti FH Jr., 2011. Impact of smoking on inflammation: overview of molecular mechanisms. Inflamm Res 60 (5), 409–424. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Heilmann C, Weihe P, Nielsen F, Mogensen UB, Budtz-Jørgensen E, 2017. Serum vaccine antibody concentrations in adolescents exposed to perfluorinated compounds. Environ. Health Perspect 125 (7), 077018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Zhang H, Sheng N, Wang J, Chen J, Dai J, 2021. Perfluorooctanoic acid (PFOA) exposure induces splenic atrophy via overactivation of macrophages in male mice. J. Hazard. Mater 407, 124862. [DOI] [PubMed] [Google Scholar]
- Gützkow KB, Haug LS, Thomsen C, Sabaredzovic A, Becher G, Brunborg G, 2012. Placental transfer of perfluorinated compounds is selective – A Norwegian Mother and Child sub-cohort study. Int. J. Hyg. Environ. Health 215 (2), 216–219. [DOI] [PubMed] [Google Scholar]
- Hampson HE, Costello E, Walker DI, Wang H, Baumert BO, Valvi D, Rock S, Jones DP, Goran MI, Gilliland FD, Conti DV, Alderete TL, Chen Z, Chatzi L, Goodrich JA, 2024. Associations of dietary intake and longitudinal measures of per- and polyfluoroalkyl substances (PFAS) in predominantly Hispanic young Adults: A multicohort study. Environ. Int 185, 108454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Nabb DL, Russell MH, Kennedy GL, Rickard RW, 2012. Renal Elimination of Perfluorocarboxylates (PFCAs). Chem. Res. Toxicol 25 (1), 35–46. [DOI] [PubMed] [Google Scholar]
- Han R, Zhang F, Wan C, Liu L, Zhong Q, Ding W, 2018. Effect of perfluorooctane sulphonate-induced Kupffer cell activation on hepatocyte proliferation through the NF-κB/TNF-α/IL-6-dependent pathway. Chemosphere 200, 283–294. [DOI] [PubMed] [Google Scholar]
- Hasnain SZ, Tauro S, Das I, Tong H, Chen AC, Jeffery PL, McDonald V, Florin TH, McGuckin MA, 2013. IL-10 promotes production of intestinal mucus by suppressing protein misfolding and endoplasmic reticulum stress in goblet cells. Gastroenterology 144 (2), 357–368.e359. [DOI] [PubMed] [Google Scholar]
- Hauke J, Kossowski T, 2011. Comparison of values of Pearson’s and Spearman’s correlation coefficients on the same sets of data. J Quaestiones Geographicae 30 (2), 87–93. [Google Scholar]
- Jiang Y, Scott AJ, Wild CJ, 2006. Secondary analysis of case-control data. Stat Med 25 (8), 1323–1339. [DOI] [PubMed] [Google Scholar]
- Keil AP, Buckley JP, O’Brien KM, Ferguson KK, Zhao S, White AJ, 2020. A Quantile-Based g-Computation Approach to Addressing the Effects of Exposure Mixtures. Environ. Health Perspect 128 (4), 047004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil A, Buckley J, O’Brien K, Ferguson K, Zhao S, White A: The qgcomp package: g-computation on exposure quantiles. In. 2021.
- Kelley AS, Banker M, Goodrich JM, Dolinoy DC, Burant C, Domino SE, Smith YR, Song PX, Padmanabhan V, 2019. Early pregnancy exposure to endocrine disrupting chemical mixtures are associated with inflammatory changes in maternal and neonatal circulation. Sci. Rep 9 (1), 5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RW, 1996. Inflammatory mediators and parturition. Reviews of Reproduction 1, 89–96. [DOI] [PubMed] [Google Scholar]
- Kirici P, Çağiran F, Kali Z, Tanriverdi E, Mavral N, Ecin S, 2023. Determination of maternal serum pro-inflammatory cytokine changes in intrauterine growth restriction. Eur. Rev. Med. Pharmacol. Sci 27 (5), 1996–2001. [DOI] [PubMed] [Google Scholar]
- Lucas JH, Wang Q, Meehan-Atrash J, Pang C, Rahman I, 2024. Developmental PFOS exposure alters lung inflammation and barrier integrity in juvenile mice. Toxicol. Sci, kfae073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley T: Thomas S Lumley - Complex Surveys_A Guide to Analysis Using R (Wiley Series in Survey Methodology; ) (2010). [Google Scholar]
- Matta K, Lefebvre T, Vigneau E, Cariou V, Marchand P, Guitton Y, Royer A-L, Ploteau S, Le Bizec B, Antignac J-P, Cano-Sancho G, 2022. Associations between persistent organic pollutants and endometriosis: A multiblock approach integrating metabolic and cytokine profiling. Environ. Int 158, 106926. [DOI] [PubMed] [Google Scholar]
- McCabe C, Anderson OS, Montrose L, Neier K, Dolinoy DC, 2017. Sexually Dimorphic Effects of Early-Life Exposures to Endocrine Disruptors: Sex-Specific Epigenetic Reprogramming as a Potential Mechanism. Curr Environ Health Rep 4 (4), 426–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor G, Cardenas I, Abrahams V, Guller S, 2011. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci 1221 (1), 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro SK, Balakrishnan B, Lissaman AC, Gujral P, Ponnampalam AP, 2021. Cytokines and pregnancy: Potential regulation by histone deacetylases. Mol. Reprod. Dev 88 (5), 321–337. [DOI] [PubMed] [Google Scholar]
- Nagelkerke NJ, Moses S, Plummer FA, Brunham RC, Fish D, 1995. Logistic regression in case-control studies: the effect of using independent as dependent variables. Stat Med 14 (8), 769–775. [DOI] [PubMed] [Google Scholar]
- NCHS: Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS_J). In.: Centers for Disease Control and Prevention; 2020. [Google Scholar]
- Nian M, Zhou W, Feng Y, Wang Y, Chen Q, Zhang J, 2022. Emerging and legacy PFAS and cytokine homeostasis in women of childbearing age. Sci Rep 12 (1), 6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsi NM, Tribe RM, 2008. Cytokine networks and the regulation of uterine function in pregnancy and parturition. J Neuroendocrinol 20 (4), 462–469. [DOI] [PubMed] [Google Scholar]
- Palaniyandi J, Bruin JE, Kumarathasan P, MacPherson S, Borghese MM, Ashley-Martin J, 2023. Prenatal exposure to perfluoroalkyl substances and inflammatory biomarker concentrations. Environ Epidemiol 7 (4), e262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey M, Chauhan M, Awasthi S, 2017. Interplay of cytokines in preterm birth. Indian J Med Res 146 (3), 316–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendeloski KPT, Ono E, Torloni MR, Mattar R, Daher S, 2017. Maternal obesity and inflammatory mediators: A controversial association. Am. J. Reprod. Immunol 77 (5), e12674. [DOI] [PubMed] [Google Scholar]
- Phelps DW, Connors AM, Ferrero G, DeWitt JC, Yoder JA, 2024. Per-and polyfluoroalkyl substances alter innate immune function: evidence and data gaps. J. Immunotoxicol 21 (1), 2343362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prairie E, Cote F, Tsakpinoglou M, Mina M, Quiniou C, Leimert K, Olson D, Chemtob S, 2021. The determinant role of IL-6 in the establishment of inflammation leading to spontaneous preterm birth. Cytokine Growth Factor Rev 59, 118–130. [DOI] [PubMed] [Google Scholar]
- Qazi MR, Bogdanska J, Butenhoff JL, Nelson BD, DePierre JW, Abedi-Valugerdi M, 2009. High-dose, short-term exposure of mice to perfluorooctanesulfonate (PFOS) or perfluorooctanoate (PFOA) affects the number of circulating neutrophils differently, but enhances the inflammatory responses of macrophages to lipopolysaccharide (LPS) in a similar fashion. Toxicology 262 (3), 207–214. [DOI] [PubMed] [Google Scholar]
- Qazi MR, Abedi MR, Nelson BD, DePierre JW, Abedi-Valugerdi M, 2010. Dietary exposure to perfluorooctanoate or perfluorooctane sulfonate induces hypertrophy in centrilobular hepatocytes and alters the hepatic immune status in mice. Int. Immunopharmacol 10 (11), 1420–1427. [DOI] [PubMed] [Google Scholar]
- Rivera-Núñez Z, Kinkade CW, Khoury L, Brunner J, Murphy H, Wang C, Kannan K, Miller RK, O’Connor TG, Barrett ES, 2023. Prenatal perfluoroalkyl substances exposure and maternal sex steroid hormones across pregnancy. Environ. Res 220, 115233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwell CE, Turley AE, Cheng X, Fields PE, Klaassen CD: Acute Immunotoxic Effects of Perfluorononanoic Acid (PFNA) in C57BL/6 Mice. LID - S4–002 [pii]. 2013(2161–1459 (Print)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwell CE, Turley AE, Cheng X, Fields PE, Klaassen CD, 2017. Persistent alterations in immune cell populations and function from a single dose of perfluorononanoic acid (PFNA) in C57Bl/6 mice. Food Chem. Toxicol 100, 24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanowska-Prochnicka K, Felis-Giemza A, Olesinska M, Wojdasiewicz P, Paradowska-Gorycka A, Szukiewicz D, 2021. The Role of TNF-alpha and Anti-TNF-alpha Agents during Preconception, Pregnancy, and Breastfeeding. Int J Mol Sci 22 (6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld CS, 2015. Sex-Specific Placental Responses in Fetal Development. Endocrinology 156 (10), 3422–3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner BA: Fundamentals of biostatistics, vol. 6: Thomson-Brooks/Cole Belmont, CA. 2006. [Google Scholar]
- Roth K, Yang Z, Agarwal M, Liu W, Peng Z, Long Z, Birbeck J, Westrick J, Liu W, Petriello MC, 2021. Exposure to a mixture of legacy, alternative, and replacement per- and polyfluoroalkyl substances (PFAS) results in sex-dependent modulation of cholesterol metabolism and liver injury. Environ. Int 157, 106843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DB, 1976. Inference and Missing Data. Biometrika 63 (3), 581–592. [Google Scholar]
- Sagiv SK, Rifas-Shiman SL, Fleisch AF, Webster TF, Calafat AM, Ye X, Gillman MW, Oken E, 2017. Early-Pregnancy Plasma Concentrations of Perfluoroalkyl Substances and Birth Outcomes in Project Viva: Confounded by Pregnancy Hemodynamics? Am. J. Epidemiol 187 (4), 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz DA, Wise LA, Bond JC, Hatch EE, Ncube CN, Wesselink AK, Willis MD, Yland JJ, Rothman KJ, 2024. Responding to Reviewers and Editors About Statistical Significance Testing. Ann Intern Med 177 (3), 385–386. [DOI] [PubMed] [Google Scholar]
- Shkoda A, Ruiz PA, Daniel H, Kim SC, Rogler G, Sartor RB, Haller D, 2007. Interleukin-10 blocked endoplasmic reticulum stress in intestinal epithelial cells: impact on chronic inflammation. Gastroenterology 132 (1), 190–207. [DOI] [PubMed] [Google Scholar]
- Siwakoti RC, Cathey A, Ferguson KK, Hao W, Cantonwine DE, Mukherjee B, McElrath TF, Meeker JD, 2023. Prenatal per- and polyfluoroalkyl substances (PFAS) exposure in relation to preterm birth subtypes and size-for-gestational age in the LIFECODES cohort 2006–2008. Environ. Res 237 (Pt 2), 116967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwakoti RC, Park S, Ferguson KK, Hao W, Cantonwine DE, Mukherjee B, McElrath TF, Meeker JD, 2024. Prenatal per- and polyfluoroalkyl substances (PFAS) and maternal oxidative stress: Evidence from the LIFECODES study. Chemosphere 360, 142363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posit Software P: RStudio: Integrated Development Environment for R. In. Edited by team P. Boston, MA. 2024. [Google Scholar]
- Spence T, Allsopp PJ, Yeates AJ, Mulhern MS, Strain JJ, McSorley EM, 2021. Maternal Serum Cytokine Concentrations in Healthy Pregnancy and Preeclampsia. J. Pregnancy 2021 (1), 6649608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y, 2007. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav. Immun 21 (7), 901–912. [DOI] [PubMed] [Google Scholar]
- Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG, 2019. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Eposure Sci. Environ. Epidemiol 29 (2), 131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda LK, Ishikawa T, Dolinoy DC, 2022. Developmental toxicant exposures and sex-specific effects on epigenetic programming and cardiovascular health across generations. Environ. Epigenet 8 (1), dvac017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y, Taibl KR, Dunlop AL, Barr DB, Panuwet P, Yakimavets V, Kannan K, Corwin EJ, Ryan PB, Eatman JA, Liang D, Eick SM, 2023. Association between a Mixture of Per- and Polyfluoroalkyl Substances (PFAS) and Inflammatory Biomarkers in the Atlanta African American Maternal-Child Cohort. Environ. Sci. Tech 57 (36), 13419–13428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson González N, Ong J, Luo L, MacKenzie D, 2022. Chronic community exposure to environmental metal mixtures is associated with selected cytokines in the navajo birth cohort study (NBCS). Int. J. Environ. Res. Public Health 19 (22), 14939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urakubo A, Jarskog LF, Lieberman JA, Gilmore JH, 2001. Prenatal exposure to maternal infection alters cytokine expression in the placenta, amniotic fluid, and fetal brain. Schizophr. Res 47 (1), 27–36. [DOI] [PubMed] [Google Scholar]
- USEPA: PFAS National Primary Drinking Water Regulation Rulemaking. In. Edited by Agency USEP; 2023. [Google Scholar]
- USEPA: Biden-Harris Administration Finalizes Critical Rule to Clean up PFAS Contamination to Protect Public Health. In. 2024.
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr., Lee D-H, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP, 2012. Hormones and Endocrine-Disrupting Chemicals: Low-Dose Effects and Nonmonotonic Dose Responses. Endocr. Rev 33 (3), 378–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanNoy BN, Lam J, Zota AR, 2018. Breastfeeding as a Predictor of Serum Concentrations of Per- and Polyfluorinated Alkyl Substances in Reproductive-Aged Women and Young Children: A Rapid Systematic Review. Curr Environ Health Rep 5 (2), 213–224. [DOI] [PubMed] [Google Scholar]
- Varner MW, Esplin MS, 2005. Current understanding of genetic factors in preterm birth. BJOG 112, 28–31. [DOI] [PubMed] [Google Scholar]
- Vilotic A, Nacka-Aleksic M, Pirkovic A, Bojic-Trbojevic Z, Dekanski D, Jovanovic Krivokuca M, 2022. IL-6 and IL-8: An Overview of Their Roles in Healthy and Pathological Pregnancies. Int J Mol Sci 23 (23). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S-Q, Fraser W, Luo Z-C, 2010. Inflammatory Cytokines and Spontaneous Preterm Birth in Asymptomatic Women: A Systematic Review, 2 Part 1 Obstet. Gynecol. 116. [DOI] [PubMed] [Google Scholar]
- Welch BM, McNell EE, Edin ML, Ferguson KK, 2022. Inflammation and oxidative stress as mediators of the impacts of environmental exposures on human pregnancy: Evidence from oxylipins. Pharmacol Ther 239, 108181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Priest N, Anderson NB, 2016. Understanding associations among race, socioeconomic status, and health: Patterns and prospects. Health Psychol 35 (4), 407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SN, 2001. mgcv: GAMs and generalized ridge regression for R. R News 1 (2), 20–25. [Google Scholar]
- Zhang Z, 2016. Multiple imputation with multivariate imputation by chained equation (MICE) package. Annals of Translational Medicine 4 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Fang W, Wang D, Gao N, Ding Y, Chen C, 2014. The role of interleukin family in perfluorooctanoic acid (PFOA)-induced immunotoxicity. J. Hazard. Mater 280, 552–560. [DOI] [PubMed] [Google Scholar]
- Zhang L, Louie A, Rigutto G, Guo H, Zhao Y, Ahn S, Dahlberg S, Sholinbeck M, Smith MT, 2023. A systematic evidence map of chronic inflammation and immunosuppression related to per-and polyfluoroalkyl substance (PFAS) exposure. Environ. Res 220, 115188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota AR, Geller RJ, Romano LE, Coleman-Phox K, Adler NE, Parry E, Wang M, Park J-S, Elmi AF, Laraia BA, Epel ES, 2018. Association between persistent endocrine-disrupting chemicals (PBDEs, OH-PBDEs, PCBs, and PFASs) and biomarkers of inflammation and cellular aging during pregnancy and postpartum. Environ. Int 115, 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors do not have permission to share data.


