Abstract
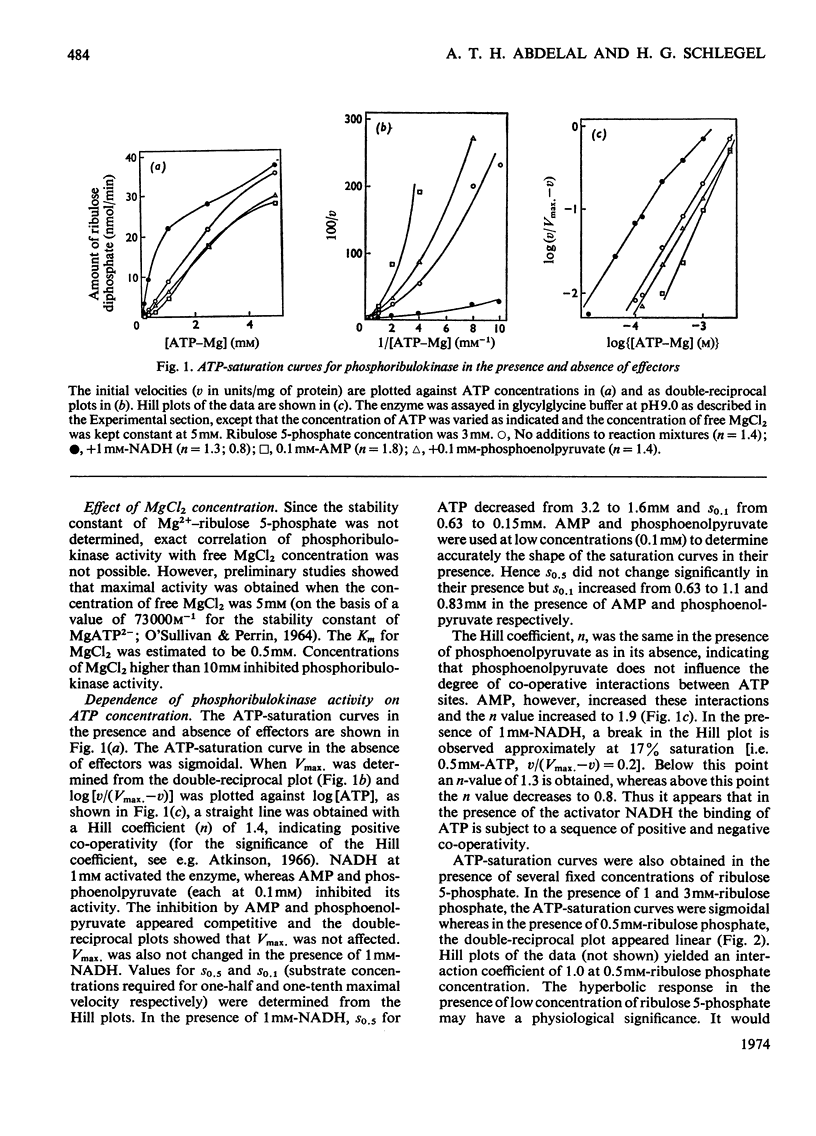
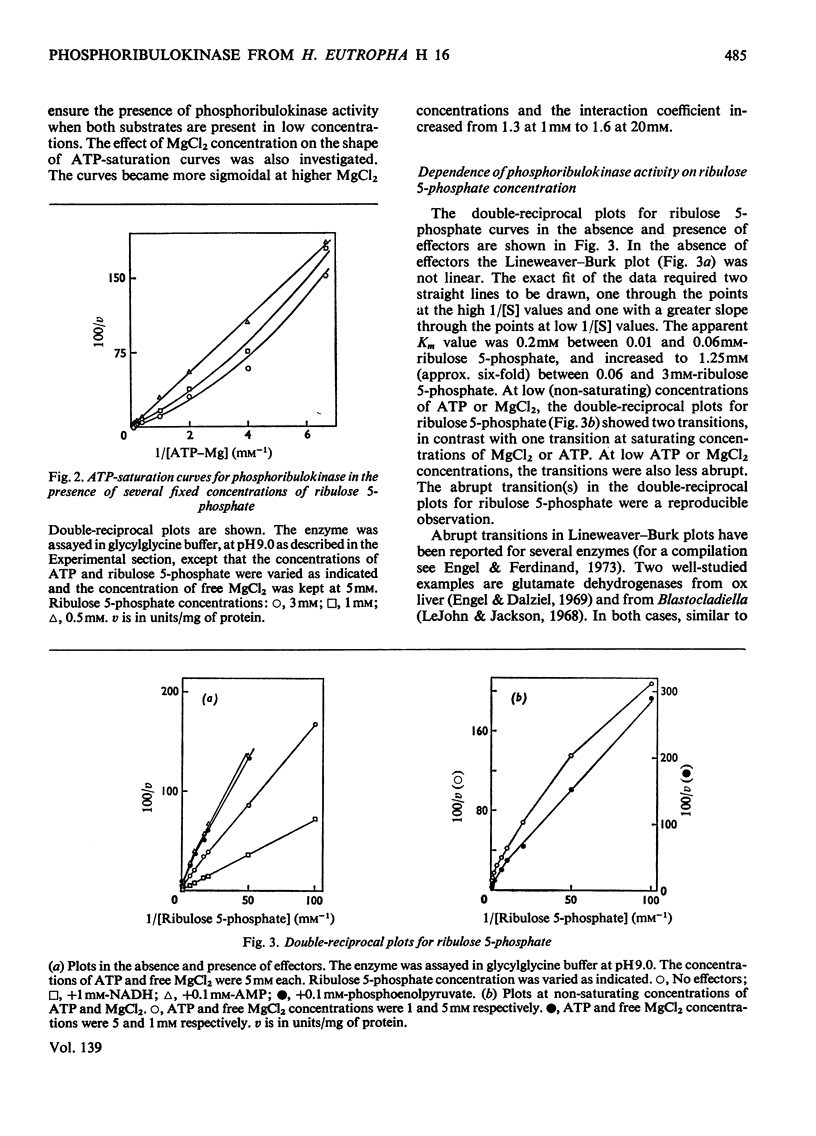
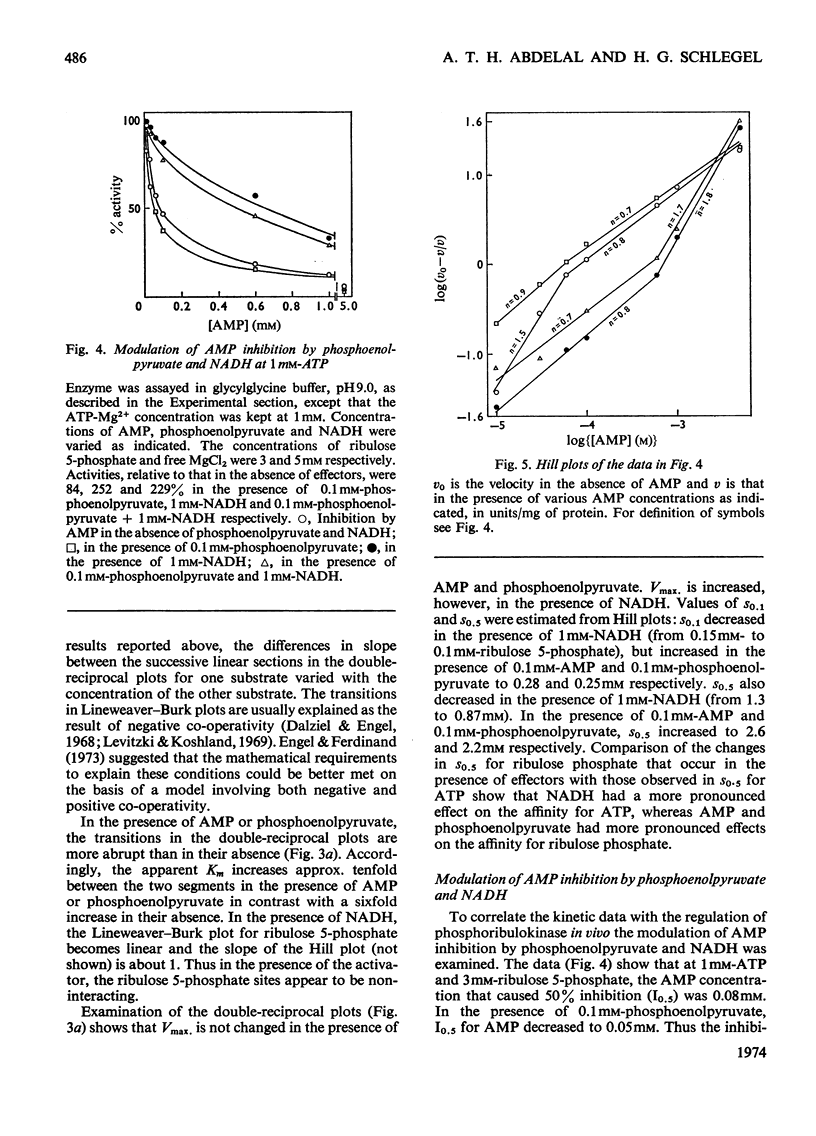
1. Phosphoribulokinase was purified 286-fold from extracts of autotrophically grown cells. 2. The enzyme had a molecular weight of 237000 and showed a pH optimum of 9.0 in both crude extracts and purified preparation. MgCl2 was required for activity; full activation was obtained at 5mm-MgCl2 and the Km was approx. 0.5mm. 3. The ATP-saturation curve was sigmoidal and the degree of positive co-operativity increased at higher MgCl2 concentrations. The ATP-binding sites appeared to be non-interacting at low ribulose 5-phosphate concentrations. 4. Lineweaver–Burk plots for ribulose 5-phosphate showed abrupt transitions between apparently linear sections. The apparent Km and Vmax. values increased with increasing concentrations of ribulose phosphate. The transitions may be explained by a sequence of negative and positive co-operativity in the catalytic rate constants. 5. Phosphoribulokinase activity was inhibited by AMP and phosphoenolpyruvate and was activated by NADH. The presence of AMP or phosphoenolpyruvate increased s0.5 (substrate concentration required for half-maximal velocity) for both ribulose 5-phosphate and ATP but Vmax. was not changed. The sigmoidicity of the ATP-saturation curve increased in the presence of AMP but was not affected by phosphoenolpyruvate. The transitions in the ribulose 5-phosphate-saturation curves were more abrupt in the presence of either inhibitor. NADH lowered the s0.5 for both ribulose 5-phosphate and ATP. The activator did not affect the degree of positive co-operativity between ATP-binding sites, but the ribulose 5-phosphate-binding sites appeared to be non-interacting in its presence. 6. A sequence of positive and negative co-operativity in the interactions of AMP-binding sites was suggested by the Hill plots. In the presence of NADH (and phosphoenolpyruvate) the sensitivity to inhibition by AMP was less below a certain AMP concentration and increased above that concentration. 7. Examination of the interactions between ligands indicated that phosphoribulokinase can be regulated effectively by changes in effector concentrations similar to those reported to occur in vivo.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdelal A. T., Schlegel H. G. Separation of phosphoribulokinase from enzymes of the Calvin cycle in Hydrogenomonas eutropha H 16. Arch Mikrobiol. 1974 Feb 1;95(2):139–143. doi: 10.1007/BF02451755. [DOI] [PubMed] [Google Scholar]
- Ballard R. W., MacElroy R. D. Phosphoenolpyruvate, a new inhibitor of phosphoribulokinase in pseudomonas facilis. Biochem Biophys Res Commun. 1971 Aug 6;44(3):614–618. doi: 10.1016/s0006-291x(71)80127-4. [DOI] [PubMed] [Google Scholar]
- Conway A., Koshland D. E., Jr Negative cooperativity in enzyme action. The binding of diphosphopyridine nucleotide to glyceraldehyde 3-phosphate dehydrogenase. Biochemistry. 1968 Nov;7(11):4011–4023. doi: 10.1021/bi00851a031. [DOI] [PubMed] [Google Scholar]
- Dalziel Keith, Engel Paul C. Antagonistic homotropic interactions as a possible explanation of coenzyme activation of glutamate dehydrogenase. FEBS Lett. 1968 Oct;1(5):349–352. doi: 10.1016/0014-5793(68)80153-x. [DOI] [PubMed] [Google Scholar]
- Engel P. C., Dalziel K. Kinetic studies of glutamate dehydrogenase with glutamate and norvaline as substrates. Coenzyme activation and negative homotropic interactions in allosteric enzymes. Biochem J. 1969 Dec;115(4):621–631. doi: 10.1042/bj1150621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel P. C., Ferdinand W. The significance of abrupt transitions in Lineweaver-Burk plots with particular reference to glutamate dehydrogenase. Negative and positive co-operativity in catalytic rate constants. Biochem J. 1973 Jan;131(1):97–105. doi: 10.1042/bj1310097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale N. L., Beck J. V. Competitive inhibition of phosphoribulokinase by AMP. Biochem Biophys Res Commun. 1966 Sep 8;24(5):792–796. doi: 10.1016/0006-291x(66)90396-2. [DOI] [PubMed] [Google Scholar]
- Hart B. A., Gibson J. Ribulose-5-phosphate kinase from Chromatium sp. strain D. Arch Biochem Biophys. 1971 May;144(1):308–321. doi: 10.1016/0003-9861(71)90483-8. [DOI] [PubMed] [Google Scholar]
- JOHNSON E. J., PECK H. D., Jr COUPLING OF PHOSPHORYLATION AND CARBON DIOXIDE FIXATION IN EXTRACTS OF THIOBACILLUS THIOPARUS. J Bacteriol. 1965 Apr;89:1041–1050. doi: 10.1128/jb.89.4.1041-1050.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. J. Occurrence of the adenosine monophosphate inhibition of carbon dioxide fixation in photosynthetic and chemosynthetic autotrophs. Arch Biochem Biophys. 1966 Apr;114(1):178–183. doi: 10.1016/0003-9861(66)90319-5. [DOI] [PubMed] [Google Scholar]
- Joint I. R., Morris I., Fuller R. C. Possible regulatory characteristics of the fructose diphosphatase--phosphoribulokinase complex from Rhodospirillum rubrum. Biochim Biophys Acta. 1972 Jul 13;276(1):333–337. doi: 10.1016/0005-2744(72)90037-x. [DOI] [PubMed] [Google Scholar]
- Joint I. R., Morris I., Fuller R. C. Purification of a complex of alkaline fructose 1,6-bisphosphatase and phosphoribulokinase from Rhodospirillum rubrum. J Biol Chem. 1972 Aug 10;247(15):4833–4838. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levitzki A., Koshland D. E., Jr Negative cooperativity in regulatory enzymes. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1121–1128. doi: 10.1073/pnas.62.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry O. H., Carter J., Ward J. B., Glaser L. The effect of carbon and nitrogen sources on the level of metabolic intermediates in Escherichia coli. J Biol Chem. 1971 Nov;246(21):6511–6521. [PubMed] [Google Scholar]
- LéJohn H. B., Jackson S. Allosteric interactions of a regulatory nicotinamide adenine dinucleotide-specific glutamate dehydrogenase from Blastocladiella. A molecular model for the enzyme. J Biol Chem. 1968 Jun 25;243(12):3447–3457. [PubMed] [Google Scholar]
- MacElroy R. D., Johnson E. J., Johnson M. K. Allosteric regulation of phosphoribulokinase activity. Biochem Biophys Res Commun. 1968 Mar 27;30(6):678–682. doi: 10.1016/0006-291x(68)90566-4. [DOI] [PubMed] [Google Scholar]
- MacElroy R. D., Johnson E. J., Johnson M. K. Characterization of ribulose diphosphate carboxylase and phosphoribulokinase from Thiobacillus thioparus and Thiobacillus neapolitanus. Arch Biochem Biophys. 1968 Sep 20;127(1):310–316. doi: 10.1016/0003-9861(68)90231-2. [DOI] [PubMed] [Google Scholar]
- MacElroy R. D., Johnson E. J., Johnson M. K. Control of ATP-dependent CO2 fixation in extracts of Hydrogenomonas facilis: NADH regulation of phosphoribulokinase. Arch Biochem Biophys. 1969 Apr;131(1):272–275. doi: 10.1016/0003-9861(69)90131-3. [DOI] [PubMed] [Google Scholar]
- MacElroy R. D., Mack H. M., Johnson E. J. Properties of phosphoribulokinase from Thiobacillus neapolitanus. J Bacteriol. 1972 Oct;112(1):532–538. doi: 10.1128/jb.112.1.532-538.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden B. A., Tu C. C. Regulation of autotrophic and heterotrophic carbon dioxide fixation in Hydrogenomonas facilis. J Bacteriol. 1967 Mar;93(3):886–893. doi: 10.1128/jb.93.3.886-893.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'SULLIVAN W. J., PERRIN D. D. THE STABILITY CONSTANTS OF METAL-ADENINE NUCLEOTIDE COMPLEXES. Biochemistry. 1964 Jan;3:18–26. doi: 10.1021/bi00889a005. [DOI] [PubMed] [Google Scholar]
- PONTREMOLI S., MANGIAROTTI G. A simple method for the preparation of D-ribulose 5-phosphate. J Biol Chem. 1962 Mar;237:643–645. [PubMed] [Google Scholar]
- Rindt K. P., Ohmann E. NADH and AMP as allosteric effectors of ribulose-5-phosphate kinase in Rhodopseudomonas spheroides. Biochem Biophys Res Commun. 1969 Aug 7;36(3):357–364. doi: 10.1016/0006-291x(69)90572-5. [DOI] [PubMed] [Google Scholar]
- SCHLEGEL H. G., KALTWASSER H., GOTTSCHALK G. [A submersion method for culture of hydrogen-oxidizing bacteria: growth physiological studies]. Arch Mikrobiol. 1961;38:209–222. [PubMed] [Google Scholar]
- WALDE E. [Studies on growth and synthesis of stored substance by Hydrogenomonas]. Arch Mikrobiol. 1962;43:109–137. [PubMed] [Google Scholar]


