Abstract
Sporadic bilateral renal cell carcinoma (BRCC) is a rare situation of RCC. The treatment for BRCC is controversial and there is a lack of authoritative guidelines about the management of BRCC. Patients diagnosed with sporadic BRCC between 2004 and 2020 were identified from Surveillance, Epidemiology, and End Results (SEER) database. The primary outcome was overall survival (OS). Kaplan–Meier survival analysis, Cox regression analysis, and competing risk regression models were used to compare survival outcomes and identify prognostic factors. A total of 20,523 patients (16,534 unilateral RCC [URCC] patients and 3989 BRCC patients) were included. The prognosis of BRCC patients is between metastatic and non-metastatic URCC patients. 3677 patients were diagnosed with localized BRCC (2180 synchronous BRCC patients and 1497 metachronous BRCC patients). Compared with metachronous BRCC, synchronous BRCC patients had relatively poor OS. However, the CSS was similar. Partial nephrectomy (PN) leads to the best OS and provides equivalent oncological outcomes to radical nephrectomy. Local tumor destruction (LTD) could also achieve an acceptable cancer-control effect. Then we developed treatment flowchart for localized BRCC patients. Additionally, we identified the prognostic factors, and analyzed the association between factors using the multivariable Cox regression method. PN should be the initial treatment for sporadic localized BRCC patients if feasible. LTD could be considered as an effective treatment alternative. This study could provide evidence for the optimization of individualized treatment for sporadic BRCC patients.
Trial registration: The trial was registered on the ClinicalTrials.gov (NCT06369519).
Supplementary Information
The online version contains supplementary material available at 10.1007/s10238-024-01535-5.
Keywords: Bilateral renal cell carcinoma, Surgery, Partial nephrectomy, Local tumor destruction, Nomogram
Background
Renal cell carcinoma (RCC) is one of the most common malignancies of the urinary system [1, 2]. Bilateral RCC (BRCC) is a rare situation with a reported incidence of 3–6% of all RCC patients [2–5]. Multiple etiologic factors have been reported to be associated with BRCC, including genetic susceptibility, hereditary cancer syndromes and environmental factors [4, 6]. Sporadic BRCC is a distinct subtype and has different biological characteristics compared with hereditary BRCC [7]. There has been a rise in the prevalence of sporadic BRCC due to a variety of contributing factors including overall growth of RCC incidence rates, increased frequencies of surveillance imaging, and longer life expectancy [8]. BRCC could be categorized into synchronous and metachronous BRCC. Synchronous BRCC is defined as the second primary contralateral RCC emerges within 6 months from the diagnosis of the first primary RCC, whereas metachronous BRCC means second primary contralateral RCC develops beyond 6 months from the diagnosis of the first primary RCC [4]. Given the different time intervals and the tumor heterogeneity, the treatment for BRCC should be individualized.
Surgery is the main treatment for localized RCC [9]. Nevertheless, the occurrence of BRCC offers challenging therapeutic dilemmas, which should balance the need for effective long-term oncological control and maximal renal functional preservation [10, 11]. The decision-making of treatments for BRCC are controversial and there is still a lack of authoritative guidelines about the management of BRCC. Currently, partial nephrectomy (PN) has been advocated as an effective treatment to maximize the preservation of renal parenchyma [12]. However, the technical challenges of PN and increased surgical complications make it inappropriate for some patients such as those with significant comorbidities [13]. In recent years, local tumor destruction (LTD) such as renal mass ablation has emerged as a treatment alternative [14]. A few studies have focused on its application in the treatment of BRCC [15, 16]. However, the relatively small sample size, the retrospective design, may inevitably introduce bias and lead to low quality of evidence. Further investigation is warranted for the optimization of surgical treatment for BRCC.
Here, based on the large-scale population from the Surveillance, Epidemiology, and End Results (SEER) database, we conducted comprehensive analyses on the clinicopathological characteristics and the long-term survival outcomes of BRCC patients. In addition, accurate risk assessment and prognosis evaluation are essential for individualized treatment decision. Due to the relatively low incidence and highly heterogeneous tumor characteristics, there is a lack of comprehensive effective risk assessment model for sporadic BRCC. Therefore, we also identified the prognostic factors, conducted multivariable Cox regression analysis, and developed treatment flowchart for sporadic localized BRCC. Our study will not only perform the comprehensive long-term survival analysis of BRCC patients from an epidemiological point of view, but also provide evidence for the optimization of the current treatment paradigm and individualized treatment strategies for sporadic BRCC.
Materials and methods
Data sources and patient population
This retrospective cohort study was based on the SEER database, which contains the population-based data from 17 Registries covering approximately 26.5% of the U.S. population [17]. RCC patients were incorporated and divided into the unilateral RCC (URCC) and BRCC cohorts. The inclusion criteria of sporadic localized BRCC were: (1) adults diagnosed with localized BRCC (M0) from 2004 to 2020; (2) active follow-up. The exclusion criteria were: (1) diagnosed by autopsy or death certificate only; (2) tending to suffer from hereditary RCC, including those complicating with pancreatic neuroendocrine tumor (pNET), pheochromocytoma, or hemangioblastoma; (3) unavailable key information. The eligible patients were randomized 7:3 into the training and validation cohort. The training set was used to screen variables, and an attempt was made to develop predictive models based on the information available from the SEER database. The validation set was used for internal validation. Given that the SEER database can be publicly accessed, the study did not require informed consent and was exempt from the review of IRB. The trial was registered on the ClinicalTrials.gov (NCT06369519).
Data acquisition and outcome measurement
Demographic and clinicopathological information was collected. The age at diagnosis of BRCC was defined as the age at the first occurrence of primary RCC. The follow-up period was defined as the time from the diagnosis of the second primary RCC to death, loss of follow-up, or last follow-up. The primary outcome was overall survival (OS), which was calculated as the time interval from the diagnosis of the second primary RCC to death or the last follow-up. The second outcome was cancer-specific survival (CSS), which was calculated as the time interval from the diagnosis of the second primary RCC to death from the same disease or the last follow-up.
Statistical analysis
Descriptive statistics student’s t test, χ2 tests, or Mann–Whitney U test were applied to compare the baseline characteristics. Propensity score matching (PSM) methods were used to minimize bias related to nonrandom assignment when comparing intergroup prognosis [18]. Propensity scores were estimated in multivariable logistic regression models based on 11 predefined covariates, then a greedy 1:1 algorithm was used to match randomly RCC patients from the two cohorts by the closest propensity score. The process was conducted using the “MatchIt” package in R software with a caliper width of 0.002.
Differences in survival between groups were compared via Kaplan–Meier (KM) analysis and log-rank tests. Cox proportional hazard models were performed to identify the variables that significantly impact OS. For CSS, considering that other cause mortality (OCM) could be competing events to cancer-specific mortality (CSM), competing risk regression models (CRR), were utilized and CSM was treated as a competing risk [19]. Fine and Gray’s proportional subdistribution hazard model was applied to test the differences in cumulative incurred function (CIF) between different groups and obtain independent prognostic factors [20]. Inclusion in the multivariable analysis was determined by a priori cut-off of p-value less than 0.1 by univariate analysis.
The potential prognostic factors were incorporated in the least absolute shrinkage and selection operator (LASSO) regression analysis to identify useful predictive factors, which could avoid overfitting to some extent and select the best weighting coefficient [21, 22]. The inclusion of covariates in the nomograms comprehensively considered the results of multivariate analysis and LASSO regression analysis, and followed Harrell’s guideline [23]. The nomograms were developed using the “rms” package in R. The receiver operating characteristic curve (ROC) was plotted. The area under the curve (AUC) and Harrell’s concordance index (C-index) were calculated to evaluate the discriminative ability [24]. KM analysis and CRR were employed to evaluate the ability of risk stratification. Calibration curves were applied to verify the consistency between the predicted values and the actual results. Moreover, decision curve analyses (DCAs) were applied to evaluate the clinical net benefit and utility of the predictive models at different risk threshold probabilities [25, 26].
All statistical tests were performed using the R software (version 4.3.1). A two-sided with P < 0.05 was considered statistically significant. The study was reported on line with the STROBE criteria [27].
Results
Baseline cohort characteristics and analysis of URCC and BRCC
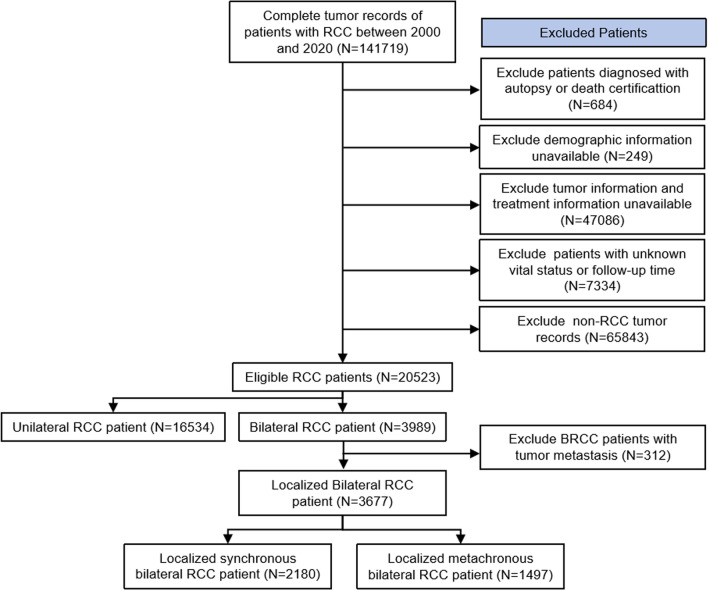
The flowchart of patient selection is shown in Fig. 1. A total of 20,523 (16,534 URCC patients and 3989 BRCC patients) were included. The demographic and clinical characteristics are summarized in Table 1. The second oncological characteristics for BRCC were exhibited. The median [interquartile range/IQR] age at diagnosis was 65 [58, 72] and 64 [55, 71] for the URCC and BRCC patients. Compared with URCC, second malignancies of BRCC had lower T stage but more M1 stage. After PSM, all clinical characteristics between the two cohorts were balanced (p > 0.05, Fig. S1).
Fig. 1.
The flow chart of study participant selection. Abbreviations: RCC, renal cell carcinoma
Table 1.
Baseline characteristics of unilateral and bilateral RCC patients
| Characteristic | Before PSM | After PSM | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall (n = 20,523) | Unilateral RCC (n = 16,534) | Bilateral RCC (n = 3989) | p-value | Overall (n = 7496) | Unilateral RCC (n = 3748) | Bilateral RCC (n = 3748) | p-value | ||
| Age (yrs) (%) | < 45 | 890 (4.3) | 606 (3.7) | 284 (7.1) | < 0.001 | 387 (5.2) | 188 (5.0) | 199 (5.3) | 0.307 |
| 45–65 | 9034 (44.0) | 7257 (43.9) | 1777 (44.5) | 3409 (45.5) | 1701 (45.4) | 1708 (45.6) | |||
| 65–85 | 10,035 (48.9) | 8226 (49.8) | 1809 (45.3) | 3441 (45.9) | 1715 (45.8) | 1726 (46.1) | |||
| > 85 | 564 (2.7) | 445 (2.7) | 119 (3.0) | 259 (3.5) | 144 (3.8) | 115 (3.1) | |||
| Sex (%) | Female | 6490 (31.6) | 5402 (32.7) | 1088 (27.3) | < 0.001 | 2069 (27.6) | 1046 (27.9) | 1023 (27.3) | 0.57 |
| Male | 14,033 (68.4) | 11,132 (67.3) | 2901 (72.7) | 5427 (72.4) | 2702 (72.1) | 2725 (72.7) | |||
| Race (%) | White | 16,639 (81.1) | 13,785 (83.4) | 2854 (71.5) | < 0.001 | 5640 (75.2) | 2848 (76.0) | 2792 (74.5) | 0.189 |
| Black | 2761 (13.5) | 1828 (11.1) | 933 (23.4) | 1508 (20.1) | 740 (19.7) | 768 (20.5) | |||
| Others | 1123 (5.5) | 921 (5.6) | 202 (5.1) | 348 (4.6) | 160 (4.3) | 188 (5.0) | |||
| Marital status (%) | Married | 12,860 (62.7) | 10,484 (63.4) | 2376 (59.6) | < 0.001 | 4572 (61.0) | 2290 (61.1) | 2282 (60.9) | 0.733 |
| Unmarried | 6722 (32.8) | 5345 (32.3) | 1377 (34.5) | 2541 (33.9) | 1274 (34.0) | 1267 (33.8) | |||
| Unknown | 941 (4.6) | 705 (4.3) | 236 (5.9) | 383 (5.1) | 184 (4.9) | 199 (5.3) | |||
| Median household income (%) | $ (0–75,000) | 12,694 (61.9) | 10,294 (62.3) | 2400 (60.2) | 0.015 | 4566 (60.9) | 2305 (61.5) | 2261 (60.3) | 0.309 |
| $ (75,000 +) | 7829 (38.1) | 6240 (37.7) | 1589 (39.8) | 2930 (39.1) | 1443 (38.5) | 1487 (39.7) | |||
| Rural/urban continuum population density (%) | Counties in metropolitan areas ge 1 million pop | 11,554 (56.3) | 9215 (55.7) | 2339 (58.6) | 0.004 | 4392 (58.6) | 2199 (58.7) | 2193 (58.5) | 0.789 |
| Counties in metropolitan areas of 0 to 1 million pop | 6170 (30.1) | 5008 (30.3) | 1162 (29.1) | 2175 (29.0) | 1089 (29.1) | 1086 (29.0) | |||
| Nonmetropolitan counties | 2765 (13.5) | 2283 (13.8) | 482 (12.1) | 920 (12.3) | 457 (12.2) | 463 (12.4) | |||
| Unknown | 34 (0.2) | 28 (0.2) | 6 (0.2) | 9 (0.1) | 3 (0.1) | 6 (0.2) | |||
| Tumor stage (%) | I | 15,024 (73.2) | 11,710 (70.8) | 3314 (83.1) | < 0.001 | 6292 (83.9) | 3178 (84.8) | 3114 (83.1) | 0.054 |
| II | 1776 (8.7) | 1600 (9.7) | 176 (4.4) | 348 (4.6) | 175 (4.7) | 173 (4.6) | |||
| III | 2685 (13.1) | 2433 (14.7) | 252 (6.3) | 479 (6.4) | 231 (6.2) | 248 (6.6) | |||
| IV | 1038 (5.1) | 791 (4.8) | 247 (6.2) | 377 (5.0) | 164 (4.4) | 213 (5.7) | |||
| Tumor T stage (%) | T1 | 15,429 (75.2) | 11,955 (72.3) | 3474 (87.1) | < 0.001 | 6515 (86.9) | 3273 (87.3) | 3242 (86.5) | 0.696 |
| T2 | 1965 (9.6) | 1759 (10.6) | 206 (5.2) | 400 (5.3) | 198 (5.3) | 202 (5.4) | |||
| T3 | 2968 (14.5) | 2683 (16.2) | 285 (7.1) | 541 (7.2) | 258 (6.9) | 283 (7.6) | |||
| T4 | 153 (0.7) | 129 (0.8) | 24 (0.6) | 40 (0.5) | 19 (0.5) | 21 (0.6) | |||
| Tumor N stage (%) | N0 | 20,090 (97.9) | 16,173 (97.8) | 3917 (98.2) | 0.093 | 7389 (98.6) | 3703 (98.8) | 3686 (98.3) | 0.153 |
| N1 | 433 (2.1) | 361 (2.2) | 72 (1.8) | 107 (1.5) | 45 (1.2) | 62 (1.6) | |||
| Tumor M stage (%) | M0 | 19,580 (95.4) | 15,828 (95.7) | 3752 (94.1) | < 0.001 | 7136 (95.2) | 3592 (95.8) | 3544 (94.6) | 0.061 |
| M1 | 943 (4.6) | 706 (4.3) | 237 (5.9) | 360 (4.8) | 156 (4.2) | 204 (5.4) | |||
| Grade (%) | Grade 1 | 2051 (10.0) | 1710 (10.3) | 341 (8.5) | < 0.001 | 632 (8.4) | 314 (8.4) | 318 (8.5) | 0.665 |
| Grade 2 | 8214 (40.0) | 6826 (41.3) | 1388 (34.8) | 2666 (35.6) | 1329 (35.5) | 1337 (35.7) | |||
| Grade 3 | 4080 (19.9) | 3474 (21.0) | 606 (15.2) | 1154 (15.4) | 566 (15.1) | 588 (15.7) | |||
| Grade 4 | 763 (3.7) | 663 (4.0) | 100 (2.5) | 176 (2.3) | 81 (2.2) | 95 (2.5) | |||
| Unknown | 5415 (26.4) | 3861 (23.4) | 1554 (39.0) | 2868 (38.3) | 1458 (38.9) | 1410 (37.6) | |||
| Histological type (%) | ccRCC | 10,793 (52.6) | 9028 (54.6) | 1765 (44.2) | < 0.001 | 3451 (46.0) | 1730 (46.2) | 1721 (45.9) | 0.505 |
| pRCC | 2899 (14.1) | 2119 (12.8) | 780 (19.6) | 1400 (18.7) | 703 (18.8) | 697 (18.6) | |||
| chRCC | 965 (4.7) | 873 (5.3) | 92 (2.3) | 167 (2.2) | 77 (2.1) | 90 (2.4) | |||
| Mixed cell adenocarcinoma | 479 (2.3) | 323 (2.0) | 156 (3.9) | 225 (3.0) | 110 (2.9) | 115 (3.1) | |||
| Others | 363 (1.8) | 308 (1.9) | 54 (1.4) | 86 (1.1) | 35 (0.9) | 51 (1.4) | |||
| Unknown | 5024 (24.5) | 3882 (23.5) | 1142 (28.6) | 2167 (28.9) | 1093 (29.2) | 1074 (28.7) | |||
KM analyses indicated that compared with URCC, BRCC correlated with worse OS and higher rates of CSM (Fig. S2A, B, E, F). There was no significant difference in OS between metachronous BRCC patients and URCC patients. Synchronous BRCC patients had worse OS than URCC patients and metachronous BRCC patients (Fig. S2C, D, p < 0.05). Regarding CSM, both synchronous and metachronous BRCC had higher rates of CSM than unilateral RCC patients (Fig. S2G, H, p < 0.001). There was no significant difference in CSM between synchronous and metachronous BRCC patients (p = 0.12). In addition, the prognosis of BRCC patients is between metastatic and non-metastatic URCC patients (Fig. S3A–D).
Clinicopathological analyses and prognostic factors identification for localized BRCC patients
Baseline characteristics and analysis of localized BRCC patients
A total of 3677 localized BRCC patients were included (2180 synchronous BRCC patients and 1497 metachronous BRCC patients, Table 2). The median follow-up was 69 months. Synchronous BRCC patients were younger (median [IQR], 63 [54, 71] years vs. 65 [57, 72] years) than metachronous BRCC patients. Of the 2389 BRCC patients with bilateral pathologic results, the overall concordance rate was 78.1%. The concordance rates for localized synchronous BRCC (lsBRCC) and localized metachronous BRCC (lmBRCC) patients were 80.8% and 74.5%.
Table 2.
Baseline characteristics of localized BRCC patients
| Characteristic | Overall (n = 3677) | Synchronous BRCC (n = 2180) | Metachronous BRCC (n = 1497) | p-value | |
|---|---|---|---|---|---|
| Age (yrs) (%) | < 45 | 278 (7.6) | 201 (9.2) | 77 (5.1) | < 0.001 |
| 45–65 | 1646 (44.8) | 992 (45.5) | 654 (43.7) | ||
| 65–85 | 1645 (44.7) | 910 (41.7) | 735 (49.1) | ||
| > 85 | 108 (2.9) | 77 (3.5) | 31 (2.1) | ||
| Sex (%) | Female | 989 (26.9) | 566 (26.0) | 423 (28.3) | 0.133 |
| Male | 2688 (73.1) | 1614 (74.0) | 1074 (71.7) | ||
| Race (%) | White | 2583 (70.2) | 1484 (68.1) | 1099 (73.4) | 0.002 |
| Black | 909 (24.7) | 573 (26.3) | 336 (22.4) | ||
| Others | 185 (5.0) | 123 (5.6) | 62 (4.1) | ||
| Marital status (%) | Unmarried/Unknown | 1496 (40.7) | 917 (42.1) | 579 (38.7) | 0.043 |
| Married | 2181 (59.3) | 1263 (57.9) | 918 (61.3) | ||
| Median household income (%) | $ (0–75,000) | 2208 (60.0) | 1333 (61.1) | 875 (58.5) | 0.108 |
| $ (75,000 +) | 1469 (40.0) | 847 (38.9) | 622 (41.5) | ||
| Rural/Urban Continuum population density (%) | Counties in metropolitan areas ge 1 million population | 2181 (59.3) | 1289 (59.1) | 892 (59.6) | 0.281 |
| Counties in metropolitan areas of 0 to 1 million population | 1053 (28.6) | 613 (28.1) | 440 (29.4) | ||
| Nonmetropolitan counties | 438 (11.9) | 276 (12.7) | 162 (10.8) | ||
| Unknown | 5 (0.1) | 2 (0.1) | 3 (0.2) | ||
| Time interval between two RCCs (months) (median [IQR]) | 3.00 [0.00, 30.00] | 0.00 [0.00, 2.00] | 42.00 [18.00, 77.00] | < 0.001 | |
| Tumor highest stage (%) | I | 2568 (69.8) | 1504 (69.0) | 1064 (71.1) | 0.366 |
| II | 467 (12.7) | 294 (13.5) | 173 (11.6) | ||
| III | 615 (16.7) | 366 (16.8) | 249 (16.6) | ||
| IV | 27 (0.7) | 16 (0.7) | 11 (0.7) | ||
| Tumor highest T stage (%) | T1 | 2578 (70.1) | 1512 (69.4) | 1066 (71.2) | 0.353 |
| T2 | 477 (13.0) | 300 (13.8) | 177 (11.8) | ||
| T3 | 598 (16.3) | 355 (16.3) | 243 (16.2) | ||
| T4 | 24 (0.7) | 13 (0.6) | 11 (0.7) | ||
| Tumor highest N stage (%) | N0 | 3624 (98.6) | 2146 (98.4) | 1478 (98.7) | 0.558 |
| N1 | 53 (1.4) | 34 (1.6) | 19 (1.3) | ||
| Tumor highest grade (%) | Grade 1 | 105 (2.9) | 67 (3.1) | 38 (2.5) | 0.137 |
| Grade 2 | 1037 (28.2) | 612 (28.1) | 425 (28.4) | ||
| Grade 3 | 792 (21.5) | 453 (20.8) | 339 (22.6) | ||
| Grade 4 | 142 (3.9) | 74 (3.4) | 68 (4.5) | ||
| Unknown | 1601 (43.5) | 974 (44.7) | 627 (41.9) | ||
| Initial RCC histological type (%) | ccRCC | 1630 (44.3) | 868 (39.8) | 762 (50.9) | < 0.001 |
| pRCC | 770 (20.9) | 445 (20.4) | 325 (21.7) | ||
| chRCC | 91 (2.5) | 54 (2.5) | 37 (2.5) | ||
| Mixed cell adenocarcinoma | 154 (4.2) | 97 (4.4) | 57 (3.8) | ||
| Others | 49 (1.3) | 32 (1.5) | 17 (1.1) | ||
| Unknown | 983 (26.7) | 684 (31.4) | 299 (20.0) | ||
| Contralateral RCC histological type (%) | ccRCC | 1789 (48.7) | 993 (45.6) | 796 (53.2) | < 0.001 |
| pRCC | 802 (21.8) | 485 (22.2) | 317 (21.2) | ||
| chRCC | 105 (2.9) | 55 (2.5) | 50 (3.3) | ||
| Mixed cell adenocarcinoma | 150 (4.1) | 100 (4.6) | 50 (3.3) | ||
| Others | 57 (1.6) | 31 (1.4) | 26 (1.7) | ||
| Unknown | 774 (21.0) | 516 (23.7) | 258 (17.2) | ||
| Histology concordance (%) | Concordence | 1865 (50.7) | 1104 (50.6) | 761 (50.8) | < 0.001 |
| Disconcordence | 524 (14.3) | 263 (12.1) | 261 (17.4) | ||
| Unknown | 1288 (35.0) | 813 (37.3) | 475 (31.7) | ||
| Surgical treatment (%) | Bilateral no surgery | 329 (8.9) | 313 (14.4) | 16 (1.1) | < 0.001 |
| Bilateral LTD | 151 (4.1) | 110 (5.0) | 41 (2.7) | ||
| Bilateral PN | 932 (25.3) | 566 (26.0) | 366 (24.4) | ||
| Bilateral RN | 524 (14.3) | 344 (15.8) | 180 (12.0) | ||
| LTD + PN | 160 (4.4) | 73 (3.3) | 87 (5.8) | ||
| LTD + RN | 287 (7.8) | 84 (3.9) | 203 (13.6) | ||
| PN + RN | 852 (23.2) | 437 (20.0) | 415 (27.7) | ||
| Unilateral LTD | 72 (2.0) | 46 (2.1) | 26 (1.7) | ||
| Unilateral PN | 150 (4.1) | 100 (4.6) | 50 (3.3) | ||
| Unilateral RN | 220 (6.0) | 107 (4.9) | 113 (7.5) | ||
| Radiotherapy (%) | None/Unknown | 3657 (99.5) | 2169 (99.5) | 1488 (99.4) | 0.87 |
| Yes | 20 (0.5) | 11 (0.5) | 9 (0.6) | ||
| Chemotherapy (%) | None/Unknown | 3567 (97.0) | 2121 (97.3) | 1446 (96.6) | 0.26 |
| Yes | 110 (3.0) | 59 (2.7) | 51 (3.4) | ||
BRCC, Bilateral renal cell carcinoma; RCC, Renal cell carcinoma; IQR, Interquartile range; ccRCC, Clear cell renal cell carcinoma; pRCC, Papillary renal cell carcinoma; chRCC, Chromophobe renal cell carcinoma; PN, Partial nephrectomy; RN, Radical nephrectomy; LTD, Local tumor destruction
Prognostic factor identification for localized BRCC patients
The Cox analysis of lsBRCC indicated that older age, higher T stage, and higher grade were independent risk factors for OS (p < 0.05), whereas married status, bilateral nccRCC histologic type, and surgical treatment were protective factors for OS (p < 0.05, Table S1). The multivariate CRR analysis demonstrated that age, race, T stage, N stage, and surgical treatment were independent prognostic factors for CSS (p < 0.05, Table S2).
Regarding lmBRCC, the Cox analysis showed that older age, black ethnicity, higher initial and contralateral RCC T stage were independent risk factors for OS (p < 0.05), whereas initial and contralateral RCC surgical treatment were protective factors (p < 0.05, Table S3). CRR analysis indicated that initial and contralateral RCC T stage, and initial and contralateral surgical treatment were independent prognostic factors for CSS (Table S4).
Association between socioeconomic factors and therapeutic characteristics of localized BRCC patients
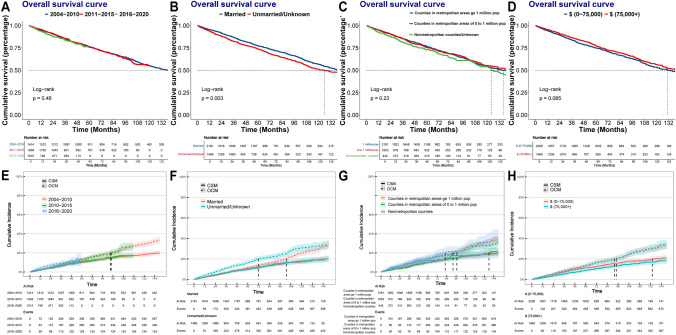
We further investigated the association between socioeconomic factors and therapeutic characteristics of localized BRCC patients. As time went on, the interval between the diagnoses of BRCC significantly shortened (median [IQR]: 2004–2010: 13 [0, 72] months, 2011–2015: 3 [0, 26] months, 2016–2020: 0 [0, 4] months, p < 0.001). Fig. S4 showed the distribution of therapeutic options. We noted that the proportion of BRCC patients accepting RN treatment decreased over time (Fig. S4A, 2004–2010: 61.45%, 2011–2015: 47.51%, 2016–2020: 41.48%), whereas the proportion of nephron-sparing treatments increased (2004–2010: 32.89%, 2011–2015: 44.16%, 2016–2020: 44.23%). There was no significant difference in prognosis of BRCC patients diagnosed in different periods (Fig. 2A, E). Survival analysis indicated that the OS of married BRCC patients was better than that of unmarried/unknown BRCC patients (Fig. 2B p = 0.003), whereas the CSM of them was similar (Fig. 2F p = 0.82). No significant difference was observed in prognoses of BRCC patients in areas with different population density (Fig. 2C, Gp > 0.05). Higher household income was related to the better OS (Fig. 2D p = 0.085) and lower CSM (Fig. 2H p = 0.030).
Fig. 2.
A Kaplan–Meier survival curves of OS for BRCC patients diagnosed in different periods. B Kaplan‒Meier survival curves of OS for BRCC patients with different marital status. C Kaplan–Meier survival curves of OS for BRCC patients living in areas with different population density. D Kaplan–Meier survival curves of OS for BRCC patients with different household income. E Cumulative incidence plots of competing risks regression models in BRCC patients diagnosed in different periods. F Cumulative incidence plots of competing risks regression models in BRCC patients diagnosed in different periods. G Cumulative incidence plots of competing risks regression models in BRCC patients living in areas with different population density. H Cumulative incidence plots of competing risks regression models in BRCC patients with different household income. Abbreviations: RCC, Renal cell carcinoma; CSM, Cancer specific mortality; OCM, Other cause mortality; OS, Overall survival
Effects of different surgical treatments on the prognosis of localized BRCC
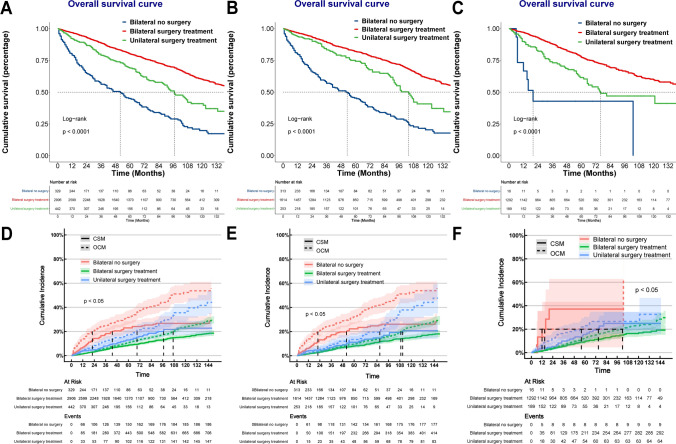
In the BRCC cohort, 2906 (79.03%) patients accepted bilateral surgical treatments, 442 patients (12.02%) received unilateral surgical treatments, and the remaining patients received nonsurgical treatment (e.g. systemic therapies) or active surveillance. Compared with lmBRCC patients, lsBRCC patients had a significantly higher proportion of bilateral nonsurgical treatment (14.4% vs. 1.1%, p < 0.001). Localized BRCC patients receiving bilateral surgical treatments had relatively good prognoses, whereas those who did not receive any surgical treatments had the worst prognoses (Fig. 3A, D). The above results remained consistent in both lsBRCC and lmBRCC (Fig. 3B, C, E, F).
Fig. 3.
A Kaplan‒Meier survival curves of OS for bilateral RCC patients receiving different treatment. B Kaplan‒Meier survival curves of OS for synchronous bilateral RCC patients receiving different treatment. C Kaplan‒Meier survival curves of OS for metachronous bilateral RCC patients receiving different treatment. D Cumulative incidence plots of competing risks regression models in bilateral RCC patients receiving different treatment. E Cumulative incidence plots of competing risks regression models in synchronous bilateral RCC patients receiving different treatment. F Cumulative incidence plots of competing risks regression models in metachronous bilateral RCC patients receiving different treatment. Abbreviations: RCC, Renal cell carcinoma; CSM, Cancer specific mortality; OCM, Other cause mortality
For lsBRCC patients, those receiving bilateral partial nephrectomy (PN) had the best OS clinical benefit, followed by patients receiving LTD + PN, patients receiving bilateral LTD, and patients receiving PN + RN. The OS clinical benefit of bilateral RN was less than bilateral nephron-sparing surgery and unilateral surgical treatment (Table S1). Regarding CSS, bilateral PN and bilateral LTD could achieve the best clinical benefits (Table S2).
The lmBRCC patients were divided into three groups according to the surgical treatment status of initial RCC. For patients without initial surgical treatments, contralateral PN or LTD could achieve the best prognosis (Fig. S5A, D). For patients with prior nephron-sparing surgical treatments, contralateral PN would lead to the best OS, and those receiving contralateral LTD and RN had similar prognoses (Fig. S5B). The three types of surgical treatment (PN, RN, LTD) had similar long-term cancer-control effects for patients with prior nephron-sparing surgical treatments (Fig. S5E). Regarding patients accepting prior RN treatment, contralateral PN was associated with the best OS and achieved the best cancer-control effects, followed by contralateral LTD treatment. (Fig. S5C, F).
Construction and evaluation of predictive models
Development and validation of predictive models for lsBRCC patients
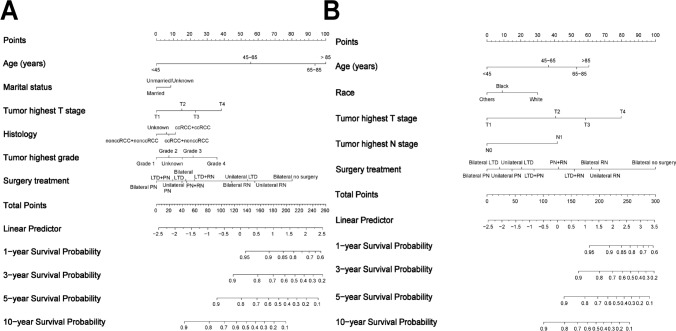
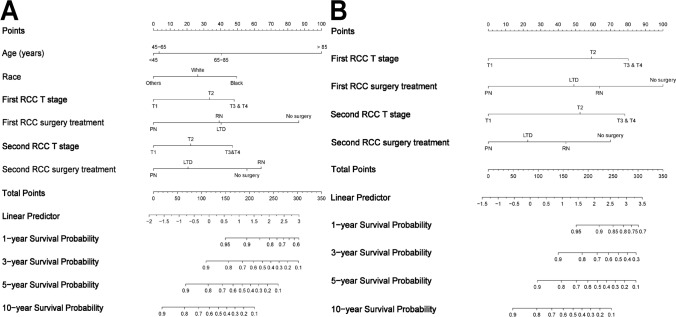
The baseline characteristics of lsBRCC patients in the training cohort and the validation cohort were well-balanced (Table S5). Variables with p-value < 0.1 in multivariate Cox regression analysis were included in LASSO regression analysis (Fig. S6A–D). Six and five variables were finally incorporated into the OS and CSS nomograms, respectively (Fig. 4A, B).
Fig. 4.
The nomogram for predicting the 1-, 3-, 5-, and 10-year OS A and CSS B of patients with localized synchronous bilateral RCC. Abbreviations: RCC, Renal cell carcinoma; OS, Overall survival; CSS, Cancer-specific survival; PN, Partial nephrectomy; RN, Radical nephrectomy; LTD, Local tumor destruction
The C-index of OS and CSS nomograms were 0.746 [95%CI: 0.723–0.769] and 0.769 [95%CI: 0.736–0.802]. The time-dependent AUCs of the ROCs were around 0.7 (Fig. S7A, B, E, F). Then patients were divided into low- and high-risk subgroups based on risk scores. A significant difference in survival was observed between the two subgroups (Fig. S7C, D, G, H, p < 0.001). Calibration plots showed high consistencies between the predicted probabilities and the actual survival outcomes (Fig. S8, S9). Moreover, DCA indicated that compared with both the treat-all-patients scheme and the treat-none scheme, the nomogram could add more net benefits across threshold probabilities between 5 and 70% for 5-year OS, between 2 and 60% for 5-year CSS, between 10 and 90% for 10-year OS, and between 2 and 95% for 10-year CSS (Fig. S10A–H).
Development and validation of predictive models for lmBRCC
The baseline characteristics of lmBRCC patients were shown in Table S6. Comprehensively considering the results of CRR analysis and the LASSO regression analysis (Fig. S11A–D), six and four variables were eventually included in the OS and CSS nomograms (Fig. 5A, B).
Fig. 5.
The nomogram for predicting the 1-, 3-, 5-, and 10-year OS A and CSS B of patients with localized metachronous bilateral RCC. Abbreviations: RCC, Renal cell carcinoma; OS, Overall survival; CSS, Cancer-specific survival; PN, Partial nephrectomy; RN, Radical nephrectomy; LTD, Local tumor destruction
The C-index of the OS and CSS nomograms were 0.751 [95%CI: 0.722–0.781] and 0.769 [95%CI 0.728–0.816]. The AUCs of the ROC curves were around 0.67 and 0.73 for the prediction of OS and CSS (Fig. S12A, B, E, F). The patients were divided into low- and high-risk subgroups based on risk scores. A significant difference in prognosis was observed between the two subgroups (Fig. S12C, D, G, H, p < 0.001). Calibration plots showed high consistencies between the predicted probabilities and the actual survival outcomes (Fig. S13, S14). Furthermore, DCA showed that the nomogram could add more net benefits compared with both the treat-all-patients scheme and the treat-none scheme across threshold probabilities between 10 and 75% for 5-year OS, between 5 and 60% for 5-year CSS, between 20 and 100% for 10-year OS, and between 10 and 100% for 10-year CSS (Fig. S15A–H).
Discussion
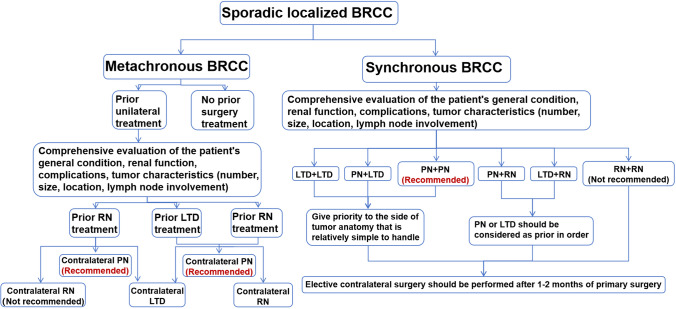
In this large-scale population-based study, we found that the prognosis of BRCC patients is between metastatic and non-metastatic URCC patients. Synchronous BRCC patients had worse OS than metachronous BRCC patients. The CSS of synchronous and metachronous BRCC patients was similar. As time went on, the interval between the diagnoses of BRCC shortened, and the proportion of BRCC patients receiving nephron-sparing treatments increased. PN should be the first choice for localized BRCC patients. LTD achieved an acceptable cancer-control effect, which could be considered an effective treatment alternative. Furthermore, we tried to construct predictive models based on the information available from the SEER database and summarize the treatment flowchart for localized BRCC patients (Fig. 6). This study comprehensively analysed the clinicopathological characteristics of the BRCC patients, identified the prognostic factors, and provided evidence for the optimization of individual treatment for sporadic BRCC patients.
Fig. 6.
Treatment flowchart for sporadic localized BRCC patients. Abbreviations: BRCC, Bilateral renal cell carcinoma; PN, Partial nephrectomy; RN, Radical nephrectomy; LTD, Local tumor destruction
The pathogenesis of BRCC remains unclear. Most previous studies supported that BRCC is an independent de novo process, representing a special type of RCC, instead of a consequence of spreading from the primary RCC [28, 29]. Comparing the prognoses of URCC and BRCC patients may provide clues for this issue. However, it remains inconsistent in previous studies [11, 30–34], which may be attributed to small sample size, different study designs, and uncontrolled bias. Our results demonstrated that the prognosis of BRCC patients was worse than that of URCC patients. Previous studies have reported that BRCC was likely to have more severe preoperative renal dysfunction than those with URCC [35, 36], and some BRCC tended to have aggressive biological behaviour [37, 38]. BRCC patients usually harbored heavier tumor burden than URCC patients [37]. The incidence of multifocality was greater in BRCC patients than in those with URCC [4, 30, 35, 37], which was an independent risk factor for RCC patients [7, 39]. BRCC patients were more likely to experience local recurrence than URCC patients [30, 37, 40]. In addition, BRCC patients may accept more treatment such as surgery and systemic therapy. The higher risk of complications and side effects caused by these treatments would reduce the quality of life and harm patients’ health. We also found that the prognosis of BRCC patients was between non-metastatic URCC patients and metastatic URCC patients, suggesting that BRCC should not be deemed either as pure primary tumors or as contralateral metastases. The biological mechanism for BRCC is different from metastatic disease and needs further exploration [7, 28, 41].
Controversy remains regarding the prognoses of synchronous and metachronous BRCC [7, 32, 37, 42, 43]. Compared with metachronous BRCC, the prognoses of synchronous BRCC was relatively poor (10-year OS: 50.2% vs. 54.3%). The CSS was comparable in synchronous and metachronous BRCC patients. We noted that the proportion of lsBRCC patients without any surgery treatment was significantly higher than those of metachronous BRCC patients (14.4% vs 1.07%), which may partly explain the relatively poor prognosis of synchronous BRCC.
We analyzed and identified prognostic factors for sporadic localized BRCC patients. We firstly investigated the association between socioeconomic factors and therapeutic characteristics of localized BRCC patients. The results indicated that BRCC patients with high income and being married exhibited better prognoses than others. The result is as expected because patients being married or with higher incomes tend to seek enhanced healthcare access and exhibit proactive health habits. Moreover, considering that the time span of the study was longer than 15 years, the diagnostic and therapeutic technology evolved, so we explored the population characteristics in different periods of diagnosis. As time went on, the interval between the diagnoses of BRCC was significantly shortened, which may be due to the development of the diagnostic tools. For example, the availability of positron emission tomography computed tomography (PET-CT) could achieve the accurate differentiation diagnosis and reflect tumor aggressiveness, which promoted the precise diagnosis of RCC [44–47]. Though no significant difference was observed in prognosis of BRCC patients diagnosed in different periods, the proportion of nephron-sparing treatments increased, which may attribute to the progression of minimally invasive surgical technology and the development of LTD technology.
Surgical treatment was a prognostic factor in all multivariate analyses. Localized BRCC patients receiving bilateral surgical treatments had the relatively good prognosis, whereas patients who did not receive any surgery had the worst prognosis. When determining the treatment strategy for localized BRCC patients, urologists should try to thoroughly balance the oncological safety, perioperative risk, and functional outcomes [10]. The essential principles are complete tumor removal, preservation of functional renal parenchyma as much as possible, avoidance of dialysis, and minimizing morbidity [8]. Based on the results of this study and the research progress in recent years [10, 43], we summarized the treatment flowchart for localized BRCC patients (Fig. 6). For both lsBRCC and lmBRCC patients, our results indicated that PN leads to the best OS. Localized BRCC patients receiving bilateral RN had poor OS. Though RN was the standard treatment for RCC in the past and could achieve good cancer-control effects, it increased the risk of chronic kidney disease (CKD) and even renal failure. The downstream sequelae of CKD would lead to excess mortality and therefore less favorable survival outcomes [48]. Consistent with previous studies [10, 48, 49], this study demonstrated that PN provided equivalent oncological outcomes to RN. Thus, in terms of better quality of life and greater longevity, PN should be the initial treatment for sporadic localized BRCC patients if feasible [41]. However, it should be noted that BRCC patients receiving RN might have more complex and aggressive tumors than those receiving PN, which might bias the survival outcomes. Despite maximal effects, not all BRCCs are amenable to PN. The benefits of PN must always be weighed against the risk of potential additional loss of renal function secondary to bilateral renal ischemia from hemorrhage, hypotension, and even acute surgical morbidity [50]. In certain scenarios such as large masses and highly complex anatomical structure, RN should still be required [10].
Moreover, LTD, including radiofrequency ablation (RFA), percutaneous microwave ablation, and cryoablation, has shown good efficacy and safety in local tumor control and emerged as a treatment alternative [51, 52]. As a minimally invasive technology, the advantages of LTD include eliminating the risk of ischemic damage, shortening operative time, and fewer complications with equivalent renal functional and oncological outcomes [52]. However, current studies focusing on the application of LTD in BRCC patients are mainly small-sample size, and the selection bias could not be avoided. In this study, lsBRCC patients receiving bilateral PN had the best prognoses, followed by patients receiving LTD and PN, and patients receiving bilateral LTD. Regarding lmBRCC patients, LTD resulted in an acceptable prognosis and achieved a comparable cancer-control effect to surgery. These results suggested that LTD could be considered as an alternative treatment option. Therefore, it’s an effective and safe treatment option for BRCC patients who are nonsurgical candidates due to comorbidities and cosmetic requirements [53]. Through judiciously expanding the use of LTD, clinicians can improve survival outcomes among a part of BRCC patients.
With the ability to generate an individual probability of a clinical event by integrating diverse prognostic and determinant variables, nomograms fulfill the drive toward individualized medicine and have been widely used as prognostic devices in the field of oncology [23, 54]. Based on the potential prognostic factors identified by multivariable analysis, we further conducted LASSO analysis and established predictive models for localized BRCC patients. The OS and CSS nomograms integrated multiple demographic and clinicopathological characteristics available from the SEER database into a quantitative model, exhibiting favorable discrimination, good accuracy, and outstanding ability for risk stratification. Furthermore, DCA analyses demonstrated that the nomograms predicted long-term survival with good clinical benefit and utility. These nomograms would facilitate individual prognosis assessment and the development of treatment strategies.
To our knowledge, this is the first large-scale population-based study comprehensively analyzing the clinicopathological characteristics of sporadic BRCC patients, identifying prognostic factors, and developing treatment flowchart for sporadic localized BRCC patients. However, the current study has some limitations. First, some bias was inevitable due to the retrospective design and the lack of randomization. Second, the SEER database lacks information on environmental factors (eg, exposure to tobacco, and chemical carcinogen), patient comorbidities, tumors multifocality, and detailed treatment information (eg, adjuvant therapy, perioperative complications). Additionally, the distinction between hereditary BRCC and sporadic BRCC is important for treatment decision-making. Though we excluded patients tending to be suffered from hereditary RCC, including those complicating with pNET or hemangioblastoma, the information of genetic testing could not be obtained from the SEER database. The few remaining hereditary BRCC patients in this study may slightly bias the results. Finally, the study is based on an American population cohort, our predictive models and nomograms for BRCC needs to be further investigated in other countries. Large-scale, well-designed prospective studies are warranted to provide more high-quality evidence for the optimization of the current treatment paradigm for sporadic BRCC.
Conclusion
The prognosis of BRCC patients is between metastatic and non-metastatic URCC patients. Synchronous BRCC patients had worse OS than metachronous BRCC patients. The CSS of synchronous and metachronous BRCC patients was comparable. With the evolvement of the diagnostic and therapeutic technology, the interval between the diagnoses of BRCC shortened, and the proportion of nephron-sparing treatments increased. PN leads to the best OS and provides equivalent oncological outcomes to RN, which should be the initial treatment for patients with localized BRCC if feasible. LTD could be considered as an effective treatment alternative. We identified independent prognostic factors through multivariate Cox regression analysis, conducted survival analyses, and developed treatment flowchart for lsBRCC and lmBRCC patients. Our study could provide evidence for the optimization of individualized treatment and contribute to the prognosis assessment for sporadic BRCC patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- AUC
Area under the curve
- BRCC
Bilateral renal cell carcinoma
- CIF
Cumulative incurred function
- CKD
Chronic kidney disease
- CRR
Competing risk regression
- CSM
Cancer-specific mortality
- CSS
Cancer-specific survival
- DCA
Decision curve analyses
- IQR
Interquartile range
- LASSO
Least absolute shrinkage and selection operator
- lsBRCC
Localized synchronous bilateral renal cell carcinoma
- lmBRCC
Localized metachronous bilateral renal cell carcinoma
- LTD
Local tumor destruction
- KM analysis
Kaplan–Meier analysis
- OCM
Other cause mortality
- OS
Overall survival
- PN
Partial nephrectomy
- pNET
Pancreatic neuroendocrine tumor
- PSM
Propensity score matching
- RCC
Renal cell carcinoma
- RFA
Radiofrequency ablation
- ROC
Receiver operating characteristic curve
- SEER
Surveillance, epidemiology, and end results
- URCC
Unilateral renal cell carcinoma
Author contributions
Ruiyi Deng, Jianhui Qiu, Jingcheng Zhou, and Kan Gong was responsible for conception, design, quality control of this study, reviewed, and edited the manuscript. Ruiyi Deng and Jianhui Qiu performed the data extraction, statistical analyses, and were major contributors in writing the manuscript. Chaojian Yu, Peidong Tian, Jiaheng Shang, Lin Cai, and Zihou Zhao contributed in data extraction, visualization, and classification criteria discussion. All authors have read and approved the final version of the manuscript.
Funding
This work was supported by Beijing Natural Science Foundation (QY23068; No. 7232176), the National High Level Hospital Clinical Research Funding (High Quality Clinical Research Project of Peking University First Hospital, 2022CR75), National Natural Science Foundation of China (No. 82141103; 82172617; 82172665; 82103153), and Capital’s Funds for Health Improvement and Research (2022–2-4074).
Data availability
We confirm that the SEER database analyzed during the current study are publicly available. These data can be downloaded from the software SEER*Stat (https://seer.cancer.gov/seerstat/). The data underlying this article will be shared on reasonable request to the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Given that the SEER database can be publicly accessed, the study did not require informed consent and was exempt from the review of IRB.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ruiyi Deng and Jianhui Qiu have contributed equally to this work and should be considered co-first authors.
Contributor Information
Jingcheng Zhou, Email: zhjc1021@126.com.
Kan Gong, Email: kan.gong@bjmu.edu.cn.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 2.Bukavina L, Bensalah K, Bray F, Carlo M, Challacombe B, Karam JA, et al. Epidemiology of renal cell carcinoma: 2022 update. Eur Urol. 2022;82(5):529–42. [DOI] [PubMed] [Google Scholar]
- 3.Grimaldi G, Reuter V, Russo P. Bilateral non-familial renal cell carcinoma. Ann Surg Oncol. 1998;5(6):548–52. [DOI] [PubMed] [Google Scholar]
- 4.Klatte T, Patard JJ, Wunderlich H, Goel RH, Lam JS, Junker K, Schubert J, Böhm M, Allhoff EP, Kabbinavar FF, Crepel M. Metachronous bilateral renal cell carcinoma: risk assessment, prognosis and relevance of the primary-free interval. J Urol. 2007;177(6):2081–7. [DOI] [PubMed] [Google Scholar]
- 5.Bani-Hani AH, Leibovich BC, Lohse CM, Cheville JC, Zincke H, Blute ML. Associations with contralateral recurrence following nephrectomy for renal cell carcinoma using a cohort of 2,352 patients. J Urol. 2005;173(2):391–4. [DOI] [PubMed] [Google Scholar]
- 6.Makino T, Kadomoto S, Izumi K, Mizokami A. Epidemiology and prevention of renal cell carcinoma. Cancers. 2022;14(16):4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qi N, Li T, Ning X, Peng X, Cai L, Gong K. Clinicopathologic features and prognosis of sporadic bilateral renal cell carcinoma: a series of 148 cases. Clin Genitourin Cancer. 2017;15(5):618–24. [DOI] [PubMed] [Google Scholar]
- 8.Sheikh NA, Khan MH, Pillai S, Lang S, Nabi G. Outcomes of synchronous and metachronous bilateral small renal masses (< 4 cm): a population-based cohort study. Int Urol Nephrol. 2018;50(4):657–63. [DOI] [PubMed] [Google Scholar]
- 9.Ljungberg B, Albiges L, Abu-Ghanem Y, Bedke J, Capitanio U, Dabestani S, et al. European Association of urology guidelines on renal cell carcinoma: the 2022 update. Eur Urol. 2022;82(4):399–410. [DOI] [PubMed] [Google Scholar]
- 10.Di Maida F, Grosso AA, Sforza S, Mari A, Lambertini L, Nardoni S, et al. Surgical management of synchronous, bilateral renal masses: a 1-decade referral center experience. Eur Urol Focus. 2022;8(5):1309–17. [DOI] [PubMed] [Google Scholar]
- 11.Simmons MN, Brandina R, Hernandez AV, Gill IS. Surgical management of bilateral synchronous kidney tumors: functional and oncological outcomes. J Urol. 2010;184(3):865–72. [DOI] [PubMed] [Google Scholar]
- 12.Ni J, Cui N, Wang Y, Liu J. Case report: bilateral renal cell carcinoma with different histological and morphological features, clear cell and cystic thyroid-like follicular subtype. Front Oncol. 2021;11:659706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dulabon LM, Lowrance WT, Russo P, Huang WC. Trends in renal tumor surgery delivery within the United States. Cancer. 2010;116(10):2316–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson RH, Atwell T, Schmit G, Lohse CM, Kurup AN, Weisbrod A, et al. Comparison of partial nephrectomy and percutaneous ablation for cT1 renal masses. Eur Urol. 2015;67(2):252–9. [DOI] [PubMed] [Google Scholar]
- 15.Mason RJ, Atwell T, Lohse C, Bhindi B, Schmit G, Schmitz J, et al. Synchronous nephron-sparing approaches for bilateral renal masses: peri-operative and renal functional outcomes. BJU Int. 2018;122(2):243–8. [DOI] [PubMed] [Google Scholar]
- 16.Zhang S, Zhao X, Ji C, Liu G, Li X, Zhang G, Gan W, Guo H. Radiofrequency ablation of synchronous bilateral renal cell carcinoma. Int J Urol. 2012;19(3):241–7. [DOI] [PubMed] [Google Scholar]
- 17.About the SEER Program [Internet]. SEER. [cited 2023 Dec 9]. Available from: https://seer.cancer.gov/about/index.html
- 18.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127(8 Pt 2):757–63. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Z. Survival analysis in the presence of competing risks. Ann Transl Med. 2017;5(3):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 21.Tibshirani R. The lasso method for variable selection in the Cox model. Stat Med. 1997;16(4):385–95. [DOI] [PubMed] [Google Scholar]
- 22.Tang G, Qi L, Sun Z, Liu J, Lv Z, Chen L, et al. Evaluation and analysis of incidence and risk factors of lower extremity venous thrombosis after urologic surgeries: a prospective two-center cohort study using LASSO-logistic regression. Int J Surg Lond Engl. 2021;89:105948. [DOI] [PubMed] [Google Scholar]
- 23.Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu J, Zhang H, Li L, Hu M, Chen L, Xu B, et al. A nomogram for predicting overall survival in patients with low-grade endometrial stromal sarcoma: a population-based analysis. Cancer Commun Lond Engl. 2020;40(7):301–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Calster B, Wynants L, Verbeek JFM, Verbakel JY, Christodoulou E, Vickers AJ, et al. Reporting and interpreting decision curve analysis: a guide for investigators. Eur Urol. 2018;74(6):796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fitzgerald M, Saville BR, Lewis RJ. Decision curve analysis. JAMA. 2015;313(4):409–10. [DOI] [PubMed] [Google Scholar]
- 27.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boorjian SA, Crispen PL, Lohse CM, Leibovich BC, Blute ML. The impact of temporal presentation on clinical and pathological outcomes for patients with sporadic bilateral renal masses. Eur Urol. 2008;54(4):855–63. [DOI] [PubMed] [Google Scholar]
- 29.Syed JS, Nguyen KA, Holford TR, Hofmann JN, Shuch B. Risk factors for metachronous bilateral renal cell carcinoma: a surveillance, epidemiology, and end results analysis. Cancer. 2019;125(2):232–8. [DOI] [PubMed] [Google Scholar]
- 30.Blute ML, Itano NB, Cheville JC, Weaver AL, Lohse CM, Zincke H. The effect of bilaterality, pathological features and surgical outcome in nonhereditary renal cell carcinoma. J Urol. 2003;169(4):1276–2181. [DOI] [PubMed] [Google Scholar]
- 31.Novick AC, Streem S, Montie JE, Pontes JE, Siegel S, Montague DK, et al. Conservative surgery for renal cell carcinoma: a single-center experience with 100 patients. J Urol. 1989;141(4):835–9. [DOI] [PubMed] [Google Scholar]
- 32.Jiang T, Wu YP, Chen SH, Ke ZB, Liang YC, Xu N. Prognosis and clinicopathological characteristics of renal cell carcinoma: does bilateral occurrence influence overall and cancer-specific survival? Transl Cancer Res. 2020;9(2):432–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pahernik S, Cudovic D, Roos F, Melchior SW, Thüroff JW. Bilateral synchronous sporadic renal cell carcinoma: surgical management, oncological and functional outcomes. BJU Int. 2007;100(1):26–9. [DOI] [PubMed] [Google Scholar]
- 34.Klatte T, Wunderlich H, Patard JJ, Kleid MD, Lam JS, Junker K, et al. Clinicopathological features and prognosis of synchronous bilateral renal cell carcinoma: an international multicentre experience. BJU Int. 2007;100(1):21–5. [DOI] [PubMed] [Google Scholar]
- 35.Takagi T, Kondo T, Izuka J, Kobayashi H, Tomita E, Hashimoto Y, et al. Prognosis and characteristics of renal cell carcinoma in hemodialysis patients: bilateral occurrence does not influence cancer-specific survival. Int J Urol Off J Jpn Urol Assoc. 2011;18(12):806–12. [DOI] [PubMed] [Google Scholar]
- 36.Lowrance WT, Yee DS, Maschino AC, Cronin AM, Bernstein M, Thompson RH, et al. Developments in the surgical management of sporadic synchronous bilateral renal tumours. BJU Int. 2010;105(8):1093–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giulioni C, Maggi M, Pirola GM, Martorana E, Cormio A, Teoh JYC, et al. The current evidence on surgical management for synchronous bilateral renal tumors: results from a scoping review. World J Urol. 2023;41(8):2107–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mari A, Di Maida F, Tellini R, Campi R, Sforza S, Cocci A, et al. Oncologic outcomes in patients treated with endoscopic robot assisted simple enucleation (ERASE) for renal cell carcinoma: results from a tertiary referral center. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2019;45(10):1977–82. [DOI] [PubMed] [Google Scholar]
- 39.Wiklund F, Tretli S, Choueiri TK, Signoretti S, Fall K, Adami HO. Risk of bilateral renal cell cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27(23):3737–41. [DOI] [PubMed] [Google Scholar]
- 40.Kim JK, Lee H, Oh JJ, Lee S, Hong SK, Lee SE, et al. Synchronous bilateral RCC is associated with poor recurrence-free survival compared with unilateral RCC: a single-center study with propensity score matching analysis. Clin Genitourin Cancer. 2019;17(3):e570–80. [DOI] [PubMed] [Google Scholar]
- 41.Wang B, Gong H, Zhang X, Li H, Ma X, Song E, et al. Bilateral synchronous sporadic renal cell carcinoma: retroperitoneoscopic strategies and intermediate outcomes of 60 patients. PLoS ONE. 2016;11(5):e0154578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berczi C, Thomas B, Bacso Z, Flasko T. Bilateral renal cancers: oncological and functional outcomes. Int Urol Nephrol. 2016;48:1617–22. [DOI] [PubMed] [Google Scholar]
- 43.Hu XY, Xu L, Guo JM, Wang H. Surgical strategy of bilateral synchronous sporadic renal cell carcinoma-experience of a Chinese university hospital. World J Surg Oncol. 2017;15(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakajima R, Abe K, Kondo T, Tanabe K, Sakai S. Clinical role of early dynamic FDG-PET/CT for the evaluation of renal cell carcinoma. Eur Radiol. 2016;26(6):1852–62. [DOI] [PubMed] [Google Scholar]
- 45.Nakajima R, Nozaki S, Kondo T, Nagashima Y, Abe K, Sakai S. Evaluation of renal cell carcinoma histological subtype and fuhrman grade using 18F-fluorodeoxyglucose-positron emission tomography/computed tomography. Eur Radiol. 2017;27(11):4866–73. [DOI] [PubMed] [Google Scholar]
- 46.Chen SH, Lin BH, Chen SM, Qiu QRS, Ruan ZT, Chen ZJ, et al. Head-to-head comparisons of enhanced CT, 68Ga-PSMA-11 PET/CT and 18F-FDG PET/CT in identifying adverse pathology of clear-cell renal cell carcinoma: a prospective study. Int Braz J Urol Off J Braz Soc Urol. 2023;49(6):716–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen S, Zhao Y, Tang Q, Wu C, Wang A, Ma L, et al. Diagnostic performance and prognostic value of preoperative 18F-FDG PET/CT in renal cell carcinoma patients with venous tumor thrombus. Cancer Imaging Off Publ Int Cancer Imaging Soc. 2022;22(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tan HJ, Norton EC, Ye Z, Hafez KS, Gore JL, Miller DC. Long-term survival following partial vs radical nephrectomy among older patients with early-stage kidney cancer. JAMA. 2012;307(15):1629–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu S, Feng C, Liu C, Wang Z. Comparison of prognosis between patients undergoing radical nephrectomy versus partial nephrectomy for renal cell carcinoma≤ 7 cm T3aN0/xM0: survival benefit is biased toward partial nephrectomy. Cancer Med. 2021;10(24):8909–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim JK, Kim H, Lee H, Oh JJ, Lee S, Hong SK, et al. Evaluation of functional outcome of bilateral kidney tumors after sequential surgery. BMC Cancer. 2021;21(1):592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhindi B, Mason RJ, Haddad MM, Boorjian SA, Leibovich BC, Atwell TD, et al. Outcomes after cryoablation versus partial nephrectomy for sporadic renal tumors in a solitary kidney: a propensity score analysis. Eur Urol. 2018;73(2):254–9. [DOI] [PubMed] [Google Scholar]
- 52.Zhang S, Zhao X, Ji C, Liu G, Li X, Zhang G, et al. Radiofrequency ablation of synchronous bilateral renal cell carcinoma. Int J Urol Off J Jpn Urol Assoc. 2012;19(3):241–7. [DOI] [PubMed] [Google Scholar]
- 53.Long JA, Bernhard JC, Bigot P, Lanchon C, Paparel P, Rioux-Leclercq N, et al. Partial nephrectomy versus ablative therapy for the treatment of renal tumors in an imperative setting. World J Urol. 2017;35(4):649–56. [DOI] [PubMed] [Google Scholar]
- 54.Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol Off J Am Soc Clin Oncol. 2008;26(8):1364–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We confirm that the SEER database analyzed during the current study are publicly available. These data can be downloaded from the software SEER*Stat (https://seer.cancer.gov/seerstat/). The data underlying this article will be shared on reasonable request to the corresponding author.