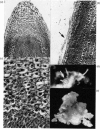
Abstract
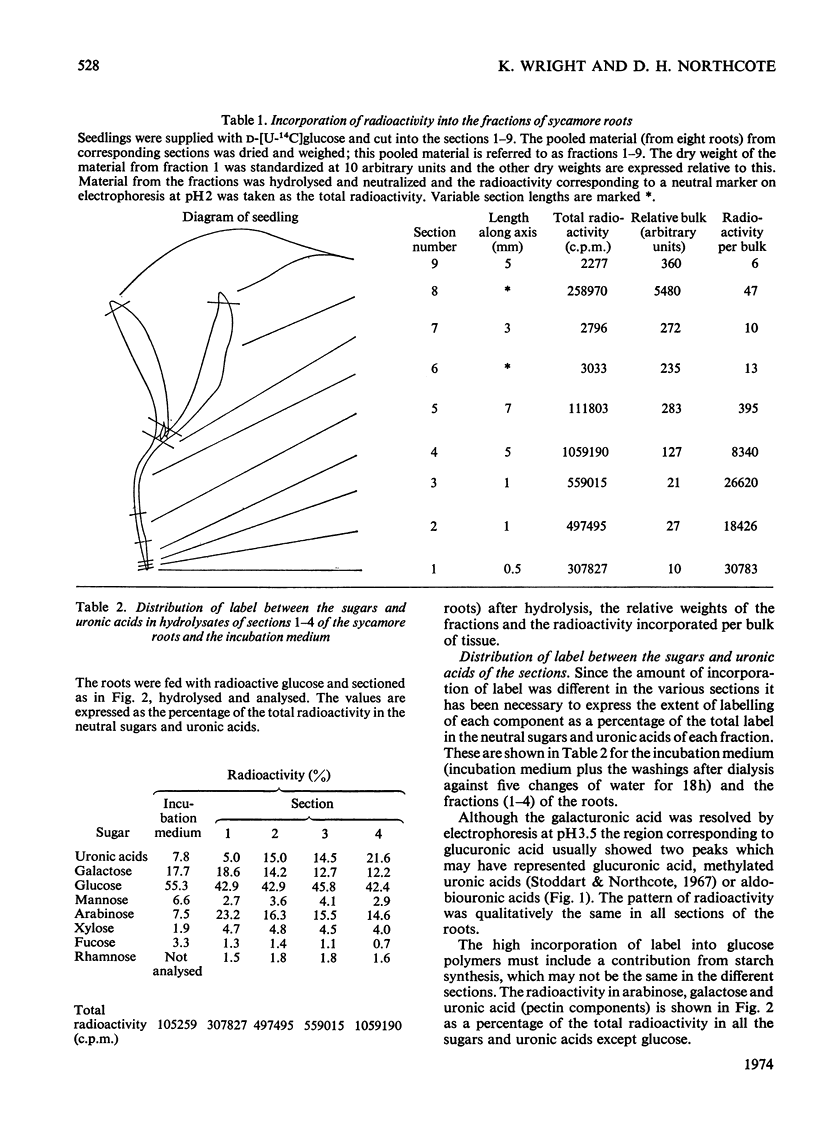
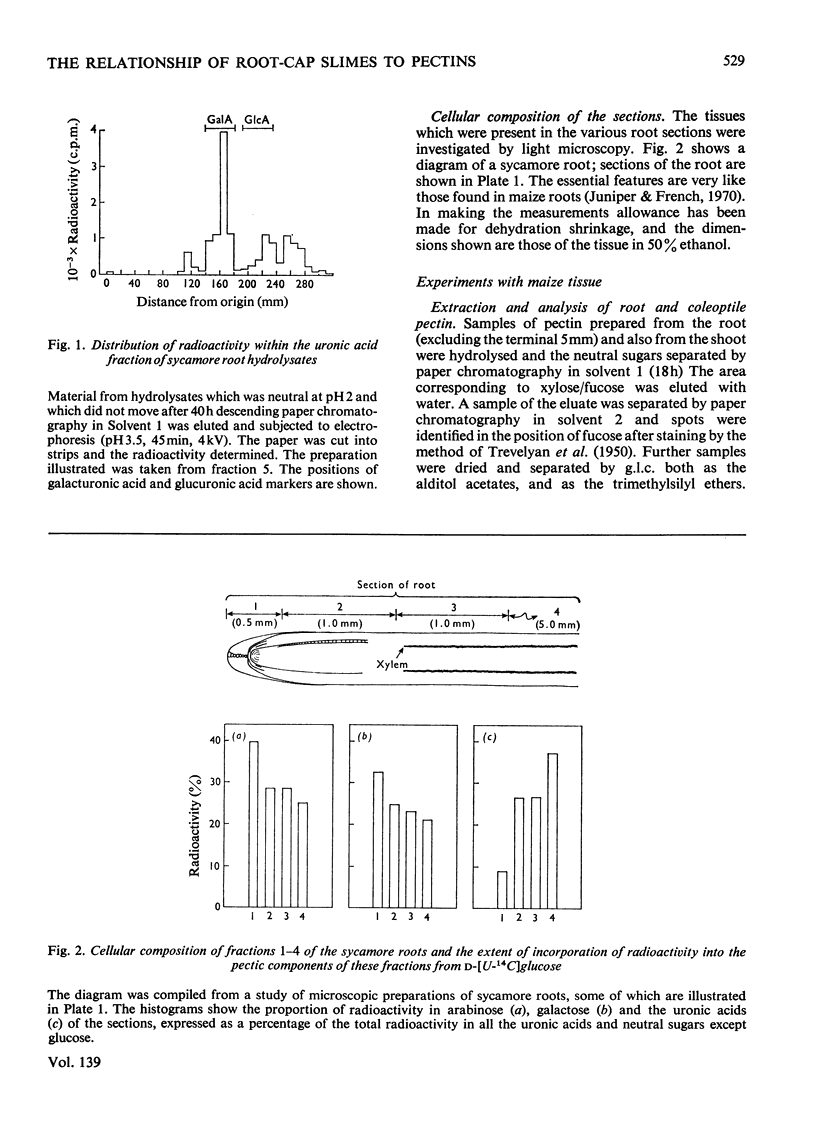
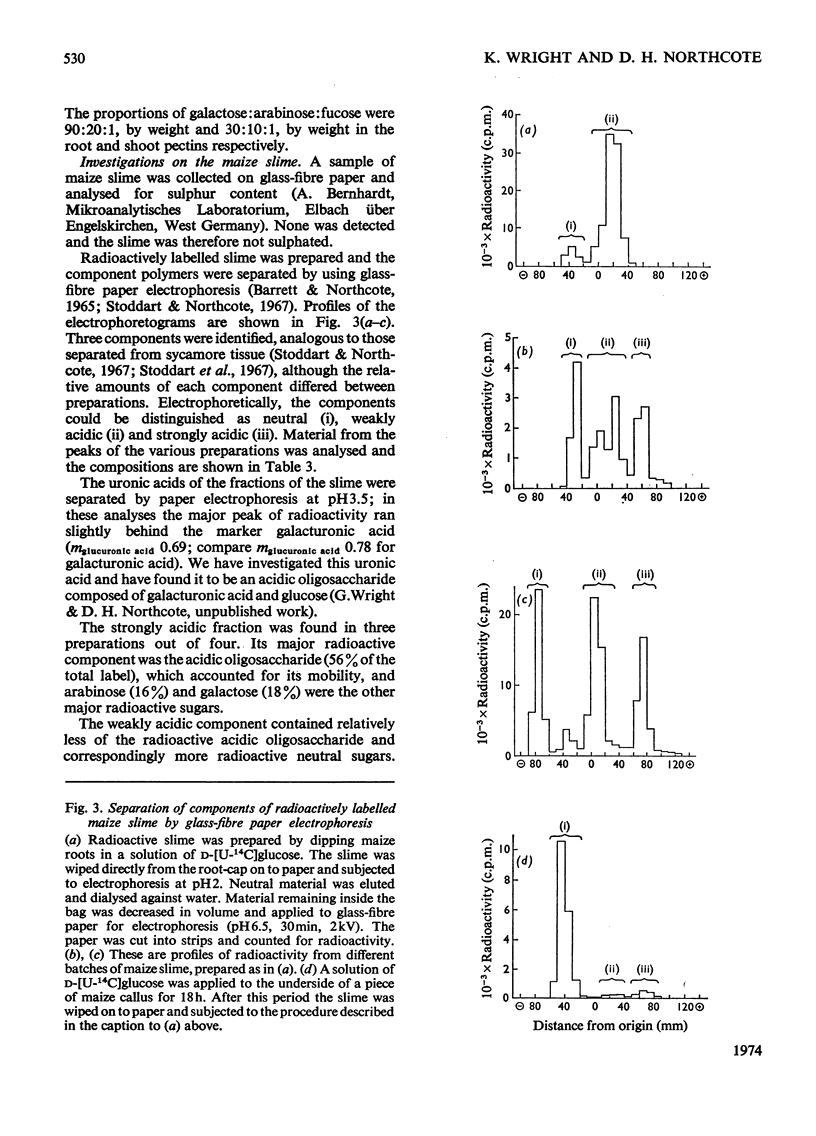
1. The patterns of incorporation of radioactivity from d-[U-14C]glucose into the pectic components of sections of sycamore roots changed so that sections nearer the tip incorporated relatively more label into arabinose and galactose compared with uronic acid. 2. Radioactive maize root-cap slime was prepared and found to contain three water-soluble component polymers which were electrophoretically (i) neutral, (ii) weakly acidic and (iii) strongly acidic at pH6.5. The neutral component was a glucan. The other components, which could be degraded by trans-elimination, consisted of an acidic backbone chain composed of galacturonic acid and glucose, attached to which were different proportions of neutral sugars. Arabinose, galactose and fucose, the main neutral sugars of the weakly and strongly acidic materials, were absent from the neutral fraction. 3. Fucose was a major sugar in maize-root slime and in a slime of similar composition synthesized by a maize callus of shoot origin. Only trace amounts were found in sycamore, pea and wheat root tips, and in pectin prepared from maize roots and coleoptiles. A high proportion of fucose is therefore a chemical characteristic of maize slime, and slime synthesis indicated a state of differentiation of the tissue. 4. The similarity between the slime and pectin is discussed; slime is a form of pectin modified in such a way as to provide a hydrated protective coating around the root tip.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aspinall G. O., Molloy J. A., Craig J. W. Extracellular polysaccharides from suspension-cultured sycamore cells. Can J Biochem. 1969 Nov;47(11):1063–1070. doi: 10.1139/o69-170. [DOI] [PubMed] [Google Scholar]
- BARRETT A. J., NORTHCOTE D. H. APPLE FRUIT PECTIC SUBSTANCES. Biochem J. 1965 Mar;94:617–627. doi: 10.1042/bj0940617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles D. J., Northcote D. H. The sites of synthesis and transport of extracellular polysaccharides in the root tissues of maize. Biochem J. 1972 Dec;130(4):1133–1145. doi: 10.1042/bj1301133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke D. E., Northcote D. H. Cell wall formation by soybean callus protoplasts. J Cell Sci. 1974 Jan;14(1):29–50. doi: 10.1242/jcs.14.1.29. [DOI] [PubMed] [Google Scholar]
- Harris P. J., Northcote D. H. Patterns of polysaccharide biosynthesis in differentiating cells of maize root-tips. Biochem J. 1970 Dec;120(3):479–491. doi: 10.1042/bj1200479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffs R. A., Northcote D. H. Experimental induction of vascular tissue in an undifferentiated plant callus. Biochem J. 1966 Oct;101(1):146–152. doi: 10.1042/bj1010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcote D. H., Pickett-Heaps J. D. A function of the Golgi apparatus in polysaccharide synthesis and transport in the root-cap cells of wheat. Biochem J. 1966 Jan;98(1):159–167. doi: 10.1042/bj0980159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLAITAN S. A., NORTHCOTE D. H. Polysaccharides of Chlorella pyrenoidosa. Biochem J. 1962 Mar;82:509–519. doi: 10.1042/bj0820509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. A. Structure, conformation, and mechanism in the formation of polysaccharide gels and networks. Adv Carbohydr Chem Biochem. 1969;24:267–332. doi: 10.1016/s0065-2318(08)60352-2. [DOI] [PubMed] [Google Scholar]
- Roberts R. M., Butt V. S. Patterns of cellulose synthesis in maize root-tips. A chemical and autoradiographic study. Exp Cell Res. 1967 Jun;46(3):495–510. doi: 10.1016/0014-4827(67)90376-x. [DOI] [PubMed] [Google Scholar]
- Rubery P. H., Northcote D. H. The effect of auxin (2,4-dichlorophenoxyacetic acid) on the synthesis of cell wall polysaccharides in cultured sycamore cells. Biochim Biophys Acta. 1970 Oct 27;222(1):95–108. doi: 10.1016/0304-4165(70)90355-7. [DOI] [PubMed] [Google Scholar]
- Stoddart R. W., Barrett A. J., Northcote D. H. Pectic polysaccharides of growing plant tissues. Biochem J. 1967 Jan;102(1):194–204. doi: 10.1042/bj1020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddart R. W., Northcote D. H. Metabolic relationships of the isolated fractions of the pectic substances of actively growing sycamore cells. Biochem J. 1967 Oct;105(1):45–59. doi: 10.1042/bj1050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORNBER J. P., NORTHCOTE D. H. Changes in the chemical composition of a cambial cell during its differentiation into xylem and phloem tissue in trees. 3. Xylan, glucomannan and alpha-cellulose fractions. Biochem J. 1962 Feb;82:340–346. doi: 10.1042/bj0820340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORNBER J. P., NORTHCOTE D. H. Changes in the chemical composition of a cambial cell during its differentiation into xylem and phloem tissue in trees. I. Main components. Biochem J. 1961 Dec;81:449–455. doi: 10.1042/bj0810449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORNBER J. P., NORTHCOTE D. H. Changes in the chemical composition of a cambial cell during its differentiation into xylem and phloem tissue in trees. II. Carbohydrate constituents of each main component. Biochem J. 1961 Dec;81:455–464. doi: 10.1042/bj0810455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Wright K., Northcote D. H. Differences in ploidy and degree of intercellular contact in differentiating and non-differentiating sycamore calluses. J Cell Sci. 1973 Jan;12(1):37–53. doi: 10.1242/jcs.12.1.37. [DOI] [PubMed] [Google Scholar]
- Wright K., Northcote D. H. Induced root differentiation in sycamore callus. J Cell Sci. 1972 Sep;11(2):319–337. doi: 10.1242/jcs.11.2.319. [DOI] [PubMed] [Google Scholar]