Abstract
Background
The relationship between gout and colorectal cancer (CRC) remains unclear, emphasizing the need for additional research to clarify the potential cumulative effect of gout on CRC development.
Methods
Leveraging a single nucleotide polymorphism-based genome-wide association study, the potential causal correlation between gout and CRC was initially analyzed using Mendelian randomization (MR). Subsequently, our analysis was expanded to include an assessment of patient survival, with the aim of evaluating the potential causal correlation between gout and CRC and the impact of gout on CRC survival outcomes.
Results
According to MR findings, a substantial relationship was observed between gout and the incidence of CRC when CRC was used as the outcome (OR = 0.954, 95% CI = 0.915–0.995). These results indicate a negative relationship between gout and the likelihood of developing CRC. In addition, when evaluating the overall survival (OS) or cancer-specific survival (CSS) of patients with CRC as outcomes, gout exhibited a significant relationship with survival. The inverse variance weighting approach demonstrated a progressive enhancement in CRC survival with the cumulative impact of gout (OS: OR = 2.000 × 10−4, 95% CI = 1.560 × 10−7–0.292; CSS: OR = 2.200 × 10−5, 95% CI = 4.660 × 10−9–0.104).
Conclusion
As gout accumulates, it exerts an inhibitory influence on CRC, indicating a potential protective effect. This study provides robust evidence that can guide the development of future clinical treatment approaches and research priorities.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12672-024-01714-6.
Keywords: Gout, Colorectal cancer, Overall survival, Cancer-specific survival, Mendelian randomization
Introduction
Gout, a condition resulting from perturbed uric acid metabolism, commonly manifests as acute joint inflammation [1, 2]. Uric acid, a metabolic byproduct, is typically eliminated through urine [3]. However, excessive uric acid levels can result in the formation of sodium urate crystals, which tend to accumulate in joints, leading to inflammation and discomfort [4, 5]. The global prevalence of gout is increasing because of the increasing consumption of purines, which act as precursors for uric acid production, in tandem with global economic advancement and shifts in dietary patterns marked by increased consumption of meat and alcohol [6]. Moreover, gout has been associated with a range of comorbidities, including hypertension [7], renal impairment [8], diabetes [9], and various cancers [10]. Therefore, understanding the intricate relationship between gout and these coexisting conditions is crucial for improving preventive and therapeutic methods.
Colorectal cancer (CRC) is the third most common cancer globally, with an increasing incidence in China in recent years. Projections indicate a significant increase in newly diagnosed CRC cases in China, which is expected to reach 0.91 million by 2040, reflecting a substantial 64% increase from 2020 [11]. Various factors, such as dietary habits [12], age [13], and lifestyle choices [14], contribute to the etiology of CRC. Investigating the risk factors associated with CRC could yield substantial advantages in the fields of cancer prevention and treatment.
Recent studies have highlighted a potential relationship between gout and specific cancer types, such as kidney cancer [15] and pancreatic cancer [16]. Some studies have shown that gout has a promoting effect, and these results suggest that uric acid can induce the production of reactive oxygen species, which may promote tumorigenesis [17, 18]. Conversely, other studies have shown that gout has a protective effect, and this protective effect may be related to the changes in the immune microenvironment and the frequent use of drugs by gout patients, such as colchicine, and anti-inflammatory drugs [19–21].
However, there is a lack of studies investigating the relationship between gout and CRC, which may contribute to conflicting findings. Thus, using the genome-wide association study (GWAS) database [22], which includes data from large sample sizes, could provide substantial evidence for examining the relationship between gout and CRC. This study examined the potential causal relationship between gout and CRC using GWAS data on single-nucleotide polymorphisms (SNPs). By employing Mendelian randomization (MR) analysis, we sought to clarify the causal correlation between gout and CRC to improve our understanding of the role of gout in CRC pathogenesis and establish a solid foundation for future clinical investigations.
Methods
Selection of genome-wide association study data for colorectal cancer
The CRC GWAS data used in the study were sourced from the UK Biobank with the specific GWAS ID of ieu-b-4965, which was published in 2021 and is the most recent available GWAS, comprising 377,673 samples (Ncase = 5657, Ncontrol = 372,016).
Genome-wide asociation study data selection for gout
Gout GWAS data were sourced from the UK Biobank, with the designated GWAS ID of ukb-b-13251, published in 2018 and included 462,933 samples (Ncase = 6543, Ncontrol = 456,390).
Selection of instrumental variables
The significance level of instrumental variables (IVs) for each gout was established at p < 1 × 10−5 based on recent research [23, 24]. SNPs were pruned using R 4.3.1 software (http:// www.Rproject.org), with linkage disequilibrium within a 10,000 kb distance and an r2 threshold of 0.001.
Effect of gout on colorectal cancer survival
The SUMMER database (http://njmu-edu.cn:3838/SUMMER/) was used to investigate the causal correlations between gout and CRC survival [25]. This study systematically evaluated the genetic effect of gout on cancer survival, including overall survival (OS) and cancer-specific survival (CSS), using data from the UK Biobanking cohort. We first select colorectal cancer as the cancer type in "Phenotype-Wide Association Analysis" and the gout related data under Miscellaneous as the exposure factor.
Statistical analysis
MR employs genetic variation to represent risk factors. The "TwoSampleMR" package (version 0.5.7) was employed to evaluate the causal correlation between gout and CRC. In addition, methods such as inverse variance weighting (IVW), weighted median, penalized weighted median, and MR Egger were used to assess the correlation between gout and CRC. Heterogeneity among the selected IVs was assessed using Cochran's Q statistic and corresponding p-values via MR Egger and IVW approaches. Furthermore, the MR-egger_intercept test was employed to exclude the influence of horizontal pleiotropy, which was identified if the intercept term was statistically significant. This study used the MR polytropic residuals and outliers (MR-presso) approach to exclude horizontal polytropic outliers in the MR-presso package that could significantly influence the estimation findings. Scatterplots and funnel plots were also employed. The scatter plots indicate that the findings are unaffected by outliers. Funnel plots demonstrate the robustness of the relationships without heterogeneity. Furthermore, the approach provided by the SUMMER database was employed to evaluate the causal correlation between gout and CRC survival.
Results
Causal effect of gout on colorectal cancer
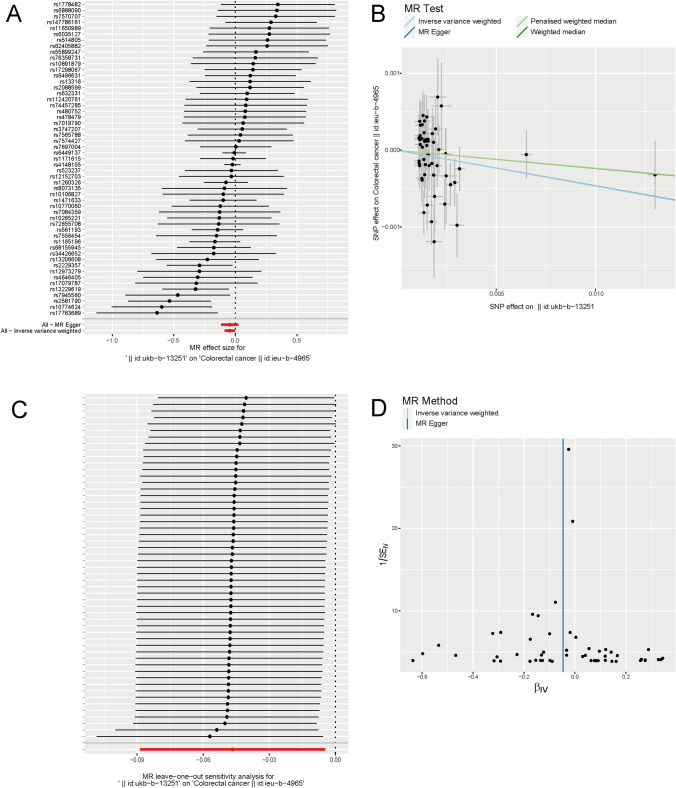
This study included a total of 53 SNPs. As shown in Table 1, the IVW approach demonstrated a gradual decrease in the risk of CRC with increasing gout levels at the criterion of p < 0.05 (p = 0.030, beta = − 0.047, OR = 0.954, 95% CI = 0.915–0.995). The other three approaches shown in Table 1 also indicated an inhibitory influence of gout on CRC (MR Egger approach: p = 0.196, beta = − 0.046, OR = 0.955, 95% CI = 0.891–1.023; weighted median approach: p = 0.421, beta = − 0.024, OR = 0.977, 95% CI = 0.922–1.035; penalized weighted median approach: p = 0.423, beta = − 0.0233, OR = 0.977, 95% CI = 0.923–1.034), although p > 0.05. These results were consistent across various representations, such as the forest plot (Fig. 1A) and scatter plots (Fig. 1B). Statistical analyses indicated that heterogeneity (pMR Egger = 0.084, pIVW = 0.100) or genetic pleiotropy (p = 0.990) did not significantly affect the findings. The stability of the models was confirmed through sensitivity analyses using the leave-one-out approach (Fig. 1C). In addition, funnel plots (Fig. 1D) illustrate the symmetrical distribution of the data points. Additionally, a Manhattan plot of SNP-based associations is shown in Figure S1. The SNP selection summary is shown in Table S1.
Table 1.
Causal associations between genetically determined gout and CRC
| Method | nsnp | Beta | SE | p |
|---|---|---|---|---|
| Inverse variance weighted | 53 | − 0.047 | 0.021 | 0.030 |
| MR Egger | 53 | − 0.046 | 0.035 | 0.196 |
| Weighted median | 53 | − 0.024 | 0.029 | 0.421 |
| Penalised weighted median | 53 | − 0.023 | 0.029 | 0.423 |
Fig. 1.
Causal association between gout and CRC. A Forest plot of single nucleotide polymorphisms (SNPs) associated with gout and the risk of CRC. B Scatter plots of SNPs associated with gout and the risk of CRC. C Leave-one-out of SNPs associated with gout and their risk of CRC. D Funnel plots of SNPs associated with gout and their risk of CRC
Causal effect of gout on colorectal cancer survival
In this investigation, a total of 29 SNPs were incorporated. By employing the IVW approach at a significance level of p < 0.05, a positive relationship between the presence of gout and improved OS and CSS was observed in patients with CRC. When OS was considered as the outcome, the IVW approach shown in Table 2 generated a statistically significant finding (p = 0.022, beta = − 8.453, OR = 0.0002, 95% CI = 1.560 × 10−7–0.292). The other three approaches in Table 2 also exhibited consistency (MR Egger approach: p = 0.027, beta = − 14.983, OR = 3.110 × 10−7, 95% CI = 1.130 × 10−12–0.0856; weighted median approach: p = 0.031, beta = − 11.314, OR = 1.220 × 10−5, 95% CI = 4.260 × 10−10–0.349; penalized weighted median approach: p = 0.023, beta = − 11.314, OR = 1.22 × 10−5, 95% CI = 7.260 × 10−10–0.205). As determined by the established methodology of the SUMMER database, these results remained unaffected by heterogeneity (p = 0.755) and genetic pleiotropy (p = 0.222).
Table 2.
Causal associations between genetically determined gout and OS of CRC
| Method | nsnp | Beta | SE | p |
|---|---|---|---|---|
| Inverse variance weighted | 29 | − 8.453 | 3.685 | 0.022 |
| MR Egger | 29 | − 14.983 | 6.390 | 0.027 |
| Weighted median | 29 | − 11.314 | 5.235 | 0.031 |
| Penalised weighted median | 29 | − 11.314 | 4.964 | 0.023 |
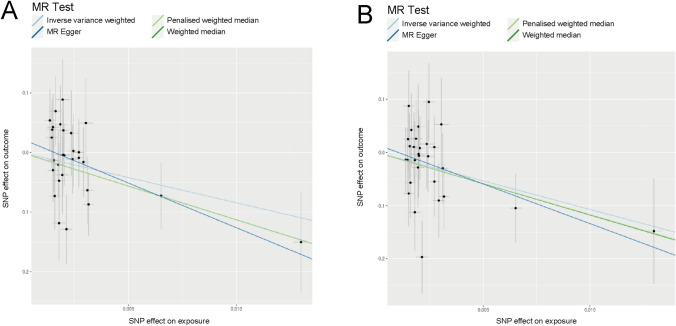
In the context of CSS, the IVW approach shown in Table 3 yielded statistically significant findings (p = 0.013, beta = − 10.726, OR = 2.200 × 10−5, 95% CI = 4.660 × 10−9–0.104). The other three approaches shown in Table 3 indicated an ameliorating impact of gout on CCS of CRC, although with p > 0.05 (MR Egger approach: p = 0.057, beta = − 14.896, OR = 3.390 × 10−7, 95% CI = 1.460 × 10−13–0.788; weighted median approach: p = 0.052, beta = − 11.787, OR = 7.600 × 10−6, 95% CI = 5.210 × 10−11–1.109; penalized weighted median approach: p = 0.060, beta = − 11.692, OR = 8.360 × 10−6, 95% CI = 4.300 × 10−11–1.627). Scatter plots for OS (Fig. 2A) and CSS (Fig. 2B) aligned with the above findings. As determined by the established methodology of the SUMMER database, these results were unaffected by heterogeneity (p = 0.821) and genetic pleiotropy (p = 0.501).
Table 3.
Causal associations between genetically determined gout and CSS of CRC
| Method | nsnp | Beta | SE | p |
|---|---|---|---|---|
| Inverse variance weighted | 29 | − 10.726 | 4.315 | 0.013 |
| MR Egger | 29 | − 14.896 | 7.478 | 0.057 |
| Weighted median | 29 | − 11.7876 | 6.067 | 0.052 |
| Penalised weighted median | 29 | − 11.692 | 6.214 | 0.060 |
Fig. 2.
Causal association between gout and survival of CRC. A Scatter plots of SNPs associated with gout and the risk of OS in CRC. B Scatter plots of SNPs associated with gout and the risk of CSS in CRC
Discussion
The incidence of gout and CRC has been increasing with economic development and lifestyle changes. Nevertheless, classical epidemiologic studies face challenges in explaining the causal sequence of exposure factors and disease outcomes due to confounding variables. MR analysis offers a straightforward and easily applicable approach that serves to minimize bias, improve comparability, and mitigate the impact of confounding variables. In this study, using GWAS data of SNPs, we discovered for the first time through MR analysis that the risk of CRC gradually decreased with the accumulation of gout, whereas OS and CSS of CRC were enhanced. These findings indicate a mitigating effect of gout on CRC outcomes.
Initially, gout was defined as the exposure factor, and CRC as the outcome. Analysis conducted using the MR approach revealed a gradual decrease in the risk of CRC with increasing gout levels (IVW approach: p = 0.030, beta = − 0.047, OR = 0.954, 95% CI = 0.915–0.995), indicating a potential protective role of gout against CRC. This is consistent with the results of a recent Mendelian randomization study on serum uric acid levels and CRC [26]. In a study conducted by Slobodnick et al., individuals diagnosed with gout demonstrated a lower prevalence of CRC, as confirmed through colonoscopy, compared with those with osteoarthritis but without gout. This observation indicates a potential protective effect of gout against CRC [19]. In addition, Kwon et al., in a study of 9920 CRC patients and 39,680 controls, found a slight decrease in the risk of CRC among patients with gout under the age of 65 years (95% CI, 0.70–0.95; p = 0.009) [20]. Various factors, including pharmaceutical interventions and the antioxidative properties associated with uric acid, may have contributed to this finding. Uric acid, the main etiological factor in gout development, exhibits antioxidant properties that help reduce the generation of reactive oxygen species resulting from prolonged inflammation. These properties also contribute to the preservation of bone metabolism, nerve function, and cardiovascular [27–29]. Wang et al. showed that uric acid accumulation plays a significant role in activating TAK1, inducing DNA damage-induced MICA/B expression, and enhancing anti-tumor immune responses [30]. Furthermore, at the onset of gout, there is an enrichment of immune cells and an upregulation of systemic inflammatory factors, including CD8+ T cells, NK cells, and inflammation-related chemokines. These processes may facilitate the body's removal of tumor cells [21].
However, numerous drugs are available for treating gout, such as colchicine (COL), indomethacin (IND), and allopurinol (ALP). COL, a naturally occurring alkaloid present in the Liliaceae family, has demonstrated efficacy in inducing cell death in SW480 cells at low doses, indicating potential antitumor properties [31]. IND is a nonsteroidal anti-inflammatory drug [32]. A previous study reported that the combination of IND and vitamin D substantially decreased the incidence and severity of colon cancer [33]. ALP is an organic compound mainly employed as a uric acid-lowering agent. According to a meta-analysis, ALP exhibits beneficial anti-colorectal cancer activity [34]. Consequently, subsequent pharmacological interventions may exert a degree of anti-tumor efficacy. However, additional clinical cohort studies are required to substantiate these findings.
It is noteworthy that certain studies have reported an association between hyperuricemia, gout, and an increased risk of colorectal cancer (CRC) [17, 18, 35], findings that contradict our results. This discrepancy may be attributed to variations in research methodologies. On the one hand, data selection is the basis of research. If the number of SNPs included in the study is different, different analysis results may result. For example, in the Mendelian randomization study on the effect of uric acid on CRC, Zhou et al. used 26 SNPs [35], while in the study of Leed et al., only 14 SNPs were included in the initial MR analysis and only 3 SNPs in the second MR analysis [26]. The number and representativeness of the selected SNPs may affect the robustness and reliability of the results. On the other hand, Mendelian randomization employs SNPs as instrumental variables, which can significantly mitigate bias in causal inference and yield more robust results. Conversely, observational studies are often susceptible to numerous confounding factors, potentially resulting in instability and bias in their outcomes.
To provide additional evidence supporting the influence of gout on CRC, further investigation into the effect of gout on survival in CRC was conducted using MR. As determined by the IVW approach, our results were consistent with previous studies, demonstrating that the presence of gout exerted a cumulative positive impact on the survival outcomes of patients with CRC, including OS and CSS (OS: p = 0.022, beta = − 8.453, OR = 0.0002, 95% CI = 1.560 × 10−7–0.292; CSS: p = 0.013, beta = − 10.726, OR = 2.200 × 10−5, 95% CI = 4.660 × 10−9–0.104).
This study provides numerous advantages. First, MR was employed to alleviate data bias and eliminate the influence of confounding variables. Second, not only is the correlation between gout and the incidence of CRC investigated but also the effect of gout on survival in patients with CRC is examined, thereby enhancing the validity of the findings of this study. However, this study has certain limitations, particularly the exclusive inclusion of samples from Europe, which limits our ability to determine potential variations across racial groups. In addition, the precise mechanism by which gout decreases the incidence of CRC and improves survival rates in patients with CRC remains unclear, highlighting the need for further comprehensive research.
Conclusion
This study presents evidence supporting the positive impact of gout on the incidence and prognosis of CRC, indicating a modulatory effect that decreases incidence rates and enhances OS and CSS outcomes. Furthermore, this study offers novel insights into the exploration of the relationship between gout and CRC.
Supplementary Information
Acknowledgements
Not applicable.
Author contributions
LQW and YBS: conceiving the study and writing the manuscript; CYL: analyzing the data; DL: Methodology; STY: Data curation; XJM: Project administration; DHL:reviewing the experimental data; XJC: Writing-review & editing.
Funding
This study was supported by funds from the Promoting Project of Basic Capacity for Young and Middle-aged University Teachers in Guangxi(2020KY03016); the Guangxi Health Department Research Project (z20190853); the Clinical Research “Climbing” Program of the First Affiliated Hospital of Guangxi Medical University (YYZS2020023); and the Guangxi Health Department Research Project (Z-A20230495).
Data availability
The data is available from the corresponding authors upon reasonable request.
Declarations
Ethics approval and consent to participate
This study does not involve ethical practices.
Consent for publication
All authors have read and agreed to the published version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Li-Qiang Wei and Yi-bei Song contributed equally to this work.
Contributor Information
Deng-He Liu, Email: liudenghe@sr.gxmu.edu.cn.
Xiao-jv Chi, Email: chixiaojv@gxmu.edu.cn.
References
- 1.Weisshaar S, et al. Cardiovascular risk and mortality in patients with hyperuricemia treated with febuxostat or allopurinol: a retrospective nation-wide cohort study in Austria 2014–2017. Rheumatol Int. 2022;42(9):1597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chi X, et al. Chinese herbal medicine for gout: a review of the clinical evidence and pharmacological mechanisms. Chin Med. 2020;15:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan Y, et al. Efficacy and safety of Qinpi Tongfeng formula in the treatment of acute gouty arthritis: a double-blind, double-dummy, multicenter, randomized controlled trial. Evid Based Complement Alternat Med. 2022;2022:7873426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin TJ, et al. Label-free uric acid estimation of spot urine using portable device based on UV spectrophotometry. Sensors (Basel). 2022. 10.3390/s22083009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zha X, et al. Combination of uric acid and pro-inflammatory cytokines in discriminating patients with gout from healthy controls. J Inflamm Res. 2022;15:1413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi HK. A prescription for lifestyle change in patients with hyperuricemia and gout. Curr Opin Rheumatol. 2010;22(2):165–72. [DOI] [PubMed] [Google Scholar]
- 7.Natsuko PD, et al. Differential gene expression of ABCG2, SLC22A12, IL-1beta, and ALPK1 in peripheral blood leukocytes of primary gout patients with hyperuricemia and their comorbidities: a case-control study. Eur J Med Res. 2022;27(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bardin T, et al. A cross-sectional study of 502 patients found a diffuse hyperechoic kidney medulla pattern in patients with severe gout. Kidney Int. 2021;99(1):218–26. [DOI] [PubMed] [Google Scholar]
- 9.Chang HW, et al. Associations between urate-lowering therapy and the risk of type 2 diabetes mellitus. PLoS ONE. 2019;14(1): e0210085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boffetta P, et al. A prospective study of gout and cancer. Eur J Cancer Prev. 2009;18(2):127–32. [DOI] [PubMed] [Google Scholar]
- 11.Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. 2021;14(10): 101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miskovic Spoljaric K, et al. Antioxidant and antiproliferative potentials of phenolic-rich extracts from biotransformed grape pomace in colorectal cancer. BMC Complement Med Ther. 2023;23(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meng W, et al. ZhenQi FuZheng formula inhibits the growth of colorectal tumors by modulating intestinal microflora-mediated immune function. Aging (Albany NY). 2022;14(11):4769–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lansdorp-Vogelaar I, et al. Risk-stratified strategies in population screening for colorectal cancer. Int J Cancer. 2022;150(3):397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bockenhauer D, Jaureguiberry G. HNF1B-associated clinical phenotypes: the kidney and beyond. Pediatr Nephrol. 2016;31(5):707–14. [DOI] [PubMed] [Google Scholar]
- 16.Huang Y, et al. Dietary sugar consumption and health: umbrella review. BMJ. 2023;381: e071609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boustany A, et al. Increased risk of colorectal cancer in patients with chronic tophaceous gout: a population-based study. Arq Gastroenterol. 2023;60(3):339–44. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, et al. Effect of serum uric acid and gout on the incidence of colorectal cancer: a meta-analysis. Am J Med Sci. 2024;367(2):119–27. [DOI] [PubMed] [Google Scholar]
- 19.Slobodnick A, et al. Colorectal cancer among gout patients undergoing colonoscopy. J Clin Rheumatol. 2019;25(8):335–40. [DOI] [PubMed] [Google Scholar]
- 20.Kwon MJ, et al. Gout and colorectal cancer likelihood: insights from a nested case-control study of the Korean population utilizing the Korean National Health Insurance Service-National Sample Cohort. Cancers (Basel). 2023. 10.3390/cancers15235602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu H, et al. Single-cell transcriptomics reveals variations in monocytes and Tregs between gout flare and remission. JCI Insight. 2023. 10.1172/jci.insight.171417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmad O, Forsti A. The complementary roles of genome-wide approaches in identifying genes linked to an inherited risk of colorectal cancer. Hered Cancer Clin Pract. 2023;21(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu B, et al. Assessing the relationship between gut microbiota and irritable bowel syndrome: a two-sample Mendelian randomization analysis. BMC Gastroenterol. 2023;23(1):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, et al. Assessment of bidirectional relationships between 98 genera of the human gut microbiota and amyotrophic lateral sclerosis: a 2-sample Mendelian randomization study. BMC Neurol. 2022;22(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xin J, et al. SUMMER: a Mendelian randomization interactive server to systematically evaluate the causal effects of risk factors and circulating biomarkers on pan-cancer survival. Nucleic Acids Res. 2023;51(D1):D1160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee M, Nam S. The causal relationship of serum uric acid on colorectal cancer: a Mendelian randomization study. Medicine (Baltimore). 2024;103(26): e38722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Si Z, et al. High-throughput metabolomics discovers metabolic biomarkers and pathways to evaluating the efficacy and exploring potential mechanisms of osthole against osteoporosis based on UPLC/Q-TOF-MS coupled with multivariate data analysis. Front Pharmacol. 2020;11:741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pakpoor J, et al. Clinical associations between gout and multiple sclerosis, Parkinson’s disease and motor neuron disease: record-linkage studies. BMC Neurol. 2015;15:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Y, et al. Is hyperuricemia a recognizable biomarker for low risk of stroke in patients with atrial fibrillation? Int J Cardiol. 2016;203:624–5. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, et al. Uric acid accumulation in DNA-damaged tumor cells induces NKG2D ligand expression and antitumor immunity by activating TGF-beta-activated kinase 1. Oncoimmunology. 2022;11(1):2016159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozmen N, Kaya-Sezginer E, Bakar-Ates F. The cellular uptake and apoptotic efficiency of colchicine is correlated with downregulation of MMP-9 mRNA expression in SW480 colon cancer cells. Anticancer Agents Med Chem. 2018;18(13):1927–33. [DOI] [PubMed] [Google Scholar]
- 32.Ceschan NE, et al. Carrier free indomethacin microparticles for dry powder inhalation. Int J Pharm. 2018;549(1–2):169–78. [DOI] [PubMed] [Google Scholar]
- 33.Okda TM, et al. Chemopreventive and anticancer activities of indomethacin and vitamin D combination on colorectal cancer induced by 1,2-dimethylhydrazine in rats. Biomed Rep. 2021;14(2):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu Q, et al. Preventive effects of 13 different drugs on colorectal cancer: a network meta-analysis. Arch Med Sci. 2023;19(5):1428–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou J, et al. Association between serum uric acid and colorectal cancer risk in European population: a two-sample Mendelian randomization study. Front Oncol. 2024;14:1394320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data is available from the corresponding authors upon reasonable request.