Abstract
Cell metabolism is crucial for orchestrating the differentiation and function of regulatory T cells (Tregs). However, the underlying mechanism that coordinates cell metabolism to regulate Treg activity is not completely understood. As a pivotal molecule in lipid metabolism, the role of SHIP-1 in Tregs remains unknown. In this study, we found SHIP-1 Treg KO mice (SHIP-1 specifically deleted in regulatory T cells) had severe autoimmunity with increased Tregs in the thymus and disrupted peripheral T cell homeostasis. Mechanistically, CD4Cre Ship-1flox/flox mice were found to have increased Treg precursors and SHIP-1 KO Tregs had reduced migration and stability, which caused decreased Tregs in the spleen. Additionally, the suppressive function of Tregs from SHIP-1 KO mice was diminished, along with their promotion of anti-tumor immunity. Interestingly, the PI3K-mTORC1, but not mTORC2, signaling axis was enhanced in SHIP-1 KO Tregs. In vivo treatment of SHIP-1 Treg KO mice with rapamycin rescued the abnormal Treg percentages and peripheral T cell homeostasis, as well as Treg suppressive function. Furthermore, the treatment of wild-type mice with SHIP-1 inhibitor enhanced anti-tumor activity. Our study highlights the SHIP-1-PI3K-mTORC1 axis that regulates Treg differentiation and function, and it is a potential target for cancer treatment.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-024-05470-2.
Keywords: Cell metabolism, Treg cells, SHIP-1, mTOR
Background
Regulatory T cells (Tregs) are an important subgroup of T lymphocytes that play a key role in a variety of diseases, including cancer. Metabolism critically regulates the activity of Treg cells. Under most circumstances of tumor-associated diseases, Treg cells undergo metabolic reprogramming, which modifies their functions to support tumor growth. Therefore, modification in these metabolic processes and functions would further amplify the benefit for the treatment of cancer [1, 2]. It was also demonstrated that activation of Treg cells in response to the tumor microenvironment (TME) is possible because the TME allows for metabolic activation, which enables selective activation, survival, and proliferation of Treg cells [3]. Such metabolic adaptations of the tumor microenvironment likely enable Treg cells to use alternative metabolites and function, respectively, to modulate tumor growth and therapeutic response [4]. The PI3K-mTOR signaling pathway is one of the critical co-regulatory metabolic signaling pathways within this repertoire of functional programming. It is among the most essential newly emerging metabolic regulators in Treg cells [5]. The mTOR complex is crucial during the maturation and differentiation of CD4+ T cell subsets specifically, in the regulation of Treg cells, without which homeostasis of the host immune and defense mechanisms would be maintained [6]. Effective immunoregulatory control on regulating Th17/Treg balance during inflammatory disease, such as colitis, was also shown with suppression of the PI3K/mTOR signaling pathway [7, 8]. Therefore, the study of PI3K signaling transduction pathways in regulating metabolic alterations of Treg cells will open up the discovery of new strategies and targets for treating cancer, immune diseases, and organ transplantation.
Src homology 2 (SH2) domain containing inositol polyphosphate 5-phosphatase 1 (SHIP-1) is a phosphatase that is encoded by the INPP5D gene in humans [9, 10], which is a member of the inositol polyphosphate-5-phosphatase (INPP5) family of proteins. SHIP-1 has an N-terminal SH2 domain, an inositol phosphatase domain, and two C-terminal protein interaction domains. SHIP-1 is expressed exclusively in cells of hematopoietic lineage. Tyrosine phosphorylation recruits SHIP-1 to the plasma membrane, where it hydrolyzes the 5′ phosphate from phosphatidylinositol (3,4,5)-trisphosphate and inositol-1,3,4,5-tetrakisphosphate, which affects downstream signaling cascades, such as inhibition of BTK and AKT activation [11–14].
The role of SHIP-1 has been studied extensively in lymphocytes, including B and CD4+ T cells. SHIP-1 is a negative regulator of BCR signaling in B cells [15–17]. SHIP-1 deficiency in activation-induced cytidine deaminase (AID+) B cells reduces the IL-10 production by B10 cells, which induces the autoimmune phenotype in Innp5dfl/flAicdaCre/+ mice [18]. SHIP-1 plays multiple roles in CD4+ T cells, including regulating immune responses and affecting cell survival. Specifically, SHIP-1 plays a central role in CD4-mediated inhibitory signaling, which can lead to T cell anergy and apoptosis in response to antigens [19]. In human CD4+ T lymphocytes, SHIP-1 negatively regulates the PI3K pathway, controlling basal PI3K signaling, and maintaining normal morphology and basal motility [20].
Currently, the role of SHIP-1 in Treg cells is not clearly understood. SHIP-1 deficiency promotes generation of the thymic Treg cell population. DOK1 and SHIP-1 are essential for development of Tregs [21]. Additionally, the colligation of CD3 and CD28 activates the SHIP-1-DOK2-GRB2 signaling complex and recruits SHIP-1 from the cytosol to the membrane [22, 23]. Mice with a germ-line deletion of SHIP-1 have an increased number of Treg cells [21]. But the number of Treg cells in the spleen of CD4creSHIP-1fl/fl mice are similar to that of wild-type mice [24]. CD4creSHIP-1fl/fl mice have a reduced humoral immune response, inefficient Th2 skewing and enhanced cytotoxicity [24]. Ex vivo Tregs from CD4creSHIP-1fl/fl mice retained normal suppressive capacity in vitro and in a T cell transfer model of colitis [25]. Deletion of SHIP-1 reduces the capacity of naive CD4+ T cells to differentiate into Th17 cells and promotes the development of induced Tregs (iTregs)[25]. This may indicate that SHIP-1 does not have an essential role in thymic selection or in T cell development in the periphery. In order to resolve this divergence and clarify the role of SHIP-1 in the development and function of Tregs, we performed studies using mice with SHIP-1 knockout only in Tregs.
In this study, we generated SHIP-1 deletion specifically in Tregs by crossing Foxp3YFP−cre mice with Ship-1fl/fl mice. Surprisingly, we found that Foxp3YFP−cre Ship-1fl/fl mice (referred to as SHIP-1 Treg KO mice for simplicity) had splenomegaly and an autoimmune phenotype characterized by IgG complex deposition in the kidney and increased dsDNA antibodies. Thymic Tregs increased markedly, driven by a rise in CD4+ T cells and Treg precursors in the thymus. The absence of SHIP-1 function enhanced thymic differentiation but impaired the suppressive capacity of Treg cells, resulting in a stronger anti-tumor response and disrupted peripheral homeostasis. Our findings also revealed that SHIP-1’s role in Treg cells operates through the PI3K-mTORC1 pathway. Additionally, SHIP-1 expression was differently regulated in Treg cells compared to conventional T cells (Tconv), suggesting that SHIP-1 orchestrates the balance between Treg and Tconv cells, ensuring an effective and controlled immune response.
Methods
Mice
Ship-1 conditional knockout mice were generated on a C57/BL6 background by crossing Foxp3YFP−cre or CD4Cre with SHIP-1fl/fl (Jackson laboratory). All mice were housed in single-ventilated cages at the animal center of Children's Hospital of Chongqing Medical University. Eight- to ten-week-old mice were used unless otherwise stated. For in vivo studies, mice were intraperitoneally injected with rapamycin at a dosage of 4 mg/kg body weight daily for 14 consecutive days. The SHIP-1 inhibitor, 3α-aminocholestane (3AC), was obtained from Echelon (Salt Lake City, UT). For these experiments, 3AC was prepared in a 0.3% Klucel/H₂O solution at a concentration of 11.46 mM. Mice in the vehicle group received 100 μL of the 0.3% Klucel/H₂O solution. In the 3AC-treated group, the final concentration of 3AC administered to the mice was 60 μM. Both vehicle- and 3AC-treated mice were subjected to daily intraperitoneal injections for 7 days before analysis or tissue collection. All animal experiments were conducted in accordance with the protocols approved by Institutional Animal Care and Usage Committee of Children’s Hospital of Chongqing Medical University.
BM chimera mice
To generate bone marrow chimeras, CD45.1 mice were sublethally irradiated (6 Gy) and then intravenously injected with 1 × 107 total BM cells containing SHIP-1 Treg KO BM (expressing CD45.2) and WT BM (expressing CD45.1) at a ratio of 1:1, respectively. As mentioned above, the recipient mice were analyzed 8 weeks after bone marrow transplantation [26].
Cell purification and flow cytometry
Lymphocytes from sex-matched Foxp3YFP−cre Ship-1fl/fl mice and Foxp3YFP−cre Ship-1+/+ mice were isolated from the thymus, spleen and lymph nodes according to published protocols [27]. For analyses of lymphocytes surface markers, the antibodies used were: PE anti-ICOS (clone 7E.17G9; eBioscience), PerCP/Cy5.5 anti-CD44 (clone: IM7), APC anti-CD25 (clone: PC61), APC-CY7 anti-TCR-β (clone: H57–597), PE-CY7 anti-CD62L (MEL-14), Pacific Blue anti-CD4 (clone: RM4-5), Alexa Fluor® 647 anti-mouse CXCR1 (clone: L138D3), and Brilliant Violet 510 anti-CD8a (clone: 53-6.7). BioLegend was the source for all antibodies unless otherwise specified. Antibody incubations were performed on ice for 30 min in PBS containing 2% FBS to stain cells. To detect intracellular proteins, cells were fixed and permeabilized with the Foxp3/transcription factor staining buffer set by eBioscience and stained using the following antibodies: PE anti-CD152 (CTLA-4) (clone UC10–4B9); APC anti-Foxp3 (clone MF14); APC anti-IL-4 (clone: 11B11), PE-Cy7 anti-IFN-γ (clone: XMG1.2), Brilliant Violet 421 anti-IL-17A (clone: TC11-18H10.1) from BioLegend, and PE-Cyanine7 Ki-67 monoclonal antibody (Clone: SolA15) from eBioscience was used to measure cell population growth. BrdU and caspase-3 staining were performed following the manufacturer’s protocol (BD Biosciences).
Treg cells were isolated using a CD4+CD25+ Treg cell isolation kit from Miltenyi Biotec and subsequently activated on plates coated with 5 μg/ml anti-mouse CD3 (clone: 145-2C11, Bio X Cell) and 5 μg/ml anti-mouse CD28 (clone: 37.51, Bio X Cell). After activation, lymphocytes were immediately fixed and permeabilized with Phosflow perm buffer (BD Biosciences), then stained with antibodies against phosphorylated AKT (pSer473), S6 (p235/236), PI3 Kinase p85 (Tyr458)/p55(Tyr199), FOXO1 (Ser256), and mTOR (Ser2448), all from Cell Signaling Technology. Flow cytometry analysis was performed using a FACS Canto (BD Biosciences), and data was analyzed using FlowJo software. The number of cells in each population was calculated by multiplying the total cell count by the percentage of each specific population from the same mouse, followed by averaging.
In vitro Treg suppression assays
For the in vitro Treg cell suppression assay, CD4⁺CD25⁺ Treg cells and CD4⁺CD62LhighCD44low naive T (Tnaive) cells were isolated using specific kits designed for CD4⁺CD25⁺ Treg cells and naive CD4⁺ T cells (Miltenyi Biotec). Tnaive cells (1 × 105) and Treg cells were co-cultured in varying ratios in 96-well flat-bottom plates. The culture included anti-CD3 (clone: 145-2C11, Bio X Cell) and anti-CD28 (clone: 37.51, Bio X Cell), each at a concentration of 5 μg/ml, and was maintained for 72 h. T cell proliferation was assessed with the CellTrace Violet Cell Proliferation Kit (C34557, Invitrogen). For drug treatment experiments, Treg cells were pre-treated with either a vehicle control or 100 nM rapamycin (AY 2298, MedChemExpress) for one hour prior to stimulation.
Colitis model
The colitis model was conducted following methods described in previous studies [28]. In summary, Rag1−/− mice received an intraperitoneal injection of 4 × 105 Tconv cells (CD45.1+), either alone or together with 2 × 105 Treg cells (CD45.2+) derived from WT and KO mice. After 9–10 weeks, the colons were fixed, stained with H&E, and an experienced pathologist performed a blind evaluation of colon pathology using a semi-quantitative scale from 0 to 5. Additionally, the production of IFN-γ by spleen CD45.1⁺CD4⁺ T cells from recipient Rag1−/− mice was assessed via flow cytometry following a 4-h stimulation with PMA and ionomycin.
Tumor model
MC38 colon adenocarcinoma cells were maintained according to previously described protocols [29], briefly, MC38 colon adenocarcinoma cells were maintained in DMEM containing 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin. In the experiment, sex-matched Foxp3YFP−cre Ship-1fl/fl and Ship-1+/+ mice were injected subcutaneously with 2 × 105 MC38 cells in the right flank region. Tumor size was monitored at three-day intervals using digital calipers, with the volume estimated according to the formula: Length × Width × [(Length × Width)^0.5] × π/6. To obtain tumor-infiltrating lymphocytes (TILs), tumors were carefully dissected, chopped into small pieces, and subjected to enzymatic digestion for one hour at 37 °C using a solution of 0.5 mg/ml collagenase IV (Roche) and 200 U/ml DNase I (Sigma). The resulting cell suspension was then subjected to density-gradient centrifugation with Percoll (Roche) to isolate the TILs.
In vivo migration assay
CD4⁺YFP-Foxp3⁺ Tregs isolated from Foxp3YFP−creShip-1+/+ and Foxp3YFP−cre Ship-1fl/fl mice were intravenously transferred into CD45.1⁺ WT mice, along with B cells from CD45.2⁺ WT mice for normalization purposes. Five hours post-injection, the ratio of CD45.2⁺CD4⁺ YFP-Foxp3⁺ Treg cells to CD45.2⁺B220⁺ B cells was assessed in the spleen, peripheral lymph nodes (PLN), mesenteric lymph nodes (MLN), and blood. This ratio was then adjusted according to the initial 1:2 proportion, following previously established methods [30].
Immunofluorescence analysis
For immunofluorescence staining of mouse kidney tissues, the kidneys were embedded in OCT compound and rapidly frozen in liquid nitrogen for 1 min. Tissue sections were cut to a thickness of 7–10 microns. The samples were then fixed with chilled acetone for 5 min and allowed to air dry at room temperature. Following PBS washes, the sections were blocked for 2 h in a solution containing 5% BSA, 1:100 Fc blocker, and PBS. They were subsequently stained overnight with AF488-conjugated anti-mouse IgG (diluted 1:500 in blocking buffer). Images were captured using a Nikon A1R confocal microscope.
Serum autoantibody analysis
Autoantibodies against double-stranded DNA (dsDNA) were detected using kits from Alpha Diagnostic International (Catalog #5120-1), following the manufacturer’s instructions.
Quantitative RT-PCR
Splenic CD4⁺YFP-Foxp3⁺ Treg cells, along with CD4SP and CD8SP T cells from WT, Foxp3YFP−cre Ship-1fl/fl, and CD4CreShip-1fl/fl mice, were sorted using a MoFlo sorter (Beckman-Coulter). RNA was subsequently extracted with the RNAPURE kit (RP1202, BioTeke). The expression of Ship-1 was assessed using the following primers: Forward 5′-GCAGCAGATGAAGAACAAG-3′ and Reverse 5′-CCAAGTGCCAATGAAGAT-3′.
Statistical analysis
Unless specified otherwise, statistical significance was evaluated using a two-tailed unpaired Student’s t-test with Prism 8 software.
Results
SHIP-1 reduces the thymic Treg population
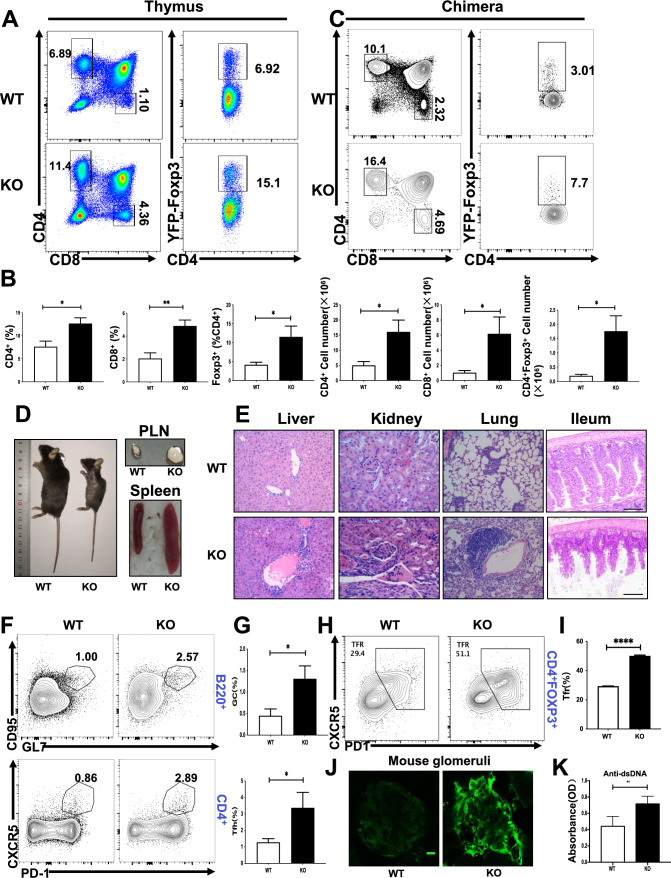
In order to study the intrinsic function of SHIP-1 in regulatory T cells, Ship-1fl/fl mice were crossed with Foxp3YFP−cre transgenic mice to delete the floxed Ship-1 allele specifically in regulatory T cells (SHIP-1 Treg KO mice). The deletion efficiency of Foxp3YFP−cre was examined by RT-PCR and we found that the expression of Ship-1 mRNA was abolished in the regulatory T cells of spleen in SHIP-1 Treg KO mice (Supplementary Fig. 1A). We also examined the expression of SHIP-1 in naive T cells (Tnaives) and Tregs and found that the expression of SHIP-1 was higher in Tregs compared to Tnaives, which indicated that SHIP-1 may have different and intrinsic roles in Treg function (Supplementary Fig. 1B, C). The thymuses of SHIP-1 Treg KO mice had increased accumulation of mature single-positive CD4+ (CD4SP) and CD8+ (CD8SP) thymocytes and CD4+YFP-Foxp3+ regulatory T cells compared to Foxp3YFP−Cre SHIP-1+/+ (WT) controls (Fig. 1A, B). To exclude environmental effects, we made WT-WT and WT-SHIP-1 Treg KO bone marrow CD45.1 (“spike”)-CD45.2 mixed chimera mice, The "spike" group functioned as an internal control for normalization, just as described in the literature [31], and CD45.1 mice were used as the recipients. After 8 weeks of reconstitution, the mice were euthanized for flow cytometry analysis. The increase of CD4SP and CD8SP thymocytes as well as Foxp3+CD4+ Treg cells in the thymus was also observed in CD45.2+ cells of WT-SHIP-1 Treg KO chimera mice, indicating these results are cell intrinsic (Fig. 1C). To exclude the deletion leakage of Foxp3YFP−cre in CD4+ and CD8+ T cells, we also examined the mRNA levels of Ship-1 in CD4+ and CD8+ T cells of SHIP-1 Treg KO mice and did not observe difference compare to WT mice (Supplementary Fig. 1A). Then we examined the CD45.1 “spike” cells in WT-SHIP-1 Treg KO chimera mice and found that the percentage of CD45.1 CD4+ and CD8+ T was also increased in thymus compared to that of WT-WT chimera mice (Supplementary Fig. 1D). These results suggested that SHIP-1 deletion in Tregs have a dominant effect on the increased accumulation of CD4+ and CD8+ T cells in the thymus. Surprisingly, SHIP-1 Treg KO mice developed profound inflammatory diseases, indicated by reduced body size, hunched posture, and crusting of ears, eyelids and tail (Fig. 1D). SHIP-1 Treg KO mice also showed extensive lymphadenopathy and splenomegaly (Fig. 1D) and infiltrations of lymphocytes and myeloid cells in ileum mucosa, lung, liver sinusoids, kidney, and other organs (Fig. 1E). We also examined the spontaneous generation of Germinal center (GC) B cell, T follicular helper(Tfh) cells and follicular regulatory T(Tfr) cell by flow cytometry, which contributes to the autoimmune phenotype. We found the percentage of GC B cells, Tfh cells and Tfr cells were significantly increased in SHIP-1 Treg KO mice (Fig. 1F–I). Additionally, glomeruli of the SHIP-1 Treg KO mice kidney contained prominent IgG deposits as systemic autoimmunity is frequently associated with renal pathology (Fig. 1J). In accordance with this, the titers of dsDNA antibodies were increased in SHIP-1 Treg KO mice (Fig. 1K). Altogether, these results suggest that SHIP-1 deficiency in Tregs causes the expansion of thymic Tregs as well as autoinflammatory disease.
Fig. 1.
SHIP-1 deficiency increases the thymic Treg population. A Flow cytometry analysis of CD4SP, CD8SP, and CD4⁺YFP-Foxp3⁺ Treg cells from the thymus of Foxp3YFP−cre Ship-1+/+ (WT) and Foxp3YFP−cre Ship-1fl/fl (SHIP-1 Treg KO) mice. B Quantification of both percentage and absolute counts of cell populations specified in (A) (n = 6). C CD45.1 mice received sublethal irradiation (6 Gy) and were then intravenously infused with a 1:1 mixture of total bone marrow (BM) cells—comprising 1 × 10⁷ cells in total—from SHIP-1 Treg KO BM (CD45.2⁺) and WT BM (CD45.1⁺). Eight weeks post-transplant, the CD4SP, CD8SP, and CD4⁺YFP-Foxp3⁺ Treg cells in the thymus of recipient mice were examined. D Photographic comparison of 8-week-old WT and SHIP-1 Treg KO mice, including their spleens and peripheral lymph nodes (n = 3). E Hematoxylin and eosin-stained sections of the liver, kidney, lung, and ileum from WT and SHIP-1 Treg KO mice. Images represent 3 mice, with scale bars at 100 μm. F, G Flow cytometric data of germinal center (GC) and T follicular helper (Tfh) cells from the spleens of WT and SHIP-1 Treg KO mice (n = 5), displaying the percentages of relevant cell subsets. H, I Flow cytometric analysis of T follicular regulatory (Tfr) cells in the spleens of WT and SHIP-1 Treg KO mice (n = 5), along with the percentages of these populations. J Immunofluorescence images showing IgG antibody deposits in the glomeruli of SHIP-1 Treg KO mice, with representative images from three mice. Scale bars are set at 25 μm. K Titers of anti-nuclear and anti-dsDNA antibodies in WT and SHIP-1 Treg KO mice (n = 6). Data are presented as the mean ± SEM. Statistical significance: ∗ p < 0.05; ∗ ∗ p < 0.01; ∗ ∗ ∗ p < 0.001
SHIP-1 is essential for maintaining the stability of Tregs
It has been shown that Foxp3+ Treg cells develop from precursors (CD4+CD25+Foxp3−) that depend on IL-2 and TGF-β or IL-15 stimulation to express FOXP3, not TCR engagement [32–35]. In order to prove whether SHIP-1 is involved in the differentiation of Treg precursors into Tregs. CD4Cre mice were crossed with SHIP-1flox/flox mice to have SHIP-1 specifically deleted in T cells (CD4Cre SHIP-1flox/flox mice). Real-time PCR analysis indicated efficient deletion of the Ship-1 gene in thymocytes (Supplementary Fig. 1E). Although the number of Tregs in the spleen of CD4Cre SHIP-1flox/flox mice was the same compared to WT mice [24], the thymic Tregs have not been analyzed in CD4Cre SHIP-1flox/flox mice yet. Notably, we found that the populations of CD4+CD25+Foxp3+ and CD4+CD25+Foxp3− were significantly increased in the thymuses of CD4Cre SHIP-1flox/flox mice (Fig. 2A, B). The increase in CD4+CD25+Foxp3− may be a compensatory response to the altered immune environment or a disruption in the equilibrium between different T cell subsets. This indicates that SHIP-1 is not involved in the differentiation of Treg precursors (CD4+CD25+Foxp3−) into Tregs.
Fig. 2.
SHIP-1 blocks differentiation of CD4+CD25+ Foxp3 cells. A Flow cytometry analysis of CD4⁺CD25⁺Foxp3⁺ and CD4⁺CD25⁺Foxp3⁻ cells, gated from CD4SP thymocytes in WT and CD4CreSHIP-1flox/flox mice. B Quantitative data showing the percentage and absolute numbers of cell populations identified in A (n = 6). C 5 × 105 sorted CD4⁺YFP-Foxp3⁺ Treg cells from WT or SHIP-1 Treg KO mice, together with 1 × 10⁶ B cells from WT mice, were transferred into wild-type congenic CD45.1⁺ mice (n = 6). Five hours post-transfer, the ratio of CD45.2⁺CD4⁺YFP-Foxp3⁺ Treg cells to CD45.2⁺B220⁺ B cells was measured in the spleen, PLN, MLN, and blood, and then normalized to the pre-transfer starting ratio of 1:2. D CD4⁺YFP-Foxp3⁺ Treg cells, sorted from the spleens of WT and SHIP-1 Treg KO mice, were labeled with Cell Trace Violet and co-cultured with anti-CD3, anti-CD28, and IL-2 for 5 days (n = 3). E The expression levels and mean fluorescence intensity (MFI) of Foxp3 in CD4⁺ single-positive (SP) cells from WT and SHIP-1 Treg KO mice were assessed after 5 days of co-culture with anti-CD3, anti-CD28, and IL-2, with data representing three independent experiments (n = 3). F Flow cytometric evaluation of IL-17 and IFN-γ production in CD4⁺Foxp3⁺ T cells from the spleens of WT and SHIP-1 Treg KO mice following a 4-h stimulation with PMA and ionomycin. G Quantification of cell populations from F (n = 4). Results are expressed as mean ± SEM. Statistical significance is denoted as follows: ∗ p < 0.05; ∗ ∗ p < 0.01; ∗ ∗ ∗ p < 0.001
To investigate whether the accumulation of thymic Tregs is also due to disrupted migration, we examined the role of SHIP-1 in the migration of Treg cells by co-transferring CD45.2+ WT or SHIP-1 deficient Treg cells from SHIP-1 Treg KO mice, together with CD45.2+B220+ (for normalization) at a 1:2 ratio into CD45.1+ congenic mice. After 5 h, we found Treg cells from WT group were lower than KO group in blood, but higher than KO group in spleen, lymph nodes and mesenteric lymph nodes (Fig. 2C), indicating a migration defect of SHIP-1 deficient Treg cells. To further analyze the stability of Tregs, isolated Tregs were labeled with Cell Trace Violet and cultured with anti-CD3 + CD28 and IL-2 for 3 days, then analyzed by flow cytometry. We found that the proliferation of SHIP KO Tregs was weakened compared to WT mice in vitro. Moreover, Tregs isolated from SHIP-1 Treg KO mice lost FOXP3 expression upon division, in contrast to control wild-type (WT) littermates (Fig. 2D, E). In summary, SHIP-1 is crucial for maintaining the proliferation and stability of Tregs in vitro. Unstable Treg cells can secrete IL-17 and IFN-γ to promote inflammatory responses [36]. Therefore, we examined the secretion of IL-17 and IFN-γ by Treg cells stimulated with PMA and ionomycin for 4 h in vitro. The results showed that IL-17 secretion by Treg cells from the spleens of KO mice was increased compared to WT mice (Fig. 2F, G).
SHIP-1 KO Treg cells have altered homeostasis and function
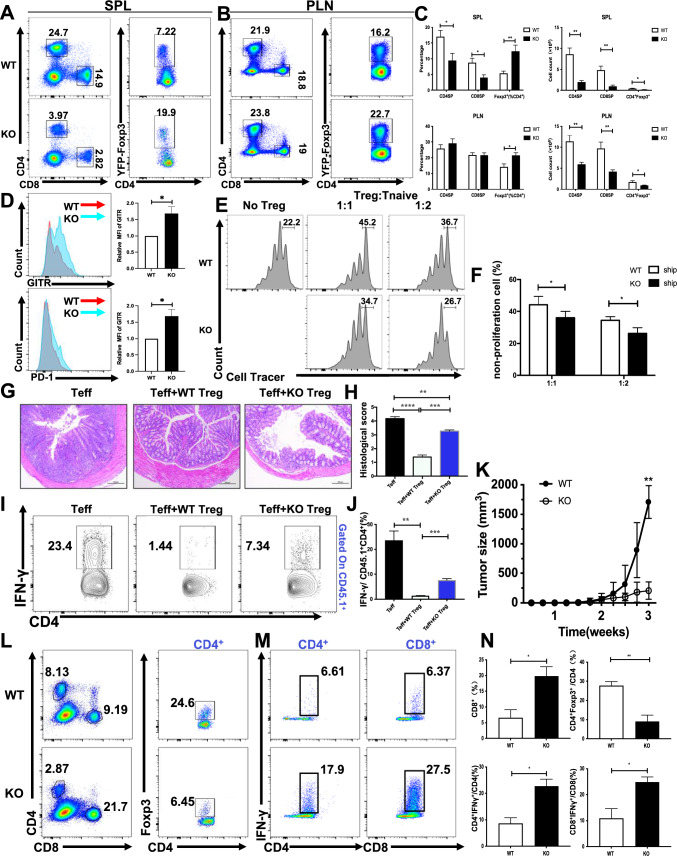
We have shown SHIP-1 negatively regulates thymic differentiation of Treg cells. Whether SHIP-1 also affects the homeostasis and suppressive function of peripheral Treg cells was investigated next. Due to SHIP-1 deficiency on the egress of thymocytes, the amount of CD4SP and CD8SP were significantly decreased in the spleen and peripheral lymph nodes (pLN) of SHIP-1 Treg KO mice (Fig. 3A–C). Although SHIP-1 deficiency resulted in an increased percentage of Foxp3+ CD4SP cells, the total number of Foxp3+ CD4SP cells was decreased in both spleen and pLN (Fig. 3C). In order to confirm that the effect of SHIP-1 deficiency on the altered homeostasis is cell autonomous, we examined the percentage of CD45.2 CD4SP, CD8SP and Tregs in the spleen and pLN. We found that the percentage of CD45.2 CD4+ and CD8+ T cells was decreased only in the spleen, but not in pLN, and the ratio of CD45.2 Tregs was increased both in spleen and pLN (Supplementary Fig. 2A, B). Interestingly the reduction of CD4+ and CD8+ T cells can be observed in CD45.1 “spike” T cells of WT-SHIP-1 Treg KO chimera mice (Supplementary Fig. 2C), which indicates the effect of SHIP-1 deficiency on the reduction of CD4+ and CD8+ T cells is a dominant effect. Altogether these results indicate that the effect of SHIP-1 deficiency on the altered homeostasis of Tregs is cell intrinsic.
Fig. 3.
Altered homeostasis and function of SHIP-1 KO Treg cells. A Flow cytometry analysis of CD4SP, CD8SP, and CD4⁺YFP-Foxp3⁺ Treg cells in the spleens of WT and SHIP-1 Treg KO mice. B Flow cytometry data of CD4SP, CD8SP, and CD4⁺YFP-Foxp3⁺ Treg cells from the peripheral lymph nodes of WT and SHIP-1 Treg KO mice (n = 6). C Percentage and absolute numbers of the cell populations identified in A, B (n = 6). D Expression analysis of GITR and PD-1 on CD4⁺YFP-Foxp3⁺ Treg cells in the spleens of WT and SHIP-1 Treg KO mice, with the fluorescence intensity ratios comparing SHIP-1 Treg KO to WT cells (n = 6). E, F CD4⁺CD62LhighCD44low Tnaive cells, sorted from WT mice and labeled with Cell Trace Violet, were co-cultured with CD4⁺CD25⁺ Treg cells from either WT or SHIP-1 Treg KO mice at ratios of 1:1 or 1:2, or without Treg cells. The proliferation of Tnaive cells was assessed via flow cytometry after 3 days (n = 4), with representative results drawn from three independent experiments. G Hematoxylin and eosin staining of the colon in recipient mice 10 weeks post-transfer of Tconv alone, Tconv with WT Treg cells, or Tconv with SHIP-1 KO Treg cells. Representative images from three mice are shown. Scale bars, 200 μm. H Histology scoring for experimental mice. I Recipient mice were evaluated for IFN-γ production via flow cytometry after 4 h of PMA and ionomycin stimulation. J Percentage analysis of cell populations highlighted in (I) (n = 3). K Tumor growth curve for WT and SHIP-1 Treg KO mice over a 30-day post-inoculation period. L Flow cytometric evaluation of CD4SP, CD8SP, and CD4⁺Foxp3⁺ Treg cells from tumor-infiltrated lymph nodes of WT and SHIP-1 Treg KO mice. M Flow cytometry analysis of IFN-γ expression in CD4SP and CD8SP cells from tumor-infiltrated lymph nodes in WT and SHIP-1 Treg KO mice. N Percentages of CD8⁺, CD4⁺Foxp3⁺, CD4⁺IFN-γ⁺, and CD8⁺IFN-γ⁺ cells in tumor-infiltrated lymph nodes from WT and SHIP-1 Treg KO mice (n = 3). Data were analyzed using one-way ANOVA (H, J) and are presented as the mean ± SEM. Statistical significance is indicated as follows: ∗ p < 0.05; ∗ ∗ p < 0.01; ∗ ∗ ∗ p < 0.001
In SHIP-1 Treg KO spleen and pLN, there was a selective increase of the Treg population marked by the expression of GITR and PD-1 (Fig. 3D) but not for other markers such as neuropilin, ICOS, CTLA4 and CD103 (Supplementary Fig. 3A). For functional studies, we sorted splenic Foxp3+ CD4SP T cells to perform an in vitro suppression assay. Proliferation of the target Foxp3− Tconv cells from wild-type mice was tested in the presence of WT or SHIP-1 deficient Foxp3+ CD4+ Treg cells. SHIP-1 KO Treg cells showed a reduced ability than WT Treg cells to inhibit Tconv cell proliferation (Fig. 3E, F). We examined the Brdu+ and caspase3+ of Treg cells alone, but no significant difference was observed in either WT or SHIP-1 Treg KO Foxp3+ cells (Supplementary Fig. 3B–D). We also examined the central Tregs (cTreg) and effector Tregs (eTreg) by using CD44 and CD62L staining, and we found that the percentage of cTreg and eTreg was not altered in the spleen of SHIP-1 Treg KO mice (Supplementary Fig. 3E, F).
We also detected the suppression function of Treg cells in vivo in colitis model, by transferring CD45.1+ Tconv and WT or SHIP-1 KO Treg cells into Rag1−/− mice, after 10 weeks, recipients of Tconv and WT Treg mice did not develop colitis, but recipients of Tconv and SHIP-1 KO Treg developed obvious colitis (Fig. 3G, H), and the IFN-γ produced by CD45.1+CD4+ T cells were significantly decreased in Tconv and WT Treg recipients compared with Tconv and SHIP-1 KO Treg recipients (Fig. 3I, J), these results suggest that the ability of SHIP1-KO Treg cells to suppress the secretion of IFN-γ by Tconv cells is weaker compared to WT Treg cells, indicating that SHIP-1 is essential for the suppressive function of Treg cells.
To determine whether SHIP-1 is required for Treg cells to inhibit anti-tumor immune responses in vivo, we inoculated SHIP-1 Treg KO mice with MC38 colon adenocarcinoma cells. Tumor growth was severely suppressed in SHIP-1 Treg KO mice (Fig. 3K), indicating that SHIP-1 KO Treg cells promotes an anti-tumor immune response. Being in line with this result, SHIP-1 Treg KO mice had a significantly increased percentage of tumor-infiltrating CD8+ T cells, but not CD4+ T cells (Fig. 3L, N) (Supplementary Fig. 4A). Additionally, there was an increase in expression of interferon-γ(IFN-γ) in effector CD4+and CD8+ T cells(Fig. 3M, N). However, SHIP-1 Treg KO mice had a profound loss of Treg cells in the tumor site, which could be due to the migration defect of SHIP-1 KO Tregs (Fig. 3L, N). To examine if SHIP-1 inhibitor can inhibit the tumor in vivo, WT mice were injected with SHIP-1 inhibitor for 14 consecutive days and then inoculated with MC38 colon without ceasing the inhibitor injection, while Vehicle-treated mice received a 200 μl injection 0.3% Klucel/H2O solution. Interestingly, we found that the percentage of splenic Tregs was enhanced but the absolute number of Tregs was decreased in WT mice treated with SHIP-1 inhibitor and tumor size was reduced compared to without treatment, although the percentage of CD4+ and CD8+ T cells was not altered (Supplementary Fig. 4B–D). These results identify a critical role of SHIP-1 in maintaining the homeostasis of Tregs and endowing Treg cells the ability to inhibit anti-tumor immune responses.
The loss of SHIP-1 in Tregs causes autoimmunity
The reduced suppressive function of Treg cells in SHIP-1 Treg KO mice made us speculate whether homeostasis of the immune system was altered in these mice. SHIP-1 Treg KO mice had reduced percentages and numbers of CD62LhighCD44low Tnaive cells, but increased activated CD62LlowCD44high effector and memory T cells in CD4+ and CD8+ T cells in spleen and pLN (Fig. 4A, B). We also observed an increase of CD62LlowCD44high effector and memory T cells in CD45.2 CD4+ and CD8+ T cells (Fig. 4C), suggesting the increase of memory T cells in SHIP-1 Treg KO mice was cell intrinsic. Furthermore, Tconv cells from SHIP-1 Treg KO mice were hyper-proliferative to TCR stimulation (Fig. 4D, E), suggesting a lower threshold for activation. Furthermore, we examined cytokine production associated with autoimmune disease [37]. SHIP-1 Treg KO CD4+ and CD8+ T cells produced more IFN-γ, IL-17, IL-2 and IL-4 (Fig. 4F–I). Interestingly, the more production of IFN-γ, IL-17 and IL-4 was also observed in CD45.2 CD4+ and CD8+ T cells (Supplementary Fig. 5A), showing the increase of inflammatory cytokine productions in SHIP-1 Treg KO mice was cell autonomous. Interestingly we found the production of IFN-γ, IL-17 and IL-4 was also increased in the CD45.1 “spike” CD4+ and CD8+ T cells of spleen in WT-SHIP-1 Treg KO chimera mice compared to that of WT-WT chimera mice (Supplementary Fig. 5B). This indicated that the deficiency of SHIP-1 in Tregs causing more cytokine productions is a dominant effect. Together, the deficiency of SHIP-1 in Treg cells leads to their spontaneous activation and differentiation and breakdown of immune tolerance.
Fig. 4.
The loss of SHIP-1 in Tregs induces autoimmunity. A Flow cytometry analysis of CD4⁺CD62LhighCD44low Tnaive cells and CD4⁺CD62LlowCD44high effector/memory T cells within the CD4⁺ and CD8⁺ T cell populations from the spleens and peripheral lymph nodes of WT and SHIP-1 Treg KO mice. B Quantitative data showing the percentages of cell populations identified in A (n = 6). C CD45.1 mice were exposed to sublethal irradiation (6 Gy) and subsequently received an intravenous injection of 1 × 10⁷ total bone marrow (BM) cells, comprising a 1:1 mix of SHIP-1 Treg KO BM (CD45.2⁺) and WT BM (CD45.1⁺). After 8 weeks, CD4⁺CD62LhighCD44low T naive cells and CD4⁺CD62LlowCD44high effector/memory T cells within the CD4⁺ and CD8⁺ T cell compartments in the spleens and peripheral lymph nodes of recipient CD45.1 mice were analyzed. D CD4⁺CD62LhighCD44low T naive cells, sorted from the spleens of WT and SHIP-1 Treg KO mice, were labeled with Cell Trace Violet and activated with anti-CD3 and anti-CD28 antibodies for 3 days. Naive T cell proliferation was assessed (n = 3), with representative data from three independent experiments. E Percentage of non-proliferating Tnaive cells from the assay in D. F Flow cytometric analysis of IL-4, IL-17, IL-2, and IFN-γ production in CD4⁺CD44⁺ T cells from the spleens of WT and SHIP-1 Treg KO mice, following 4-h stimulation with PMA and ionomycin. G Quantitative analysis of cell populations from F (n = 6). H Cytokine expression in CD8⁺CD44⁺ T cells from the spleens of WT and SHIP-1 Treg KO mice, assessed via flow cytometry after 4-h PMA and ionomycin stimulation. I Percentages of cell populations identified in H (n = 6). Data are reported as the mean ± SEM. Statistical significance is indicated as follows: ∗ p < 0.05; ∗ ∗ p < 0.01; ∗ ∗ ∗ p < 0.001
SHIP-1 signaling inhibits mTORC1 to decrease Treg differentiation and function
To investigate mechanisms mediating SHIP-1 function, we stimulated peripheral Tregs with CD3 and CD28 coated plates and determined the phosphorylation levels of mTORC1 and mTORC2 signaling molecules by flow cytometry. We found that the phosphorylation levels of PI3K, S6 and mTOR were significantly enhanced, but that of FOXO-1 and AKT(Ser 473) were not altered in SHIP-1 KO Tregs either in resting or activated state (Fig. 5A). These results suggest that SHIP-1 may affect the Tregs differentiation by inhibiting the mTORC1 signaling. To test this hypothesis, SHIP-1 Treg KO mice were injected with rapamycin by IP injection for 12 consecutive days and then euthanized for flow cytometry analysis. Interestingly, the frequency of CD4+Foxp3+T cells in SHIP-1 Treg KO mice treated with rapamycin was reduced to the degree of WT littermates. Furthermore, the percentages of naive or effector CD4+ and CD8+ T cells were also rescued. And the cytokine productions including IL-4, IL-17 and IFN-γ in CD44+ T cells of SHIP-1 Treg KO mice treated with rapamycin were rescued to the degree of WT mice (Fig. 5B). Furthermore, the suppressive function of SHIP-1 KO Tregs treated with rapamycin was elevated greatly compared to the vehicle treatment (Fig. 5C). Altogether these results imply that SHIP-1 regulates Treg development and differentiation via inhibiting the mTORC1 activity.
Fig. 5.
SHIP-1 signaling inhibits mTORC1 to decrease Treg differentiation and function. A Flow cytometry analysis of phosphorylated signaling proteins—p-S6, p-MTOR, p-FOXO1, p-AKT, and p-PI3K—in CD4⁺Foxp3⁺ Treg cells from WT and SHIP-1 Treg KO mice, both with and without 4-h stimulation using anti-CD3 and anti-CD28. Representative data, including mean fluorescence intensity (MFI), were obtained from three independent experiments (n = 3). B SHIP-1 Treg KO mice received intraperitoneal injections of rapamycin daily for 14 days. Subsequent analysis included the expression of CD4⁺YFP-Foxp3⁺ in splenocytes, proportions of CD4⁺CD62LhighCD44low Tnaive cells and CD4⁺CD62LlowCD44high effector/memory T cells in CD4⁺ and CD8⁺ splenocytes, as well as levels of IL-4, IL-17, and IFN-γ in CD4⁺CD44⁺ and CD8⁺CD44⁺ T cells following 4-h stimulation with PMA and ionomycin (n = 5). Data were analyzed using one-way ANOVA and are presented as the mean ± SEM. Statistical significance: ∗ p < 0.05; ∗ ∗ p < 0.01; ∗ ∗ ∗ p < 0.001. C CD4⁺CD25⁺ Treg cells were isolated from untreated WT and SHIP-1 Treg KO mice, as well as from SHIP-1 Treg KO mice treated with rapamycin for 1 h. These cells were then co-cultured with CD4⁺CD62LhighCD44low Tnaive cells from WT mice at a 1:2 ratio. The proliferation of naive T cells was measured by flow cytometry after 3 days (n = 3), with representative results drawn from three independent experiments.
Discussion
Phosphatase activity is generally thought to negatively regulate TCR signaling in the resting or stimulated state. But the role of phosphatase activity is not completely understood in the function of Tregs. Previous research has shown the deletion of PTEN specifically in Tregs leads to increased Tfh and GC responses as well as inflammatory disease [38]. These abnormalities can be rescued by knock-out of IFN-γ. Mechanistically, PTEN is critical to maintain Treg stability and metabolic balance between glycolysis and mitochondrial fitness. Moreover, the loss of PTEN enhances mTORC2-AKT activity, and the deficiency of this activity restores PTEN-deficient Treg function. The activity of PI3K is downregulated by two lipid phosphatases: PTEN and SHIP-1. Both hydrolyze PIP3, generating the lipid products, PI4,5P2 and PI3,4P2, respectively. Plasma membrane recruitment of SHIP-1 expressed hematopoietically requires binding of its SH2 domain to proteins with specific phosphotyrosine motifs [39]. PTEN, which is expressed ubiquitously and highly active, regulates basal and induced PIP3 levels via dynamic interactions with the plasma membrane [40]. It is unknown whether these two phosphatases regulate the Treg differentiation and function in different ways. In Ptenfl/flFoxp3Cre mice, there was a noticeable increase in both the proportion and absolute number of Foxp3⁺ Treg cells in the spleen and lymph nodes, accompanied by the spontaneous development of Tfh cells and germinal centers (GCs). These Treg cells exhibited elevated levels of ICOS, PD-1, and GITR, but showed a marked decrease in CD25 expression. Furthermore, Ptenfl/flFoxp3Cre mice demonstrated higher levels of IFN-γ, while the production of IL-17 and IL-4 remained unaltered. This indicated a spontaneous tendency of T cells in these mice to differentiate into a TH1 phenotype, alongside heightened mTORC2 signaling activity within Treg cells [38]. Similarly, in SHIP-1 Treg KO mice, there was an increased percentage of Foxp3⁺ Treg cells in the spleen and lymph nodes, though a decline was noted in Treg numbers within peripheral lymph nodes. These mice also exhibited a lower percentage of CD4⁺ cells and an elevated presence of memory/effector T cells (CD44highCD62Llow). Additionally, there was an increase in the proportion of CD4⁺ and CD8⁺ T cells producing IFN-γ, IL-4, IL-2, and IL-17, a pattern that closely resembles the characteristics observed in scurfy mice [41]. However, the regulation pathway is different in these two mouse models. In Ptenfl/flFoxp3Cre Treg cells, mTORC2 activity was upregulated but mTORC1 activity was upregulated in SHIP-1 KO Treg cells. Therefore, we have established another different regulation pathway by SHIP-1 via mTORC1 compared to that of PTEN through mTORC2. To further confirm that SHIP-1 regulates the Tregs via mTORC1, it is necessary to cross Foxp3YFP−cre Ship-1fl/fl mice with Raptorfl/fl mice for confirmation on a genetic level. Additionally, the production of cytokines was elevated in SHIP-1 Treg KO mice unlike the Th1 cytokines in Ptenfl/flFoxp3Cre mice.
The role of SHIP-1 in the development and function of Tregs remains controversial. By using the germline deletion mice, SHIP-1−/− mice also have an increase in the proportion, but not the absolute number of thymic Foxp3+ Tregs (Supplementary Table 1). Moreover, T cell-specific deletion of SHIP-1 with CD4-Cre does not result in an increase in the number of splenic Foxp3+ cells [24]. In SHIP-1−/− mice, the percentage and number of thymic and splenic Foxp3+ Tregs were both increased. Wild-type and SHIP-1−/− Tregs suppress Teff cell proliferation in the same degree in vitro [25]. These results are in line with previous findings that SHIP-1 deficient Tregs retain their suppressive function intact [21, 42]. Furthermore, SHIP-1 deficient Tregs in CD4creSHIP-1fl/fl mice suppress the adoptive transfer of colitis [25]. These results indicate that the suppressive function of SHIP-1 deficient Tregs in germline deletion mice or CD4creSHIP-1fl/fl mice is intact. But in our mouse model, the suppressive function of SHIP-1 deficient Tregs has a defect both in vitro and in vivo. It is noteworthy that there is a decrease in total splenic T cells when SHIP is only deleted in FoxP3+ cells (Fig. 3A–C). Although SHIP is specifically deleted in FoxP3+ regulatory T cells (Tregs), the dysregulation of these cells can indirectly affect the homeostasis of the broader T cell population (Fig. 4A, B). We also observed that the lack of SHIP in Tregs leads to changes in the cytokine environment or affects interactions between T cells (Fig. 4F–I), potentially impacting the overall number of CD4 + T cells. Additionally, compensatory regulatory mechanisms might come into play in response to the disrupted Treg function, influencing the general T cell milieu. Considering the deletion specificity and the impact of the environment, our model has explained the effect of SHIP-1 deficiency on the differentiation and function of Tregs.
Another significant finding is why Foxp3-specific SHIP-1 deletion causes cells to behave differently than CD4-specific deletion. This may be due to several reasons. Firstly, the differential effects of Foxp3-specific and CD4-specific SHIP-1 deletion are probably due to the different roles of both proteins play in T cell biology. The transcription factor Foxp3 plays a central role in regulatory T cells (Tregs) biology, development, and function [43]. Deletion of SHIP-1 in Foxp3+ cells might modify the regulatory pathways required for Treg maintenance and/or function that would lead to an alteration of immune homeostasis and responses. In addition, CD4 is more widely expressed on T cells and participates in the transduction of activation and differentiation signals in these cells [44]. Deletion of SHIP-1 specifically in CD4+ cells may have different effects on these processes, potentially affecting the T cell activation or differentiation pathways in a pattern distinct from what is seen in Tregs. What’s more, it is important to consider that CD4 is expressed on a subset of cells within the myeloid lineage including monocytes and dendritic cell (DC) types where some CD4-Cre recombination may take place. The involvement of macrophages and DCs, which play significant roles in immune responses and pathology, could also contribute to the observed differences. Third, this may be a consequence of differences in the signaling pathways and transcriptional program of Foxp3+ Tregs compared to CD4+ T cells. The outcome of SHIP-1 deletion on PI3K/Akt signaling may vary depending on the cellular context and downstream pathways involved, suggesting that SHIP-1 modulates PI3K/Akt-signaling. In Tregs, this could result in a loss of suppressive function or stability, whereas in CD4+ T cells, it would lead to altered activation or differentiation. Further experimental work, possibly involving transcriptomic and proteomic analyses, could shed more light on the specific mechanisms and pathways altered by SHIP-1 deletion in these different T cell subsets. These studies would go far in understanding the strong interplay between SHIP-1 signaling and T cell functionality.
The mTORC activity is correlated with the function of Tregs. mTORC1 signaling is essential for Treg cell function in mice and enhanced in Treg cells compared to Tnaive cells. Treg-specific deletion of the signature component of mTORC1-raptor leads to a significantly reduced suppressive activity in vivo and inflammatory disorders [45]. Our results have also shown that Tregs with higher mTORC1 activity also leads to the diminished suppressive function of Tregs and inflammatory disease. T cells lacking mTORC differentiate into Foxp3+ regulatory cells. However, T cells with mTORC1 deletion do not divert to a regulatory T cell pathway, indicating mTORC2 signaling in preventing the generation of regulatory T cells. Altogether these suggest that mTORC1 and mTORC2 signaling regulate decisions between effector and Treg cells [46]. Foxp3-Cre Raptor KO mice have abolished mTORC1 activity but enhanced mTORC2 activity. However, SHIP-1 Treg KO mice have increased mTORC1 activity, but normal mTORC2 activity. S1P1 transgenic mice have increased mTORC1 activity that promotes the accumulation of Treg precursors-CD25+Foxp3− populations, which is in line with the results of CD4CreSHIP-1 flox/flox mice [28]. Our findings further enrich the understanding of Treg cell function and offer new insights for diseases associated with abnormal Treg function [47].
In conclusion, our study has established the intrinsic role of SHIP-1 on the development, differentiation and function in Tregs, which is different from what has been previously reported, especially that SHIP-1 is the pivotal positive determinant of Treg-cell function in mice. Furthermore, we have revealed the underlying regulatory molecular mechanism of the SHIP-1-PI3K-mTORC1 axis, which is essential for the differentiation and function of Tregs. More excitingly, the fact that SHIP-1 inhibition increases anti-tumor activity can facilitate the advancement of cancer therapeutic treatment.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Thanks for the critical reading of the manuscript by Dr.Xiaoping Zhong from Duke University and Hao Shi from St. Jude Children’s Research Hospital.
Abbreviations
- Tregs
Regulatory T cells
- SHIP-1
Src homology 2 (SH2) domain containing inositol polyphosphate 5-phosphatase1
- INPP5
Inositol polyphosphate-5-phosphatase
- AKT
AKT serine/threonine kinase
- APC
Allophycocyanin
- BM
Bone marrow
- FITC
Fluorescein isothiocyanate
- GCs
Germinal centers
- PTEN
Phosphatase and tensin homologue
- PI3K
Phosphatidylinositol-4,5-bisphosphate 3-kinase
- Tconv
Conventional T cells
- Foxp3
Forkhead box protein P3
- mTOR
Mammalian target of rapamycin
- WT
Wild type
- KO
Knocked out
- PLN
Peripheral lymph node
- MLN
Mesenteric lymph node
- PMA
Phorbol myristate acetate
Author contributions
Z.D. carried out the initial analyses and drafted the initial manuscript. Z.D.,D.Y.,L.H.,Q.L. performed the flow cytometry assay, J.W., X.S performed the chimera and histopathology analysis, Z.D. performed MC38 tumor model and immunofluorescence, P.H., L.Y.,X.D., L.W.,Y.A. and X.T reviewed and revised the manuscript. C.L. conceptualized and designed the study, reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding
This study was supported by the National Natural Science Foundation of China (32100710 and 32360184), China Postdoctoral Science Foundation (2024MD754045) and Guizhou Provincial Science and Technology Projects (QKHCG[2024]ZD012, QKHZK-2022-YB641).
Data availability
All data generated and analyzed during the current study are included in this published article and its additional files.
Declarations
Conflict of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval and consent to participate
All animal experiments were performed according to protocols approved by the Institutional Animal Care and Usage Committee of Children’s Hospital of Chongqing Medical University.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zuochen Du, Jinzhi Wang and Qian Liu Dhas been contributed equally to this work.
References
- 1.Siska PJ, Rathmell JC (2015) T cell metabolic fitness in antitumor immunity. Trends Immunol 36(4):257–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Myers DR, Wheeler B, Roose JP (2019) mTOR and other effector kinase signals that impact T cell function and activity. Immunol Rev 291(1):134–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei J, Zheng W, Chapman NM, Geiger TL, Chi H (2021) T cell metabolism in homeostasis and cancer immunity. Curr Opin Biotechnol 68:240–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bulygin AS, Khantakova JN, Shkaruba NS, Shiku H, Sennikov SS (2022) The role of metabolism on regulatory T cell development and its impact in tumor and transplantation immunity. Front Immunol 13:1016670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu C, Chapman NM, Karmaus PW, Zeng H, Chi H (2015) mTOR and metabolic regulation of conventional and regulatory T cells. J Leucoc Biol 97(5):837–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma S, Ming Y, Wu J, Cui G (2024) Cellular metabolism regulates the differentiation and function of T-cell subsets. Cell Mol Immunol 21:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L, Ruan G, Cheng Y, Yi A, Chen D, Wei Y (2023) The role of Th17 cells in inflammatory bowel disease and the research progress. Front Immunol 13:1055914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q, Zheng S, Niu K, Qiao Y, Liu Y, Zhang Y et al (2024) Paeoniflorin improves ulcerative colitis via regulation of PI3K-AKT based on network pharmacology analysis. Exp Ther Med 27(4):1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damen JE, Liu L, Rosten P, Humphries RK, Jefferson AB, Majerus PW et al (1996) The 145-kDa protein induced to associate with Shc by multiple cytokines is an inositol tetraphosphate and phosphatidylinositol 3,4,5-triphosphate 5-phosphatase. Proc Natl Acad Sci USA 93(4):1689–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ware MD, Rosten P, Damen JE, Liu L, Humphries RK, Krystal G (1996) Cloning and characterization of human SHIP, the 145-kD inositol 5-phosphatase that associates with SHC after cytokine stimulation. Blood 88(8):2833–2840 [PubMed] [Google Scholar]
- 11.Carver DJ, Aman MJ, Ravichandran KS (2000) SHIP inhibits Akt activation in B cells through regulation of Akt membrane localization. Blood 96(4):1449–1456 [PubMed] [Google Scholar]
- 12.Aman MJ, Lamkin TD, Okada H, Kurosaki T, Ravichandran KS (1998) The inositol phosphatase SHIP inhibits Akt/PKB activation in B cells. J Biol Chem 273(51):33922–33928 [DOI] [PubMed] [Google Scholar]
- 13.Bolland S, Pearse RN, Kurosaki T, Ravetch JV (1998) SHIP modulates immune receptor responses by regulating membrane association of Btk. Immunity 8(4):509–516 [DOI] [PubMed] [Google Scholar]
- 14.Chacko GW, Tridandapani S, Damen JE, Liu L, Krystal G, Coggeshall KM (1996) Negative signaling in B lymphocytes induces tyrosine phosphorylation of the 145-kDa inositol polyphosphate 5-phosphatase. SHIP J Immunol 157(6):2234–2238 [PubMed] [Google Scholar]
- 15.Liu Q, Oliveira-Dos-Santos AJ, Mariathasan S, Bouchard D, Jones J, Sarao R et al (1998) The inositol polyphosphate 5-phosphatase ship is a crucial negative regulator of B cell antigen receptor signaling. J Exp Med 188(7):1333–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui B, Chen L, Zhang S, Mraz M, Fecteau JF, Yu J et al (2014) MicroRNA-155 influences B-cell receptor signaling and associates with aggressive disease in chronic lymphocytic leukemia. Blood 124(4):546–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ono M, Bolland S, Tempst P, Ravetch JV (1996) Role of the inositol phosphatase SHIP in negative regulation of the immune system by the receptor Fc(gamma)RIIB. Nature 383(6597):263–266 [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Hu F, Dong X, Zhao M, Wang J, Sun X et al (2017) SHIP-1 deficiency in AID(+) B cells leads to the impaired function of B10 cells with spontaneous autoimmunity. J Immunol 199(9):3063–3073 [DOI] [PubMed] [Google Scholar]
- 19.Waterman PM, Marschner S, Brandl E, Cambier JC (2012) The inositol 5-phosphatase SHIP-1 and adaptors Dok-1 and 2 play central roles in CD4-mediated inhibitory signaling. Immunol Lett 143(1):122–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris SJ, Parry RV, Foster JG, Blunt MD, Wang A, Marelli-Berg F et al (2011) Evidence that the lipid phosphatase SHIP-1 regulates T lymphocyte morphology and motility. J Immunol 186(8):4936–4945 [DOI] [PubMed] [Google Scholar]
- 21.Kashiwada M, Cattoretti G, McKeag L, Rouse T, Showalter BM, Al-Alem U et al (2006) Downstream of tyrosine kinases-1 and Src homology 2-containing inositol 5′-phosphatase are required for regulation of CD4+CD25+ T cell development. J Immunol 176(7):3958–3965 [DOI] [PubMed] [Google Scholar]
- 22.Edmunds C, Parry RV, Burgess SJ, Reaves B, Ward SG (1999) CD28 stimulates tyrosine phosphorylation, cellular redistribution and catalytic activity of the inositol lipid 5-phosphatase SHIP. Eur J Immunol 29(11):3507–3515 [DOI] [PubMed] [Google Scholar]
- 23.Dong S, Corre B, Foulon E, Dufour E, Veillette A, Acuto O et al (2006) T cell receptor for antigen induces linker for activation of T cell-dependent activation of a negative signaling complex involving Dok-2, SHIP-1, and Grb-2. J Exp Med 203(11):2509–2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tarasenko T, Kole HK, Chi AW, Mentink-Kane MM, Wynn TA, Bolland S (2007) T cell-specific deletion of the inositol phosphatase SHIP reveals its role in regulating Th1/Th2 and cytotoxic responses. Proc Natl Acad Sci USA 104(27):11382–11387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Locke NR, Patterson SJ, Hamilton MJ, Sly LM, Krystal G, Levings MK (2009) SHIP regulates the reciprocal development of T regulatory and Th17 cells. J Immunol 183(2):975–983 [DOI] [PubMed] [Google Scholar]
- 26.Du Z, Huang L, Dai X, Yang D, Niu L, Miller H et al (2022) Progranulin regulates the development and function of NKT2 cells through EZH2 and PLZF. Cell Death Differ 29(10):1901–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma S, Orlowski G, Song W (2009) Btk regulates B cell receptor-mediated antigen processing and presentation by controlling actin cytoskeleton dynamics in B cells. J Immunol 182(1):329–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu G, Burns S, Huang G, Boyd K, Proia RL, Flavell RA et al (2009) The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat Immunol 10(7):769–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei J, Long L, Yang K, Guy C, Shrestha S, Chen Z et al (2016) Autophagy enforces functional integrity of regulatory T cells by coupling environmental cues and metabolic homeostasis. Nat Immunol 17(3):277–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi H, Liu C, Tan H, Li Y, Nguyen TM, Dhungana Y et al (2018) Hippo kinases Mst1 and Mst2 sense and amplify IL-2R-STAT5 signaling in regulatory T cells to establish stable regulatory activity. Immunity 49(5):899-914.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang K, Neale G, Green DR, He W, Chi H (2011) The tumor suppressor Tsc1 enforces quiescence of naive T cells to promote immune homeostasis and function. Nat Immunol 12(9):888–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lio CW, Hsieh CS (2008) A two-step process for thymic regulatory T cell development. Immunity 28(1):100–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burchill MA, Yang J, Vang KB, Moon JJ, Chu HH, Lio CW et al (2008) Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity 28(1):112–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng SG, Gray JD, Ohtsuka K, Yamagiwa S, Horwitz DA (2002) Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25− precursors. J Immunol 169(8):4183–9 [DOI] [PubMed] [Google Scholar]
- 35.Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA (2004) Natural and induced CD4+CD25+ cells educate CD4+CD25- cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J Immunol 172(9):5213–21 [DOI] [PubMed] [Google Scholar]
- 36.Arterbery AS, Osafo-Addo A, Avitzur Y, Ciarleglio M, Deng Y, Lobritto SJ et al (2016) Production of proinflammatory cytokines by monocytes in liver-transplanted recipients with de novo autoimmune hepatitis is enhanced and induces TH1-like regulatory T cells. J Immunol 196(10):4040–4051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Theofilopoulos AN, Koundouris S, Kono DH, Lawson BR (2001) The role of IFN-gamma in systemic lupus erythematosus: a challenge to the Th1/Th2 paradigm in autoimmunity. Arthritis Res 3(3):136–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shrestha S, Yang K, Guy C, Vogel P, Neale G, Chi H (2015) Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat Immunol 16(2):178–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sattler M, Verma S, Pride YB, Salgia R, Rohrschneider LR, Griffin JD (2001) SHIP1, an SH2 domain containing polyinositol-5-phosphatase, regulates migration through two critical tyrosine residues and forms a novel signaling complex with DOK1 and CRKL. J Biol Chem 276(4):2451–2458 [DOI] [PubMed] [Google Scholar]
- 40.Vazquez F, Devreotes P (2006) Regulation of PTEN function as a PIP3 gatekeeper through membrane interaction. Cell Cycle 5(14):1523–1527 [DOI] [PubMed] [Google Scholar]
- 41.Kanangat S, Blair P, Reddy R, Daheshia M, Godfrey V, Rouse BT et al (1996) Disease in the scurfy (sf) mouse is associated with overexpression of cytokine genes. Eur J Immunol 26(1):161–165 [DOI] [PubMed] [Google Scholar]
- 42.Collazo MM, Wood D, Paraiso KH, Lund E, Engelman RW, Le CT et al (2009) SHIP limits immunoregulatory capacity in the T-cell compartment. Blood 113(13):2934–2944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ono M (2020) Control of regulatory T-cell differentiation and function by T-cell receptor signalling and Foxp3 transcription factor complexes. Immunology 160(1):24–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hwang J-R, Byeon Y, Kim D, Park S-G (2020) Recent insights of T cell receptor-mediated signaling pathways for T cell activation and development. Exp Mol Med 52(5):750–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeng H, Yang K, Cloer C, Neale G, Vogel P, Chi H (2013) mTORC1 couples immune signals and metabolic programming to establish T(reg)-cell function. Nature 499(7459):485–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B et al (2009) The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 30(6):832–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang L, Jing Y, Kang D, Jiang P, Li N, Zhou X et al (2021) Ubiquitin-specific peptidase 18 regulates the differentiation and function of Treg cells. Genes Dis 8(3):344–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated and analyzed during the current study are included in this published article and its additional files.